Evaluation of a Simple and Accurate Method for Intraocular Lens Constant Optimization Using Linear Interpolation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. IOL Power Calculation Formula Protocol
2.3.1. EVO (Emmetropia Verifying Optical) Formula
2.3.2. Hill-RBF (Hill Radial Basis Function) Formula
2.3.3. Kane Formula
2.4. Device Protocol
2.5. Optimization of IOL Constants Using Linear Interpolation
2.6. Correction of Predicted Refractions for Each Case Using Linear Interpolation
2.7. Inclusion and Exclusion Criteria
2.8. Primary Outcome Measures
2.9. Statistical Analysis
2.10. Use of Generative AI
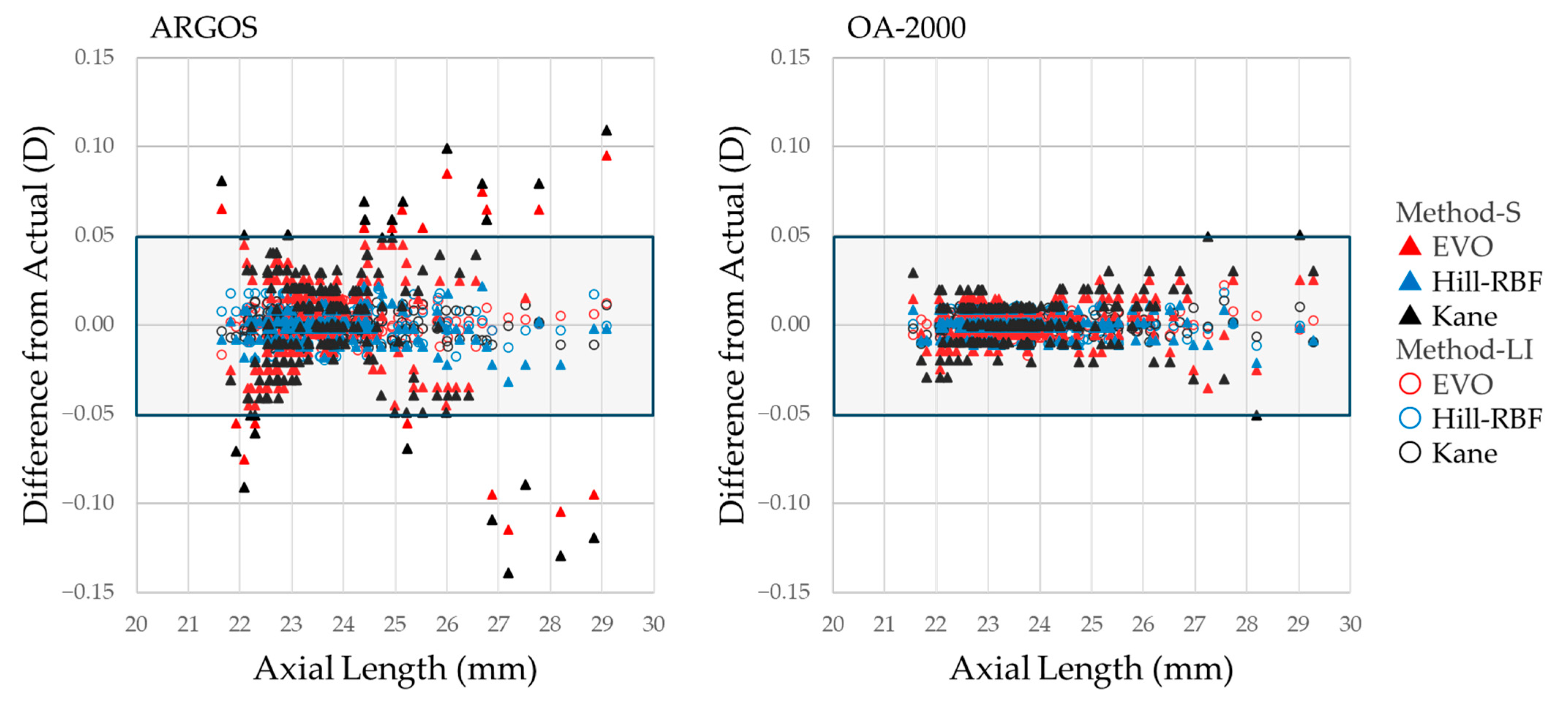
3. Results
3.1. Participant Characteristics and Measurements
3.2. Optimized A-Constants and Mean Prediction Errors for Each Formula and Device
3.3. Comparative Analysis of Absolute Prediction Errors and Correction Methods for Each Formula and Device
3.3.1. For All Cases
3.3.2. For Short-Axial-Length Cases
3.3.3. For Long-Axial-Length Cases
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fedorov, S.N.; Kolinko, A.I. A method of calculating the optical power of the intraocular lens. Vestn. Oftalmol. 1967, 80, 27–31. [Google Scholar] [PubMed]
- Montés-Micó, R.; Pastor-Pascual, F.; Ruiz-Mesa, R.; Taná-Rivero, P. Ocular biometry with swept-source optical coherence tomography. J. Cataract Refract. Surg. 2021, 47, 802–814. [Google Scholar] [CrossRef]
- Barrett, G.D. An improved universal theoretical formula for intraocular lens power prediction. J. Cataract Refract. Surg. 1993, 19, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Yeo, T.K. Emmetropia Verifying Optical (EVO) Formula. In Intraocular Lens Calculations; Springer: Cham, Switzerland, 2024; pp. 617–623. [Google Scholar] [CrossRef]
- Hill, W.E.; Haehnle, J. Hill-RBF: Improving IOL power selection by artificial intelligence. In Intraocular Lens Calculations; Aramberri, J., Savini, G., Eds.; Springer: Cham, Switzerland, 2024; pp. 637–648. [Google Scholar]
- Darcy, K.; Gunn, D.; Tavassoli, S.; Sparrow, J.; Kane, J.X. Assessment of the accuracy of new and updated intraocular lens power calculation formulas in 10,930 eyes from the UK National Health Service. J. Cataract Refract. Surg. 2020, 46, 2–7. [Google Scholar]
