Insights on the Pathogenesis of Mycobacterium abscessus Infection in Patients with Cystic Fibrosis
Abstract
1. Introduction
2. The Immune Response to Mab Infection
3. Mab Virulence Factors
3.1. Type VII Secretions Systems
3.2. Mab Rough (R) Morphotype
3.3. Biofilm Formation
4. The CF Host
Hyperinflammation and Immune Dysregulation
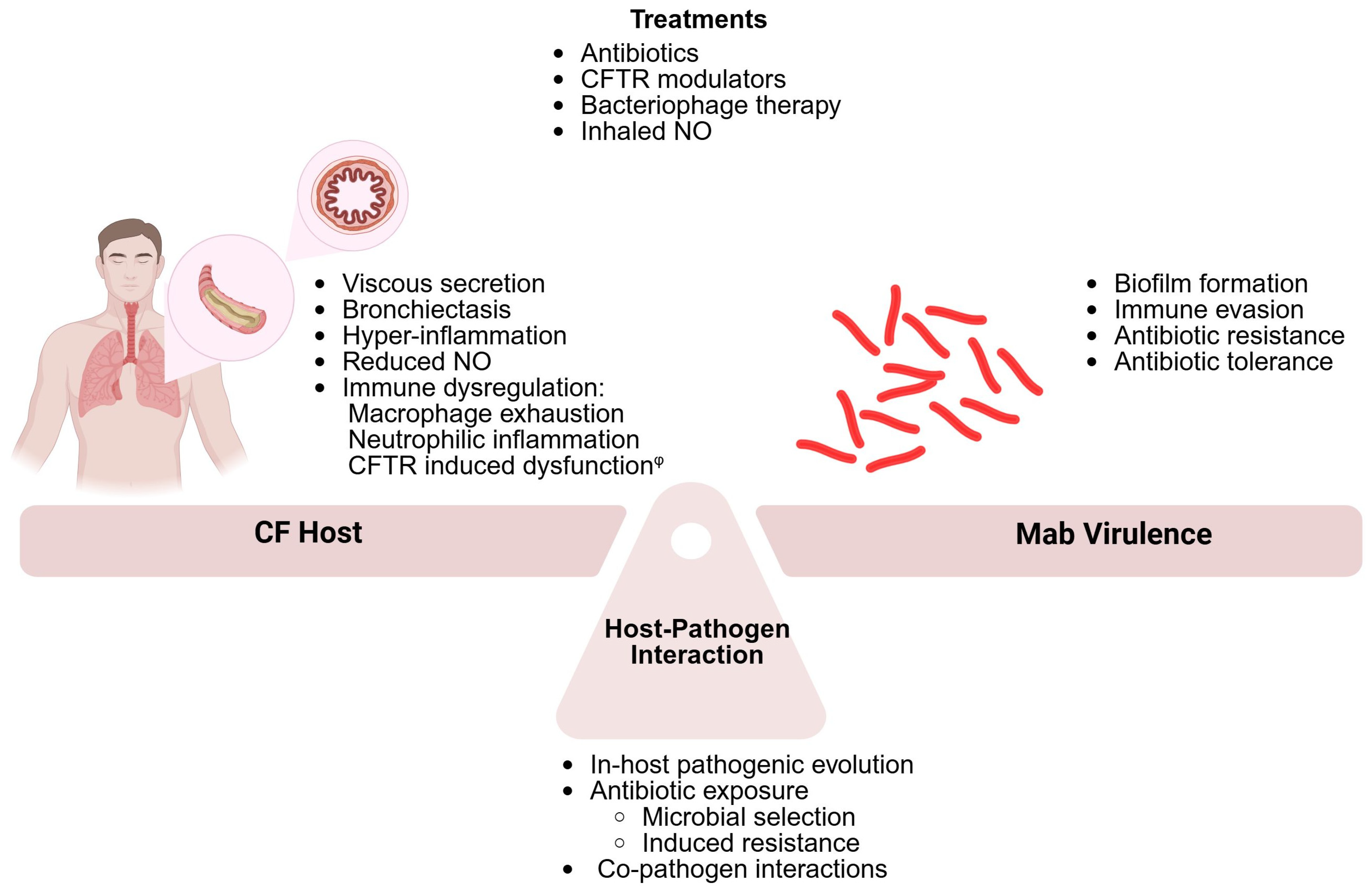
5. The Host–Pathogen Interaction
5.1. Within-Host Pathogenic Adaptation
5.2. Co-Pathogens in CF Airways
6. Antibiotic Treatment in Mab Infection
7. Novel Therapies
7.1. Nitric Oxide (NO)
7.2. Bacteriophage Therapy
7.3. Other Therapies
7.3.1. Host-Directed Therapies (HDTs)
7.3.2. Drug Repurposing
8. Omics in NTM Lung Disease in CF
9. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- Novosad, S.A.; Beekmann, S.E.; Polgreen, P.M.; Mackey, K.; Winthrop, K.L. Treatment of Mycobacterium abscessus Infection. Emerg. Infect. Dis. 2016, 22, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Prevots, D.R.; Marras, T.K. Epidemiology of Human Pulmonary Infection with Nontuberculous Mycobacteria. Clin. Chest Med. 2015, 36, 13–34. [Google Scholar] [CrossRef]
- Nessar, R.; Cambau, E.; Reyrat, J.M.; Murray, A.; Gicquel, B. Mycobacterium abscessus: A new antibiotic nightmare. J. Antimicrob. Chemother. 2012, 67, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.D.; Herrmann, J.-L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 18, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Collins, F. Cystic fibrosis: Molecular biology and therapeutic implications. Science 1992, 256, 774–779. [Google Scholar] [CrossRef]
- Chen, Q.; Shen, Y.; Zheng, J. A review of cystic fibrosis: Basic and clinical aspects. Anim. Model. Exp. Med. 2021, 4, 220–232. [Google Scholar] [CrossRef]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Lynch, S.V.; Goldfarb, K.C.; Wild, Y.K.; Kong, W.; De Lisle, R.C.; Brodie, E.L. Cystic fibrosis transmembrane conductance regulator knockout mice exhibit aberrant gastrointestinal microbiota. Gut Microbes 2013, 4, 41–47. [Google Scholar] [CrossRef]
- Saint-Criq, V.; Gray, M.A. Role of CFTR in epithelial physiology. Cell. Mol. Life Sci. 2017, 74, 93–115. [Google Scholar] [CrossRef]
- Livraghi, A.; Randell, S.H. Cystic Fibrosis and Other Respiratory Diseases of Impaired Mucus Clearance. Toxicol. Pathol. 2007, 35, 116–129. [Google Scholar] [CrossRef]
- Martiniano, S.L.; Nick, J.A.; Daley, C.L. Nontuberculous Mycobacterial Infections in Cystic Fibrosis. Clin. Chest Med. 2022, 43, 697–716. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.D.; Alam, M.E.; Franciosi, A.N.; Quon, B.S. Global burden of nontuberculous mycobacteria in the cystic fibrosis population: A systematic review and meta-analysis. ERJ Open Res. 2023, 9, 00336–02022. [Google Scholar] [CrossRef]
- Esther, C.R.J.; Esserman, D.A.; Gilligan, P.; Kerr, A.; Noone, P.G. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J. Cyst. Fibros. 2010, 9, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Nick, J.A.; Pohl, K.; Martiniano, S.L. Nontuberculous mycobacterial infections in cystic fibrosis. Curr. Opin. Pulm. Med. 2016, 22, 629–636. [Google Scholar] [CrossRef]
- Keefe, B.F.; Bermudez, L.E. Environment in the lung of cystic fibrosis patients stimulates the expression of biofilm phenotype in Mycobacterium abscessus. J. Med. Microbiol. 2022, 71, 001467. [Google Scholar] [CrossRef] [PubMed]
- Jean-Pierre, V.; Boudet, A.; Sorlin, P.; Menetrey, Q.; Chiron, R.; Lavigne, J.P.; Marchandin, H. Biofilm Formation by Staphylococcus aureus in the Specific Context of Cystic Fibrosis. Int. J. Mol. Sci. 2022, 24, 597. [Google Scholar] [CrossRef]
- Guillaume, O.; Butnarasu, C.; Visentin, S.; Reimhult, E. Interplay between biofilm microenvironment and pathogenicity of Pseudomonas aeruginosa in cystic fibrosis lung chronic infection. Biofilm 2022, 4, 100089. [Google Scholar] [CrossRef]
- Bar-Oz, M.; Meir, M.; Barkan, D. Virulence-Associated Secretion in Mycobacterium abscessus. Front. Immunol. 2022, 13, 938895. [Google Scholar] [CrossRef]
- Hedin, W.; Fröberg, G.; Fredman, K.; Chryssanthou, E.; Selmeryd, I.; Gillman, A.; Orsini, L.; Runold, M.; Jönsson, B.; Schön, T.; et al. A Rough Colony Morphology of Mycobacterium abscessus Is Associated With Cavitary Pulmonary Disease and Poor Clinical Outcome. J. Infect. Dis. 2023, 227, 820–827. [Google Scholar] [CrossRef]
