Updates in Contemporary Management of Singleton Pregnancies Complicated by a Short Cervix
Abstract
1. Introduction
2. Vaginal Progesterone
3. Cervical Cerclage
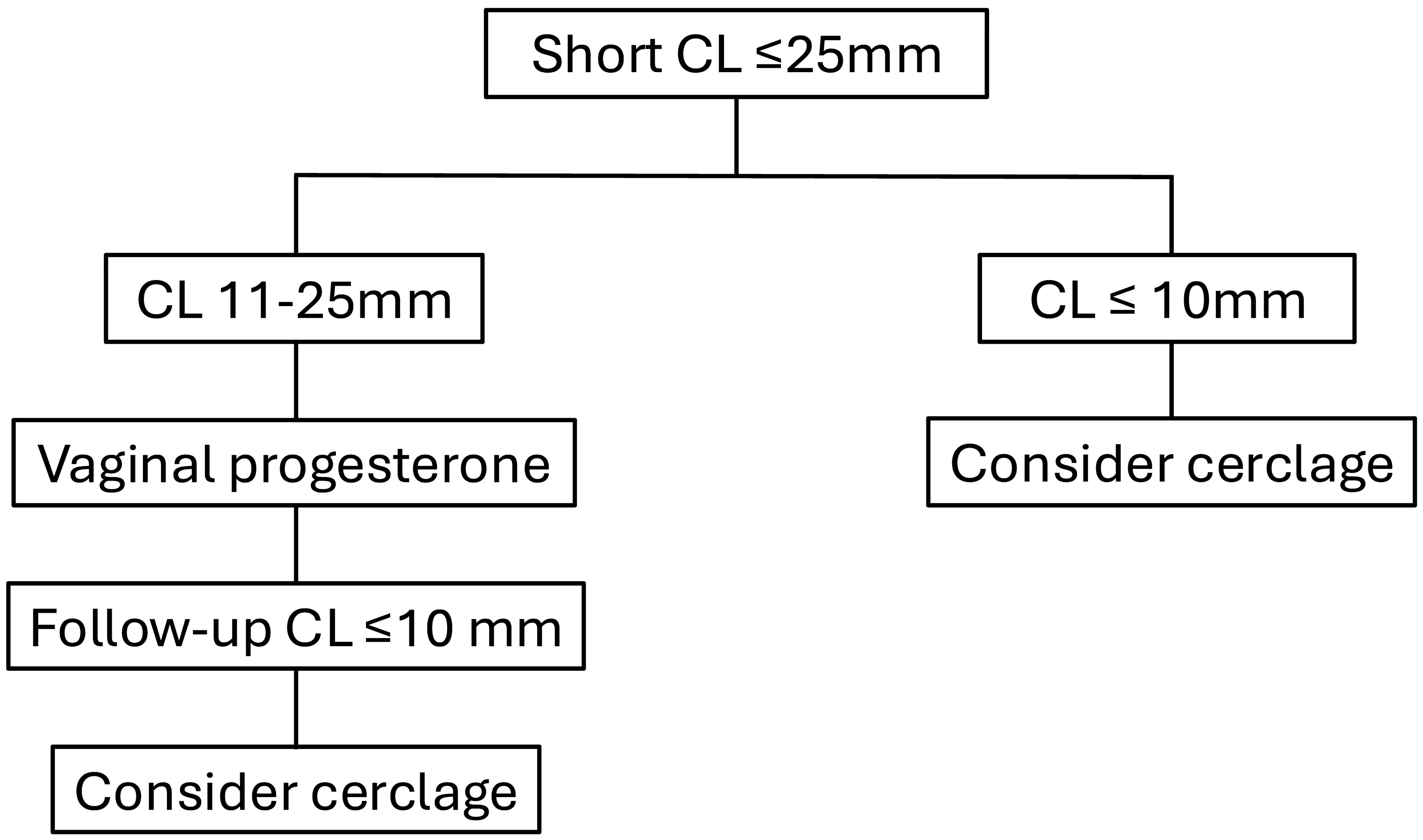
4. Short Cervix and Prior Preterm Birth
5. Short Cervix and No Prior Spontaneous Preterm Birth
6. Cervical Pessary
7. Future Research
8. Conclusions
Funding
Conflicts of Interest
References
- Liang, X.; Lyu, Y.; Li, J.; Li, Y.; Chi, C. Global, regional, and national burden of preterm birth, 1990-2021: A systematic analysis from the global burden of disease study 2021. EClinicalMedicine 2024, 76, 102840. [Google Scholar] [CrossRef]
- Iams, J.D.; Goldenberg, R.L.; Meis, P.J.; Mercer, B.M.; Moawad, A.; Das, A.; Thom, E.; McNellis, D.; Copper, R.L.; Johnson, F.; et al. The length of the cervix and risk of spontaneous premature delivery. N. Engl. J. Med. 1996, 334, 567–572. [Google Scholar] [CrossRef]
- Hoffman, M.K. Prediction and prevention of spontaneous preterm birth. ACOG Practice Bulletin No. 234. American College of Obstetricians and Gynecologists. Obstet. Gynecol. 2021, 138, e65–e90. [Google Scholar] [CrossRef]
- Hessami, K.; D’Alberti, E.; Mascio, D.D.; Berghella, V. Universal cervical length screening and risk of spontaneous preterm birth: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2024, 6, 101343. [Google Scholar] [CrossRef] [PubMed]
- Society for Maternal-Fetal Medicine (SMFM); Biggio, J. SMFM Consult Series #70: Management of short cervix in individuals without a history of spontaneous preterm birth. Am. J. Obstet. Gynecol. 2024, 231, B2–B13. [Google Scholar] [CrossRef]
- Khalifeh, A.; Berghella, V. Not transabdominal! Am. J. Obstet. Gynecol. 2016, 215, 739–744.e1. [Google Scholar] [CrossRef]
- Orzechowski, K.M.; Boelig, R.C.; Berghella, V. Cervical length screening in asymptomatic women at high risk and low risk for spontaneous preterm birth. Clin. Obstet. Gynecol. 2016, 59, 241–251. [Google Scholar] [CrossRef]
- Society for Maternal-Fetal Medicine (SMFM); McIntosh, J.; Feltovich, H.; Berghella, V.; Manuck, T. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am. J. Obstet. Gynecol. 2016, 215, B2–B7. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.M.; Hutchens, D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: A systematic review. Ultrasound Obstet. Gynecol. 2008, 31, 579–587. [Google Scholar] [CrossRef]