- Shajari, M.; Kolb, C.M.; Petermann, K.; Böhm, M.; Herzog, M.; de’Lorenzo, N.; Schönbrunn, S.; Kohnen, T. Comparison of 9 modern intraocular lens power calculation formulas for a quadrifocal intraocular lens. J. Cataract Refract. Surg. 2018, 44, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, L.; Mo, E.; Zhu, K.; Zhu, Y.; Feng, K.; Wu, Z.; Zheng, Y.; Huang, F.; Gong, X.; et al. The effect of corneal power on the accuracy of 14 IOL power formulas. BMC Ophthalmol. 2024, 24, 126. [Google Scholar] [CrossRef]
- Stopyra, W.; Langenbucher, A.; Grzybowski, A. Intraocular lens power calculation formulas—A systematic review. Ophthalmol. Ther. 2023, 12, 2881–2902. [Google Scholar] [CrossRef]
- Moshirfar, M.; Sulit, C.A.; Brown, A.H.; Irwin, C.; Ronquillo, Y.C.; Hoopes, P.C. Comparing the accuracy of the Kane, Barrett universal II, hill-radial basis function, emmetropia verifying optical, and Ladas super formula intraocular lens power calculation formulas. Clin. Ophthalmol. 2023, 17, 2643–2652. [Google Scholar] [CrossRef]
- Stopyra, W.; Cooke, D.L.; Grzybowski, A. A Review of Intraocular Lens Power Calculation Formulas Based on Artificial Intelligence. J. Clin. Med. 2024, 13, 498. [Google Scholar] [CrossRef]
- Hoffer, K.J.; Aramberri, J.; Haigis, W.; Olsen, T.; Savini, G.; Shammas, H.J.; Bentow, S. Protocols for studies of intraocular lens formula accuracy. Am. J. Ophthalmol. 2015, 160, 403–405. [Google Scholar] [CrossRef]
- Hoffer, K.J.; Savini, G. Update on intraocular lens power calculation study protocols: The better way to design and report clinical trials. Ophthalmology 2021, 128, e115–e120. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, T.; Mori, H.; Nakajima, F.; Ohji, M. Comparison of a new biometer using swept-source optical coherence tomography and a conventional biometer using partial coherence interferometry. PLoS ONE 2018, 13, e0196401. [Google Scholar] [CrossRef] [PubMed]
- Omoto, M.K.; Torii, H.; Masui, S.; Ayaki, M.; Tsubota, K.; Negishi, K. Ocular biometry and refractive outcomes using two swept-source optical coherence tomography-based biometers with segmental or equivalent refractive indices. Sci. Rep. 2019, 9, 6557. [Google Scholar] [CrossRef]
- Yang, C.M.; Lim, D.H.; Kim, H.J.; Chung, T.Y. Comparison of two swept-source optical coherence tomography biometers and a partial coherence interferometer. PLoS ONE 2019, 14, e0223114. [Google Scholar] [CrossRef]
- Blehm, C.; Hall, B. Comparing predictive accuracy of a swept source optical coherence tomography biometer and an optical low coherence reflectometry biometer. Clin. Ophthalmol. 2023, 17, 2125–2131. [Google Scholar] [CrossRef]