- Caverly, L.J.; Zimbric, M.; Azar, M.; Opron, K.; LiPuma, J.J. Cystic fibrosis airway microbiota associated with outcomes of nontuberculous mycobacterial infection. ERJ Open Res. 2021, 7, 00578–2020. [Google Scholar] [CrossRef] [PubMed]
- Caverly, L.J.; Zhao, J.; LiPuma, J.J. Cystic fibrosis lung microbiome: Opportunities to reconsider management of airway infection. Pediatr. Pulmonol. 2015, 50 (Suppl. S4), S31–S38. [Google Scholar] [CrossRef]
- Widder, S.; Carmody, L.A.; Opron, K.; Kalikin, L.M.; Caverly, L.J.; LiPuma, J.J. Microbial community organization designates distinct pulmonary exacerbation types and predicts treatment outcome in cystic fibrosis. Nat. Commun. 2024, 15, 4889. [Google Scholar] [CrossRef]
- Parmar, S.; Tocheva, E.I. The cell envelope of Mycobacterium abscessus and its role in pathogenesis. PLoS Pathog. 2023, 19, e1011318. [Google Scholar] [CrossRef] [PubMed]
- Qvist, T.; Eickhardt, S.; Kragh, K.N.; Andersen, C.B.; Iversen, M.; Høiby, N.; Bjarnsholt, T. Chronic pulmonary disease with Mycobacterium abscessus complex is a biofilm infection. Eur. Respir. J. 2015, 46, 1823–1826. [Google Scholar] [CrossRef]
- Fennelly, K.P.; Ojano-Dirain, C.; Yang, Q.; Liu, L.; Lu, L.; Progulske-Fox, A.; Wang, G.P.; Antonelli, P.; Schultz, G. Biofilm Formation by Mycobacterium abscessus in a Lung Cavity. Am. J. Respir. Crit. Care Med. 2016, 193, 692–693. [Google Scholar] [CrossRef]
- Leestemaker-Palmer, A.L.; Bermudez, L.E. Mycobacterium abscessus infection results in decrease of oxidative metabolism of lung airways cells and relaxation of the epithelial mucosal tight junctions. Tuberculosis 2023, 138, 102303. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, K.C.; Caceres, S.M.; Pohl, K.; Poch, K.R.; Bernut, A.; Kremer, L.; Bratton, D.L.; Herrmann, J.-L.; Nick, J.A. Neutrophil killing of Mycobacterium abscessus by intra- and extracellular mechanisms. PLoS ONE 2018, 13, e0196120. [Google Scholar] [CrossRef] [PubMed]
- Caverly, L.J.; Caceres, S.M.; Fratelli, C.; Happoldt, C.; Kidwell, K.M.; Malcolm, K.C.; Nick, J.A.; Nichols, D.P. Mycobacterium abscessus morphotype comparison in a murine model. PLoS ONE 2015, 10, e0117657. [Google Scholar] [CrossRef]
- Bernut, A.; Nguyen-Chi, M.; Halloum, I.; Herrmann, J.-L.; Lutfalla, G.; Kremer, L. Mycobacterium abscessus-Induced Granuloma Formation Is Strictly Dependent on TNF Signaling and Neutrophil Trafficking. PLoS Pathog. 2016, 12, e1005986. [Google Scholar] [CrossRef]
- Lee, S.J.; Shin, S.J.; Lee, S.J.; Lee, M.H.; Kang, T.H.; Noh, K.T.; Shin, Y.K.; Kim, H.W.; Yun, C.-H.; Jung, I.D.; et al. Mycobacterium abscessus MAB2560 induces maturation of dendritic cells via Toll-like receptor 4 and drives Th1 immune response. BMB Rep. 2014, 47, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.-M.; Yang, C.-S.; Yuk, J.-M.; Lee, J.-Y.; Kim, K.H.; Shin, S.J.; Takahara, K.; Lee, S.J.; Jo, E.-K. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell. Microbiol. 2008, 10, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, A.E.; Congel, J.H.; Corley, J.M.; Janssen, W.J.; Nick, J.A.; Malcolm, K.C.; Hisert, K.B. Dectin-1-Independent Macrophage Phagocytosis of Mycobacterium abscessus. Int. J. Mol. Sci. 2023, 24, 11062. [Google Scholar] [CrossRef]
- Kam, J.Y.; Hortle, E.; Krogman, E.; Warner, S.E.; Wright, K.; Luo, K.; Cheng, T.; Manuneedhi Cholan, P.; Kikuchi, K.; Triccas, J.A.; et al. Rough and smooth variants of Mycobacterium abscessus are differentially controlled by host immunity during chronic infection of adult zebrafish. Nat. Commun. 2022, 13, 952. [Google Scholar] [CrossRef]
- Ferrell, K.C.; Johansen, M.D.; Triccas, J.A.; Counoupas, C. Virulence Mechanisms of Mycobacterium abscessus: Current Knowledge and Implications for Vaccine Design. Front. Microbiol. 2022, 13, 842017. [Google Scholar] [CrossRef]