- Esplin, M.S.; Elovitz, M.A.; Iams, J.D.; Parker, C.B.; Wapner, R.J.; Grobman, W.A.; Simhan, H.N.; Wing, D.A.; Haas, D.M.; Silver, R.M.; et al. Predictive accuracy of serial transvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA 2017, 317, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Berghella, V.; Roman, A.; Daskalakis, C.; Ness, A.; Baxter, J.K. Gestational age at cervical length measurement and incidence of preterm birth. Obstet. Gynecol. 2007, 110, 311–317. [Google Scholar] [CrossRef]
- Patberg, E.T.; Wells, M.; Vahanian, S.A.; Zavala, J.; Bhattacharya, S.; Richmond, D.; Akerman, M.; Demishev, M.; Kinzler, W.L.; Chavez, M.R.; et al. Use of cervical elastography at 18 to 22 weeks’ gestation in the prediction of spontaneous preterm birth. Am. J. Obstet. Gynecol. 2021, 225, e1–e525. [Google Scholar] [CrossRef] [PubMed]
- Hrubaru, I.; Motoc, A.; Moise, M.L.; Miutescu, B.; Citu, I.M.; Pingilati, R.A.; Popescu, D.-E.; Dumitru, C.; Gorun, F.; Olaru, F.; et al. The predictive role of maternal biological markers and inflammatory scores NLR, PLR, MLR, SII, and SIRI for the risk of preterm delivery. J. Clin. Med. 2022, 11, 6982. [Google Scholar] [CrossRef] [PubMed]
- Giles, M.L.; Krishnaswamy, S.; Metlapalli, M.; Roman, A.; Jin, W.; Li, W.; Mol, B.W.; Sheehan, P.; Said, J. Azithromycin treatment for short cervix with or without amniotic fluid sludge: A matched cohort study. Aust. N. Z. J. Obstet. Gynaecol. 2023, 63, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Conde-Agudelo, A.; Da Fonseca, E.; O’bRien, J.M.; Cetingoz, E.; Creasy, G.W.; Hassan, S.S.; Nicolaides, K.H. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: A meta-analysis of individual patient data. Am. J. Obstet. Gynecol. 2018, 218, 161–180. [Google Scholar] [CrossRef]
- EPPPIC Group. Evaluating progestogens for preventing preterm birth international collaborative (EPPPIC): Meta-analysis of individual participant data from randomized controlled trials. Lancet 2021, 397, 1183–1194. [Google Scholar] [CrossRef]
- Csapo, A.I.; Pulkkinen, M.O.; Ruttner, B.; Sauvage, J.P.; Weist, W.G. The significance of the human corpus luteum in pregnancy maintenance: I. Preliminary studies. Am. J. Obstet. Gynecol. 1972, 112, 1061–1067. [Google Scholar] [CrossRef]
- Romero, R. Prevention of spontaneous preterm birth: The role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound. Obstet. Gynecol. 2007, 30, 675–686. [Google Scholar] [CrossRef]
- Romero, R.; Yeo, L.; Chaemsaithong, P.; Chaiworapongsa, T.; Hassan, S.S. Progesterone to prevent spontaneous preterm birth. Semin. Fetal Neonatal. Med. 2014, 19, 15–26. [Google Scholar] [CrossRef]
- Norman, J.E.; Marlow, N.; McConnachie, A.; Petrou, S.; Sebire, N.J.; Lavender, T.; Whyte, S.; Norrie, J.; Messow, C.-M.; Shennan, A.; et al. Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): A multicentre, randomised, double-blind trial. Lancet 2016, 387, 2106–2116. [Google Scholar] [CrossRef]
