Hemolysis and Its Clinical Implications in Septic Patients with Acute Respiratory Failure
Abstract
1. Introduction
2. Materials and Methods
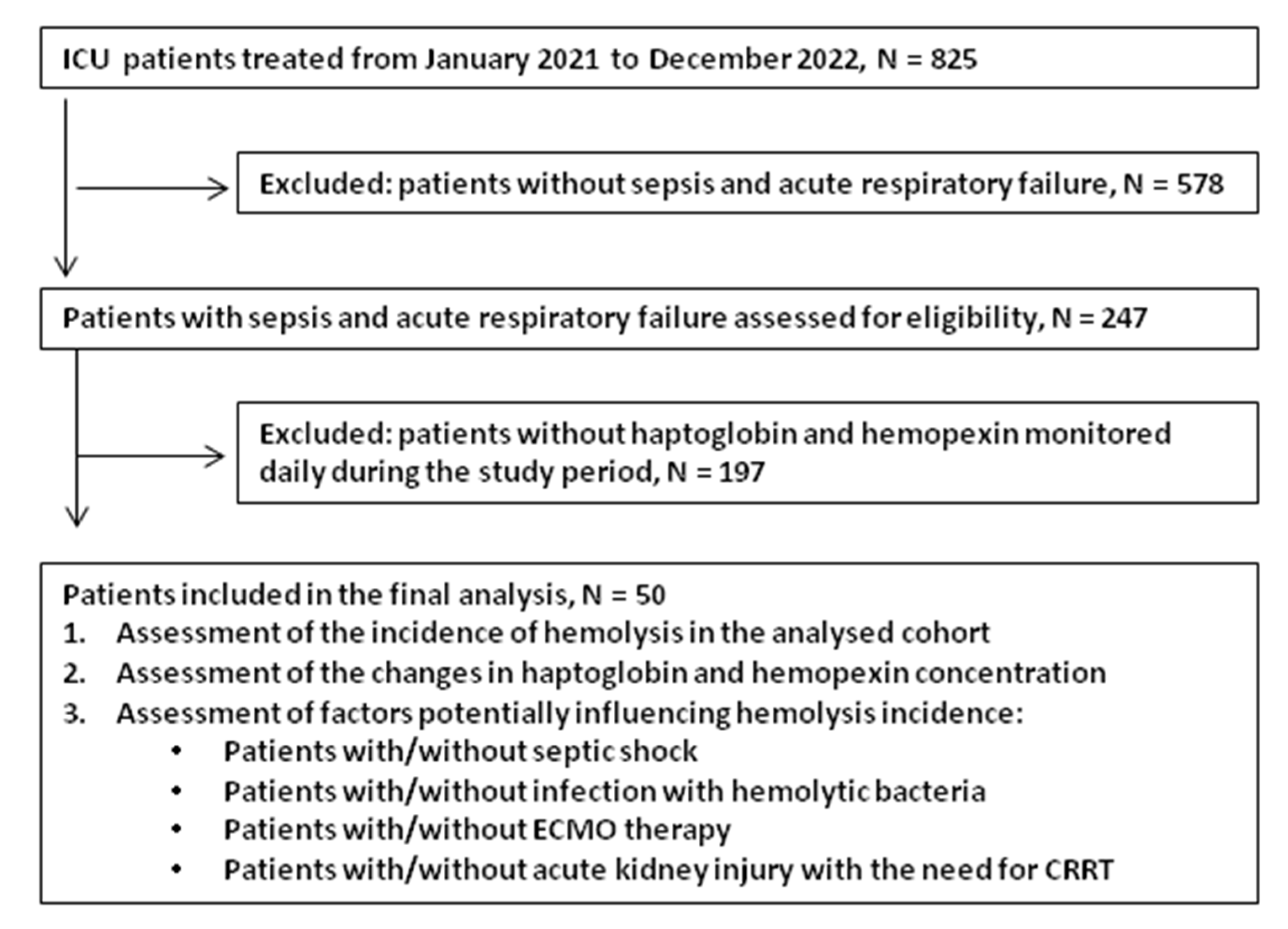
2.1. Patients
2.2. Data Collection
2.3. Hemolysis Measurements
2.4. Patient Management
2.5. Statistical Analysis
3. Results
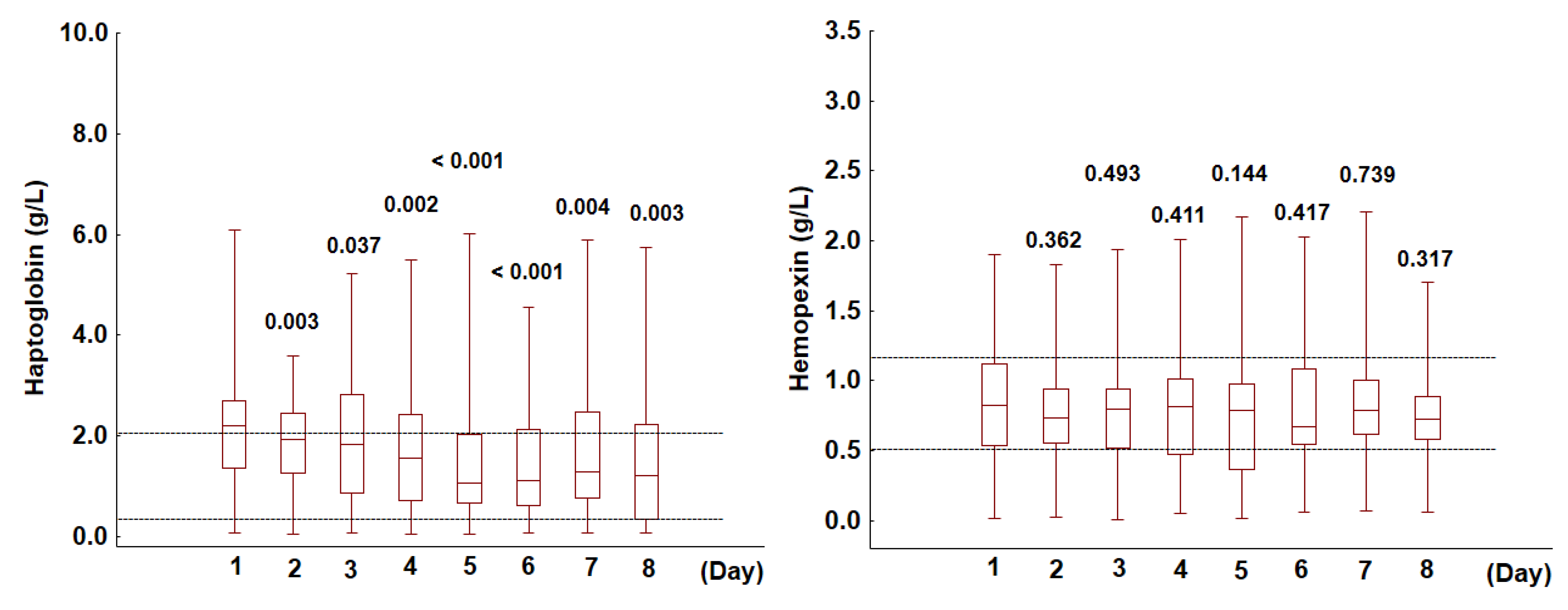
3.1. Haptoglobin and Hemopexin in the Analyzed Cohort
3.2. Evaluation of Factors Potentially Influencing Hemolysis Incidence
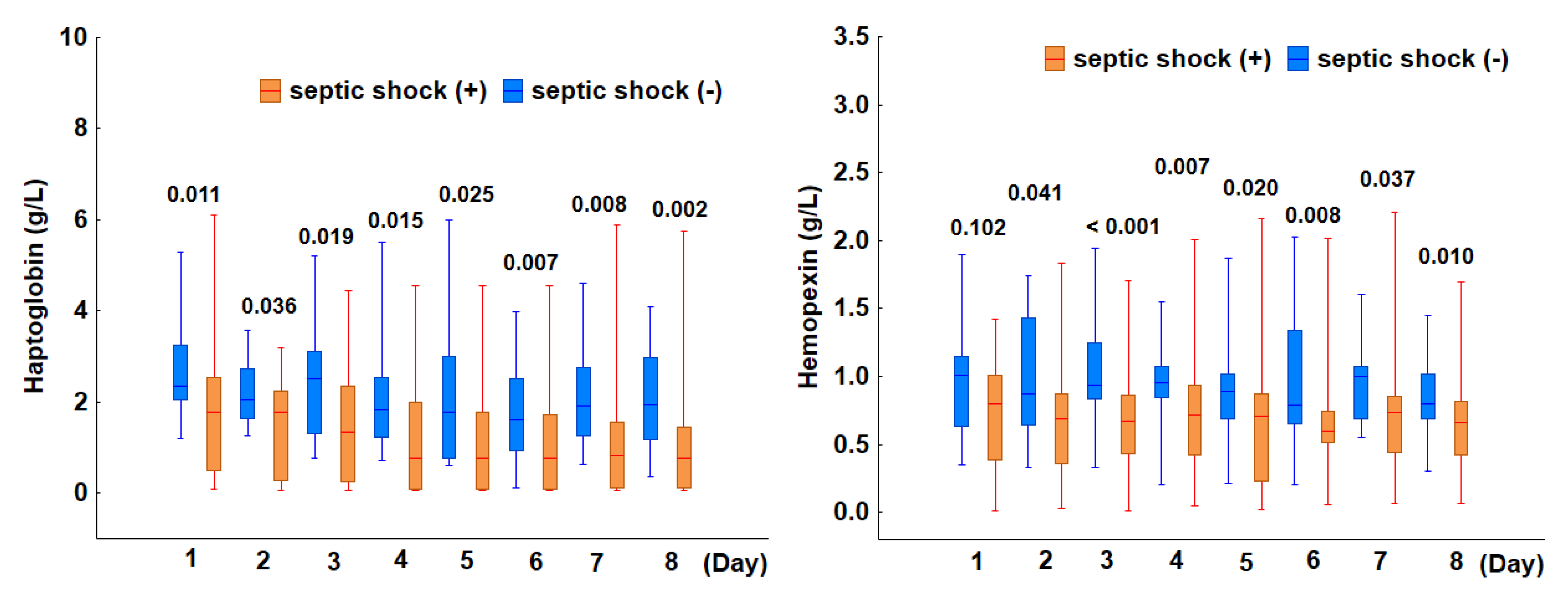
3.2.1. Incidence of Hemolysis in Patients with Septic Shock
3.2.2. Incidence of Hemolysis in Patients with Bacterial Infections
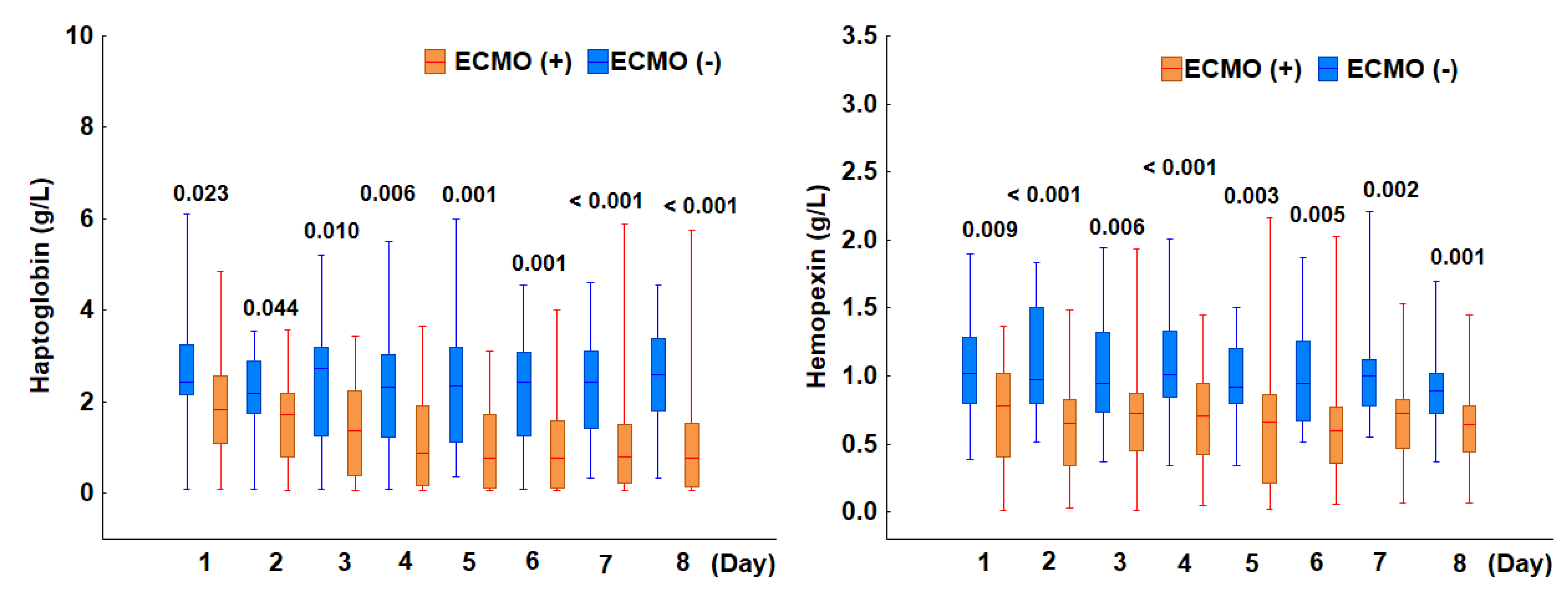
3.2.3. Incidence of Hemolysis in Patients Treated with ECMO
3.2.4. Incidence of Hemolysis in Patients Undergoing Continuous Renal Replacement Therapy
3.3. Prediction of ICU Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.J.; Law, S.K.A.; Moestrup, S.K. Identification of the Haemoglobin Scavenger Receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Drvenica, I.T.; Stančić, A.Z.; Maslovarić, I.S.; Trivanović, D.I.; Ilić, V.L. Extracellular Hemoglobin: Modulation of Cellular Functions and Pathophysiological Effects. Biomolecules 2022, 12, 1708. [Google Scholar] [CrossRef]
- Effenberger-Neidnicht, K.; Hartmann, M. Mechanisms of Hemolysis During Sepsis. Inflammation 2018, 41, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lin, K.; Huang, L.; Jiang, Y.; Ding, X.; Luo, W.; Samorodov, A.V.; Pavlov, V.N.; Liang, G.; Qian, J.; et al. Heme Induces Inflammatory Injury by Directly Binding to the Complex of Myeloid Differentiation Protein 2 and Toll-like Receptor 4. Toxicol. Lett. 2022, 370, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kerchberger, V.E.; Ware, L.B. The Role of Circulating Cell-Free Hemoglobin in Sepsis-Associated Acute Kidney Injury. Semin. Nephrol. 2020, 40, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Yu, P.; Ni, J.; Zhou, J. Higher Serum Haptoglobin Levels Were Associated with Improved Outcomes of Patients with Septic Shock. Crit. Care 2022, 26, 131. [Google Scholar] [CrossRef]
