Altered Autonomic Function in Metabolic Syndrome: Interactive Effects of Multiple Components
Abstract
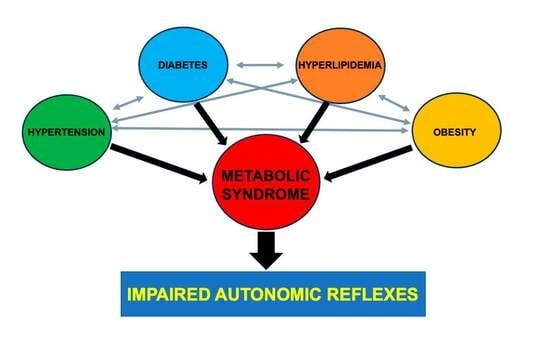
1. Introduction
1.1. Metabolic Syndrome: Clinical Presentation and Effects
1.2. Current Challenges to Animal Models of Metabolic Syndrome
2. Metabolic Syndrome and Hypertension
3. Autonomic Function: Hypertension
4. Metabolic Syndrome and Impaired Glucose Control
5. Autonomic Function: Type 2 Diabetes
6. Metabolic Syndrome and Obesity
7. Autonomic Function: Obesity
8. Effect of Metabolic Syndrome on Autonomic Dysfunction
9. Metabolic Syndrome Known vs. Unknown Syndrome Component Interactions and Autonomic Function
10. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef]
- Ostchega, Y.; Fryar, C.D.; Nwankwo, T.; Nguyen, D.T. Hypertension Prevalence among Adults Aged 18 and Over: United States, 2017–2018. NCHS Data Brief 2020, 1–8. [Google Scholar]
- Carlson, S.H.; Shelton, J.; White, C.R.; Wyss, J.M. Elevated Sympathetic Activity Contributes to Hypertension and Salt Sensitivity in Diabetic Obese Zucker Rats. Hypertension 2000, 35, 403–408. [Google Scholar] [CrossRef]
- Huber, D.A.; Schreihofer, A.M. Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. J. Physiol. 2010, 588, 1515–1525. [Google Scholar] [CrossRef]
- Landsberg, L. Insulin-mediated sympathetic stimulation- role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why). J. Hypertens. 2001, 19, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.E.; Triscari, J.; Sullivan, A.C. Altered sympathetic activity during development of diet-induced obesity in rat. Am. J. Physiol. 1983, 244, R347–R355. [Google Scholar] [CrossRef] [PubMed]
- Thorp, A.A.; Schlaich, M.P. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J. Diabetes Res. 2015, 2015, 341583. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, I.C.; Batalha, L.T.; Rondon, M.U.; Laterza, M.C.; Kuniyoshi, F.H.; Gowdak, M.M.; Barretto, A.C.; Halpern, A.; Villares, S.M.; Negrao, C.E. Weight loss improves neurovascular and muscle metaboreflex control in obesity. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H974–H982. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Jennings, G.; Turner, A.; Cox, H.; Lambert, G.; Esler, M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 1997, 96, 3423–3429. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Dell’Oro, R.; Quarti-Trevano, F.; Scopelliti, F.; Seravalle, G.; Paleari, F.; Gamba, P.L.; Mancia, G. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia 2005, 48, 1359–1365. [Google Scholar] [CrossRef]
- Heusser, K.; Tank, J.; Engeli, S.; Diedrich, A.; Menne, J.; Eckert, S.; Peters, T.; Sweep, F.C.; Haller, H.; Pichlmaier, A.M.; et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension 2010, 55, 619–626. [Google Scholar] [CrossRef]
- Kawada, T.; Shimizu, S.; Kamiya, A.; Sata, Y.; Uemura, K.; Sugimachi, M. Dynamic characteristics of baroreflex neural and peripheral arcs are preserved in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R155–R165. [Google Scholar] [CrossRef]
- Choi, H.M.; Stebbins, C.L.; Lee, O.T.; Nho, H.; Lee, J.H.; Chun, J.M.; Kim, K.A.; Kim, J.K. Augmentation of the exercise pressor reflex in prehypertension: Roles of the muscle metaboreflex and mechanoreflex. Appl. Physiol. Nutr. Metab. 2013, 38, 209–215. [Google Scholar] [CrossRef]
- Greaney, J.L.; Matthews, E.L.; Boggs, M.E.; Edwards, D.G.; Duncan, R.L.; Farquhar, W.B. Exaggerated exercise pressor reflex in adults with moderately elevated systolic blood pressure: Role of purinergic receptors. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H132–H141. [Google Scholar] [CrossRef]
- Grotle, A.K.; Macefield, V.G.; Farquhar, W.B.; O’Leary, D.S.; Stone, A.J. Recent advances in exercise pressor reflex function in health and disease. Auton. Neurosci. 2020, 228, 102698. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Murphy, M.N.; Mitchell, J.H.; Smith, S.A. Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J. Physiol. 2011, 589, 6191–6204. [Google Scholar] [CrossRef] [PubMed]
- Spranger, M.D.; Kaur, J.; Sala-Mercado, J.A.; Krishnan, A.C.; Abu-Hamdah, R.; Alvarez, A.; Machado, T.M.; Augustyniak, R.A.; O’Leary, D.S. Exaggerated coronary vasoconstriction limits muscle metaboreflex-induced increases in ventricular performance in hypertension. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H68–H79. [Google Scholar] [CrossRef] [PubMed]