- Romero, R.; Conde-Agudelo, A.; da Fonseca, E.; O’Brien, J.M.; Creasy, G.W.; Hassan, S.S.; Nicolaides, K.H. Vaginal progesterone reduces the risk of preterm birth and adverse perinatal outcomes in singleton gestations with a midtrimester sonographic short cervix (25 mm): An updated individual patient data meta-analysis. Am. J. Obstet. Gynecol. 2025, 223, e1–e5. [Google Scholar] [CrossRef]
- O’Brien, J.M.; Steichen, J.J.; Phillips, J.A.; Creasy, G.W. Two year infant outcomes for children exposed to supplemental intravaginal progesterone gel in utero: Secondary analysis of a multicenter, randomized, double-blind, placebo-controlled trial. Am. J. Obstet. Gynecol. 2012, 206, S223. [Google Scholar] [CrossRef]
- Jain, V.; McDonald, S.D.; Mundle, W.R.; Farine, D. Guideline No. 398: Progesterone for prevention of spontaneous preterm birth. J. Obstet. Gynecol. Can. 2020, 42, 806–812. [Google Scholar] [CrossRef]
- Shennan, A.; Suff, N.; Simpson, J.L.; Jacobsson, B.; Mol, B.W.; Grobman, W.A.; The FIGO Working Group for Preterm Birth. FIGO good practice recommendations on progestogens for prevention of preterm delivery. Int. J. Gynaecol. Obstet. 2021, 155, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Shirodkar, V.N. Surgical treatment of female fertility. J. Indian Med. Assoc. 1957, 29, 56–57. [Google Scholar] [PubMed]
- McDonald, I.A. Suture of the cervix for inevitable miscarriage. J. Obstet. Gynaecol. Br. Emp. 1957, 64, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Espinoza, J.; Erez, O.; Hassan, S. The role of cervical cerclage in obstetric practice: Can the patient who could benefit from this procedure be identified? Am. J. Obstet. Gynecol. 2006, 194, 1–9. [Google Scholar] [CrossRef]
- House, M.; Socrate, S. The cervix as a biomechanical structure. Ultrasound Obstet. Gynecol. 2006, 28, 745–749. [Google Scholar] [CrossRef]
- Berghella, V.; Rafael, T.J.; Szychowski, J.M.; Rust, O.A.; Owen, J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: A meta-analysis. Obstet. Gynecol. 2011, 117, 663–671. [Google Scholar] [CrossRef]
- ACOG Practice Bulletin No. 142: Cerclage for the management of cervical insufficiency. Obstet. Gynecol. 2014, 123, 372–379. [CrossRef] [PubMed]
- Berghella, V.; Ciardulli, A.; Rust, O.A.; To, M.; Otsuki, K.; Althuisius, S.; Nicolaides, K.H.; Roman, A.; Saccone, G. Cerclage for sonographic short cervix in singleton gestations without prior spontaneous preterm birth: Systematic review and meta-analysis of randomized controlled trials using individual patient-level data. Ultrasound Obstet. Gynecol. 2017, 50, 569–577. [Google Scholar] [CrossRef]
