Diagnostic Imaging of Agminated Blue Lesions and Blue Lesions with Satellitosis: Case Series with a Concise Review of the Current Literature
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Upshaw, B.Y.; Ghormley, R.K.; Montgomery, H. Extensive blue nevus of Jadassohn-Tièche; report of a case. Surgery 1947, 22, 761–765. [Google Scholar] [PubMed]
- Dorsey, C.S.; Montgomery, H. Blue nevus and its distinction from Mongolian spot and the nevus of Ota. J. Investig. Dermatol. 1954, 22, 225–236. [Google Scholar] [CrossRef]
- Pittman, J.L.; Fisher, B.K. Plaque-type blue nevus. Arch. Dermatol. 1976, 112, 1127–1128. [Google Scholar] [CrossRef]
- Shenfield, H.T.; Maize, J.C. Multiple and agminated blue nevi. J. Dermatol. Surg. Oncol. 1980, 6, 725–728. [Google Scholar] [CrossRef]
- Atherton, D.J.; Wells, R.S. Confluent and reticulate papillomatosis. Clin. Exp. Dermatol. 1980, 5, 465–469. [Google Scholar] [CrossRef]
- Hendricks, W.M. Eruptive blue nevi. J. Am. Acad. Dermatol. 1981, 4, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Tuthill, R.J.; Clark, W.H., Jr.; Levene, A. Pilar neurocristic hamartoma: Its relationship to blue nevus and equine melanotic disease. Arch. Dermatol. 1982, 118, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, A.; Kimura, K.; Kukita, A. Plaque-type blue nevus combined with lentigo (nevus spilus). J. Cutan. Pathol. 1990, 17, 241–245. [Google Scholar] [CrossRef]
- Hofmann, U.; Schacht, B.; Megahed, M. Gruppiert angeordnete und kombinierte blaue Nävi [Grouped and combined blue nevi]. Hautarzt 1992, 43, 517–519. (In German) [Google Scholar]
- Misago, N.; Narisawa, Y.; Kohda, H. A combination of speckled lentiginous nevus with patch-type blue nevus. J. Dermatol. 1993, 20, 643–647. [Google Scholar] [CrossRef]
- Vélez, A.; del-Río, E.; Martín-de-Hijas, C.; Furió, V.; Sánchez Yus, E. Agminated blue nevi: Case report and review of the literature. Dermatology 1993, 186, 144–148. [Google Scholar] [CrossRef]
- Kiene, P.; Brodersen, J.P.; Fölster-Holst, R. “Blaue” Variante eines Naevus spilus [“Blue” variant of naevus spilus]. Hautarzt 1995, 46, 349–351. (In German) [Google Scholar] [CrossRef]
- El-Ansary, M.S.; Al-Fouzan, A.S.; Hassab-El-Naby, H.M.M. Agminated blue nevus: A case report. GJDV 2001, 8, 50–52. [Google Scholar]
- Pizzichetta, M.A.; Soyer, H.P.; Massone, C.; Cerroni, L. Clinical and dermoscopic features of agminated blue nevus. Arch. Dermatol. 2007, 143, 1225–1226. [Google Scholar] [CrossRef]
- Chen, T.; Kurwa, H.A.; Trotter, M.J.; Haber, R.M. Agminated blue nevi in a patient with dermatomyositis. J. Am. Acad. Dermatol. 2013, 68, e52–e53. [Google Scholar] [CrossRef]