- Spranger, M.D.; Kaur, J.; Sala-Mercado, J.A.; Machado, T.M.; Krishnan, A.C.; Alvarez, A.; O’Leary, D.S. Attenuated muscle metaboreflex-induced pressor response during postexercise muscle ischemia in renovascular hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R650–R658. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.P.; Martinez, D.; Faria, C.C.; de Carli, L.A.; de Souza, W.I.; Meinhardt, N.G.; Souto, K.E.; Trindade, M.R.; Ribeiro, J.P. Improvement of exercise capacity and peripheral metaboreflex after bariatric surgery. Obes. Surg. 2013, 23, 1835–1841. [Google Scholar] [CrossRef]
- Dipla, K.; Zafeiridis, A.; Koidou, I.; Geladas, N.; Vrabas, I.S. Altered hemodynamic regulation and reflex control during exercise and recovery in obese boys. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H2090–H2096. [Google Scholar] [CrossRef]
- Limberg, J.; Morgan, B.; Schrage, W. Mechanical and metabolic reflex activation of the sympathetic nervous system in younger adults with metabolic syndrome. Auton. Neurosci. 2014, 183, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Milia, R.; Velluzzi, F.; Roberto, S.; Palazzolo, G.; Sanna, I.; Sainas, G.; Pusceddu, M.; Mulliri, G.; Loviselli, A.; Crisafulli, A. Differences in hemodynamic response to metaboreflex activation between obese patients with metabolic syndrome and healthy subjects with obese phenotype. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H779–H789. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.A.; Hoffman, R.P.; Balon, T.W.; Sinkey, C.A.; Mark, A.L. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J. Clin. Investig. 1991, 87, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Daubert, D.L.; Chung, M.Y.; Brooks, V.L. Insulin resistance and impaired baroreflex gain during pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R2188–R2195. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.M. Insulin resistance and the sympathetic nervous system. Curr. Hypertens. Rep. 2003, 5, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Muntzel, M.S.; Anderson, E.A.; Johnson, A.K.; Mark, A.L. Mechanisms of insulin action on sympathetic nerve activity. Clin. Exp. Hypertens. 1995, 17, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Silva, L.; Vangipurapu, J.; Laakso, M. The “Common Soil Hypothesis” Revisited-Risk Factors for Type 2 Diabetes and Cardiovascular Disease. Metabolites 2021, 11, 691. [Google Scholar] [CrossRef]
- Dombrowski, M.; Mannozzi, J.; O’Leary, D.S. Neural Control of Cardiovascular Function During Exercise in Hypertension. Front. Physiol. 2018, 9, 1829. [Google Scholar] [CrossRef]
- Laterza, M.C.; de Matos, L.D.; Trombetta, I.C.; Braga, A.M.; Roveda, F.; Alves, M.J.; Krieger, E.M.; Negrao, C.E.; Rondon, M.U. Exercise training restores baroreflex sensitivity in never-treated hypertensive patients. Hypertension 2007, 49, 1298–1306. [Google Scholar] [CrossRef]
- O’Leary, D.S.; Mannozzi, J.; Augustyniak, R.A.; Ichinose, M.; Spranger, M.D. Hypertension depresses arterial baroreflex control of both heart rate and cardiac output during rest, exercise, and metaboreflex activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 323, R720–R727. [Google Scholar] [CrossRef]
- Davis, G. Baroreflex and somato-reflex control of blood pressure, heart rate and renal sympathetic nerve activity in the obese Zucker rat. Exp. Physiol. 2011, 96, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Tuck, M.L. Obesity, the sympathetic nervous system, and essential hypertension. Hypertension 1992, 19, I67–I77. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.D.; Brechtel-Hook, G.; Johnson, A.; Hardin, D. Skeletal muscle blood flow. A possible link between insulin resistance and blood pressure. Hypertension 1993, 21, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-S.; Doherty, J.U.; Faillace, R.; Maekawa, K.; Arnold, S.; Gavras, H.; Hood, W.B. Insulin Infusion in Conscious Dogs. J. Clin. Investig. 1982, 69, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Fairus, A.; Ima-Nirwana, S. Animal models of metabolic syndrome: A review. Nutr. Metab. 2016, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- de Moura, E.D.M.; Dos Reis, S.A.; da Conceicao, L.L.; Sediyama, C.; Pereira, S.S.; de Oliveira, L.L.; Gouveia Peluzio, M.D.C.; Martinez, J.A.; Milagro, F.I. Diet-induced obesity in animal models: Points to consider and influence on metabolic markers. Diabetol. Metab. Syndr. 2021, 13, 32. [Google Scholar] [CrossRef]