- Tamaoki, A.; Kojima, T.; Hasegawa, A.; Yamamoto, M.; Kaga, T.; Tanaka, K.; Ichikawa, K. Clinical evaluation of a new swept-source optical coherence biometer that uses individual refractive indices to measure axial length in cataract patients. Ophthalmic Res. 2019, 62, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Kamiya, K. Accuracy validation of the new Barrett True Axial Length formula and the optimized lens factor using sum-of-segment biometry. J. Clin. Med. 2024, 13, 4639. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.; Li, Y.; Chen, Z.; Gao, R.; Yu, J.; Zhao, Y.; Lu, W.; McAlinden, C.; Wang, Q. Comprehensive Comparison of Axial Length Measurement With Three Swept-Source OCT-Based Biometers and Partial Coherence Interferometry. J. Refract. Surg. 2019, 35, 115–120. [Google Scholar] [CrossRef]
- Haigis, W.; Lege, B.; Miller, N.; Schneider, B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch. Clin. Exp. Ophthalmol. 2000, 238, 765–773. [Google Scholar] [CrossRef]
- Shammas, H.J.; Taroni, L.; Pellegrini, M.; Shammas, M.C.; Jivrajka, R.V. Accuracy of newer intraocular lens power formulas in short and long eyes using sum-of-segments biometry. J. Cataract Refract. Surg. 2022, 48, 1113–1120. [Google Scholar] [CrossRef]
- Kato, Y.; Ayaki, M.; Tamaoki, A.; Tanaka, Y.; Ichikawa, K.; Ichikawa, K. Accuracy of new intraocular lens power calculation formula for short and long eyes using segmental refractive indices. J. Cataract Refract. Surg. 2024, 50, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Blehm, C.; Hall, B. Refractive predictability of a swept source optical coherence tomography biometer in long and short eyes implanted with extended depth of focus intraocular lenses. Clin. Ophthalmol. 2023, 17, 3525–3530. [Google Scholar] [CrossRef] [PubMed]
- Retzlaff, J.A.; Sanders, D.R.; Kraff, M.C. Development of the SRK/T intraocular lens implant power calculation formula. J. Cataract Refract. Surg. 1990, 16, 333–340. [Google Scholar] [CrossRef] [PubMed]
- De Bernardo, M.; Cione, F.; Capasso, L.; Coppola, A.; Rosa, N. A formula to improve the reliability of optical axial length measurement in IOL power calculation. Sci. Rep. 2022, 12, 18845. [Google Scholar] [CrossRef]