- Milkova, L.; Treudler, R.; Simon, J.C.; Kunz, M. Agminated blue naevi in a patient with EMO syndrome. Acta Derm. Venereol. 2013, 93, 104–105. [Google Scholar] [CrossRef]
- Rocha, C.R.; Grazziotin, T.C.; Rey, M.C.; Luzzatto, L.; Bonamigo, R.R. Congenital agminated melanocytic nevus-case report. An. Bras. Dermatol. 2013, 88, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Spring, P.; Perrier, P.; Erba, P.; Hagmann, P.; Mihm, M.C.; Hohl, D. Large agminated cellular ‘plaque-type’ blue nevus surrounding the ear: A case and review. Dermatology 2013, 227, 21–25. [Google Scholar] [CrossRef]
- Koba, S.; Mori, M.; Misago, N.; Narisawa, Y. Agminated blue naevus on the sole. J. Eur. Acad Dermatol. Venereol. 2014, 30, 334–335. [Google Scholar] [CrossRef] [PubMed]
- Paolino, G.; Didona, D.; Lopez, T.; Alesini, F.; Cantisani, C.; Richetta, A.G.; Soda, G.; Calvieri, S. Agminated Blue Nevus: Two Case Reports and a Mini-review of the Literature. Acta Dermatovenerol. Croat. 2016, 24, 37–41. [Google Scholar] [PubMed]
- Lisboa, A.P.; Silvestre, K.J.; Pedreira, R.L.; Alves, N.R.; Obadia, D.L.; Azulay-Abulafia, L. Agminated blue nevus—Case report. An. Bras. Dermatol. 2016, 91, 658–660. [Google Scholar] [CrossRef]
- Oliveira, A.H.K.; Shiraishi, A.F.M.C.; Kadunc, B.V.; Sotero, P.C.; Stelini, R.F.; Mendes, C. Blue nevus with satellitosis: Case report and literature review. An. Bras. Dermatol. 2017, 92, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.A.; Crandall, M.; Spring, L.K. Agminated heterogeneous papules on the neck. Cutis 2018, 102, E24–E26. [Google Scholar] [PubMed]
- Woo, W.A.; Coe, J.; Springgay, G.; Gupta, P. A rare combination of agminated blue naevus and naevus spilus. Clin. Exp. Dermatol. 2019, 44, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, C.; Magri, F.; Iacovino, C.; Soda, G.; Bergler-Czop, B.B.; Marino, R.; Tornese, A.; Cantoresi, F. Blue nevus with satellitosis in a pregnant patient. Ital. J. Dermatol. Venerol. 2021, 156, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Jiménez, P.; Mayor-Sanabria, F.; Rütten, A.; Fraga, J.; Llamas-Velasco, M. Agminated Blue Nevus: GNAQ Mutations and Beyond. Actas Dermosifiliogr. (Engl. Ed.) 2021, 112, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Sławińska, M.; Balicka, U.; Kamińska-Winciorek, G.; Sikorska, M.; Nowicki, R.J.; Sobjanek, M. Agminated blue nevi: A case series and updated dermoscopic review. Postepy Dermatol. Alergol. 2022, 39, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Hassab-El-Naby, H.M.M.; Rageh, M.A. Agminated Halo Nevus: A Novel Presentation. Am. J. Dermatopathol. 2023, 45, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.C.; Paver, E.C.; Agar, N.; Scolyer, R.A.; Moloney, F.J. A sheep in wolf’s clothing: Agminated blue naevi masquerading as in-transit melanoma metastases. Australas. J. Dermatol. 2023, 64, e196–e199. [Google Scholar] [CrossRef]