- Janz, D.R.; Bastarache, J.A.; Sills, G.; Wickersham, N.; May, A.K.; Bernard, G.R.; Ware, L.B. Association between Haptoglobin, Hemopexin and Mortality in Adults with Sepsis. Crit. Care 2013, 17, R272. [Google Scholar] [CrossRef]
- Verheij, M.W.; Bulder, I.; Wuillemin, W.A.; Voermans, C.; Zeerleder, S.S. Scavengers of Hemoproteins as Potential Biomarkers for Severe Sepsis and Septic Shock. Transl. Med. Commun. 2021, 6, 8. [Google Scholar] [CrossRef]
- Rezoagli, E.; Bombino, M.; Ware, L.B.; Carlesso, E.; Rona, R.; Grasselli, G.; Pesenti, A.; Bellani, G.; Foti, G. Signs of Hemolysis Predict Mortality and Ventilator Associated Pneumonia in Severe Acute Respiratory Distress Syndrome Patients Undergoing Veno-Venous Extracorporeal Membrane Oxygenation. ASAIO J. 2025, 71, 82–91. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). J. Am. Med. Assoc. 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Osmancik, P.; Bacova, B.; Herman, D.; Hozman, M.; Fiserova, I.; Hassouna, S.; Melenovsky, V.; Karch, J.; Vesela, J.; Benesova, K.; et al. Periprocedural Intravascular Hemolysis During Atrial Fibrillation Ablation: A Comparison of Pulsed Field With Radiofrequency Ablation. JACC. Clin. Electrophysiol. 2024, 10, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Fraering, J.; Salnot, V.; Gautier, E.F.; Ezinmegnon, S.; Argy, N.; Peoc’h, K.; Manceau, H.; Alao, J.; Guillonneau, F.; Migot-Nabias, F.; et al. Infected Erythrocytes and Plasma Proteomics Reveal a Specific Protein Signature of Severe Malaria. EMBO Mol. Med. 2024, 16, 319. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. J. Am. Med. Assoc. 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Kellum, J.A. Diagnostic Criteria for Acute Kidney Injury: Present and Future. Crit. Care Clin. 2015, 31, 621. [Google Scholar] [CrossRef]
- Qian, Q.; Nath, K.A.; Wu, Y.; Daoud, T.M.; Sethi, S. Hemolysis and Acute Kidney Failure. Am. J. Kidney Dis. 2010, 56, 780. [Google Scholar] [CrossRef]
- Baldetti, L.; Labanca, R.; Belletti, A.; Dias-Frias, A.; Peveri, B.; Kotani, Y.; Fresilli, S.; Calvo, F.; Fominskiy, E.; Pieri, M.; et al. Haptoglobin Administration for Intravascular Hemolysis: A Systematic Review. Blood Purif. 2024, 53, 851–859. [Google Scholar] [CrossRef]