- Shammas, H.J.; Shammas, M.C.; Jivrajka, R.V.; Cooke, D.L.; Potvin, R. Effects on IOL power calculation and expected clinical outcomes of axial length measurements based on multiple vs single refractive indices. Clin. Ophthalmol. 2020, 14, 1511–1519. [Google Scholar] [CrossRef]


| Patient Characteristics | |||
|---|---|---|---|
| Age | 73.8 ± 7.1 | ||
| Sex (men, %) | 35.6 | ||
| Follow-up duration (days) | 94.9 ± 8.3 | ||
| Measurements | |||
| ARGOS | OA-2000 | p-Value | |
| AL (mm) | 23.81 ± 1.34 | 23.78 ± 1.37 | <0.001 |
| ACD (mm) | 3.17 ± 0.34 | 3.08 ± 0.33 | <0.001 |
| LT (mm) | 4.67 ± 0.40 | 4.70 ± 0.39 | <0.001 |
| CCT (μm) | 528 ± 33 | 521 ± 33 | <0.001 * |
| HCD (mm) | 11.66 ± 0.38 | 11.56 ± 0.40 | <0.001 |
| K values (D) | 44.47 ± 1.38 | 44.36 ± 1.38 | <0.001 * |
| Device/Formula | Recommended A-Constant (CNA0T0) | Optimized A-Constant | A-Constant (119.1) MPE (D) | A-Constant (119.33) MPE (D) | Optimized A-Constant Actual MPE (D) |
|---|---|---|---|---|---|
| ARGOS | |||||
| EVO | 119.1 | 119.540 | −0.3456 | −0.1649 | −0.0006 |
| Hill-RBF | 119.26 | 119.733 | −0.4431 | −0.2820 | −0.0012 |
| Kane | 119.33 | 119.563 | −0.3763 | −0.1893 | −0.0006 |
| OA-2000 | |||||
| EVO | 119.1 | 119.388 | −0.2259 | −0.0453 | +0.0003 |
| Hill-RBF | 119.26 | 119.532 | −0.3025 | −0.1413 | −0.0002 |
| Kane | 119.33 | 119.417 | −0.2572 | −0.0705 | −0.0001 |
| Correction Method | EVO | Hill-RBF | Kane | P-Formula | Post Hoc | |
|---|---|---|---|---|---|---|
| ARGOS (n = 188) | ||||||
| MAE (D) ± SD | Method-S | 0.243 ± 0.193 | 0.249 ± 0.205 | 0.252 ± 0.198 | 0.234 | |
| Method-LI | 0.245 ± 0.193 | 0.248 ± 0.204 | 0.254 ± 0.194 | <0.05 (1) | (1) H vs. K (<0.05) | |
| Actual | 0.245 ± 0.193 | 0.248 ± 0.204 | 0.254 ± 0.193 | <0.05 (2) | (2) H vs. K (<0.05) | |
| P-method | 0.143 | 0.315 | 0.407 | |||
| The rate < 0.25 D (%) | Method-S | 60.1 | 59 | 56.9 | 0.529 | |
| Method-LI | 59.6 | 58.5 | 58 | 0.864 | ||
| Actual | 60.1 | 59.6 | 58.5 | 0.862 | ||
| P-method | 0.92 | 0.651 | 0.497 | |||
| The rate < 0.5 D (%) | Method-S | 91 | 86.2 | 87.8 | <0.05 (3) | (3) E vs. K (<0.05) |
| Method-LI | 91.5 | 86.2 | 88.3 | <0.05 (4) | (4) E vs. H, E vs. K (<0.05) | |
| Actual | 91.5 | 86.2 | 88.3 | <0.05 (5) | (5) E vs. H, E vs. K (<0.05) | |
| P-method | 0.368 | 1 | 0.716 | |||
| Standard Error | Method-S | 0.0141 | 0.015 | 0.0144 | ||
| Method-LI | 0.0141 | 0.0149 | 0.0141 | |||
| Actual | 0.0141 | 0.0149 | 0.0141 | |||
| MedAE (D) | Method-S | 0.205 | 0.208 | 0.209 | ||