- Enakpene, C.A.; DiGiovanni, L.; Jones, T.N.; Marshalla, M.; Mastrogiannis, D.; Della Torre, M. Cervical cerclage for singleton pregnant patients on vaginal progesterone with progressive cervical shortening. Am. J. Obstet. Gynecol. 2018, 219, e1–e397. [Google Scholar] [CrossRef]
- Gulersen, M.; Bornstein, E.; Domney, A.; Blitz, M.J.; Rafael, T.J.; Li, X.; Krantz, D.; Rochelson, B. Cerclage in singleton gestations with an extremely short cervix (≤10 mm) and no history of spontaneous preterm birth. Am. J. Obstet. Gynecol. MFM 2021, 3, 100430. [Google Scholar] [CrossRef] [PubMed]
- Boelig, R.C.; Tersigni, C.; Di Simone, N.; Saccone, G.; Facchinetti, F.; Scambia, G.; Berghella, V. Cerclage in singleton pregnancies with no prior spontaneous preterm birth and short cervix: A randomized controlled trial. Am. J. Obstet. Gynecol. MFM 2025, 7, 101602. [Google Scholar] [CrossRef]
- Arabin, B.; Alfirevic, Z. Cervical pessaries for prevention of spontaneous preterm birth: Past, present, and future. Ultrasound Obstet. Gynecol. 2013, 42, 390–399. [Google Scholar] [CrossRef]
- Goya, M.; Pratcorona, L.; Merced, C.; Rodó, C.; Valle, L.; Romero, A.; Juan, M.; Rodríguez, A.; Muñoz, B.; Santacruz, B.; et al. Cervical pessary in pregnant women with a short cervix (PECEP): An open-label randomised controlled trial. Lancet 2012, 379, 1800–1806. [Google Scholar] [CrossRef]
- Nicolaides, K.H.; Syngelaki, A.; Poon, L.C.; Picciarelli, G.; Tul, N.; Zamprakou, A.; Skyfta, E.; Parra-Cordero, M.; Palma-Dias, R.; Calvo, J.R. A randomized trial of a cervical pessary to prevent preterm singleton birth. N. Engl. J. Med. 2016, 374, 1044–1052. [Google Scholar] [CrossRef]
- Saccone, G.; Maruotti, G.M.; Giudicepietro, A.; Martinelli, P.; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: A randomized clinical trial. JAMA 2017, 318, 2317–2324. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Romero, R.; Nicolaides, K.H. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2020, 223, 42–65.e2. [Google Scholar] [CrossRef]
- Hoffman, M.K.; Clifton, R.G.; Biggio, J.R.; Saade, G.R.; Ugwu, L.G.; Longo, M.; Bousleiman, S.Z.; Clark, K.; Grobman, W.A.; Frey, H.A.; et al. Cervical pessary for prevention of preterm birth in individuals with a short cervix: The TOPS randomized clinical trial. JAMA 2023, 330, 340–348. [Google Scholar] [CrossRef]