- Vinchi, F.; De Franceschi, L.; Ghigo, A.; Townes, T.; Cimino, J.; Silengo, L.; Hirsch, E.; Altruda, F.; Tolosano, E. Hemopexin Therapy Improves Cardiovascular Function by Preventing Heme-Induced Endothelial Toxicity in Mouse Models of Hemolytic Diseases. Circulation 2013, 127, 1317–1329. [Google Scholar] [CrossRef]
- Belcher, J.D.; Chen, C.; Nguyen, J.; Milbauer, L.; Abdulla, F.; Alayash, A.I.; Smith, A.; Nath, K.A.; Hebbel, R.P.; Vercellotti, G.M. Heme Triggers TLR4 Signaling Leading to Endothelial Cell Activation and Vaso-Occlusion in Murine Sickle Cell Disease. Blood 2014, 123, 377–390. [Google Scholar] [CrossRef]
- Liang, X.; Lin, T.; Sun, G.; Beasley-Topliffe, L.; Cavaillon, J.-M.; Warren, H.S. Hemopexin Down-Regulates LPS-Induced Proinflammatory Cytokines from Macrophages. J. Leukoc. Biol. 2009, 86, 229–235. [Google Scholar] [CrossRef]
- Kubota, K.; Egi, M.; Mizobuchi, S. Haptoglobin Administration in Cardiovascular Surgery Patients: Its Association With the Risk of Postoperative Acute Kidney Injury. Anesth. Analg. 2017, 124, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Bünger, V.; Hunsicker, O.; Krannich, A.; Balzer, F.; Spies, C.D.; Kuebler, W.M.; Weber-Carstens, S.; Menk, M.; Graw, J.A. Potential of Cell-Free Hemoglobin and Haptoglobin as Prognostic Markers in Patients with ARDS and Treatment with Veno-Venous ECMO. J. Intensive Care 2023, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Schaer, D.J.; Buehler, P.W.; Alayash, A.I.; Belcher, J.D.; Vercellotti, G.M. Hemolysis and Free Hemoglobin Revisited: Exploring Hemoglobin and Hemin Scavengers as a Novel Class of Therapeutic Proteins. Blood 2012, 121, 1276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kinzie, E.; Berger, F.G.; Lim, S.K.; Baumann, H. Haptoglobin, an Inflammation-Inducible Plasma Protein. Redox Rep. 2001, 6, 379–385. [Google Scholar] [CrossRef]
- Huntoon, K.M.; Wang, Y.; Eppolito, C.A.; Barbour, K.W.; Berger, F.G.; Shrikant, P.A.; Baumann, H. The Acute Phase Protein Haptoglobin Regulates Host Immunity. J. Leukoc. Biol. 2008, 84, 170–181. [Google Scholar] [CrossRef]
- Gorecki, G.; Cochior, D.; Moldovan, C.; Rusu, E. Molecular Mechanisms in Septic Shock (Review). Exp. Ther. Med. 2021, 22, 1161. [Google Scholar] [CrossRef]