- Aydin, S.; Aksoy, A.; Aydin, S.; Kalayci, M.; Yilmaz, M.; Kuloglu, T.; Citil, C.; Catak, Z. Today’s and yesterday’s of pathophysiology: Biochemistry of metabolic syndrome and animal models. Nutrition 2014, 30, 1–9. [Google Scholar] [CrossRef]
- Rantala, A.O.; Kauma, H.; Lilja, M.; Savolainen, M.J.; Reunanen, A.; Kesaniemi, Y.A. Prevalence of the metabolic syndrome in drug-treated hypertensive patients and control subjects. J. Intern. Med. 1999, 245, 163–174. [Google Scholar] [CrossRef]
- Duvnjak, L. Hypertension and the Metabolic Syndrome. EJIFCC 2007, 18, 55–60. [Google Scholar]
- Ribeiro, M.J.; Sacramento, J.F.; Gonzalez, C.; Guarino, M.P.; Monteiro, E.C.; Conde, S.V. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes 2013, 62, 2905–2916. [Google Scholar] [CrossRef]
- Schillaci, G.; Pirro, M.; Vaudo, G.; Gemelli, F.; Marchesi, S.; Porcellati, C.; Mannarino, E. Prognostic value of the metabolic syndrome in essential hypertension. J. Am. Coll. Cardiol. 2004, 43, 1817–1822. [Google Scholar] [CrossRef]
- Stanciu, S.; Rusu, E.; Miricescu, D.; Radu, A.C.; Axinia, B.; Vrabie, A.M.; Ionescu, R.; Jinga, M.; Sirbu, C.A. Links between Metabolic Syndrome and Hypertension: The Relationship with the Current Antidiabetic Drugs. Metabolites 2023, 13, 87. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Ceron, J.J.; Holden, S.L.; Cuthbertson, D.J.; Biourge, V.; Morris, P.J.; German, A.J. Obesity-related metabolic dysfunction in dogs: A comparison with human metabolic syndrome. BMC Vet. Res. 2012, 8, 147. [Google Scholar] [CrossRef]
- Lee, D.C.; Sui, X.; Church, T.S.; Lavie, C.J.; Jackson, A.S.; Blair, S.N. Changes in fitness and fatness on the development of cardiovascular disease risk factors hypertension, metabolic syndrome, and hypercholesterolemia. J. Am. Coll. Cardiol. 2012, 59, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, H.; Yamada, T.; Oka, Y. Adiposity and cardiovascular disorders: Disturbance of the regulatory system consisting of humoral and neuronal signals. Circ. Res. 2007, 101, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Tomono, Y.; Ito, K.; Furutani, N.; Yoshida, H.; Tada, N. The underlying mechanisms for development of hypertension in the metabolic syndrome. Nutr. J. 2008, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, D.; Despres, J.P.; Landry, J.F.; Moorjani, S.; Lupien, P.J.; Tremblay, A.; Nadeau, A.; Bouchard, C. Systolic blood pressure during submaximal exercise: An important correlate of cardiovascular disease risk factors in normotensive obese women. Metabolism 1994, 43, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.X.; Lamers, F.; Hiles, S.A.; Penninx, B.W.; de Geus, E.J. Basal autonomic activity, stress reactivity, and increases in metabolic syndrome components over time. Psychoneuroendocrinology 2016, 71, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Esler, M.; Straznicky, N.; Eikelis, N.; Masuo, K.; Lambert, G.; Lambert, E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension 2006, 48, 787–796. [Google Scholar] [CrossRef]
- Pierdomenico, S.D.; Lapenna, D.; Di Tommaso, R.; Di Carlo, S.; Caldarella, M.P.; Neri, M.; Mezzetti, A.; Cuccurullo, F. Prognostic relevance of metabolic syndrome in hypertensive patients at low-to-medium risk. Am. J. Hypertens. 2007, 20, 1291–1296. [Google Scholar] [CrossRef][Green Version]
- Dubey, P.; Tiwari, S.; Bajpai, M.; Singh, K.; Jha, P. Effect of Metaboreflex on Cardiovascular System in Subjects of Metabolic Syndrome. J. Clin. Diagn. Res. 2017, 11, CC01–CC04. [Google Scholar] [CrossRef]
- Sharman, J.E.; Boutouyrie, P.; Perier, M.C.; Thomas, F.; Guibout, C.; Khettab, H.; Pannier, B.; Laurent, S.; Jouven, X.; Empana, J.P. Impaired baroreflex sensitivity, carotid stiffness, and exaggerated exercise blood pressure: A community-based analysis from the Paris Prospective Study III. Eur. Heart J. 2018, 39, 599–606. [Google Scholar] [CrossRef]
