Abstract
Background/Objectives: The prevalence of Achromobacter xylosoxidans is increasing in people with Cystic Fibrosis (pwCF), yet its clinical pathogenicity remains controversial. The objective of this study was to chart the longitudinal prevalence and examine clinical associations before and after infection. Methods: This observational, retrospective study was conducted at a single CF center over a 14-year period. Data were collated from patient charts and clinic databases. Patients with Achromobacter sputum cultures were compared to those without the bacterium and analyzed according to whether they had single, intermittent, or chronic infections. Results: During the study period, an annual average of 124 pwCF were followed up at our clinic, with a median age of 13.6 years (IQR = 7.6–27.7). The Achromobacter detection rate increased from 0 to 6.1%. Twenty-three percent (29/124) of patients had at least one positive culture. The median age at acquisition was 17 years (IQR = 14.5–33). At the time of acquisition, the median FEV1 was 81% (IQR = 46–94), compared to 90% (IQR = 72–99) for patients without Achromobacter, p < 0.001. Patients with Achromobacter tended to demonstrate more chronic Pseudomonas (55% vs. 27%, p = 0.06) and pancreatic insufficiency (66% vs. 47%, p = 0.07). At two years post-acquisition, the median FEV1 for patients with intermittent and chronically infected decreased by 11.5% (IQR = −3.75–7.5), compared to 1.5% (IQR = −2.5–12.5) for those with a single positive culture, p = 0.03. Similarly, pulmonary exacerbations per year became more frequent post-acquisition in intermittent and chronically infected patients: Median (range) 2.5 (0–8) pre-, versus 3.0 (0–9) post-acquisition, p = 0.036. Conclusions: Chronic and intermittent infection with Achromobacter were associated with accelerated lung function decline and increased exacerbation frequency. Larger prospective studies are needed to confirm these findings and examine the effect of eradication on the clinical course.
1. Introduction
Bacterial airway infections continue to be the main cause of morbidity and mortality in Cystic Fibrosis (CF) [1]. The most common pathogens are Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus aureus, and Haemophilus influenza. But as life expectancy in individuals with CF rises, the complexity of airway infections increases, with the emergence of further bacterial pathogens, including Burkholderia, Stenotrophomonas, non-tuberculous mycobacteria, Achromobacter, and others [2].
Achromobacter xylosoxidans (A. xylosoxidans) is increasingly isolated from the sputum of people with CF (pwCF), with prevalence ranging from 2.3% to 29.3% in different centers around the world [3,4,5,6]. The European CF registry only started monitoring A. xylosoxidans infection in 2018 [7] and reported an average prevalence of 3.75% across Europe, while the 2019 United States CF registry reported a 6.1% prevalence [8]. These figures are likely an underestimation, as taxonomy has been evolving over the years [9] due to difficulties with exact laboratory identification [10]; for instance, A. xylosoxidans used to be confused with other Gram-negative bacteria, especially P. aeruginosa [11].
Recent in vitro and animal studies have shown that A. xylosoxidans shares important pathophysiological features with better-known CF pathogens, likely creating a competitive survival advantage in the ecological niche of the CF lung and contributing to the vicious circle of infection and inflammation [12,13].
Nevertheless, the clinical impact of A. xylosoxidans infection remains controversial. Some reports indicate no effect on lung function [3,4,14], while others have demonstrated a more rapid decline in lung function following infection, as well as more frequent exacerbations, including fatal ones [4,5,15]. A recent analysis of clinical outcomes associated with Achromobacter species from the European CF Society Patient Registry also suggested association with disease severity, of similar magnitude to infection with P. aeruginosa [16].
The aims of our study were to describe the longitudinal prevalence of A. xylosoxidans infection in our CF cohort and evaluate the clinical status of pwCF with A. xylosoxidans airway infection before and after its acquisition. Of note, our observation period occurred prior to the widespread use of highly effective CFTR modulators.
2. Methods
2.1. Study Design and Population
This observational, longitudinal, retrospective study included all patients with CF attending the Graub CF Center at Schneider Children’s Medical Center of Israel from January 2007 to December 2020. During this period, the clinic population consisted of both pediatric and adult patients. Subjects were diagnosed with CF according to accepted criteria [17]. Data were retrieved from charts of patients with CF and the annual CF clinic database. Patients who ever had any sputum cultures positive for A. xylosoxidans during the observation period were compared with those who never grew A. xylosoxidans. Parameters examined included age, gender, CFTR mutation class, forced expiratory volume in 1 s, percent of predicted (FEV1%pp), Pseudomonas status, body mass index (BMI), pancreatic enzyme replacement therapy, the presence of CF-related diabetes (CFRD), and intravenous (IV) antibiotic courses administered.