- Rottman, M.; Catherinot, E.; Hochedez, P.; Emile, J.-F.; Casanova, J.-L.; Gaillard, J.-L.; Soudais, C. Importance of T cells, gamma interferon, and tumor necrosis factor in immune control of the rapid grower Mycobacterium abscessus in C57BL/6 mice. Infect. Immun. 2007, 75, 5898–5907. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Lee, M.-S.; Kim, D.-J.; Yang, S.-J.; Lee, S.-J.; Noh, E.-J.; Shin, S.J.; Park, J.-H. Nucleotide-Binding Oligomerization Domain 2 Contributes to Limiting Growth of Mycobacterium abscessus in the Lung of Mice by Regulating Cytokines and Nitric Oxide Production. Front. Immunol. 2017, 8, 1477. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Park, J.-Y.; Kim, D.-Y.; Lee, T.-S.; Jung, D.-H.; Kim, Y.-J.; Lee, Y.-J.; Lee, Y.-J.; Seo, I.-S.; Song, E.-J.; et al. Type I Interferons Are Involved in the Intracellular Growth Control of Mycobacterium abscessus by Mediating NOD2-Induced Production of Nitric Oxide in Macrophages. Front. Immunol. 2021, 12, 738070. [Google Scholar] [CrossRef]
- Xia, L.; Liu, X.-H.; Yuan, Y.; Lowrie, D.B.; Fan, X.-Y.; Li, T.; Hu, Z.-D.; Lu, S.-H. An Updated Review on MSMD Research Globally and A Literature Review on the Molecular Findings, Clinical Manifestations, and Treatment Approaches in China. Front. Immunol. 2022, 13, 926781. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Chang, E.; Yamashita, S.; Iademarco, M.F.; LoBue, P.A. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-alpha therapy. Emerg. Infect. Dis. 2009, 15, 1556–1561. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yang, C.-S.; Nguyen, L.T.; Kim, J.K.; Jin, H.S.; ho Choe, J.; Kim, S.Y.; Lee, H.-M.; Jung, M.; Kim, J.-M.; et al. Mycobacterium abscessus ESX-3 plays an important role in host inflammatory and pathological responses during infection. Microbes Infect. 2017, 19, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Daher, W.; Le Moigne, V.; Tasrini, Y.; Parmar, S.; Sexton, D.L.; Aguilera-Correa, J.J.; Berdal, V.; Tocheva, E.I.; Herrmann, J.-L.; Kremer, L. Deletion of ESX-3 and ESX-4 secretion systems in Mycobacterium abscessus results in highly impaired pathogenicity. Commun. Biol. 2025, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Laencina, L.; Dubois, V.; Le Moigne, V.; Viljoen, A.; Majlessi, L.; Pritchard, J.; Bernut, A.; Piel, L.; Roux, A.-L.; Gaillard, J.-L.; et al. Identification of genes required for Mycobacterium abscessus growth in vivo with a prominent role of the ESX-4 locus. Proc. Natl. Acad. Sci. USA 2018, 115, E1002–E1011. [Google Scholar] [CrossRef]
- Howard, S.T.; Rhoades, E.; Recht, J.; Pang, X.; Alsup, A.; Kolter, R.; Lyons, C.R.; Byrd, T.F. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology 2006, 152, 1581–1590. [Google Scholar] [CrossRef]
- Jönsson, B.E.; Gilljam, M.; Lindblad, A.; Ridell, M.; Wold, A.E.; Welinder-Qlsson, C. Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis. J. Clin. Microbiol. 2007, 45, 1497–1504. [Google Scholar] [CrossRef]
- Bernut, A.; Viljoen, A.; Dupont, C.; Sapriel, G.; Blaise, M.; Bouchier, C.; Brosch, R.; de Chastellier, C.; Herrmann, J.; Kremer, L. Insights into the smooth-to-rough transitioning in Mycobacterium bolletii unravels a functional Tyr residue conserved in all mycobacterial MmpL family members. Mol. Microbiol. 2016, 99, 866–883. [Google Scholar] [CrossRef]
- Bernut, A.; Herrmann, J.-L.; Kissa, K.; Dubremetz, J.-F.; Gaillard, J.-L.; Lutfalla, G.; Kremer, L. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl. Acad. Sci. USA 2014, 111, E943–E952. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-R.; Kim, B.-J.; Kook, Y.-H.; Kim, B.-J. Mycobacterium abscessus infection leads to enhanced production of type 1 interferon and NLRP3 inflammasome activation in murine macrophages via mitochondrial oxidative stress. PLoS Pathog. 2020, 16, e1008294. [Google Scholar] [CrossRef]
- Le Moigne, V.; Bernut, A.; Cortès, M.; Viljoen, A.; Dupont, C.; Pawlik, A.; Gaillard, J.L.; Misguich, F.; Crémazy, F.; Kremer, L.; et al. Lsr2 is an important determinant of intracellular growth and virulence in Mycobacterium abscessus. Front. Microbiol. 2019, 10, 905. [Google Scholar] [CrossRef]
- Touré, H.; Galindo, L.A.; Lagune, M.; Glatigny, S.; Waterhouse, R.M.; Guénal, I.; Herrmann, J.-L.; Girard-Misguich, F.; Szuplewski, S. Mycobacterium abscessus resists the innate cellular response by surviving cell lysis of infected phagocytes. PLoS Pathog. 2023, 19, e1011257. [Google Scholar] [CrossRef]