- Chang, J.C. Sepsis and Septic Shock: Endothelial Molecular Pathogenesis Associated with Vascular Microthrombotic Disease. Thromb. J. 2019, 17, 10. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and Septic Shock. Nat. Rev. Dis. Prim. 2016, 2, 16045. [Google Scholar] [CrossRef]
- Charchaflieh, J.; Wei, J.; Labaze, G.; Hou, Y.J.; Babarsh, B.; Stutz, H.; Lee, H.; Worah, S.; Zhang, M. The Role of Complement System in Septic Shock. J. Immunol. Res. 2012, 2012, 407324. [Google Scholar] [CrossRef]
- Schaer, C.A.; Jeger, V.; Gentinetta, T.; Spahn, D.R.; Vallelian, F.; Rudiger, A.; Schaer, D.J. Haptoglobin Treatment Prevents Cell-Free Hemoglobin Exacerbated Mortality in Experimental Rat Sepsis. Intensive Care Med. Exp. 2021, 9, 22. [Google Scholar] [CrossRef]
- Remy, K.E.; Cortés-Puch, I.; Solomon, S.B.; Sun, J.; Pockros, B.M.; Feng, J.; Lertora, J.J.; Hantgan, R.R.; Liu, X.; Perlegas, A.; et al. Haptoglobin Improves Shock, Lung Injury, and Survival in Canine Pneumonia. JCI Insight 2018, 3, e123013. [Google Scholar] [CrossRef]
- Bastarache, J.A.; Roberts, L.J.; Ware, L.B. Thinking Outside the Cell: How Cell-Free Hemoglobin Can Potentiate Acute Lung Injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L231–L232. [Google Scholar] [CrossRef]
- Shaver, C.M.; Upchurch, C.P.; Janz, D.R.; Grove, B.S.; Putz, N.D.; Wickersham, N.E.; Dikalov, S.I.; Ware, L.B.; Bastarache, J.A. Cell-Free Hemoglobin: A Novel Mediator of Acute Lung Injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L532–L541. [Google Scholar] [CrossRef]
- Janz, D.R.; Ware, L.B. The Role of Red Blood Cells and Cell-Free Hemoglobin in the Pathogenesis of ARDS. J. Intensive Care 2015, 3, 20. [Google Scholar] [CrossRef]
- Materne, L.A.; Hunsicker, O.; Menk, M.; Graw, J.A. Hemolysis in Patients with Extracorporeal Membrane Oxygenation Therapy for Severe Acute Respiratory Distress Syndrome—A Systematic Review of the Literature. Int. J. Med. Sci. 2021, 18, 1730–1738. [Google Scholar] [CrossRef]
- Lou, S.; MacLaren, G.; Best, D.; Delzoppo, C.; Butt, W. Hemolysis in Pediatric Patients Receiving Centrifugal-Pump Extracorporeal Membrane Oxygenation: Prevalence, Risk Factors, and Outcomes. Crit. Care Med. 2014, 42, 1213–1220. [Google Scholar] [CrossRef]