- Valensi, P. Autonomic nervous system activity changes in patients with hypertension and overweight: Role and therapeutic implications. Cardiovasc. Diabetol. 2021, 20, 170. [Google Scholar] [CrossRef]
- Delaney, E.P.; Greaney, J.L.; Edwards, D.G.; Rose, W.C.; Fadel, P.J.; Farquhar, W.B. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: Role of the muscle metaboreflex. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1318–H1327. [Google Scholar] [CrossRef]
- Sausen, M.T.; Delaney, E.P.; Stillabower, M.E.; Farquhar, W.B. Enhanced metaboreflex sensitivity in hypertensive humans. Eur. J. Appl. Physiol. 2009, 105, 351–356. [Google Scholar] [CrossRef]
- Smith, S.A.; Leal, A.K.; Williams, M.A.; Murphy, M.N.; Mitchell, J.H.; Garry, M.G. The TRPv1 receptor is a mediator of the exercise pressor reflex in rats. J. Physiol. 2010, 588, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Mammen, P.P.; Mitchell, J.H.; Garry, M.G. Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation 2003, 108, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Mitchell, J.H.; Garry, M.G. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J. Physiol. 2001, 537, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.K.; Williams, M.A.; Garry, M.G.; Mitchell, J.H.; Smith, S.A. Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1429–H1438. [Google Scholar] [CrossRef]
- Kaur, J.; Spranger, M.D.; Hammond, R.L.; Krishnan, A.C.; Alvarez, A.; Augustyniak, R.A.; O’Leary, D.S. Muscle metaboreflex activation during dynamic exercise evokes epinephrine release resulting in beta2-mediated vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H524–H529. [Google Scholar] [CrossRef]
- O’Leary, D.S. Autonomic mechanisms of muscle metaboreflex control of heart rate. J. Appl. Physiol. 1993, 74, 1748–1754. [Google Scholar] [CrossRef]
- O’Leary, D.S.; Augustyniak, R.A. Muscle metaboreflex increases ventricular performance in conscious dogs. Am. J. Physiol. 1998, 275, H220–H224. [Google Scholar] [CrossRef]
- Sala-Mercado, J.A.; Hammond, R.L.; Kim, J.K.; Rossi, N.F.; Stephenson, L.W.; O’Leary, D.S. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H751–H757. [Google Scholar] [CrossRef]
- Mannozzi, J.; Al-Hassan, M.H.; Lessanework, B.; Alvarez, A.; Senador, D.; O’Leary, D.S. Chronic Ablation of TRPV1 Sensitive Skeletal Muscle Afferents Attenuates the Muscle Metaboreflex. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R385–R395. [Google Scholar] [CrossRef]
- Mannozzi, J.; Kaur, J.; Spranger, M.D.; Al-Hassan, M.H.; Lessanework, B.; Alvarez, A.; Chung, C.S.; O’Leary, D.S. Muscle Metaboreflex-Induced Increases in Effective Arterial Elastance: Effect of Heart Failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R1–R10. [Google Scholar] [CrossRef] [PubMed]
- Mannozzi, J.; Massoud, L.; Kaur, J.; Coutsos, M.; O’Leary, D.S. Ventricular contraction and relaxation rates during muscle metaboreflex activation in heart failure: Are they coupled? Exp. Physiol. 2021, 106, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Brum, P.C.; Da Silva, G.J.; Moreira, E.D.; Ida, F.; Negrao, C.E.; Krieger, E.M. Exercise training increases baroreceptor gain sensitivity in normal and hypertensive rats. Hypertension 2000, 36, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Bugenhagen, S.M.; Cowley, A.W., Jr.; Beard, D.A. Identifying physiological origins of baroreflex dysfunction in salt-sensitive hypertension in the Dahl SS rat. Physiol. Genom. 2010, 42, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Greaney, J.L.; Wenner, M.M.; Farquhar, W.B. Exaggerated increases in blood pressure during isometric muscle contraction in hypertension: Role for purinergic receptors. Auton. Neurosci. 2015, 188, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, D.D.; O’Leary, D.S.; Scher, A.M.; Rowell, L.B. Baroreflex attenuates pressor response to graded muscle ischemia in exercising dogs. Am. J. Physiol. 1990, 258, H305–H310. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Sala-Mercado, J.A.; Hammond, R.L.; Rodriguez, J.; Scislo, T.J.; O’Leary, D.S. Attenuated arterial baroreflex buffering of muscle metaboreflex in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2416–H2423. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Sala-Mercado, J.A.; Rodriguez, J.; Scislo, T.J.; O’Leary, D.S. Arterial baroreflex alters strength and mechanisms of muscle metaboreflex during dynamic exercise. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1374–H1380. [Google Scholar] [CrossRef] [PubMed]