Data for pwCF with A. xylosoxidans in sputum cultures were analyzed in more detail, comparing the period of two years prior to two years after acquisition. For the sake of comparison, data for pwCF with sputum cultures negative for A. xylosoxidans were taken from the annual clinic CF database of 2014, the mid-point of data collection.
2.2. Definitions
We adapted the European CF Society patient registry’s definition of chronic infection [7]: “Patient should be regarded as chronically infected if they fulfill the criteria now (or in recent years and the status is expected to be preserved), when a. >50% of respiratory samples during the last 12 months are positive; at least 4 samples during that period (modified Leeds criteria) are positive; and/or b. significantly raised bacteria-specific antibodies are present”. To accommodate the longitudinal design of our study, patients were classified as chronically infected when, in any of the years during the observation period, the above criteria were met. Since our center does not test for A. xylosoxidans specific antibodies, only criteria “a” above was considered.
We defined a “single infection” as one in which incidental sputum culture was positive during the entire observation period. We defined “intermittent infection” as having 2 or more positive cultures for A. xylosoxidans during the entire observation period but not meeting the above criteria of “chronic infection”, that is, 2 or more positive cultures recurring during the observation period, with months and years of negative cultures in between. Patients who met the “chronic infection” criteria during any given year remained classified as such for the purpose of the analysis.
2.3. CFTR Genotype
Using the standard classification of CFTR mutations [18], patients were classified as having “minimal CFTR function” if they had 2 mutations from classes I, II, or III; or “residual CFTR function” if they had at least one mutation from class IV–V.
2.4. Sputum Cultures
As part of the routine clinic protocol, patients with CF were seen at 1–6 monthly intervals, usually every 3 months, as well as during periods of clinical deterioration. During each visit, sputum for culture was either expectorated or induced as previously described [19] and was transferred immediately to the department of microbiology.
Sputum specimens were processed following the recommendations of the Clinical Microbiology Procedures Handbook [20]. Gram staining was performed for all samples. Then, automated identification and susceptibility testing were performed. Each isolate was identified using the VITEK 2 system (bioMérieux) or the MALDI Biotyper System (Bruker Daltonics Inc., Billerica, MA, USA), according to the manufacturer’s instructions for bacteria identification. Antimicrobial susceptibility profiles of the isolates were determined using the disk diffusion method, E-test, or VITEK II (bioMérieux) as needed and according to CLSI criteria. Of note, microbiology laboratory techniques have not significantly changed during the observation period with regard to A. xylosoxidans identification.
We calculated the absolute change in FEV1 after A. xylosoxidans acquisition by taking the FEV1 percent predicted measured as close as possible to the acquisition date, and the FEV1 percent predicted, measured as close as possible to 1 year and 2 years after acquisition, and calculating the difference between them. We calculated pulmonary exacerbations by documenting the number of intravenous antibiotic courses.
2.5. Statistical Analysis
Patient demographic and clinical characteristics were summarized using median and interquartile range, or mean and standard deviation, as appropriate. Proportions were calculated for categorical variables. Parameters were compared between A. xylosoxidans positive and negative subjects using paired student’s t-tests, or chi-squared test, as appropriate. Linear regression analysis was performed to test for associations between P. aeruginosa infection and pulmonary outcomes in A. xylosoxidans-infected subjects. All analyses were 2-tailed, and a p-value < 0.05 was considered significant.
2.6. Ethics Board Approval
This study was approved by the local ethics Institution Review Board (IRB), RMC-0284-20 on 12 June 2020. This study is a retrospective analysis based on de-identified data extracted from patients’ medical records. As such, the IRB granted an exemption from the requirement for obtaining informed consent.
3. Results
During the 14-year observation period, an annual average of 124 patients were followed at our clinic, gradually increasing over time, from 86 in 2007 to 148 in 2020. The observation period occurred before the state approval and widespread usage of highly effective CFTR modulators.