- Sniderman, J.; Monagle, P.; Annich, G.M.; MacLaren, G. Hematologic Concerns in Extracorporeal Membrane Oxygenation. Res. Pract. Thromb. Haemost. 2020, 4, 455–468. [Google Scholar] [CrossRef]
- Zangrillo, A.; Landoni, G.; Biondi-Zoccai, G.; Greco, M.; Greco, T.; Frati, G.; Patroniti, N.; Antonelli, M.; Pesenti, A.; Pappalardo, F. A Meta-Analysis of Complications and Mortality of Extracorporeal Membrane Oxygenation. Crit. Care Resusc. 2013, 15, 172–178. [Google Scholar] [CrossRef]
- Gbadegesin, R.; Zhao, S.; Charpie, J.; Brophy, P.D.; Smoyer, W.E.; Lin, J.J. Significance of Hemolysis on Extracorporeal Life Support after Cardiac Surgery in Children. Pediatr. Nephrol. 2009, 24, 589–595. [Google Scholar] [CrossRef]
- Betrus, C.; Remenapp, R.; Charpie, J.; Kudelka, T.; Brophy, P.; Smoyer, W.E.; Lin, J.J. Enhanced Hemolysis in Pediatric Patients Requiring Extracorporeal Membrane Oxygenation and Continuous Renal Replacement Therapy. Ann. Thorac. Cardiovasc. Surg. 2007, 13, 378–383. [Google Scholar]
- Graw, J.A.; Hildebrandt, P.; Krannich, A.; Balzer, F.; Spies, C.; Francis, R.C.; Kuebler, W.M.; Weber-Carstens, S.; Menk, M.; Hunsicker, O. The Role of Cell-Free Hemoglobin and Haptoglobin in Acute Kidney Injury in Critically Ill Adults with ARDS and Therapy with VV ECMO. Crit. Care 2022, 26, 50. [Google Scholar] [CrossRef]
- Bierer, P.; Holt, A.W.; Bersten, A.D.; Plummer, J.L.; Chalmers, A.H. Haemolysis Associated with Continuous Venovenous Renal Replacement Circuits. Anaesth. Intensive Care 1998, 26, 272–275. [Google Scholar] [CrossRef]
- Taghavi, M.; Jacobs, L.; Kaysi, S.; Do Carmo Filomena Mesquita, M. Hemolysis in a Patient during Hemodialysis. Case Rep. Nephrol. Dial. 2021, 11, 348–354. [Google Scholar] [CrossRef]
- Boretti, F.S.; Baek, J.H.; Palmer, A.F.; Schaer, D.J.; Buehler, P.W. Modeling Hemoglobin and Hemoglobin:Haptoglobin Complex Clearance in a Non-Rodent Species–Pharmacokinetic and Therapeutic Implications. Front. Physiol. 2014, 5, 385. [Google Scholar] [CrossRef]
- Deuel, J.W.; Schaer, C.A.; Boretti, F.S.; Opitz, L.; Garcia-Rubio, I.; Baek, J.H.; Spahn, D.R.; Buehler, P.W.; Schaer, D.J. Hemoglobinuria-Related Acute Kidney Injury Is Driven by Intrarenal Oxidative Reactions Triggering a Heme Toxicity Response. Cell Death Dis. 2016, 7, e2064. [Google Scholar] [CrossRef]