- Harthmann, A.D.; De Angelis, K.; Costa, L.P.; Senador, D.; Schaan, B.D.; Krieger, E.M.; Irigoyen, M.C. Exercise training improves arterial baro- and chemoreflex in control and diabetic rats. Auton. Neurosci. 2007, 133, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.C.; Arce-Alvarez, A.; Toledo, C.; Diaz, H.S.; Lucero, C.; Schultz, H.D.; Marcus, N.J.; Del Rio, R. Exercise training improves cardiac autonomic control, cardiac function, and arrhythmogenesis in rats with preserved-ejection fraction heart failure. J. Appl. Physiol. 2017, 123, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Iellamo, F.; Legramante, J.M.; Massaro, M.; Raimondi, G.; Galante, A. Effects of a Residential Exercise Training on Baroreflex Sensitivity and Heart Rate Variability in Patients With Coronary Artery Disease: A Randomized, Controlled Study. Circulation 2000, 102, 2588–2592. [Google Scholar] [CrossRef]
- Iellamo, F.; Manzi, V.; Caminiti, G.; Sposato, B.; Massaro, M.; Cerrito, A.; Rosano, G.; Volterrani, M. Dose-response relationship of baroreflex sensitivity and heart rate variability to individually-tailored exercise training in patients with heart failure. Int. J. Cardiol. 2013, 166, 334–339. [Google Scholar] [CrossRef]
- Loimaala, A.; Huikuri, H.V.; Koobi, T.; Rinne, M.; Nenonen, A.; Vuori, I. Exercise training improves baroreflex sensitivity in type 2 diabetes. Diabetes 2003, 52, 1837–1842. [Google Scholar] [CrossRef]
- Mameletzi, D.; Kouidi, E.; Koutlianos, N.; Deligiannis, A. Effects of long-term exercise training on cardiac baroreflex sensitivity in patients with coronary artery disease: A randomized controlled trial. Clin. Rehabil. 2011, 25, 217–227. [Google Scholar] [CrossRef]
- Wang, H.J.; Pan, Y.X.; Wang, W.Z.; Gao, L.; Zimmerman, M.C.; Zucker, I.H.; Wang, W. Exercise training prevents the exaggerated exercise pressor reflex in rats with chronic heart failure. J. Appl. Physiol. 2010, 108, 1365–1375. [Google Scholar] [CrossRef]
- Doneddu, A.; Roberto, S.; Pinna, V.; Magnani, S.; Ghiani, G.; Sainas, G.; Mulliri, G.; Serra, S.; Kakhak, S.A.H.; Milia, R.; et al. Effect of Combined Mental Task and Metaboreflex Activation on Hemodynamics and Cerebral Oxygenation in Patients with Metabolic Syndrome. Front. Physiol. 2020, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Meshkani, R.; Adeli, K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin. Biochem. 2009, 42, 1331–1346. [Google Scholar] [CrossRef] [PubMed]
- Reynisdottir, S.; Ellerfeldt, K.; Wahrenberg, H.; Lithell, H.; Arner, P. Multiple lipolysis defects in the insulin resistance (metabolic) syndrome. J. Clin. Investig. 1994, 93, 2590–2599. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S.; Essien, U.N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diabetes Metab. Disord. 2013, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.C.; Warram, J.H.; Krolewski, A.S.; Bergman, R.N.; Soeldner, J.S.; Kahn, C.R. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: Results of a 25-year follow-up study. Lancet 1992, 340, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Marselli, L.; Piron, A.; Suleiman, M.; Colli, M.L.; Yi, X.; Khamis, A.; Carrat, G.R.; Rutter, G.A.; Bugliani, M.; Giusti, L.; et al. Persistent or Transient Human beta Cell Dysfunction Induced by Metabolic Stress: Specific Signatures and Shared Gene Expression with Type 2 Diabetes. Cell Rep. 2020, 33, 108466. [Google Scholar] [CrossRef]
- Poitout, V.; Robertson, R.P. Minireview: Secondary beta-cell failure in type 2 diabetes--a convergence of glucotoxicity and lipotoxicity. Endocrinology 2002, 143, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Sone, H.; Kagawa, Y. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia 2005, 48, 58–67. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, H.; Ren, T.Y.S. Repair of Damaged Pancreatic beta Cells: New Hope for a Type 2 Diabetes Reversal? J. Transl. Int. Med. 2021, 9, 150–151. [Google Scholar] [CrossRef]