Table 1 displays the demographic and clinical characteristics of pwCF who were ever positive for A. xylosoxidans versus those who were never positive. With the exception of FEV1%pp, which was lower in the A. xylosoxidans-positive group, there were no differences, although patients with A. xylosoxidans tended to have a higher proportion of pancreatic insufficiency, CF-related diabetes, and chronic P. aeruginosa infection, without reaching statistical significance.

Table 1.
Demographic and clinical characteristics of patients ever positive for A. xylosoxidans versus patients never positive (n = 124).
3.1. A. xylosoxidans Prevalence and Chronicity
A total of 29 patients of our entire CF cohort had at least one positive culture with A. xylosoxidans throughout the study period. During the first two years of data collection (2007–2008), no sputum cultures were positive for A. xylosoxidans. Thereafter, a fluctuating increase was observed, stabilizing from 2014 until the end of the observation period, with a mean (SD) annual prevalence of 6.2% (±0.5) during these seven years (Figure 1).
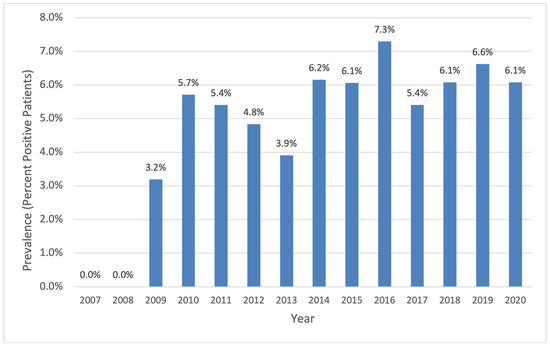
Figure 1.
Percent of patients with CF with any A. xylosoxidans-positive sputum culture (single, intermittent, and chronic) of total CF clinic population per year.
Nine patients (31% of patients ever infected with A. xylosoxidans) met the criteria of “chronic infection”, seven (24% of all A. xylosoxidans positive patients) had “intermittent infection” and thirteen (45% of patients infected with A. xylosoxidans) only one single positive culture during the study period.
3.2. Change In Clinical Status Following Acquisition of A. xylosoxidans
The frequency of pulmonary exacerbations requiring a course of IV antibiotics for all A. xylosoxidans-infected patients did not differ before versus after acquisition, with a median of two courses (range 0–9) in the two years before, compared to a median of two courses (range 0–9) in the two years after acquisition, p = 0.38. However, subgroup analysis demonstrates that patients with intermittent and chronic infection taken together suffered more exacerbations post-acquisition, with a median (range) 2.5 (0–8) exacerbations pre- and 3 (0–9) post-acquisition (p = 0.036). Patients with a single sporadic infection had a median of 2 (0–9) exacerbations pre and 1 (1–8) post-acquisition, p = 0.10.
When comparing the lung function of all pwCF ever infected with A. xylosoxidans versus all negative patients, there was no excessive deterioration in FEV1% at 1 year (−0.2%) or 2 years (−0.6%) post-acquisition, p = 0.83 and p = 0.25, respectively. However, subgroup analysis demonstrates that chronically and intermittently infected patients taken together had a more significant median FEV1% decline of 11.5% (IQR = −3.5–7.5) at 2 years post-acquisition, compared to a median decline of only 1.5% (IQR = −2.5–12.5) for patients with a single positive culture, p = 0.03 (Figure 2).
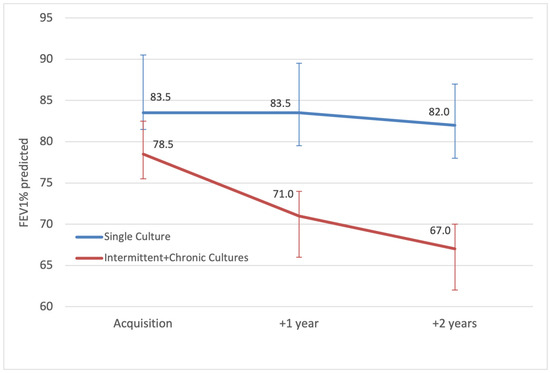
Figure 2.
Median annual FEV1 percent predicted (error bars showing IQR) of patients with intermittent and chronic A. xylosoxidans, compared to patients with a single culture, from acquisition until 2 years after.