- Bar-Oz, M.; Martini, M.C.; Alonso, M.N.; Meir, M.; Lore, N.I.; Miotto, P.; Riva, C.; Angala, S.K.; Xiao, J.; Masiello, C.S.; et al. The small non-coding RNA B11 regulates multiple facets of Mycobacterium abscessus virulence. PLoS Pathog. 2023, 19, e1011575. [Google Scholar] [CrossRef]
- Roux, A.-L.; Ray, A.; Pawlik, A.; Medjahed, H.; Etienne, G.; Rottman, M.; Catherinot, E.; Coppée, J.-Y.; Chaoui, K.; Monsarrat, B.; et al. Overexpression of proinflammatory TLR-2-signalling lipoproteins in hypervirulent mycobacterial variants. Cell. Microbiol. 2011, 13, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Nessar, R.; Reyrat, J.-M.; Davidson, L.B.; Byrd, T.F. Deletion of the mmpL4b gene in the Mycobacterium abscessus glycopeptidolipid biosynthetic pathway results in loss of surface colonization capability, but enhanced ability to replicate in human macrophages and stimulate their innate immune response. Microbiology 2011, 157, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Yam, Y.-K.; Alvarez, N.; Go, M.-L.; Dick, T. Extreme Drug Tolerance of Mycobacterium abscessus “Persisters”. Front. Microbiol. 2020, 11, 359. [Google Scholar] [CrossRef]
- Hunt-Serracin, A.C.; Parks, B.J.; Boll, J.; Boutte, C.C. Mycobacterium abscessus Cells Have Altered Antibiotic Tolerance and Surface Glycolipids in Artificial Cystic Fibrosis Sputum Medium. Antimicrob. Agents Chemother. 2019, 63, e02488-18. [Google Scholar] [CrossRef] [PubMed]
- Nahid, P.; Dorman, S.E.; Alipanah, N.; Barry, P.M.; Brozek, J.L.; Cattamanchi, A.; Chaisson, L.H.; Chaisson, R.E.; Daley, C.L.; Grzemska, M.; et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin. Infect. Dis. 2016, 63, e147–e195. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Marras, T.K.; Adjemian, J.; Zhang, H.; Wang, P.; Zhang, Q. Incidence and Prevalence of Nontuberculous Mycobacterial Lung Disease in a Large U.S. Managed Care Health Plan, 2008–2015. Ann. Am. Thorac. Soc. 2020, 17, 178–185. [Google Scholar] [CrossRef]
- Schäfer, J.; Griese, M.; Chandrasekaran, R.; Chotirmall, S.H.; Hartl, D. Pathogenesis, imaging and clinical characteristics of CF and non-CF bronchiectasis. BMC Pulm. Med. 2018, 18, 79. [Google Scholar] [CrossRef]
- Strassburg, C.P.; Manns, M.P. Autoimmune hepatitis. Best Pract. Res. Clin. Gastroenterol. 2003, 17, 291–306. [Google Scholar] [CrossRef]
- Turcios, N.L. Cystic Fibrosis Lung Disease: An Overview. Respir. Care 2020, 65, 233–251. [Google Scholar] [CrossRef]
- Tay, G.; Reid, D.; Bell, S. Inhaled Antibiotics in Cystic Fibrosis (CF) and Non-CF Bronchiectasis. Semin. Respir. Crit. Care Med. 2015, 36, 267–286. [Google Scholar] [CrossRef]
- Liessi, N.; Pesce, E.; Braccia, C.; Bertozzi, S.M.; Giraudo, A.; Bandiera, T.; Pedemonte, N.; Armirotti, A. Distinctive lipid signatures of bronchial epithelial cells associated with cystic fibrosis drugs, including Trikafta. JCI Insight 2020, 5, e138722. [Google Scholar] [CrossRef] [PubMed]
- Schnitker, F.; Liu, Y.; Keitsch, S.; Soddemann, M.; Verhasselt, H.L.; Kehrmann, J.; Grassmé, H.; Kamler, M.; Gulbins, E.; Wu, Y. Reduced Sphingosine in Cystic Fibrosis Increases Susceptibility to Mycobacterium abscessus Infections. Int. J. Mol. Sci. 2023, 24, 14004. [Google Scholar] [CrossRef] [PubMed]
- Trinh, N.T.N.; Bardou, O.; Privé, A.; Maillé, E.; Adam, D.; Lingée, S.; Ferraro, P.; Desrosiers, M.-Y.Y.; Coraux, C.; Brochiero, E. Improvement of defective cystic fibrosis airway epithelial wound repair after CFTR rescue. Eur. Respir. J. 2012, 40, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.Z.; Wagener, J.S.; Bost, T.; Martinez, J.; Accurso, F.J.; Riches, D.W. Early pulmonary inflammation in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1995, 151, 1075–1082. [Google Scholar] [CrossRef]
- Elizur, A.; Cannon, C.L.; Ferkol, T.W. Airway Inflammation in Cystic Fibrosis. Chest 2008, 133, 489–495. [Google Scholar] [CrossRef]
- Cohen, T.S.; Prince, A. Cystic fibrosis: A mucosal immunodeficiency syndrome. Nat. Med. 2012, 18, 509–519. [Google Scholar] [CrossRef]
- Nichols, D.P.; Chmiel, J.F. Inflammation and its genesis in cystic fibrosis. Pediatr. Pulmonol. 2015, 50, S39–S56. [Google Scholar] [CrossRef]
- Montanini, L.; Smerieri, A.; Gullì, M.; Cirillo, F.; Pisi, G.; Sartori, C.; Amarri, S.; Bernasconi, S.; Marmiroli, N.; Street, M.E. miR-146a, miR-155, miR-370, and miR-708 Are CFTR-Dependent, Predicted FOXO1 Regulators and Change at Onset of CFRDs. J. Clin. Endocrinol. Metab. 2016, 101, 4955–4963. [Google Scholar] [CrossRef]