| Method-LI | 0.197 | 0.21 | 0.205 | |||
| Actual | 0.198 | 0.2 | 0.205 | |||
| 95% CI (MAE, %) | Method-S | 0.216–0.271 | 0.220–0.279 | 0.224–0.281 | ||
| Method-LI | 0.217–0.273 | 0.219–0.278 | 0.226–0.281 | |||
| Actual | 0.217–0.273 | 0.219–0.277 | 0.226–0.281 | |||
| OA-2000 (n = 188) | ||||||
| MAE (D) ± SD | Method-S | 0.251 ± 0.192 | 0.244 ± 0.207 | 0.256 ± 0.198 | 0.147 | |
| Method-LI | 0.252 ± 0.193 | 0.243 ± 0.206 | 0.258 ± 0.197 | 0.56 | ||
| Actual | 0.253 ± 0.194 | 0.244 ± 0.207 | 0.258 ± 0.198 | 0.553 | ||
| P-method | 0.114 | 0.616 | 0.894 | |||
| The rate < 0.25 D (%) | Method-S | 60.1 | 59.6 | 57.4 | 0.692 | |
| Method-LI | 58.5 | 59.6 | 57.4 | 0.804 | ||
| Actual | 59 | 61.2 | 59 | 0.744 | ||
| P-method | 0.247 | 0.223 | 0.223 | |||
| The rate < 0.5 D (%) | Method-S | 89.4 | 88.8 | 88.8 | 0.931 | |
| Method-LI | 89.4 | 88.3 | 88.8 | 0.819 | ||
| Actual | 89.4 | 88.8 | 89.4 | 0.931 | ||
| P-method | NaN | 0.368 | 0.607 | |||
| Standard Error | Method-S | 0.014 | 0.0151 | 0.0145 | ||
| Method-LI | 0.0141 | 0.015 | 0.0144 | |||
| Actual | 0.0141 | 0.0151 | 0.0144 | |||
| MedAE (D) | Method-S | 0.215 | 0.204 | 0.217 | ||
| Method-LI | 0.213 | 0.205 | 0.219 | |||
| Actual | 0.207 | 0.2 | 0.22 | |||
| 95% CI (MAE, %) | Method-S | 0.224–0.279 | 0.214–0.274 | 0.227–0.284 | ||
| Method-LI | 0.224–0.280 | 0.214–0.273 | 0.230–0.287 | |||
| Actual | 0.225–0.280 | 0.214–0.273 | 0.230–0.287 | |||
| Correction Method | EVO | Hill-RBF | Kane | P-Formula | Post Hoc | |
|---|---|---|---|---|---|---|
| ARGOS (n = 17) | ||||||
| MAE (D) ± SD | Method-S | 0.313 ± 0.186 | 0.373 ± 0.214 | 0.319 ± 0.194 | 0.251 | (1, 2) NS. * |
| Method-LI | 0.293 ± 0.188 | 0.367 ± 0.213 | 0.296 ± 0.189 | 0.165 | ||
| Actual | 0.292 ± 0.190 | 0.369 ± 0.214 | 0.296 ± 0.190 | 0.147 | ||
| P-method | <0.05 (1) | 0.066 | <0.05 (2) | |||
| The rate < 0.25 D (%) | Method-S | 41.2 | 35.3 | 35.3 | 0.819 | |
| Method-LI | 47.1 | 35.3 | 41.2 | 0.449 | ||
| Actual | 47.1 | 35.3 | 35.3 | 0.549 | ||
| P-method | 0.368 | NaN | 0.368 | |||
| The rate < 0.5 D (%) | Method-S | 82.4 | 64.7 | 76.5 | 0.368 | |
| Method-LI | 88.2 | 64.7 | 82.4 | 0.197 | ||
| Actual | 88.2 | 64.7 | 82.4 | 0.197 | ||
| P-method | 0.368 | NaN | 0.368 | |||
| Standard Error | Method-S | 0.045 | 0.052 | 0.047 | ||
| Method-LI | 0.0456 | 0.0517 | 0.0459 | |||
| Actual | 0.0462 | 0.0518 | 0.0462 | |||