- Huggett, R.J.; Scott, E.M.; Gilbey, S.G.; Stoker, J.B.; Mackintosh, A.F.; Mary, D.A. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation 2003, 108, 3097–3101. [Google Scholar] [CrossRef]
- Mancusi, C.; Izzo, R.; di Gioia, G.; Losi, M.A.; Barbato, E.; Morisco, C. Insulin Resistance the Hinge Between Hypertension and Type 2 Diabetes. High Blood Press. Cardiovasc. Prev. 2020, 27, 515–526. [Google Scholar] [CrossRef]
- Zhou, M.S.; Wang, A.; Yu, H. Link between insulin resistance and hypertension: What is the evidence from evolutionary biology? Diabetol. Metab. Syndr. 2014, 6, 12. [Google Scholar] [CrossRef]
- Russo, B.; Menduni, M.; Borboni, P.; Picconi, F.; Frontoni, S. Autonomic Nervous System in Obesity and Insulin-Resistance-The Complex Interplay between Leptin and Central Nervous System. Int. J. Mol. Sci. 2021, 22, 5187. [Google Scholar] [CrossRef]
- Roberto, S.; Milia, R.; Doneddu, A.; Pinna, V.; Palazzolo, G.; Serra, S.; Orru, A.; Hosseini Kakhak, S.A.; Ghiani, G.; Mulliri, G.; et al. Hemodynamic abnormalities during muscle metaboreflex activation in patients with type 2 diabetes mellitus. J. Appl. Physiol. 2019, 126, 444–453. [Google Scholar] [CrossRef]
- Figueroa, A.; Maharaj, A.; Johnson, S.A.; Fischer, S.M.; Arjmandi, B.H.; Jaime, S.J. Exaggerated Aortic Pulse Pressure and Wave Amplitude During Muscle Metaboreflex Activation in Type 2 Diabetes Patients. Am. J. Hypertens. 2020, 33, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Pinna, V.; Doneddu, A.; Roberto, S.; Magnani, S.; Ghiani, G.; Mulliri, G.; Sanna, I.; Serra, S.; Hosseini Kakhak, S.A.; Milia, R.; et al. Combined mental task and metaboreflex impair cerebral oxygenation in patients with type 2 diabetes mellitus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R488–R499. [Google Scholar] [CrossRef] [PubMed]
- McMillan, N.J.; Soares, R.N.; Harper, J.L.; Shariffi, B.; Moreno-Cabanas, A.; Curry, T.B.; Manrique-Acevedo, C.; Padilla, J.; Limberg, J.K. Role of the arterial baroreflex in the sympathetic response to hyperinsulinemia in adult humans. Am. J. Physiol. Endocrinol. Metab. 2022, 322, E355–E365. [Google Scholar] [CrossRef]
- Young, B.E.; Padilla, J.; Shoemaker, J.K.; Curry, T.B.; Fadel, P.J.; Limberg, J.K. Sympathetic transduction to blood pressure during euglycemic-hyperinsulinemia in young healthy adults: Role of burst amplitude. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 324, R536–R546. [Google Scholar] [CrossRef] [PubMed]
- Pricher, M.P.; Freeman, K.L.; Brooks, V.L. Insulin in the brain increases gain of baroreflex control of heart rate and lumbar sympathetic nerve activity. Hypertension 2008, 51, 514–520. [Google Scholar] [CrossRef]
- Ryan, J.P.; Sheu, L.K.; Verstynen, T.D.; Onyewuenyi, I.C.; Gianaros, P.J. Cerebral blood flow links insulin resistance and baroreflex sensitivity. PLoS ONE 2013, 8, e83288. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harrison, N.A.; Cooper, E.; Voon, V.; Miles, K.; Critchley, H.D. Central autonomic network mediates cardiovascular responses to acute inflammation: Relevance to increased cardiovascular risk in depression? Brain Behav. Immun. 2013, 31, 189–196. [Google Scholar] [CrossRef]
- Morrison, S.F. Differential control of sympathetic outflow. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R683–R698. [Google Scholar] [CrossRef] [PubMed]
- Lucini, D.; Cusumano, G.; Bellia, A.; Kozakova, M.; Difede, G.; Lauro, R.; Pagani, M. Is reduced baroreflex gain a component of the metabolic syndrome? Insights from the LINOSA study. J. Hypertens. 2006, 24, 361–370. [Google Scholar] [CrossRef] [PubMed]
- James, P.T.; Rigby, N.; Leach, R.; International Obesity Task, F. The obesity epidemic, metabolic syndrome and future prevention strategies. Eur. J. Cardiovasc. Prev. Rehabil. 2004, 11, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.E.; Beske, S.D.; Ballard, T.P.; Davy, K.P. Sympathetic neural activation in visceral obesity. Circulation 2002, 106, 2533–2536. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Dell’Oro, R.; Facchini, A.; Quarti Trevano, F.; Bolla, G.B.; Mancia, G. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J. Hypertens. 2004, 22, 2363–2369. [Google Scholar] [CrossRef]