- Palazzo, J.P.; Duray, P.H. Congenital agminated Spitz nevi: Immunoreactivity with a melanoma-associated monoclonal antibody. J. Cutan. Pathol. 1988, 15, 166–170. [Google Scholar] [CrossRef]
- Renfro, L.; Grant-Kels, J.M.; Brown, S.A. Multiple agminate Spitz nevi. Pediatr. Dermatol. 1989, 6, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Abramovits, W.; Gonzalez-Serva, A. Multiple agminated pigmented Spitz nevi (mimicking acral lentiginous malignant melanoma and dysplastic nevus) in an African-American girl. Int. J. Dermatol. 1993, 32, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Herd, R.M.; Allan, S.M.; Biddlestone, L.; Buxton, P.K.; Mclaran, K.M. Agminate Spitz naevi arising on hyperpigmented patches. Clin. Exp. Dermatol. 1994, 19, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Bullen, R.; Snow, S.N.; Larson, P.O.; Kircik, L.H.; Nychay, S.; Briggs, P. Multiple agminated Spitz nevi: Report of two cases and review of the literature. Pediatr. Dermatol. 1995, 12, 156–158. [Google Scholar] [CrossRef]
- Krasovec, M.; Gianadda, B.; Hohl, D. Giant recurrence of a multiple agminated Spitz nevus. J. Am. Acad. Dermatol. 1995, 33, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Aloi, F.; Tomasini, C.; Pippione, M. Agminated Spitz nevi occurring within a congenital speckled lentiginous nevus. Am. J. Dermatopathol. 1995, 17, 594–598. [Google Scholar] [CrossRef]
- Sabroe, R.A.; Vaingankar, N.V.; Rigby, H.S.; Peachey, R.D. Agminate Spitz naevi occurring in an adult after the excision of a solitary Spitz naevus--report of a case and review of the literature. Clin. Exp. Dermatol. 1996, 21, 197–200. [Google Scholar] [CrossRef]
- Hulshof, M.M.; van Haeringen, A.; Gruis, N.A.; Snels, D.C.; Bergman, W. Multiple agminate Spitz naevi. Melanoma Res. 1998, 8, 156–160. [Google Scholar] [CrossRef]
- Akyürek, M.; Kayikçioğlu, A.; Ozkan, O.; Güler, G.; Mavili, E.; Erk, Y. Multiple agminated Spitz nevi of the scalp. Ann. Plast. Surg. 1999, 43, 459–460. [Google Scholar] [CrossRef]
- Böer, A.; Wolter, M.; Kneisel, L.; Kaufmann, R. Multiple agminated Spitz nevi arising on a café au lait macule: Review of the literature with contribution of another case. Pediatr. Dermatol. 2001, 18, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, M.A.; Lain, E.L.; Kincannon, J.M. Agminated Spitz nevi: Report of a child with a unique dermatomal distribution. Pediatr. Dermatol. 2005, 22, 546–549. [Google Scholar] [CrossRef]