| MedAE (D) | Method-S | 0.305 | 0.432 | 0.301 | ||
| Method-LI | 0.287 | 0.43 | 0.306 | |||
| Actual | 0.28 | 0.44 | 0.3 | |||
| 95% CI (MAE, %) | Method-S | 0.217–0.408 | 0.263–0.483 | 0.219–0.418 | ||
| Method-LI | 0.196–0.389 | 0.257–0.476 | 0.199–0.394 | |||
| Actual | 0.194–0.380 | 0.259–0.479 | 0.199–0.394 | |||
| OA-2000 (n = 22) | ||||||
| MAE (D) ± SD | Method-S | 0.267 ± 0.154 | 0.260 ± 0.183 | 0.268 ± 0.151 | 0.967 | |
| Method-LI | 0.265 ± 0.156 | 0.259 ± 0.182 | 0.265 ± 0.154 | 0.979 | ||
| Actual | 0.263 ± 0.156 | 0.260 ± 0.181 | 0.264 ± 0.154 | 0.992 | ||
| P-method | 0.197 | 0.511 | 0.446 | |||
| The rate < 0.25 D (%) | Method-S | 50 | 54.5 | 54.5 | 0.913 | |
| Method-LI | 54.5 | 59.1 | 54.5 | 0.895 | ||
| Actual | 54.5 | 63.6 | 54.5 | 0.607 | ||
| P-method | 0.368 | 0.223 | 1 | |||
| The rate < 0.5 D (%) | Method-S | 95.5 | 77.3 | 90.9 | <0.05 (1) | (1–3) NS. * |
| Method-LI | 95.5 | 77.3 | 95.5 | <0.05 (2) | ||
| Actual | 95.5 | 77.3 | 95.5 | <0.05 (3) | ||
| P-method | NaN | NaN | 0.368 | |||
| MedAE (D) | Method-S | 0.245 | 0.245 | 0.237 | ||
| Method-LI | 0.244 | 0.241 | 0.233 | |||
| Actual | 0.24 | 0.24 | 0.24 | |||
| 95% CI (MAE, %) | Method-S | 0.199–0.335 | 0.179–0.341 | 0.201–0.335 | ||
| Method-LI | 0.195–0.334 | 0.179–0.340 | 0.197–0.333 | |||
| Actual | 0.194–0.332 | 0.180–0.340 | 0.195–0.332 | |||
| Correction Method | EVO | Hill-RBF | Kane | P-Formula | Post Hoc | |
|---|---|---|---|---|---|---|
| ARGOS (n = 21) | ||||||
| MAE (D) ± SD | Method-S | 0.318 ± 0.278 | 0.378 ± 0.317 | 0.327 ± 0.278 | 0.538 | (1) Method-S vs. -LI, Method-S vs. Actual (<0.01) |
| Method-LI | 0.311 ± 0.270 | 0.370 ± 0.316 | 0.315 ± 0.266 | 0.165 | ||
| Actual | 0.314 ± 0.268 | 0.371 ± 0.214 | 0.314 ± 0.264 | 0.147 | ||
| P-method | 0.18 | <0.01 (1) | 0.651 | |||
| The rate < 0.25 D (%) | Method-S | 52.4 | 38.1 | 47.6 | 0.247 | |
| Method-LI | 57.1 | 42.9 | 52.4 | 0.368 | ||
| Actual | 57.1 | 42.9 | 57.1 | 0.277 | ||
| P-method | 0.717 | 0.368 | 0.223 | |||
| The rate < 0.5 D (%) | Method-S | 81 | 66.7 | 81 | <0.05 (2) | (2) NS. * |
| Method-LI | 81 | 66.7 | 76.2 | 0.174 | ||
| Actual | 81 | 66.7 | 76.2 | 0.174 | ||
| P-method | NaN | NaN | 0.368 | |||
| Standard Error | Method-S | 0.0607 | 0.0691 | 0.0606 | ||
| Method-LI | 0.0589 | 0.0689 | 0.058 | |||
| Actual | 0.0585 | 0.0685 | 0.0575 | |||
| MedAE (D) | Method-S | 0.225 | 0.262 | 0.269 | ||