- Gulersen, M.; Divon, M.Y.; Krantz, D.; Chervenak, F.A.; Bornstein, E. The risk of spontaneous preterm birth in asymptomatic women with a short cervix (≤ 25mm) at 23-28 weeks’ gestation. Am. J. Obstet. Gynecol. MFM 2020, 2, 100059. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet. Gynecol. 2017, 130, e187–e199. [Google Scholar] [CrossRef] [PubMed]
- Koops, B.L.; Morgan, L.J.; Battaglia, F.C. Neonatal mortality risk in relation to birth weight and gestational age: Update. J. Pediatr. 1982, 101, 969–977. [Google Scholar] [CrossRef]
- Kabiri, D.; Nesher, D.R.; Luxenbourg, D.; Rottenstreich, A.; Rosenbloom, J.I.; Ezra, Y.; Yagel, S.; Porat, S.; Romero, R. The role of vaginal progesterone for preterm birth prevention in women with threatened labor and shortened cervix diagnosed after 24 weeks of pregnancy. Int. J. Gynaecol. Obstet. 2023, 161, 423–431. [Google Scholar] [CrossRef]
- Gulersen, M.; Lenchner, E.; Nicolaides, K.H.; Otsuki, K.; Rust, O.A.; Althuisius, S.; Bornstein, E.; Berghella, V. Cervical cerclage for short cervix at 24 to 26 weeks of gestation: Systematic review and meta-analysis of randomized controlled trials using individual patient-level data. Am. J. Obstet. Gynecol. MFM 2023, 5, 100930. [Google Scholar] [CrossRef]
- Tolosa, J.E.; Boelig, R.C.; Bell, J.; Martínez-Baladejo, M.; Stoltzfus, J.; Mateus, J.; Quiñones, J.N.; Galeano-Herrera, S.; Pereira, L.; Burwick, R.; et al. Concurrent progestogen and cerclage to reduce preterm birth: A multicenter international retrospective cohort. Am. J. Obstet. Gynecol. MFM 2024, 6, 101351. [Google Scholar] [CrossRef]
- Kim, H.Y.; Cho, G.J.; Kwon, H.S. Applications of artificial intelligence in obstetrics. Ultrasonography 2023, 42, 2–9. [Google Scholar] [CrossRef] [PubMed]

| Outcome | Number of Trials Included | Vaginal Progesterone Group | Placebo Group | Relative Risk (95% Confidence Interval) |
|---|---|---|---|---|
| Preterm birth < 35 weeks | 4 | 105/494 (21%) | 138/472 (29%) | 0.73 (0.58–0.90) |
| Preterm birth < 34 weeks | 4 | 85/494 (17%) | 125/472 (26%) | 0.65 (0.51–0.83) |
| Preterm birth < 32 weeks | 4 | 62/494 (13%) | 91/472 (19%) | 0.65 (0.48–0.87) |
| Respiratory distress syndrome | 3 | 16/361 (4%) | 36/354 (10%) | 0.45 (0.26–0.80) |
| Necrotizing enterocolitis | 4 | 11/491 (2%) | 12/471 (3%) | 0.89 (0.41–1.93) |
| Intraventricular hemorrhage | 4 | 5/490 (1%) | 10/471 (2%) | 0.50 (0.18–1.38) |
| Perinatal death | 4 | 15/494 (3%) | 22/472 (5%) | 0.64 (0.34–1.22) |
| NICU admission | 4 | 82/492 (17%) | 115/470 (24%) | 0.69 (0.53–0.88) |
| Composite neonatal morbidity and mortality * | 3 | 28/361 (8%) | 48/354 (14%) | 0.58 (0.37–0.90) |
| Outcome | Number of Trials Included | Cerclage Group | No Cerclage Group | Relative Risk (95% Confidence Interval) |