- Bergman, R.N.; Kim, S.P.; Catalano, K.J.; Hsu, I.R.; Chiu, J.D.; Kabir, M.; Hucking, K.; Ader, M. Why visceral fat is bad: Mechanisms of the metabolic syndrome. Obesity 2006, 14 (Suppl. S1), 16S–19S. [Google Scholar] [CrossRef]
- Jensen, M.D. Is visceral fat involved in the pathogenesis of the metabolic syndrome? Human model. Obesity 2006, 14 (Suppl. S1), 20S–24S. [Google Scholar] [CrossRef]
- Kalil, G.Z.; Haynes, W.G. Sympathetic nervous system in obesity-related hypertension: Mechanisms and clinical implications. Hypertens. Res. 2012, 35, 4–16. [Google Scholar] [CrossRef]
- Shibao, C.; Gamboa, A.; Diedrich, A.; Ertl, A.C.; Chen, K.Y.; Byrne, D.W.; Farley, G.; Paranjape, S.Y.; Davis, S.N.; Biaggioni, I. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension 2007, 49, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Latchman, P.L.; Mathur, M.; Bartels, M.N.; Axtell, R.S.; De Meersman, R.E. Impaired autonomic function in normotensive obese children. Clin. Auton. Res. 2011, 21, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Derella, C.C.; Blanks, A.M.; Wang, X.; Tucker, M.A.; Horsager, C.; Jeong, J.H.; Rodriguez-Miguelez, P.; Looney, J.; Thomas, J.; Pollock, D.M.; et al. Endothelin receptor blockade blunts the pressor response to acute stress in men and women with obesity. J. Appl. Physiol. 2022, 132, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Licht, C.M.; de Geus, E.J.; Penninx, B.W. Dysregulation of the autonomic nervous system predicts the development of the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Flaa, A.; Aksnes, T.A.; Kjeldsen, S.E.; Eide, I.; Rostrup, M. Increased sympathetic reactivity may predict insulin resistance: An 18-year follow-up study. Metabolism 2008, 57, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Bousquet, P.; Elghozi, J.L.; Esler, M.; Grassi, G.; Julius, S.; Reid, J.; Van Zwieten, P.A. The sympathetic nervous system and the metabolic syndrome. J. Hypertens. 2007, 25, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Stavres, J.; Aultman, R.A.; Brandner, C.F.; Newsome, T.Q.A.; Vallecillo-Bustos, A.; Wise, H.L.; Henderson, A.; Stanfield, D.; Mannozzi, J.; Graybeal, A.J. Hemodynamic responses to handgrip and metaboreflex activation are exaggerated in individuals with metabolic syndrome independent of resting blood pressure, waist circumference, and fasting blood glucose. Front. Physiol. 2023, 14, 1212775. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef]
- Lindgren, K.; Hagelin, E.; Hansen, N.; Lind, L. Baroreceptor sensitivity is impaired in elderly subjects with metabolic syndrome and insulin resistance. J. Hypertens. 2006, 24, 143–150. [Google Scholar] [CrossRef]
- Zanoli, L.; Empana, J.P.; Estrugo, N.; Escriou, G.; Ketthab, H.; Pruny, J.F.; Castellino, P.; Laude, D.; Thomas, F.; Pannier, B.; et al. The Neural Baroreflex Pathway in Subjects With Metabolic Syndrome: A Sub-Study of the Paris Prospective Study III. Medicine 2016, 95, e2472. [Google Scholar] [CrossRef]
- Kaur, J.; Machado, T.M.; Alvarez, A.; Krishnan, A.C.; Hanna, H.W.; Altamimi, Y.H.; Senador, D.; Spranger, M.D.; O’Leary, D.S. Muscle metaboreflex activation during dynamic exercise vasoconstricts ischemic active skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H2145–H2151. [Google Scholar] [CrossRef]
- Kaur, J.; Alvarez, A.; Hanna, H.W.; Krishnan, A.C.; Senador, D.; Machado, T.M.; Altamimi, Y.H.; Lovelace, A.T.; Dombrowski, M.D.; Spranger, M.D.; et al. Interaction between the muscle metaboreflex and the arterial baroreflex in control of arterial pressure and skeletal muscle blood flow. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1268–H1276. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Senador, D.; Krishnan, A.C.; Hanna, H.W.; Alvarez, A.; Machado, T.M.; O’Leary, D.S. Muscle Metaboreflex-Induced Vasoconstriction in the Ischemic Active Muscle is Exaggerated in Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2017, 314, H11–H18. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Blain, G.M.; Proctor, L.T.; Sebranek, J.J.; Pegelow, D.F.; Dempsey, J.A. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J. Physiol. 2011, 589, 5299–5309. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Blain, G.M.; Proctor, L.T.; Sebranek, J.J.; Pegelow, D.F.; Dempsey, J.A. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J. Appl. Physiol. 2010, 109, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Proctor, L.T.; Sebranek, J.J.; Pegelow, D.F.; Dempsey, J.A. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J. Physiol. 2009, 587, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Sidhu, S.K.; Weavil, J.C.; Mangum, T.S.; Venturelli, M. Autonomic responses to exercise: Group III/IV muscle afferents and fatigue. Auton. Neurosci. 2015, 188, 19–23. [Google Scholar] [CrossRef]