- Aida, K.; Monia, K.; Ahlem, S.; Dominique, H.T.; Becima, F.; Sylvie, F.; Ridha, K.M. Agminated Spitz nevi arising on a nevus spilus after chemotherapy. Pediatr. Dermatol. 2010, 27, 411–413. [Google Scholar] [CrossRef]
- Hassanein, A.H.; Gellis, S.E.; Schmidt, B.A.; Greene, A.K. Agminated atypical Spitz tumor: Large nasal lesion in a child with Down syndrome. J. Pediatr. Surg. 2011, 46, 1435–1437. [Google Scholar] [CrossRef]
- Zeng, M.H.; Kong, Q.T.; Sang, H.; Deng, D.Q.; Xie, Q.M. Agminated spitz nevi: Case report and review of the literature. Pediatr. Dermatol. 2013, 30, e104–e105. [Google Scholar] [CrossRef]
- Gupta, R.; Gautam, R.K.; Bhardwaj, M. Agminated spitz nevus on sole of elderly. Indian. J. Dermatol. 2015, 60, 217. [Google Scholar] [PubMed]
- Adachi, A.; Maekawa, T.; Komine, M.; Murata, S.; Ueda, Y.; Ohtsuki, M. Case of a penile atypical Spitz tumor with an agminated appearance. J. Dermatol. 2017, 44, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Porubsky, C.; Teer, J.K.; Zhang, Y.; Deschaine, M.; Sondak, V.K.; Messina, J.L. Genomic analysis of a case of agminated Spitz nevi and congenital-pattern nevi arising in extensive nevus spilus. J. Cutan. Pathol. 2018, 45, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Pontoizeau, J.; Stefan, A.; Comoz, F.; Houlier, A.; Haddad, V.; Pissaloux, D.; de la Fouchardiere, A. Agminated Spitz nevus arising in normal skin with redundant HRAS mutation. Eur. J. Dermatol. 2017, 27, 73–74. [Google Scholar] [CrossRef] [PubMed]
- van Kester, M.S.; Eggen, C.; Beishuizen, A.; Kukutsch, N.A. Agminated Spitz naevi or metastatic spitzoid melanoma? Australas. J. Dermatol. 2018, 59, e234–e235. [Google Scholar] [CrossRef] [PubMed]
- Adler, N.R.; Kelly, J.W.; Dowling, J.P.; Saunders, H.; Mar, V.J. An unusual onset of agminated Spitz naevi in an adult patient. Clin. Exp. Dermatol. 2018, 43, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Nevares-Pomales, O.W.; Carrasquillo, O.Y.; Santiago-Vazquez, M.; Colon-Fontanez, F. Rare Variant of Agminated Spitz Nevi on a Hypopigmented Background and Segmental Distribution: Case Report and Review of Literature. Am. J. Dermatopathol. 2018, 40, 686–689. [Google Scholar] [CrossRef]
- Nemeth, K.; Szabo, S.; Cottrell, C.E.; McNulty, S.M.; Segura, A.; Sokumbi, O.; Browning, M.; Siegel, D.H. Mosaic pathogenic HRAS variant in a patient with nevus spilus with agminated Spitz nevi and parametrial-uterine rhabdomyosarcoma. Br. J. Dermatol. 2018, 178, 804–806. [Google Scholar] [CrossRef]