- Windsant, I.C.V.; Snoeijs, M.G.; Hanssen, S.J.; Altintas, S.; Heijmans, J.H.; Koeppel, T.A.; Schurink, G.W.H.; Buurman, W.A.; Jacobs, M.J. Hemolysis Is Associated with Acute Kidney Injury during Major Aortic Surgery. Kidney Int. 2010, 77, 913–920. [Google Scholar] [CrossRef]
- Vermeulen Windsant, I.C.; de Wit, N.C.J.; Sertorio, J.T.C.; van Bijnen, A.A.; Ganushchak, Y.M.; Heijmans, J.H.; Tanus-Santos, J.E.; Jacobs, M.J.; Maessen, J.G.; Buurman, W.A. Hemolysis during Cardiac Surgery Is Associated with Increased Intravascular Nitric Oxide Consumption and Perioperative Kidney and Intestinal Tissue Damage. Front. Physiol. 2014, 5, 340. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.L.; Morine, K.J.; Annamalai, S.K.; O’Kelly, R.; Aghili, N.; Pedicini, R.; Breton, C.; Mullin, A.; Hamadeh, A.; Kiernan, M.S.; et al. Increased Plasma-Free Hemoglobin Levels Identify Hemolysis in Patients With Cardiogenic Shock and a Trans Valvular Micro-Axial Flow Pump. Artif. Organs 2019, 43, 125–131. [Google Scholar] [CrossRef]
- Adamzik, M.; Hamburger, T.; Petrat, F.; Peters, J.; de Groot, H.; Hartmann, M. Free Hemoglobin Concentration in Severe Sepsis: Methods of Measurement and Prediction of Outcome. Crit. Care 2012, 16, R125. [Google Scholar] [CrossRef]
- Komolafe, O.; Pereira, S.P.; Davidson, B.R.; Gurusamy, K.S. Serum C-Reactive Protein, Procalcitonin, and Lactate Dehydrogenase for the Diagnosis of Pancreatic Necrosis. Cochrane Database Syst. Rev. 2017, 4, CD012645. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Allemailem, K.S.; Alhumaydhi, F.A.; Gowder, S.J.T.; Rahmani, A.H. The Biochemical and Clinical Perspectives of Lactate Dehydrogenase: An Enzyme of Active Metabolism. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Brauckmann, S.; Effenberger-Neidnicht, K.; De Groot, H.; Nagel, M.; Mayer, C.; Peters, J.; Hartmann, M. Lipopolysaccharide-Induced Hemolysis: Evidence for Direct Membrane Interactions. Sci. Rep. 2016, 6, 35508. [Google Scholar] [CrossRef]