|---|---|---|---|---|
| Prior preterm birth | ||||
| Preterm birth < 37 weeks | 5 | 105/250 (42.0) | 154/254 (60.6) | 0.70 (0.58–0.83) |
| Preterm birth < 35 weeks | 5 | 71/250 (28.4) | 105/254 (41.3) | 0.70 (0.55–0.89) |
| Preterm birth < 32 weeks | 5 | 48/250 (19.2) | 75/254 (29.5) | 0.66 (0.48–0.91) |
| Preterm birth < 28 weeks | 5 | 32/250 (12.8) | 51/254 (20.1) | 0.64 (0.43–0.96) |
| Respiratory distress syndrome | 4 | 13/207 (6.3) | 21/196 (10.7) | 0.61 (0.32–1.19) |
| Necrotizing enterocolitis | 4 | 1/207 (0.5) | 2/196 (1.0) | 0.62 (0.08–4.67) |
| Intraventricular hemorrhage | 4 | 0/207 (0) | 4/196 (2.0) | 0.28 (0.05–1.64) |
| Perinatal mortality | 5 | 22/250 (8.8) | 35/254 (13.8) | 0.65 (0.40–1.07) |
| NICU admission | 4 | 57/207 (27.5) | 67/196 (34.2) | 0.63 (0.34–1.18) |
| Composite perinatal mortality or morbidity | 5 | 39/250 (15.6) | 63/254 (24.8) | 0.64 (0.45–0.91) |
| No prior preterm birth | ||||
| Preterm birth < 37 weeks | 5 | 81/224 (36.2) | 80/195 (41.0) | 0.93 (0.73–1.18) |
| Preterm birth < 35 weeks | 5 | 49/224 (21.9) | 54/195 (27.7) | 0.88 (0.63–1.23) |
| Preterm birth < 32 weeks | 5 | 38/224 (17.0) | 39/195 (20.0) | 0.96 (0.64–1.42) |
| Preterm birth < 28 weeks | 5 | 26/224 (11.6) | 22/195 (11.3) | 1.15 (0.68–1.93) |
| Respiratory distress syndrome | 2 | 2/14 (14.3) | 2/16 (12.5) | 1.33 (0.23–7.74) |
| Necrotizing enterocolitis | 2 | 0/14 (0) | 0/16 (0) | ––– |
| Intraventricular hemorrhage | 2 | 1/14 (7.1) | 0/16 (0) | 3.90 (0.18–85.93) |
| Neonatal death | 4 | 7/118 (5.9) | 6/92 (6.5) | 1.08 (0.41–2.86) |
| NICU admission | 3 | 3/67 (4.5) | 4/38 (10.5) | 0.80 (0.26–2.47) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulersen, M.; Berghella, V.; Bornstein, E. Updates in Contemporary Management of Singleton Pregnancies Complicated by a Short Cervix. J. Clin. Med. 2025, 14, 5544. https://doi.org/10.3390/jcm14155544
Gulersen M, Berghella V, Bornstein E. Updates in Contemporary Management of Singleton Pregnancies Complicated by a Short Cervix. Journal of Clinical Medicine. 2025; 14(15):5544. https://doi.org/10.3390/jcm14155544
Chicago/Turabian StyleGulersen, Moti, Vincenzo Berghella, and Eran Bornstein. 2025. "Updates in Contemporary Management of Singleton Pregnancies Complicated by a Short Cervix" Journal of Clinical Medicine 14, no. 15: 5544. https://doi.org/10.3390/jcm14155544
APA StyleGulersen, M., Berghella, V., & Bornstein, E. (2025). Updates in Contemporary Management of Singleton Pregnancies Complicated by a Short Cervix. Journal of Clinical Medicine, 14(15), 5544. https://doi.org/10.3390/jcm14155544