- Robertson, S.J.; Orme, L.; Teixeira, R.; Shamassi, M.; Newell, F.; Patch, A.M.; Yeh, I.; Gard, G.; Wilmott, J.; Jackett, L.; et al. Evaluation of Crizotinib Treatment in a Patient With Unresectable GOPC-ROS1 Fusion Agminated Spitz Nevi. JAMA Dermatol. 2021, 157, 836–841. [Google Scholar] [CrossRef]
- De Giorgi, V.; Venturi, F.; Scarfì, F.; Trane, L.; Silvestri, F.; Savarese, I.; Facchini, F.; Buccoliero, A.M.; Massi, D. Clinical and dermoscopic polymorphisms in agminated Spitz nevi: Ugly presentation but benign behavior. Pediatr. Dermatol. 2021, 38, 461–463. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Wong, H.; Tang, X.; He, D.; Han, J.; Zhou, H. Successful treatment of adult-onset auricular agminated Spitz nevi with cryotherapy: A case report with an 11-year follow-up and literature review. JAAD Case Rep. 2022, 23, 49–51. [Google Scholar] [CrossRef]
- Fumero-Velázquez, M.; Hagstrom, M.; Dhillon, S.; Olivares, S.; Jennings, L.J.; Dittman, D.; Sukhanova, M.; Arva, N.C.; Goldstein, S.D.; Theos, A.; et al. Agminated presentation of fusion-driven melanocytic neoplasms. J. Cutan. Pathol. 2023, 50, 913–921. [Google Scholar] [CrossRef]
- Ferrara, G.; Soyer, H.P.; Malvehy, J.; Piccolo, D.; Puig, S.; Sopena, J.; Zalaudek, I.; Argenziano, G. The many faces of blue nevus: A clinicopathologic study. J. Cutan. Pathol. 2007, 34, 543–551. [Google Scholar] [CrossRef]
- Knoell, K.A.; Nelson, K.C.; Patterson, J.W. Familial multiple blue nevi. J. Am. Acad. Dermatol. 1998, 39 Pt 2, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Blackford, S.; Roberts, D.L. Familial multiple blue naevi. Clin. Exp. Dermatol. 1991, 16, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, D.Z.; Cotter, D.; Thorson, J.; Hinds, B.; Sun, B.K. Agminated blue nevus with a GNAQ mutation: A case report and review of the literature. J. Cutan. Pathol. 2019, 46, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.; Fang, Y.; Busam, K.J. Melanoma arising in a large plaque-type blue nevus with subcutaneous cellular nodules. Am. J. Surg. Pathol. 2012, 36, 1258–1263. [Google Scholar] [CrossRef]
- Sanada, S.; Higaki, K.; Torii, Y.; Higashi, T.; Yamaguchi, R.; Nakamura, Y.; Yano, H. Malignant melanoma arising in a plaque-type blue nevus. Pathol. Int. 2012, 62, 749–753. [Google Scholar] [CrossRef]
- Fernandez-Flores, A.; Cassarino, D. Genetic Studies on a Case of Eruptive Disseminated Spitz Nevus and Review of Other 33 Cases. Am. J. Dermatopathol. 2022, 44, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Masdemont, B.; Pérez-Tato, B.; Zamora-Martínez, E.; Rodríguez-Lomba, E. Regressing eruptive disseminated pigmented Spitz (Reed) nevi in a young adult. An. Bras. Dermatol. 2021, 96, 768–770. [Google Scholar] [CrossRef]