- Roussel, L.; Farias, R.; Rousseau, S. IL-33 is expressed in epithelia from patients with cystic fibrosis and potentiates neutrophil recruitment. J. Allergy Clin. Immunol. 2013, 131, 913–916. [Google Scholar] [CrossRef]
- Bernut, A.; Dupont, C.; Ogryzko, N.V.; Neyret, A.; Herrmann, J.-L.; Floto, R.A.; Renshaw, S.A.; Kremer, L. CFTR Protects against Mycobacterium abscessus Infection by Fine-Tuning Host Oxidative Defenses. Cell Rep. 2019, 26, 1828–1840.e4. [Google Scholar] [CrossRef]
- Wang, G.; Nauseef, W.M. Neutrophil dysfunction in the pathogenesis of cystic fibrosis. Blood 2022, 139, 2622–2631. [Google Scholar] [CrossRef]
- Ng, H.P.; Jennings, S.; Wellems, D.; Sun, F.; Xu, J.; Nauseef, W.M.; Wang, G. Myeloid CFTR loss-of-function causes persistent neutrophilic inflammation in cystic fibrosis. J. Leukoc. Biol. 2020, 108, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Pohl, K.; Grimm, X.A.; Caceres, S.M.; Poch, K.R.; Rysavy, N.; Saavedra, M.; Nick, J.A.; Malcolm, K.C. Mycobacterium abscessus Clearance by Neutrophils Is Independent of Autophagy. Infect. Immun. 2020, 88, e00024-20. [Google Scholar] [CrossRef]
- Lévêque, M.; Le Trionnaire, S.; Del Porto, P.; Martin-Chouly, C. The impact of impaired macrophage functions in cystic fibrosis disease progression. J. Cyst. Fibros. 2017, 16, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Ratner, D.; Mueller, C. Immune responses in cystic fibrosis: Are they intrinsically defective? Am. J. Respir. Cell Mol. Biol. 2012, 46, 715–722. [Google Scholar] [CrossRef]
- Button, B.; Anderson, W.H.; Boucher, R.C. Mucus Hyperconcentration as a Unifying Aspect of the Chronic Bronchitic Phenotype. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S2), S156–S162. [Google Scholar]
- Esther, C.R.J.; Muhlebach, M.S.; Ehre, C.; Hill, D.B.; Wolfgang, M.C.; Kesimer, M.; Ramsey, K.A.; Markovetz, M.R.; Garbarine, I.C.; Forest, M.G.; et al. Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci. Transl. Med. 2019, 11, eaav3488. [Google Scholar] [CrossRef]
- Margaroli, C.; Horati, H.; Garratt, L.W.; Giacalone, V.D.; Schofield, C.; Dittrich, A.S.; Rosenow, T.; Dobosh, B.S.; Lim, H.S.; Frey, D.L.; et al. Macrophage PD-1 associates with neutrophilia and reduced bacterial killing in early cystic fibrosis airway disease. J. Cyst. Fibros. 2022, 21, 967–976. [Google Scholar] [CrossRef]
- Roesch, E.A.; Nichols, D.P.; Chmiel, J.F. Inflammation in cystic fibrosis: An update. Pediatr. Pulmonol. 2018, 53, S30–S50. [Google Scholar] [CrossRef]
- Zhang, S.; Shrestha, C.L.; Kopp, B.T. Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have differential effects on cystic fibrosis macrophage function. Sci. Rep. 2018, 8, 17066. [Google Scholar] [CrossRef] [PubMed]
- Aridgides, D.S.; Mellinger, D.L.; Gwilt, L.L.; Hampton, T.H.; Mould, D.L.; Hogan, D.A.; Ashare, A. Comparative effects of CFTR modulators on phagocytic, metabolic and inflammatory profiles of CF and nonCF macrophages. Sci. Rep. 2023, 13, 11995. [Google Scholar] [CrossRef]
- Wiesel, V.; Aviram, M.; Mei-Zahav, M.; Dotan, M.; Prais, D.; Cohen-Cymberknoh, M.; Gur, M.; Bar-Yoseph, R.; Livnat, G.; Goldbart, A.; et al. Eradication of Nontuberculous Mycobacteria in People with Cystic Fibrosis Treated with Elexacaftor/Tezacaftor/Ivacaftor: A Multicenter Cohort Study. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2024, 23, 41–49. [Google Scholar] [CrossRef]
- Bryant, J.M.; Brown, K.P.; Burbaud, S.; Everall, I.; Belardinelli, J.M.; Rodriguez-Rincon, D.; Grogono, D.M.; Peterson, C.M.; Verma, D.; Evans, I.E.; et al. Stepwise pathogenic evolution of Mycobacterium abscessus. Science 2021, 372, eabb8699. [Google Scholar] [CrossRef]
- Louw, G.E.; Warren, R.M.; Gey van Pittius, N.C.; McEvoy, C.R.E.; Van Helden, P.D.; Victor, T.C. A Balancing Act: Efflux/Influx in Mycobacterial Drug Resistance. Antimicrob. Agents Chemother. 2009, 53, 3181–3189. [Google Scholar] [CrossRef] [PubMed]
- Leestemaker-Palmer, A.; Bermudez, L.E. Mycobacteroides abscessus ability to interact with the host mucosal cells plays an important role in pathogenesis of the infection. Crit. Rev. Microbiol. 2024, 25, 1–13. [Google Scholar] [CrossRef]