After A. xylosoxidans acquisition, there was no statistically significant BMI decline, from 20.1 kg/m2 at baseline to 20.5 kg/m2 at 1 year and at 20 kg/m2 at 2 years post-acquisition, p = 0.10 and p = 0.13, respectively. The mean BMI of patients without A. xylosoxidans also remained stable during this period, at 20.4 kg/m2, p = NS. Subgroup analysis comparing intermittently and chronically infected patients taken together versus patients with a single infection was also non-significant.
No patient with A. xylosoxidans underwent a lung transplant. One patient with A. xylosoxidans died during the observation period. This patient had a single isolation, and his demise was deemed unrelated to the A. xylosoxidans infection.
3.3. The Effect of Concurrent P. aeruginosa Infection
Chronic A. xylosoxidans infection was not associated with chronic P. aeruginosa infection (p = 0.46). Linear regression analyses were performed to assess the impact of chronic P. aeruginosa infection on the decline in lung function, as measured by FEV1.
There was no significant correlation with a decline greater than 4% in one year (p = 0.52) or greater than 5% in two years (p = 0.89). When analyzing the effect of chronic co-infection with A. xylosoxidans and P. aeruginosa, there was equally no significant association with a decline greater than 4% in one year (p = 0.66) or greater than 5% in two years (p = 0.53). Among the 29 patients with A. xylosoxidans infection, 11 experienced an increased need for IV antibiotic courses in the two years following A. xylosoxidans acquisition compared to the two years prior. Notably, 10 of these 11 patients also had chronic P. aeruginosa infection. A borderline significant positive association was observed between P. aeruginosa infection and the need for IV treatment (p = 0.052).
3.4. Antibiotic Susceptibility
The antibiogram results of all 296 sputum cultures collected during the observation period demonstrated very high in vitro sensitivity to beta-lactams, including Carbapenems (especially Ertapenem and Imipenem), Minocycline, and extended-spectrum Penicillins (Piperacillin, Piperacillin–Tazobactam, Amoxicillin–Clavulonate), and low susceptibility to Cephalosporins evaluated. Antibiogram results are presented in Table 2.

Table 2.
Antibiogram of all Achromobacter xylosoxidans cultures, showing the percentage of cultures sensitive, resistant, or intermediate to the respective antibiotics tested.
4. Discussion
4.1. Association of A. xylosoxidans with More Severe Lung Disease
In this single-center study spanning a 14-year observation period, patients with A. xylosoxidans infection appeared to have more severe disease at baseline, as evident from lower FEV1, as well as a tendency to present with a higher proportion of pancreatic insufficiency, CFRD, and chronic Pseudomonas infection. A similarly designed French retrospective case–control study also found A. xylosoxidans-infected patients to have had more frequent pulmonary exacerbations, hospitalizations, and intravenous and oral antibiotic courses over the 2 years prior to acquisition [21]. As opposed to those with a single episode of infection, patients in our cohort with chronic and intermittent infection experienced accelerated lung function decline and an increase in the frequency of pulmonary exacerbations, another finding mirrored by the French study [21].
4.2. Prevalence and Age at Acquisition
During our observation period, about one-fifth of all pwCF were infected at least once with A. xylosoxidans, in line with reports for pwCF in other centers [12,22]. The prevalence increased with fluctuations during the first half and appeared to have reached a plateau during the second half. The initial rise in prevalence may be due to patient-to-patient spread [23,24]. Due to the lack of genotyping in our laboratory, we can only speculate that the observed plateau in A. xylosoxidans prevalence in later years may be attributed to more rigorous isolation practices”.
The median age at acquisition was late adolescence, which was also shown by others [3,4,6,14,25] and similar to the age of acquisition of other emerging CF bacteria, such a non-tuberculous mycobacteria [26]. The predilection of these pathogens for the CF lung with more advanced disease may be explained by favorable metabolic niche conditions, such as thick mucus, nutrient availability, altered oxygen levels, chronic inflammation, biofilm formation, and an impaired immune response [12].
4.3. European Cystic Fibroses Society Registry Data
Kerem et al. recently examined the European CF Society Patient Registry data for cross-sectional demographic and clinical characteristics, as well as outcomes associated with Achromobacter species [16]. In a total of 38,795 eligible pwCF, Achromobacter infection was associated with disease severity similar to infection with Pseudomonas aeruginosa. Being infected with both bacteria was associated with even more severe disease. Despite the inherent limitations of this registry study, such as its cross-sectional design, variable data quality and consistency, and potential selection bias, the examination of such a large patient cohort provides additional support for the hypothesis that Achromobacter may indeed contribute to disease severity rather than just serve as a surrogate marker. Our data, from a smaller sample but with the benefit of longitudinal follow-up and more granularity concerning the frequency of bacterial growths, are in line with the registry findings. Definitive claims regarding this relationship would require evidence from different types of trial designs, such as randomized controlled trials, which may not be considered ethical given the current state of evidence.