- Cerejeira, D.; Bonito, F.; António, A.M.; Alves, J. Eruptive disseminated Spitz nevi: A case report. Int. J. Dermatol. 2021, 60, 773–774. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.S.; Kapler, E.S.; Dinges, M.M.; Bastian, B.C.; Yeh, I. Eruptive Spitz nevus, a striking example of benign metastasis. Sci. Rep. 2020, 10, 16216. [Google Scholar] [CrossRef] [PubMed]
- Vargas, P.; Cárdenas, R.; Cullen, R.; Figueroa, A. Eruptive disseminated Spitz nevi—Case report. An. Bras. Dermatol. 2020, 95, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Paradisi, A.; Annessi, G.; Paradisi, M.; Abeni, D. Eruptive disseminated Spitz nevi. Eur. J. Dermatol. 2017, 27, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Scheiba, N.; Hartschuh, W. Image Gallery: Eruptive disseminated lentiginous Spitz naevi. Br. J. Dermatol. 2016, 175, e113. [Google Scholar] [CrossRef] [PubMed]
- Bhoyrul, B.; Tang, D.Y.; Carling, E.E.; Harikumar, C.; Newton-Bishop, J.; Carmichael, A.J. Regressing Eruptive Disseminated Spitz Nevi. Pediatr. Dermatol. 2015, 32, e181–e183. [Google Scholar] [CrossRef]
- Sharma, N.; Ho, S.; Bing, T.K.; McCormack, C.; Scolyer, R.; Lee, J. Eruptive disseminated Spitz naevus (EDSN) in a young girl of Indian origin. Australas. J. Dermatol. 2015, 56, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Mazzurco, J.; Menssen, K.; Schapiro, B.; Ramirez, J.; Heidelberg, K. Eruptive disseminated Spitz nevi in a 26-year-old African-American woman. Int. J. Dermatol. 2012, 51, 1270–1271. [Google Scholar] [CrossRef]
- Feito-Rodríguez, M.; de Lucas-Laguna, R.; Bastian, B.C.; Leboit, P.; González-Beato, M.J.; López-Gutiérrez, J.C.; Requena, L.; Pizarro, A. Nodular lesions arising in a large congenital melanocytic naevus in a newborn with eruptive disseminated Spitz naevi. Br. J. Dermatol. 2011, 165, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Saggini, A.; Di Stefani, A.; Saraceno, R.; Chimenti, S. “Bathing trunk” eruption of papules and nodules in a young woman. Eruptive disseminated Spitz naevi. Acta Derm. Venereol. 2011, 91, 738–739. [Google Scholar] [CrossRef]
- Kilinc Karaarslan, I.; Ozdemir, F.; Akalin, T.; Ozturk, G.; Turk, B.G.; Kandiloglu, G. Eruptive disseminated Spitz naevi: Dermatoscopic features. Clin. Exp. Dermatol. 2009, 34, e807–e810. [Google Scholar] [CrossRef] [PubMed]
- Boone, S.L.; Busam, K.J.; Marghoob, A.A.; Fang, Y.; Guitart, J.; Martini, M.; Gerami, P. Two cases of multiple spitz nevi: Correlating clinical, histologic, and fluorescence in situ hybridization findings. Arch. Dermatol. 2011, 147, 227–231. [Google Scholar] [CrossRef][Green Version]