- Miranda-CasoLuengo, A.A.; Staunton, P.M.; Dinan, A.M.; Lohan, A.J.; Loftus, B.J. Functional characterization of the Mycobacterium abscessus genome coupled with condition specific transcriptomics reveals conserved molecular strategies for host adaptation and persistence. BMC Genom. 2016, 17, 553. [Google Scholar] [CrossRef]
- Rojony, R.; Danelishvili, L.; Campeau, A.; Wozniak, J.M.; Gonzalez, D.J.; Bermudez, L.E. Exposure of Mycobacterium abscessus to Environmental Stress and Clinically Used Antibiotics Reveals Common Proteome Response among Pathogenic Mycobacteria. Microorganisms 2020, 8, 698. [Google Scholar] [CrossRef]
- LiPuma, J.J. The Changing Microbial Epidemiology in Cystic Fibrosis. Clin. Microbiol. Rev. 2010, 23, 299–323. [Google Scholar] [CrossRef]
- Wu, W.; Jin, Y.; Bai, F.; Jin, S. Pseudomonas aeruginosa. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 753–767. [Google Scholar]
- Rodríguez-Sevilla, G.; Crabbé, A.; García-Coca, M.; Aguilera-Correa, J.J.; Esteban, J.; Pérez-Jorge, C. Antimicrobial Treatment Provides a Competitive Advantage to Mycobacterium abscessus in a Dual-Species Biofilm with Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 63, e01547-19. [Google Scholar] [CrossRef] [PubMed]
- Idosa, A.W.; Wozniak, D.J.; Hall-Stoodley, L. Surface Dependent Inhibition of Mycobacterium abscessus by Diverse Pseudomonas aeruginosa Strains. Microbiol. Spectr. 2022, 10, e0247122. [Google Scholar] [CrossRef]
- Long, Y.; Li, Z.; Li, M.; Lu, P.; Deng, Y.; Wu, P.; Li, X.; Qin, G.; Huang, J.; Gao, W.; et al. Pseudomonas aeruginosa pqs Quorum Sensing Mediates Interaction with Mycobacterium abscessus In Vitro. Microorganisms 2025, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Birmes, F.S.; Säring, R.; Hauke, M.C.; Ritzmann, N.H.; Drees, S.L.; Daniel, J.; Treffon, J.; Liebau, E.; Kahl, B.C.; Fetzner, S. Interference with Pseudomonas aeruginosa Quorum Sensing and Virulence by the Mycobacterial Pseudomonas Quinolone Signal Dioxygenase AqdC in Combination with the N-Acylhomoserine Lactone Lactonase QsdA. Infect. Immun. 2019, 87, e00278-19. [Google Scholar] [CrossRef]
- Birmes, F.S.; Wolf, T.; Kohl, T.A.; Rüger, K.; Bange, F.; Kalinowski, J.; Fetzner, S. Mycobacterium abscessus subsp. abscessus Is Capable of Degrading Pseudomonas aeruginosa Quinolone Signals. Front. Microbiol. 2017, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J.J.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur. Respir. J. 2020, 56, e1–e36. [Google Scholar] [CrossRef]
- Floto, R.A.; Olivier, K.N.; Saiman, L.; Daley, C.L.; Herrmann, J.-L.; Nick, J.A.; Noone, P.G.; Bilton, D.; Corris, P.; Gibson, R.L.; et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 2016, 71 (Suppl. 1), i1–i22. [Google Scholar] [CrossRef]
- Deppisch, C.; Herrmann, G.; Graepler-Mainka, U.; Wirtz, H.; Heyder, S.; Engel, C.; Marschal, M.; Miller, C.C.; Riethmüller, J. Gaseous nitric oxide to treat antibiotic resistant bacterial and fungal lung infections in patients with cystic fibrosis: A phase I clinical study. Infection 2016, 44, 513–520. [Google Scholar] [CrossRef]
- Vitiello, A.; Blasi, F.; Sabbatucci, M.; Zovi, A.; Miele, F.; Ponzo, A.; Langella, R.; Boccellino, M. The Impact of Antimicrobial Resistance in Cystic Fibrosis. J. Clin. Med. 2024, 13, 1711. [Google Scholar] [CrossRef]
- Nash, K.A.; Brown-Elliott, A.B.; Wallace, R.J. A Novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob. Agents Chemother. 2009, 53, 1367–1376. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Luthra, S.; Rominski, A.; Sander, P. The role of antibiotic-target-modifying and antibiotic-modifying enzymes in Mycobacterium abscessus drug resistance. Front. Microbiol. 2018, 9, 2179. [Google Scholar] [CrossRef] [PubMed]
- Wivagg, C.N.; Bhattacharyya, R.P.; Hung, D.T. Mechanisms of β-lactam killing and resistance in the context of Mycobacterium tuberculosis. J. Antibiot. 2014, 67, 645–654. [Google Scholar] [CrossRef]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014, 12, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Rudra, P.; Hurst-Hess, K.; Lappierre, P.; Ghosh, P. High Levels of Intrinsic Tetracycline Resistance in Mycobacterium abscessus Are Conferred by a Tetracycline-Modifying Monooxygenase. Antimicrob. Agents Chemother. 2018, 62, e00119-18. [Google Scholar] [CrossRef]