4.4. Possible Confounding Through Co-Infection with P. aeruginosa
Achromobacter species and P. aeruginosa exhibit several pathophysiological similarities in the context of CF, which contribute to their persistence in the CF lung. These include their capability to produce biofilms [27], complex resistance mechanisms [28], and their possession of various virulence factors that enhance their pathogenicity [29]. We examined whether co-infection with P. aeruginosa may indicate more severe disease. No accelerated decline in lung function was observed with either isolated chronic P. aeruginosa infection or in combination with Achromobacter. Sample size constraints might account for the failure to demonstrate this. Another possible explanation is the aggressive treatment and eradication protocols implemented upon detection of P. aeruginosa, which may mitigate its impact on lung function. Conversely, Achromobacter, which does not always prompt intensive antibiotic treatment upon isolation, might exert a more subtle effect that is not readily captured in short-term lung function analyses. The borderline significant association between P. aeruginosa and the increased need for IV antibiotics suggests a possible link; however, as IV therapy is also used for eradication purposes—even in the absence of clinical exacerbation—this association should be interpreted with caution.
4.5. Antibiotic Resistance Patterns
In our cohort, sputum cultures demonstrated high sensitivity of A. xylosoxidans to Carbapenems (especially Ertapenem and Imipenem) and high sensitivity to Minocycline, a tetracycline antibiotic. This is in contrast to other reports in which only about 50% of isolates were sensitive to Minocycline [10]. Piperacillin, Piperacillin–Tazobactam, and Amoxicillin–Clavulonate also demonstrated very high sensitivity against A. xylosoxidans. Conversely, as expected, the lowest in vitro sensitivity was found for Aminoglycosides and quinolones. None of our A. xylosoxidans strains exhibited particular antibiotic resistance.
4.6. Study Limitations
Our study is limited by its retrospective design and a relatively small sample size. To compare culture-positive patients with those who are culture-negative, an arbitrary cohort had to be chosen. We elected to use data from our CF clinic database, collected in 2014, which is the midpoint of our observation period (excluding all patients who were ever positive for Achromobacter). We are aware that this comparison is artificial and may have skewed the results; however, it was primarily used for basic demographic and clinical features. Regarding highly effective CFTR modulators, these were introduced only toward the end of our study period and in a limited number of patients. As a result, isolating their impact on A. xylosoxidans prevalence and associated morbidity was neither feasible nor within the scope of the study.
5. Conclusions
The present study contributes to the growing body of evidence regarding the increasing prevalence and emerging pathogenicity of A. xylosoxidans in CF. Intermittent and chronic infection had a detrimental influence on lung function and pulmonary exacerbations. Despite the inability to demonstrate causality, we infer that it may be reasonable to attempt eradication whilst awaiting larger prospective longitudinal studies to confirm our findings. Future research ought to better characterize strain dynamics and transmission patterns, in particular considering the effect of highly effective CFTR modulators on the prevalence and clinical impact of A. xylosoxidans.
Author Contributions
Conceptualization, O.B.-O., M.M.-Z., H.M. and H.B.; methodology, O.B.-O. and H.M.; validation, M.M.-Z., H.L., H.B., H.B.Z. and D.P.; formal analysis, O.B.-O. and H.B.; investigation, M.M.-Z., H.L. and D.P.; resources, P.S.; data curation, O.B.-O., H.L., H.B. and H.B.Z.; writing—original draft, O.B.-O.; writing—review & editing, O.B.-O., M.M.-Z., H.L., H.M., H.B., H.B.Z., D.P. and P.S.; supervision, H.M., D.P. and P.S.; project administration, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the local ethics Institution Review Board (IRB), RMC-0284-20, on 12 June 2020.