- Levy, R.M.; Ming, M.E.; Shapiro, M.; Tucker, M.; Guerry, D., IV; Cirillo-Hyland, V.A.; Elenitsas, R. Eruptive disseminated Spitz nevi. J. Am. Acad. Dermatol. 2007, 57, 519–523. [Google Scholar] [CrossRef]
- Morgan, C.J.; Nyak, N.; Cooper, A.; Pees, B.; Friedmann, P.S. Multiple Spitz naevi: A report of both variants with clinical and histopathological correlation. Clin. Exp. Dermatol. 2006, 31, 368–371. [Google Scholar] [CrossRef]
- Dardano, F.; Colombo, E.; Tacchini, G.A.; Silvestri, T.; Flora, F.; Ottinetti, A. Nevo di Spitz multiplo [Multiple Spitz nevus]. Minerva Pediatr. 2003, 55, 75–78. (In Italian) [Google Scholar]
- Rim, J.H.; Won, C.H.; Lee, J.S.; Cho, K.H. A case of multiple disseminated eruptive Spitz nevi. J. Dermatol. 2002, 29, 380–382. [Google Scholar] [CrossRef]
- Onsun, N.; Saraçoğlu, S.; Demirkesen, C.; Kural, Y.B.; Atilganoğlu, U. Eruptive widespread Spitz nevi: Can pregnancy be a stimulating factor? J. Am. Acad. Dermatol. 1999, 40, 866–867. [Google Scholar]
- Salmon-Ehr, V.; Belaïch, S.; Tran, C.; Biaunié, G.; Serpier, H.; Bressieux, J.M.; Kalis, B. Naevus de Spitz éruptifs multiples disséminés: Un cas [Multiple disseminated eruptive Spitz nevi: A case]. Ann. Dermatol. Venereol. 1993, 120, 822–824. (In French) [Google Scholar]
- Del Río-Sancho, S.; Gallay, C.; Ventéjou, S.; Christen-Zaech, S. Non-invasive imaging of agminated Spitz nevi with line-field confocal optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e658–e659. [Google Scholar] [CrossRef]
- Kervarrec, T.; Briand, C.; Pissaloux, D.; Tirode, F.; Abasq-Thomas, C.; Fraitag, S.; de la Fouchardière, A. Agminated Spitz naevus with an activating HRAS Q61R mutation. Pathology 2022, 54, 374–376. [Google Scholar] [CrossRef]
- Goto, K.; Pissaloux, D.; Kauer, F.; Huriet, V.; Tirode, F.; de la Fouchardière, A. GOPC-ROS1 mosaicism in agminated Spitz naevi: Report of two cases. Virchows Arch. 2021, 479, 559–564. [Google Scholar] [CrossRef]
- Goto, K.; Pissaloux, D.; Durand, L.; Tirode, F.; Guillot, B.; de la Fouchardière, A. Novel three-way complex rearrangement of TRPM1-PUM1-LCK in a case of agminated Spitz nevi arising in a giant congenital hyperpigmented macule. Pigment. Cell Melanoma Res. 2020, 33, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Agulló, A.; Hinds, B.; Santesteban, R.; Mitxelena, J.M.; Yanguas, I. Agminated melanocytic nevus status post dabrafenib therapy for metastatic melanoma. Dermatol. Online J. 2016, 22. [Google Scholar] [CrossRef]