- Gupta, G.S. The Lactate and the Lactate Dehydrogenase in Inflammatory Diseases and Major Risk Factors in COVID-19 Patients. Inflammation 2022, 45, 2091–2123. [Google Scholar] [CrossRef]
- Li, C.; Ye, J.; Chen, Q.; Hu, W.; Wang, L.; Fan, Y.; Lu, Z.; Chen, J.; Chen, Z.; Chen, S.; et al. Elevated Lactate Dehydrogenase (LDH) Level as an Independent Risk Factor for the Severity and Mortality of COVID-19. Aging 2020, 12, 15670–15681. [Google Scholar] [CrossRef]
- Kuang, Z.S.; Yang, Y.L.; Wei, W.; Wang, J.L.; Long, X.Y.; Li, K.Y.; Tong, C.Y.; Sun, Z.; Song, Z.J. Clinical Characteristics and Prognosis of Community-Acquired Pneumonia in Autoimmune Disease-Induced Immunocompromised Host: A Retrospective Observational Study. World J. Emerg. Med. 2020, 11, 145–151. [Google Scholar] [CrossRef]

| Variable | All | Group 1 | Group 2 | p-Value |
|---|---|---|---|---|
| n = 50 | n = 30 | n = 20 | ||
| Age (years) | 47 (38–59) | 46 (38–57) | 50 (38–62) | 0.469 |
| Gender, male n (%) | 33 (66) | 23 (70) | 10 (30) | 0.050 |
| APACHE II score | 14 (11–19) | 15 (12–21) | 14 (11–18) | 0.481 |
| SOFA score | 9 (8–11) | 9 (8–12) | 8 (7–10) | 0.036 |
| Comorbidities, n (%): | ||||
| Hypertension | 10 (20) | 6 (60) | 4 (40) | 0.645 |
| Coronary heart disease | 6 (12) | 3 (50) | 3 (50) | 0.455 |
| Diabetes | 6 (12) | 4 (67) | 3 (33) | 0.544 |
| Obesity | 14 (28) | 10 (71) | 4 (29) | 0.241 |
| Malignancy | 2 (4) | 1 (50) | 1 (50) | 0.644 |
| Laboratory parameters: | ||||
| Leukocytes, 103/uL | 15 (11–20) | 17 (11–24) | 14 (10–18) | 0.238 |
| Hemoglobin, (g/dL) | 11.8 (10.4–12.8) | 11.6 (10.4–12.2) | 12.0 (10.2–13.7) | 0.519 |
| Platelets, (109/L) | 233 (173–302) | 204 (170–274) | 271 (201–304) | 0.246 |
| Fibrynogen, (g/L) | 4.5 (3.1–6.7) | 4.8 (2.6–6.9) | 4.5 (3.9–6.1) | 0.613 |
| D-dimers, (mg/L) | 5.1 (1.9–17.6) | 5.1 (1.9–17.6) | 5.0 (2.0–18.7) | 0.858 |
| ATIII, (%) | 80 (67–95) | 79 (65–93) | 83 (67–104) | 0.319 |
| CRP, mg/L | 115 (61–218) | 112 (37–199) | 126 (66–254) | 0.227 |
| PCT, (ng/mL) | 0.37 (0.15–1.07) | 0.5 (0.16–2.00) | 0.31 (0.14–0.88) | 0.254 |
| LDH, (U/L) | 664 (498–889) | 610 (479–865) | 747 (553–1030) | 0.258 |
| Blood transfusion, n (%) | 29 (58) | 19 (63) | 10 (50) | 0.349 |
| ICU length of stay, (day) | 23 (12–39) | 22 (12–39) | 28 (15–41) | 0.613 |