Informed Consent Statement
This study is a retrospective analysis based on de-identified data extracted from patients’ medical records. As such, the IRB granted an exemption from the requirement for obtaining informed consent.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
During the preparation of this work, the authors used Chat GPT-4 in order to improve language and readability. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
References
- Ciofu, O.; Hansen, C.R.; Hoiby, N. Respiratory bacterial infections in cystic fibrosis. Curr. Opin. Pulm. Med. 2013, 19, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Parkins, M.D.; Floto, R.A. Emerging bacterial pathogens and changing concepts of bacterial pathogenesis in cystic fibrosis. J. Cyst. Fibros. 2015, 14, 293–304. [Google Scholar] [PubMed]
- Tan, K.; Conway, S.P.; Brownlee, K.G.; Etherington, C.; Peckham, D.G. Alcaligenes infection in cystic fibrosis. Pediatr. Pulmonol. 2002, 34, 101–104. [Google Scholar] [CrossRef]
- De Baets, F.; Schelstraete, P.; Van Daele, S.; Haerynck, F.; Vaneechoutte, M. Achromobacter xylosoxidans in cystic fibrosis: Prevalence and clinical relevance. J. Cyst. Fibros. 2007, 6, 75–78. [Google Scholar] [CrossRef]
- Firmida, M.C.; Pereira, R.H.; Silva, E.A.; Marques, E.A.; Lopes, A.J. Clinical impact of Achromobacter xylosoxidans colonization/infection in patients with cystic fibrosis. Braz. J. Med. Biol. Res. 2016, 49, e5097. [Google Scholar]
- Raso, T.; Bianco, O.; Grosso, B.; Zucca, M.; Savoia, D. Achromobacter xylosoxidans respiratory tract infections in cystic fibrosis patients. Apmis 2008, 116, 837–841. [Google Scholar]
- Zolin, A.; Bossi, A.; Cirilli, N.; Kashirskaya, N.; Padoan, R. Cystic Fibrosis Mortality in Childhood. Data from European Cystic Fibrosis Society Patient Registry. Int. J. Environ. Res. Public Health 2018, 15, 2020. [Google Scholar] [CrossRef]
- Foundation, C.F. Patient Registry Annual Data Report. Cyst Fibros Found Patient Regist. 2019. Available online: https://www.cff.org/sites/default/files/2021-10/2019-Annual-Report.pdf (accessed on 30 March 2025).
- Swenson, C.E.; Sadikot, R.T. Achromobacter respiratory infections. Ann. Am. Thorac. Soc. 2015, 12, 252–258. [Google Scholar] [PubMed]
- Saiman, L.; Chen, Y.; Tabibi, S.; San Gabriel, P.; Zhou, J.; Liu, Z.; Lai, L.; Whittier, S. Identification and antimicrobial susceptibility of Alcaligenes xylosoxidans isolated from patients with cystic fibrosis. J. Clin. Microbiol. 2001, 39, 3942–3945. [Google Scholar]
- Kidd, T.J.; Ramsay, K.A.; Hu, H.; Bye, P.T.; Elkins, M.R.; Grimwood, K.; Harbour, C.; Marks, G.B.; Nissen, M.D.; Robinson, P.J.; et al. Low rates of Pseudomonas aeruginosa misidentification in isolates from cystic fibrosis patients. J. Clin. Microbiol. 2009, 47, 1503–1509. [Google Scholar]
- Menetrey, Q.; Sorlin, P.; Jumas-Bilak, E.; Chiron, R.; Dupont, C.; Marchandin, H. Achromobacter xylosoxidans and Stenotrophomonas maltophilia: Emerging Pathogens Well-Armed for Life in the Cystic Fibrosis Patients’ Lung. Genes 2021, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- Billiot, C.E.; McDaniel, M.S.; Lindgren, N.R.; Swords, W.E. Pathogenesis of Achromobacter xylosoxidans respiratory infections: Colonization and persistence of airway epithelia and differential gene expression in synthetic cystic fibrosis sputum medium. bioRxiv 2023. Update in: Infect. Immun. 2023, 91, e0041623. https://doi.org/10.1128/iai.00416-23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lambiase, A.; Catania, M.R.; Del Pezzo, M.; Rossano, F.; Terlizzi, V.; Sepe, A.; Raia, V. Achromobacter xylosoxidans respiratory tract infection in cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 973–980. [Google Scholar] [PubMed]