- Mudde, S.E.; Schildkraut, J.A.; Ammerman, N.C.; de Vogel, C.P.; de Steenwinkel, J.E.M.; van Ingen, J.; Bax, H.I. Unraveling antibiotic resistance mechanisms in Mycobacterium abscessus: The potential role of efflux pumps. J. Glob. Antimicrob. Resist. 2022, 31, 345–352. [Google Scholar] [CrossRef]
- Hooper, D.C. Mechanisms of Action and Resistance of Older and Newer Fluoroquinolones. Clin. Infect. Dis. 2000, 31, S24–S28. [Google Scholar] [CrossRef]
- Hooper, D.C. Mechanisms of fluoroquinolone resistance. Drug Resist. Updat. 1999, 2, 38–55. [Google Scholar] [CrossRef]
- Bozdogan, B.; Appelbaum, P.C. Oxazolidinones: Activity, mode of action, and mechanism of resistance. Int. J. Antimicrob. Agents 2004, 23, 113–119. [Google Scholar] [CrossRef]
- Sköld, O. Sulfonamide resistance: Mechanisms and trends. Drug Resist. Updat. 2000, 3, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Recchia, D.; Stelitano, G.; Stamilla, A.; Gutierrez, D.L.; Degiacomi, G.; Chiarelli, L.R.; Pasca, M.R. Mycobacterium abscessus Infections in Cystic Fibrosis Individuals: A Review on Therapeutic Options. Int. J. Mol. Sci. 2023, 24, 4635. [Google Scholar] [CrossRef]
- Witek, J.; Lakhkar, A.D. Nitric Oxide. In Encyclopedia of Toxicology, Fourth Edition Volume 1–9; Academic Press: Cambridge, MA, USA, 2023; Volume 6, pp. V6-817–V6-820. [Google Scholar] [CrossRef]
- Bentur, L.; Gur, M.; Ashkenazi, M.; Livnat-Levanon, G.; Mizrahi, M.; Tal, A.; Ghaffari, A.; Geffen, Y.; Aviram, M.; Efrati, O. Pilot study to test inhaled nitric oxide in cystic fibrosis patients with refractory Mycobacterium abscessus lung infection. J. Cyst. Fibros. 2020, 19, 225–231. [Google Scholar] [CrossRef]
- Yaacoby-Bianu, K.; Gur, M.; Toukan, Y.; Nir, V.; Hakim, F.; Geffen, Y.; Bentur, L. Compassionate Nitric Oxide Adjuvant Treatment of Persistent Mycobacterium Infection in Cystic Fibrosis Patients. Pediatr. Infect. Dis. J. 2018, 37, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.J.; Olivier, K.N. Nontuberculous Mycobacteria in Cystic Fibrosis. Semin. Respir. Crit. Care Med. 2019, 40, 737–750. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Smith, B.E.; Cristinziano, M.; Freeman, K.G.; Jacobs-Sera, D.; Belessis, Y.; Brown, A.W.; Cohen, K.A.; Davidson, R.M.; van Duin, D.; et al. Phage Therapy of Mycobacterium Infections: Compassionate Use of Phages in 20 Patients With Drug-Resistant Mycobacterial Disease. Clin. Infect. Dis. 2023, 76, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Gorzynski, M.; De Ville, K.; Week, T.; Jaramillo, T.; Danelishvili, L. Understanding the Phage–Host Interaction Mechanism toward Improving the Efficacy of Current Antibiotics in Mycobacterium abscessus. Biomedicines 2023, 11, 1379. [Google Scholar] [CrossRef]
- Palucci, I.; Delogu, G. Alternative therapies against Mycobacterium abscessus infections. Clin. Microbiol. Infect. 2024, 30, 732–737. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, S.H.; Jeon, S.M.; Silwal, P.; Seo, J.Y.; Hanh, B.T.B.; Park, J.W.; Whang, J.; Lee, M.J.; Heo, J.Y.; et al. Sirtuin 3 is essential for host defense against Mycobacterium abscessus infection through regulation of mitochondrial homeostasis. Virulence 2020, 11, 1225–1239. [Google Scholar] [CrossRef]
- Kim, J.S.; Cha, S.H.; Kim, W.S.; Han, S.J.; Cha, S.B.; Kim, H.M.; Kwon, K.W.; Kim, S.J.; Choi, H.H.; Lee, J.; et al. A Novel Therapeutic Approach Using Mesenchymal Stem Cells to Protect Against Mycobacterium abscessus. Stem Cells 2016, 34, 1957–1970. [Google Scholar] [CrossRef]
- Dong, K.; Jang, J.; Shannon, C.P.; Ng, R.; Tebbutt, S.J.; Quon, B.S. Blood Transcriptomic and Inflammatory Protein Biomarkers Associated with Imminent Pulmonary Exacerbation Risk in Cystic Fibrosis. Ann. Am. Thorac. Soc. 2024, 21, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Nick, J.A.; Malcolm, K.C.; Hisert, K.B.; Wheeler, E.A.; Rysavy, N.M.; Poch, K.; Caceres, S.; Lovell, V.K.; Armantrout, E.; Saavedra, M.T.; et al. Culture independent markers of nontuberculous mycobacterial (NTM) lung infection and disease in the cystic fibrosis airway. Tuberculosis 2023, 138, 102276. [Google Scholar] [CrossRef] [PubMed]