| Hospital length of stay, (day) | 29 (16–51) | 25 (12–51) | 32 (18–50) | 0.451 |
| ICU mortality, n (%) | 27 (54) | 18 (60) | 9 (45) | 0.297 |
| Hospital mortality, n (%) | 29 (58) | 19 (63) | 10 (50) | 0.349 |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95%CI | p-Value | OR | 95%CI | p-Value |
| Age | 1.1 | 1.0–1.2 | <0.001 | 1.2 | 1.01–1.3 | 0.002 |
| Gender | 1.5 | 0.5–4.9 | 0.481 | |||
| Septic shock | 2.3 | 0.7–7.7 | 0.198 | |||
| APACHEII | 1.2 | 1.1–1.4 | 0.004 | |||
| SOFA | 1.4 | 1.1–1.9 | 0.018 | |||
| ECMO | 0.4 | 0.1–1.5 | 0.181 | |||
| AKI stage 3 | 17.6 | 2.1–150.0 | 0.008 | 22.2 | 1.4–358.1 | 0.029 |
| Haptoglobin < 0.3 mg/L | 4.6 | 1.1–19.3 | 0.037 | 27.1 | 2.7–276.9 | 0.005 |
| Hemopexin < 0.5 mg/L | 1.9 | 0.6–5.8 | 0.267 | |||
| Hb | 1.1 | 0.8–1.5 | 0.562 | |||
| LDH | 1.1 | 1.0–1.0 | 0.189 | |||
| Secondary infection | 0.7 | 0.2–2.1 | 0.487 | |||
| Hypertension | 2.3 | 0.5–10.3 | 0.246 | |||
| Diabetes | 5.0 | 0.5–46.4 | 0.156 | |||
| Obesity | 1.8 | 0.5–6.4 | 0.365 | |||
| Coronary artery disease | 5.0 | 0.5–46.4 | 0.157 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bąkowski, W.; Śmiechowicz, J.; Lemańska-Perek, A.; Dragan, B.; Goździk, W.; Adamik, B. Hemolysis and Its Clinical Implications in Septic Patients with Acute Respiratory Failure. J. Clin. Med. 2025, 14, 3493. https://doi.org/10.3390/jcm14103493
Bąkowski W, Śmiechowicz J, Lemańska-Perek A, Dragan B, Goździk W, Adamik B. Hemolysis and Its Clinical Implications in Septic Patients with Acute Respiratory Failure. Journal of Clinical Medicine. 2025; 14(10):3493. https://doi.org/10.3390/jcm14103493
Chicago/Turabian StyleBąkowski, Wojciech, Jakub Śmiechowicz, Anna Lemańska-Perek, Barbara Dragan, Waldemar Goździk, and Barbara Adamik. 2025. "Hemolysis and Its Clinical Implications in Septic Patients with Acute Respiratory Failure" Journal of Clinical Medicine 14, no. 10: 3493. https://doi.org/10.3390/jcm14103493
APA StyleBąkowski, W., Śmiechowicz, J., Lemańska-Perek, A., Dragan, B., Goździk, W., & Adamik, B. (2025). Hemolysis and Its Clinical Implications in Septic Patients with Acute Respiratory Failure. Journal of Clinical Medicine, 14(10), 3493. https://doi.org/10.3390/jcm14103493