| Method-LI | 0.202 | 0.26 | 0.209 | |||
| Actual | 0.19 | 0.26 | 0.2 | |||
| 95% CI (MAE, %) | Method-S | 0.191–0.445 | 0.234–0.522 | 0.200–0.453 | ||
| Method-LI | 0.188–0.434 | 0.226–0.514 | 0.194–0.436 | |||
| Actual | 0.192–0.436 | 0.228–0.514 | 0.194–0.434 | |||
| OA-2000 (n = 21) | ||||||
| MAE (D) ± SD | Method-S | 0.325 ± 0.287 | 0.334 ± 0.330 | 0.293 ± 0.290 | 0.538 | |
| Method-LI | 0.327 ± 0.288 | 0.334 ± 0.327 | 0.301 ± 0.283 | 0.953 | ||
| Actual | 0.330 ± 0.288 | 0.333 ± 0.325 | 0.301 ± 0.281 | 0.313 | ||
| P-method | 0.097 | 0.953 | 0.084 | |||
| The rate < 0.25 D (%) | Method-S | 52.4 | 42.9 | 57.1 | 0.497 | |
| Method-LI | 47.6 | 38.1 | 57.1 | 0.301 | ||
| Actual | 47.6 | 38.1 | 61.9 | 0.178 | ||
| P-method | 0.368 | 0.368 | 0.368 | |||
| The rate < 0.5 D (%) | Method-S | 81 | 90.5 | 90.5 | 0.135 | |
| Method-LI | 81 | 90.5 | 90.5 | 0.135 | ||
| Actual | 81 | 90.5 | 90.5 | 0.135 | ||
| P-method | NaN | NaN | NaN | |||
| Standard Error | Method-S | 0.0627 | 0.072 | 0.0632 | ||
| Method-LI | 0.0628 | 0.0714 | 0.0617 | |||
| Actual | 0.0628 | 0.0709 | 0.0613 | |||
| MedAE (D) | Method-S | 0.235 | 0.279 | 0.22 | ||
| Method-LI | 0.25 | 0.288 | 0.245 | |||
| Actual | 0.255 | 0.28 | 0.24 | |||
| 95% CI (MAE, %) | Method-S | 0.194–0.456 | 0.184–0.484 | 0.161–0.424 | ||
| Method-LI | 0.196–0.458 | 0.185–0.483 | 0.173–0.430 | |||
| Actual | 0.199–0.461 | 0.185–0.481 | 0.173–0.429 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyamoto, S.; Kamiya, K. Evaluation of a Simple and Accurate Method for Intraocular Lens Constant Optimization Using Linear Interpolation. J. Clin. Med. 2025, 14, 4543. https://doi.org/10.3390/jcm14134543
Miyamoto S, Kamiya K. Evaluation of a Simple and Accurate Method for Intraocular Lens Constant Optimization Using Linear Interpolation. Journal of Clinical Medicine. 2025; 14(13):4543. https://doi.org/10.3390/jcm14134543
Chicago/Turabian StyleMiyamoto, Sumitaka, and Kazutaka Kamiya. 2025. "Evaluation of a Simple and Accurate Method for Intraocular Lens Constant Optimization Using Linear Interpolation" Journal of Clinical Medicine 14, no. 13: 4543. https://doi.org/10.3390/jcm14134543
APA StyleMiyamoto, S., & Kamiya, K. (2025). Evaluation of a Simple and Accurate Method for Intraocular Lens Constant Optimization Using Linear Interpolation. Journal of Clinical Medicine, 14(13), 4543. https://doi.org/10.3390/jcm14134543