- Bolden, N.; Mell, J.C.; Logan, J.B.; Planet, P.J. Phylogenomics of nontuberculous mycobacteria respiratory infections in people with cystic fibrosis. Paediatr. Respir. Rev. 2023, 46, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Breen, P.; Zimbric, M.; Opron, K.; Caverly, L.J. Sputum Metabolites Associated with Nontuberculous Mycobacterial Infection in Cystic Fibrosis. mSphere 2022, 7, e0010422. [Google Scholar] [CrossRef]
- Plebani, R.; Potla, R.; Soong, M.; Bai, H.; Izadifar, Z.; Jiang, A.; Travis, R.N.; Belgur, C.; Dinis, A.; Cartwright, M.J.; et al. Modeling pulmonary cystic fibrosis in a human lung airway-on-a-chip. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2022, 21, 606–615. [Google Scholar] [CrossRef]
- Mattoscio, D.; Baeza, L.A.; Bai, H.; Colangelo, T.; Castagnozzi, S.; Marzotto, M.; Cufaro, M.C.; Lotti, V.; Yuan, Y.-C.; Mucci, M.; et al. Inflammation and epithelial-mesenchymal transition in a CFTR-depleted human bronchial epithelial cell line revealed by proteomics and human organ-on-a-chip. FEBS J. 2025. [Google Scholar] [CrossRef] [PubMed]
| Antibiotics Active Against Mab | Antibiotic Class | Mechanisms of Action | Resistance Mechanisms |
|---|---|---|---|
| Azithromycin Clarithromycin | Macrolides | Inhibit bacterial protein synthesis by binding to the 50S subunit of the bacterial ribosome | Synthesis of erythromycin resistance methylase 41 (erm 41)—modifies the ribosomal binding site through methylation of the 23S rRNA [101] |
| Amikacin | Aminoglycosides | Inhibits bacterial protein synthesis by binding to the 16S rRNA of the 30S ribosomal subunit [98,102,103] | Aminoglycoside-modifying enzymes, such as Eis2 and AAC(2′)-II—prevent binding to the ribosomal unit [104] |
| Cefoxitin | Cephalosporins | β-lactam antibiotics—inhibit cell wall synthesis | Decreased cell wall permeability; production of β-lactamase enzymes [105] |
| Imipenem Meropenem | Carbapenems | ||
| Tigecycline Minocycline | Tetracyclines | Inhibit bacterial protein synthesis, by binding to the 30S ribosomal subunit [106] | Tetracycline-modifying enzymes, e.g., monooxygenases—inactivate the antibiotic [107]; efflux pumps—actively expel antibiotics from the bacterial cell [108] |
| Moxifloxacin | Fluoroquinolones | Inhibits bacterial DNA replication by binding to the DNA gyrase and topoisomerase IV enzyme complexes [109] | Mutations in the antibiotic target enzymes—reduce binding affinity, limiting antibiotic access to the binding site [110] |
| Linezolid | Oxazolidinones | Inhibit bacterial protein synthesis by binding to the P site of the 50S ribosomal subunit. | Alterations in the 23S rRNA—reduce binding affinity [111] |
| TMP-SMX | Sulfonamides | Bind the enzyme dihydropteroate synthase enzyme therefore preventing synthesis of folic acid | Modifications in the dihydropteroate synthase enzyme—reduce binding affinity [112] |
| Knowledge Gaps | Research Questions |
|---|---|
| Pathogenicity of Mab Is Mab virulence primarily driven by genetic factors, epigenetic regulation, or a combination of both? |
|
| Host Immune Response in CF Is immune dysfunction in pwCF an intrinsic feature of the disease, or is it a consequence of the altered airway environment?? |
|
| Pathogen interactions within the CF airway |
|
| Advanced and Novel Therapies |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basher, M.; Gur, M.; Meir, M. Insights on the Pathogenesis of Mycobacterium abscessus Infection in Patients with Cystic Fibrosis. J. Clin. Med. 2025, 14, 3492. https://doi.org/10.3390/jcm14103492
Basher M, Gur M, Meir M. Insights on the Pathogenesis of Mycobacterium abscessus Infection in Patients with Cystic Fibrosis. Journal of Clinical Medicine. 2025; 14(10):3492. https://doi.org/10.3390/jcm14103492
Chicago/Turabian StyleBasher, Mai, Michal Gur, and Michal Meir. 2025. "Insights on the Pathogenesis of Mycobacterium abscessus Infection in Patients with Cystic Fibrosis" Journal of Clinical Medicine 14, no. 10: 3492. https://doi.org/10.3390/jcm14103492
APA StyleBasher, M., Gur, M., & Meir, M. (2025). Insights on the Pathogenesis of Mycobacterium abscessus Infection in Patients with Cystic Fibrosis. Journal of Clinical Medicine, 14(10), 3492. https://doi.org/10.3390/jcm14103492