- Recio, R.; Branas, P.; Martinez, M.T.; Chaves, F.; Orellana, M.A. Effect of respiratory Achromobacter spp. infection on pulmonary function in patients with cystic fibrosis. J. Med. Microbiol. 2018, 67, 952–956. [Google Scholar] [PubMed]
- Kerem, E.; Orenti, A.; Zolin, A.; Annicchiarico, L.; Drevinek, P. Clinical outcomes associated with Achromobacter species infection in people with cystic fibrosis. J. Cyst. Fibros. 2023, 22, 334–343. [Google Scholar]
- Farrell, P.M.; Rosenstein, B.J.; White, T.B.; Accurso, F.J.; Castellani, C.; Cutting, G.R.; Durie, P.R.; Legrys, V.A.; Massie, J.; Parad, R.B.; et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J. Pediatr. 2008, 153, S4–S14. [Google Scholar]
- Ratjen, F.; Bell, S.C.; Rowe, S.M.; Goss, C.H.; Quittner, A.L.; Bush, A. Cystic fibrosis. Nat. Rev. Dis. Primers 2015, 1, 15010. [Google Scholar]
- Blau, H.; Linnane, B.; Carzino, R.; Tannenbaum, E.L.; Skoric, B.; Robinson, P.J.; Robertson, C.; Ranganathan, S.C. Induced sputum compared to bronchoalveolar lavage in young, non-expectorating cystic fibrosis children. J. Cyst. Fibros. 2014, 13, 106–110. [Google Scholar]
- Leber, A.L. Clinical Microbiology Procedures Handbook. Sons, J.W., Ed.; 2016. Available online: https://www.wiley.com/en-ae/Clinical+Microbiology+Procedures+Handbook%2C+4th+Edition-p-9781683673255 (accessed on 30 March 2025).
- Marsac, C.; Berdah, L.; Thouvenin, G.; Sermet-Gaudelus, I.; Corvol, H. Achromobacter xylosoxidans airway infection is associated with lung disease severity in children with cystic fibrosis. ERJ Open Res. 2021, 7. [Google Scholar] [CrossRef]
- Mahenthiralingam, E. Emerging cystic fibrosis pathogens and the microbiome. Paediatr. Respir. Rev. 2014, 15 (Suppl. 1), 13–15. [Google Scholar] [CrossRef]
- Hansen, C.R.; Pressler, T.; Ridderberg, W.; Johansen, H.K.; Skov, M. Achromobacter species in cystic fibrosis: Cross-infection caused by indirect patient-to-patient contact. J. Cyst. Fibros. 2013, 12, 609–615. [Google Scholar] [PubMed]
- Cools, P.; Ho, E.; Vranckx, K.; Schelstraete, P.; Wurth, B.; Franckx, H.; Ieven, G.; Van Simaey, L.; Van Daele, S.; Verhulst, S.; et al. Epidemic Achromobacter xylosoxidans strain among Belgian cystic fibrosis patients and review of literature. BMC Microbiol. 2016, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Ronne Hansen, C.; Pressler, T.; Hoiby, N.; Gormsen, M. Chronic infection with Achromobacter xylosoxidans in cystic fibrosis patients; a retrospective case control study. J. Cyst. Fibros. 2006, 5, 245–251. [Google Scholar]
- Bar-On, O.; Mussaffi, H.; Mei-Zahav, M.; Prais, D.; Steuer, G.; Stafler, P.; Hananya, S.; Blau, H. Increasing nontuberculous mycobacteria infection in cystic fibrosis. J. Cyst. Fibros. 2015, 14, 53–62. [Google Scholar]
- Veschetti, L.; Boaretti, M.; Saitta, G.M.; Passarelli Mantovani, R.; Lleo, M.M.; Sandri, A.; Malerba, G. Achromobacter spp. prevalence and adaptation in cystic fibrosis lung infection. Microbiol. Res. 2022, 263, 127140. [Google Scholar] [CrossRef]
- Dupont, C.; Jumas-Bilak, E.; Michon, A.L.; Chiron, R.; Marchandin, H. Impact of High Diversity of Achromobacter Populations within Cystic Fibrosis Sputum Samples on Antimicrobial Susceptibility Testing. J. Clin. Microbiol. 2017, 55, 206–215. [Google Scholar]
- Li, X.; Hu, Y.; Gong, J.; Zhang, L.; Wang, G. Comparative genome characterization of Achromobacter members reveals potential genetic determinants facilitating the adaptation to a pathogenic lifestyle. Appl. Microbiol. Biotechnol. 2013, 97, 6413–6425. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).