- Amann, M.; Venturelli, M.; Ives, S.J.; Morgan, D.E.; Gmelch, B.; Witman, M.A.; Jonathan Groot, H.; Walter Wray, D.; Stehlik, J.; Richardson, R.S. Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int. J. Cardiol. 2014, 174, 368–375. [Google Scholar] [CrossRef]
- Ali, A.; Ganai, J.; Muthukrishnan, S.; Kohli, S. Evaluation of Autonomic Dysfunction in Obese and Non-Obese Hypertensive Subjects. J. Clin. Diagn. Res. 2016, 10, YC01–YC03. [Google Scholar] [CrossRef]
- Min, K.B.; Min, J.Y.; Paek, D.; Cho, S.I. The impact of the components of metabolic syndrome on heart rate variability: Using the NCEP-ATP III and IDF definitions. Pacing Clin. Electrophysiol. 2008, 31, 584–591. [Google Scholar] [CrossRef]
- Jarczok, M.N.; Li, J.; Mauss, D.; Fischer, J.E.; Thayer, J.F. Heart rate variability is associated with glycemic status after controlling for components of the metabolic syndrome. Int. J. Cardiol. 2013, 167, 855–861. [Google Scholar] [CrossRef]
- Altuncu, M.E.; Baspinar, O.; Keskin, M. The use of short-term analysis of heart rate variability to assess autonomic function in obese children and its relationship with metabolic syndrome. Cardiol. J. 2012, 19, 501–506. [Google Scholar] [CrossRef][Green Version]
- Balcioglu, A.S.; Akinci, S.; Cicek, D.; Eldem, H.O.; Coner, A.; Bal, U.A.; Muderrisoglu, H. Which is responsible for cardiac autonomic dysfunction in non-diabetic patients with metabolic syndrome: Prediabetes or the syndrome itself? Diabetes Metab. Syndr. 2016, 10, S13–S20. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Yang, Y.C.; Lu, F.H.; Lin, T.S.; Chen, J.J.; Yeh, T.L.; Wu, C.H.; Wu, J.S. Altered cardiac autonomic function may precede insulin resistance in metabolic syndrome. Am. J. Med. 2010, 123, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tseng, P.H.; Ahn, A.; Wu, M.S.; Ho, Y.L.; Chen, M.F.; Peng, C.K. Cardiac Autonomic Alteration and Metabolic Syndrome: An Ambulatory ECG-based Study in A General Population. Sci. Rep. 2017, 7, 44363. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Krishnan, A.C.; Senador, D.; Alvarez, A.; Hanna, H.W.; O’Leary, D.S. Altered arterial baroreflex-muscle metaboreflex interaction in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1383–H1392. [Google Scholar] [CrossRef]
| Component | Range |
|---|---|
| Waist Circumference | >102 cm for males or >88 cm for females (>80 cm for Asian females) |
| HDL Cholesterol | <40 mg/dL for males and <50 mg/dL for females |
| Blood Pressure | >130 mmHg systolic or >85 mmHg diastolic |
| Fasted Blood Glucose | ≥100 mg/dL |
| Triglycerides | ≥150 mg/dL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannozzi, J.; Massoud, L.; Stavres, J.; Al-Hassan, M.-H.; O’Leary, D.S. Altered Autonomic Function in Metabolic Syndrome: Interactive Effects of Multiple Components. J. Clin. Med. 2024, 13, 895. https://doi.org/10.3390/jcm13030895
Mannozzi J, Massoud L, Stavres J, Al-Hassan M-H, O’Leary DS. Altered Autonomic Function in Metabolic Syndrome: Interactive Effects of Multiple Components. Journal of Clinical Medicine. 2024; 13(3):895. https://doi.org/10.3390/jcm13030895
Chicago/Turabian StyleMannozzi, Joseph, Louis Massoud, Jon Stavres, Mohamed-Hussein Al-Hassan, and Donal S. O’Leary. 2024. "Altered Autonomic Function in Metabolic Syndrome: Interactive Effects of Multiple Components" Journal of Clinical Medicine 13, no. 3: 895. https://doi.org/10.3390/jcm13030895
APA StyleMannozzi, J., Massoud, L., Stavres, J., Al-Hassan, M.-H., & O’Leary, D. S. (2024). Altered Autonomic Function in Metabolic Syndrome: Interactive Effects of Multiple Components. Journal of Clinical Medicine, 13(3), 895. https://doi.org/10.3390/jcm13030895