- Sarin, K.Y.; Sun, B.K.; Bangs, C.D.; Cherry, A.; Swetter, S.M.; Kim, J.; Khavari, P.A. Activating HRAS mutation in agminated Spitz nevi arising in a nevus spilus. JAMA Dermatol. 2013, 149, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Cervigón González, I.; Palomo Arellano, A.; Torres Iglesias, L.M.; Serrano Egea, A.; Moreno Gómez, E.; Palomero Domínguez, M.A. Nevus agminados de Spitz [Agminated Spitz nevi]. An Pediatr. (Barc.) 2012, 76, 373–374. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Berk, D.R.; Lane, A.T. Acquired bilateral agminated Spitz nevi in a child with Langerhans cell histiocytosis. Pediatr. Dermatol. 2010, 27, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Hueso, L.; Hernández, A.; Torrelo, A.; Colmenero, I.; Zambrano, A. Nevos de Spitz agrupados sobre una mácula hiperpigmentada [Agminated Spitz nevi on a hyperpigmented macule]. Actas Dermosifiliogr. 2008, 99, 69–72. (In Spanish) [Google Scholar] [CrossRef]
- Menni, S.; Betti, R.; Boccardi, D.; Gualandri, L. Both unilateral naevus achromicus and congenital agminated Spitz naevi in a checkerboard mosaic pattern. Br. J. Dermatol. 2001, 144, 187–188. [Google Scholar] [CrossRef]
- Betti, R.; Inselvini, E.; Palvarini, M.; Crosti, C. Agminated intradermal Spitz nevi arising on an unusual speckled lentiginous nevus with localized lentiginosis: A continuum? Am. J. Dermatopathol. 1997, 19, 524–527. [Google Scholar] [CrossRef]
- van Leeuwen, R.L.; Vink, J.; Bergman, W.; Herfst, M.; Bruijn, J.A. Agminate-type combined nevus consisting of a common blue nevus with a junctional Spitz nevus. Arch. Dermatol. 1994, 130, 1074–1075. [Google Scholar] [CrossRef] [PubMed]
- Sourreil, P.; Beylot, C.; Carrard, X. Mélanome de Spitz (forme multiple agminée). Deux observations [Spitz’s melanoma (multiple agminated form). 2 cases]. Bull. Soc. Fr. Dermatol. Syphiligr. 1969, 76, 77–79. (In French) [Google Scholar]
- Piérard, G.E.; Piérard-Franchimont, C. Cutaneous Melanocytomas: Variants and Caveats. Austin J. Cancer Clin. Res. 2015, 2, 1035. [Google Scholar]
- Costa, J.; Ortiz-Ibañez, K.; Salerni, G.; Borges, V.; Carrera, C.; Puig, S.; Malvehy, J. Dermoscopic patterns of melanoma metastases: Interobserver consistency and accuracy for metastasis recognition. Br. J. Dermatol. 2013, 169, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Bono, R.; Giampetruzzi, A.R.; Concolino, F.; Puddu, P.; Scoppola, A.; Sera, F.; Marchetti, P. Dermoscopic patterns of cutaneous melanoma metastases. Melanoma Res. 2004, 14, 367–373. [Google Scholar] [CrossRef] [PubMed]
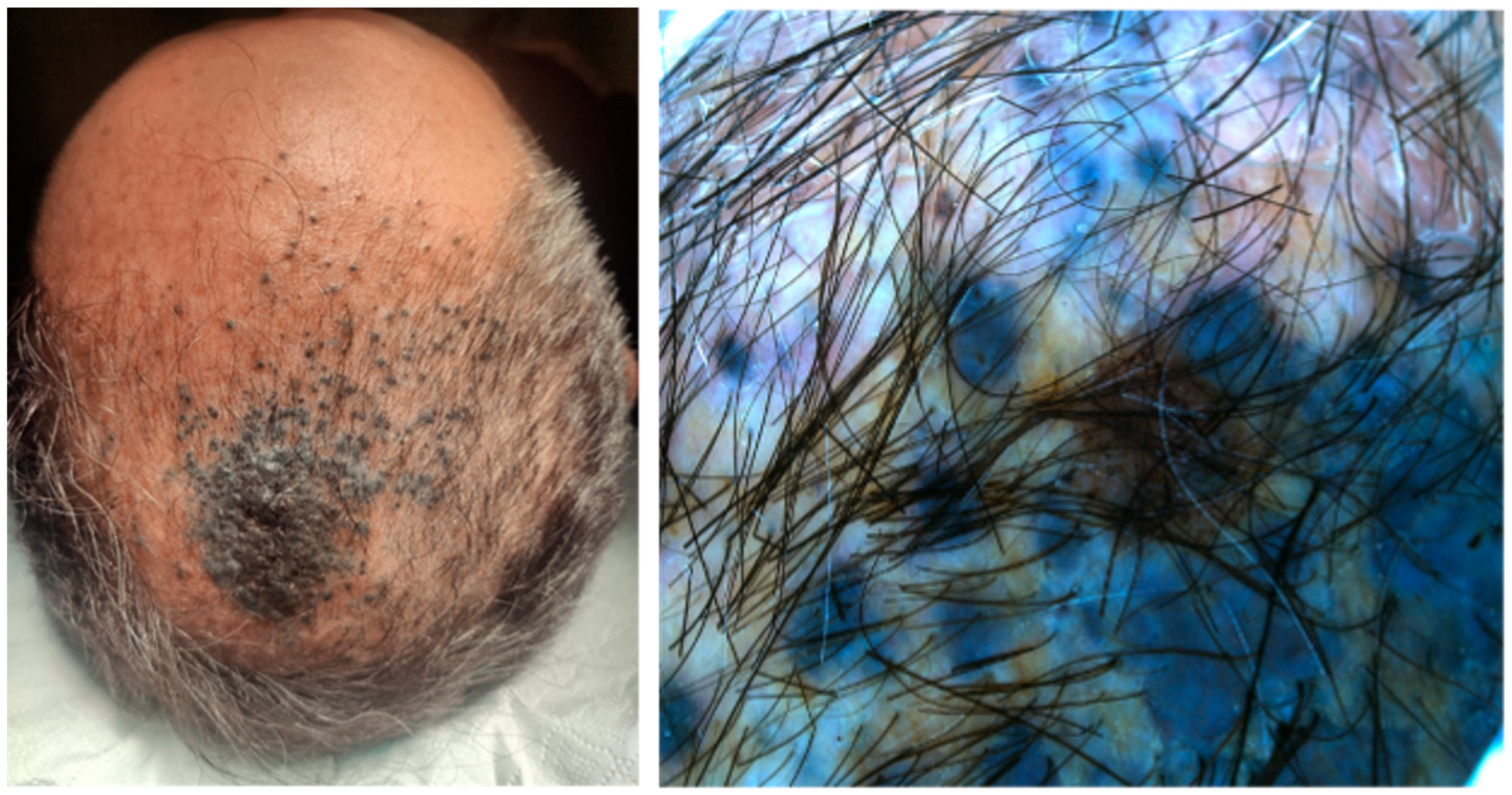
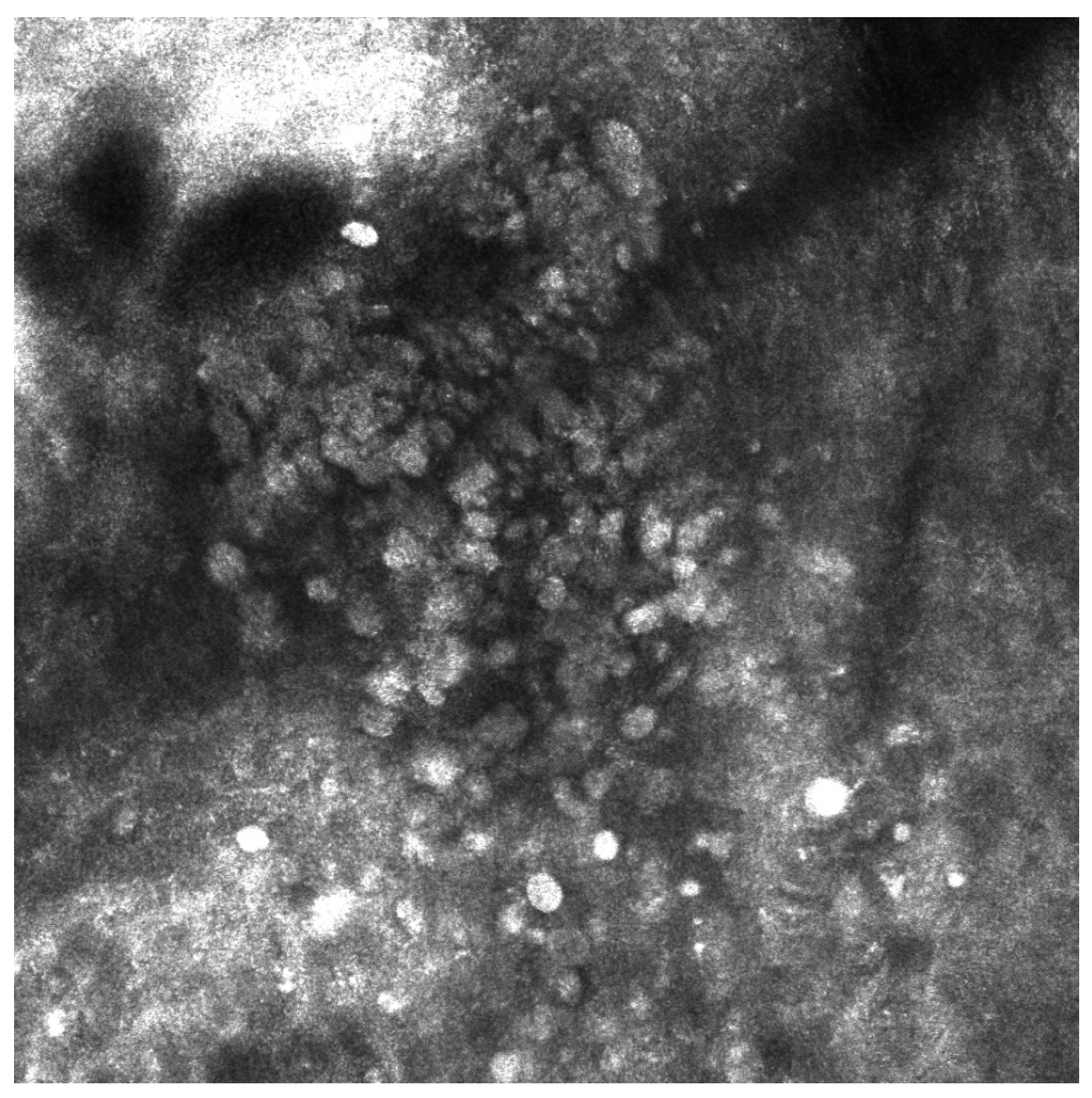
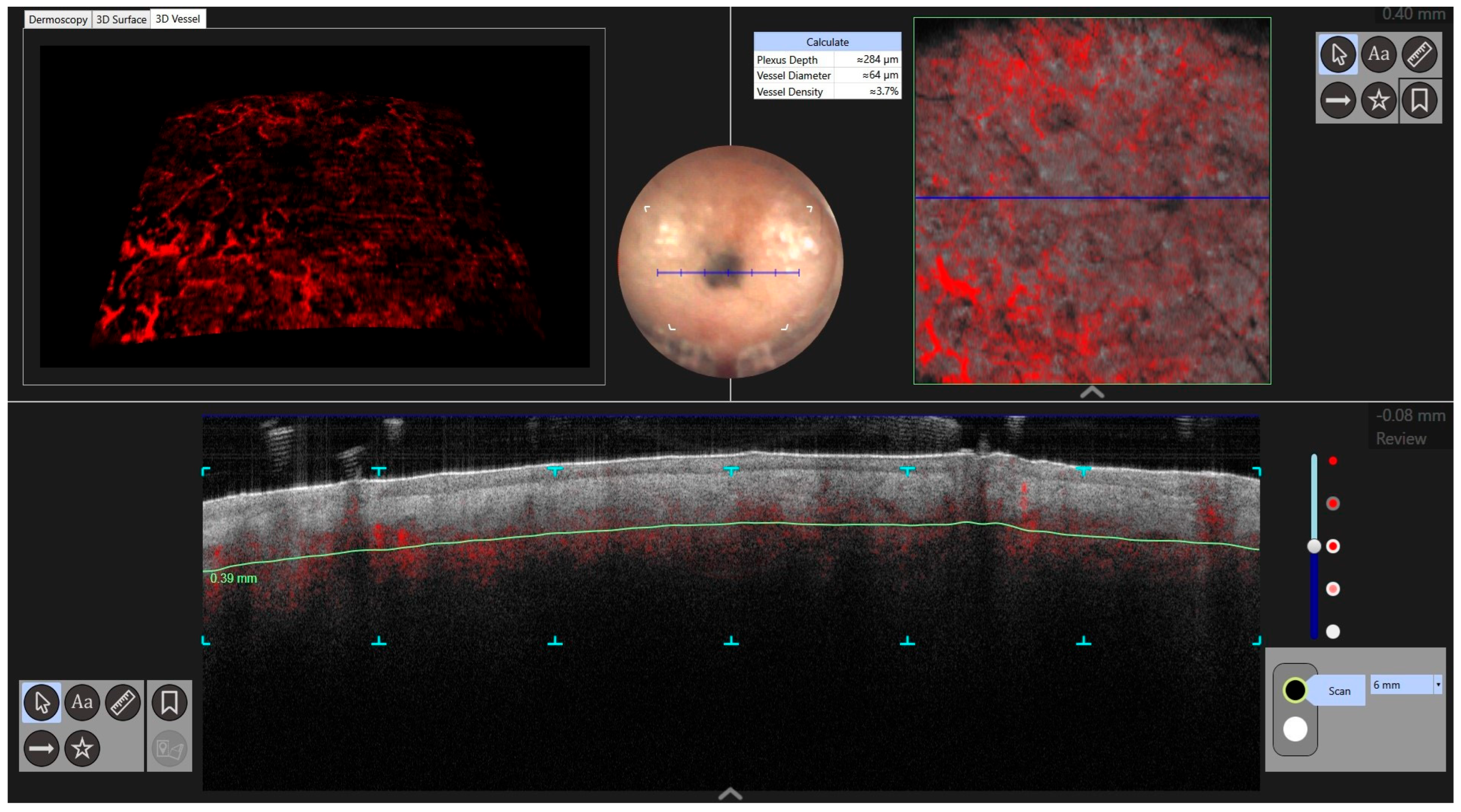
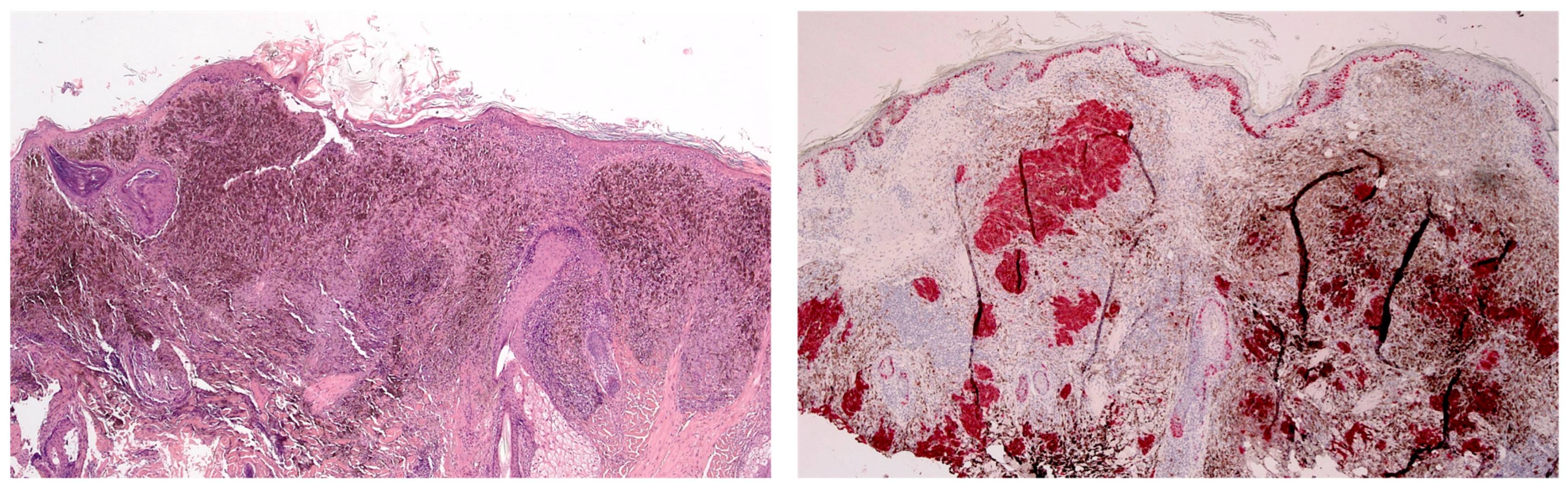

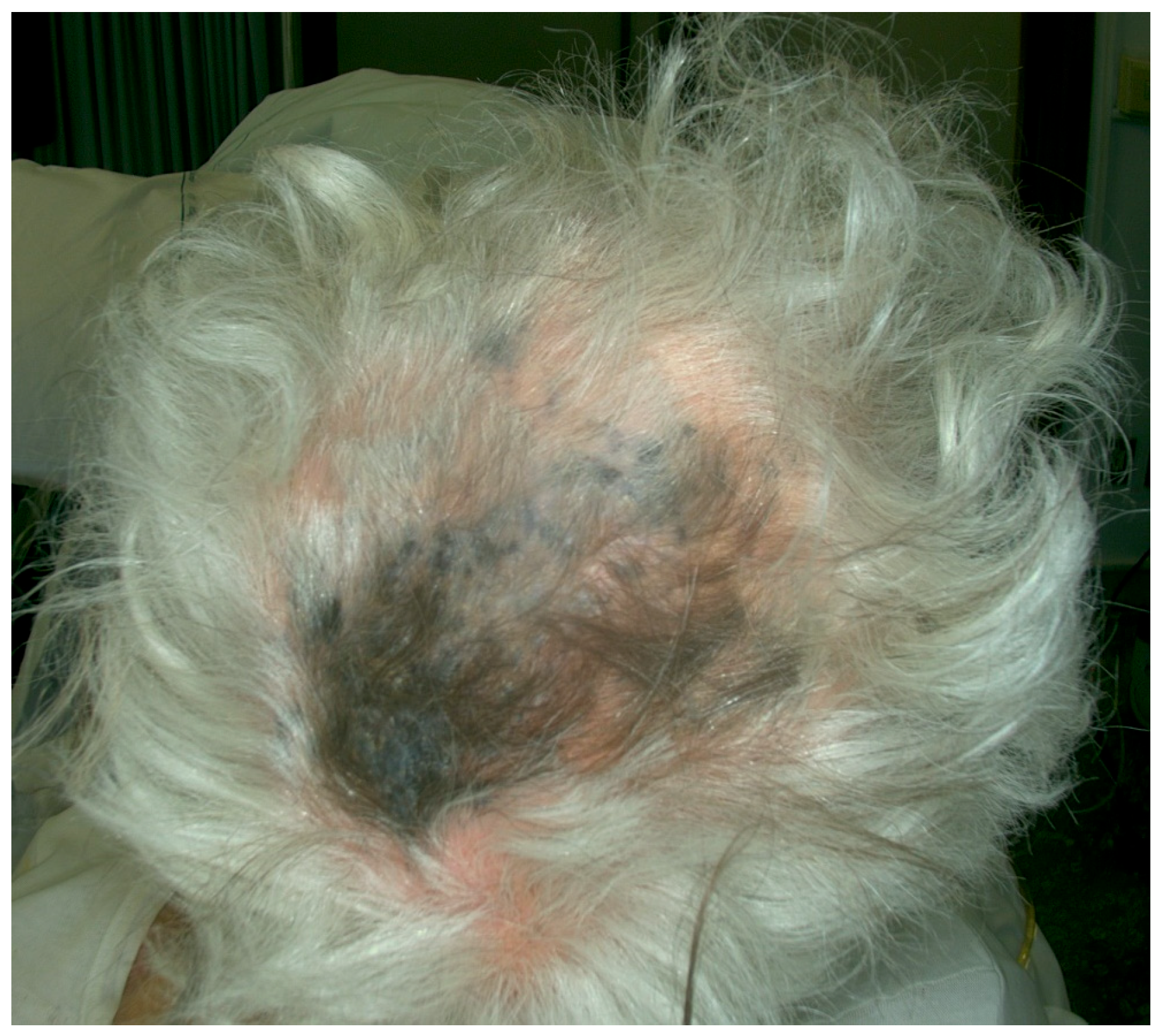
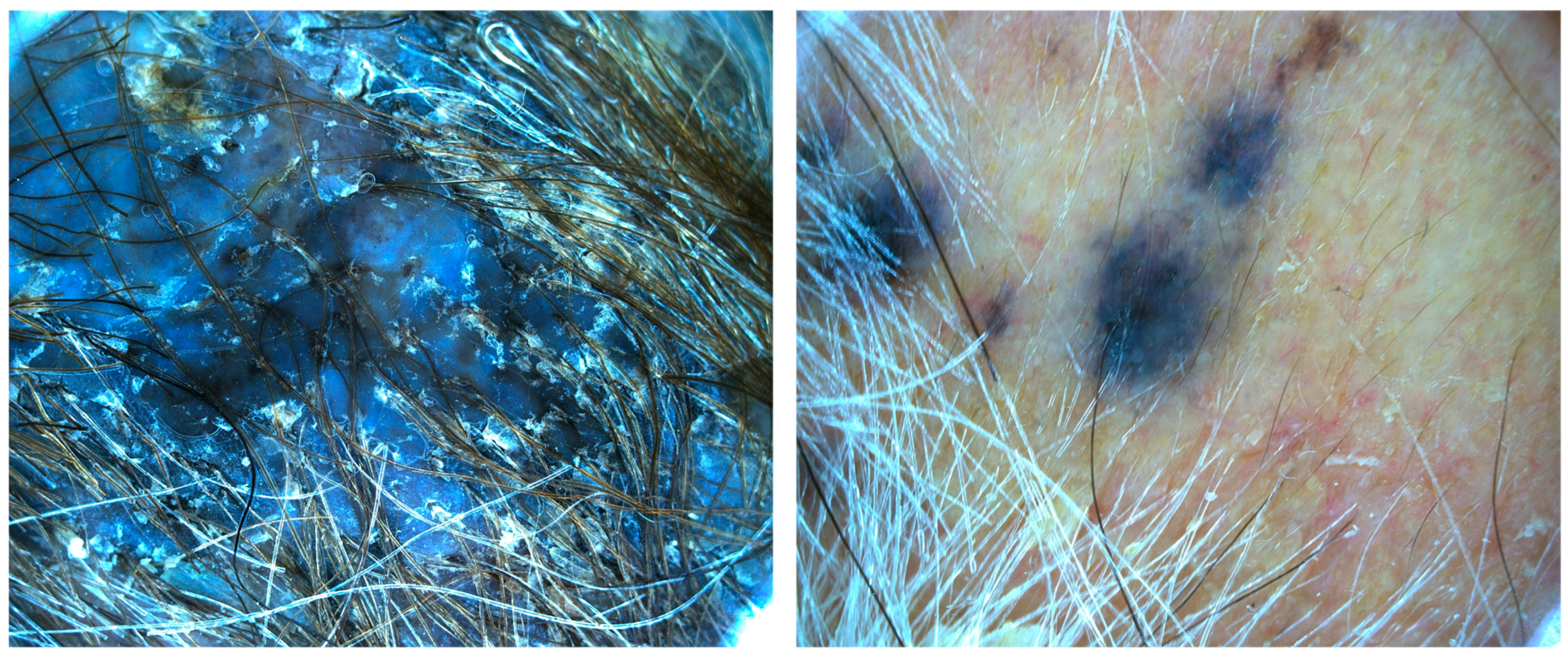
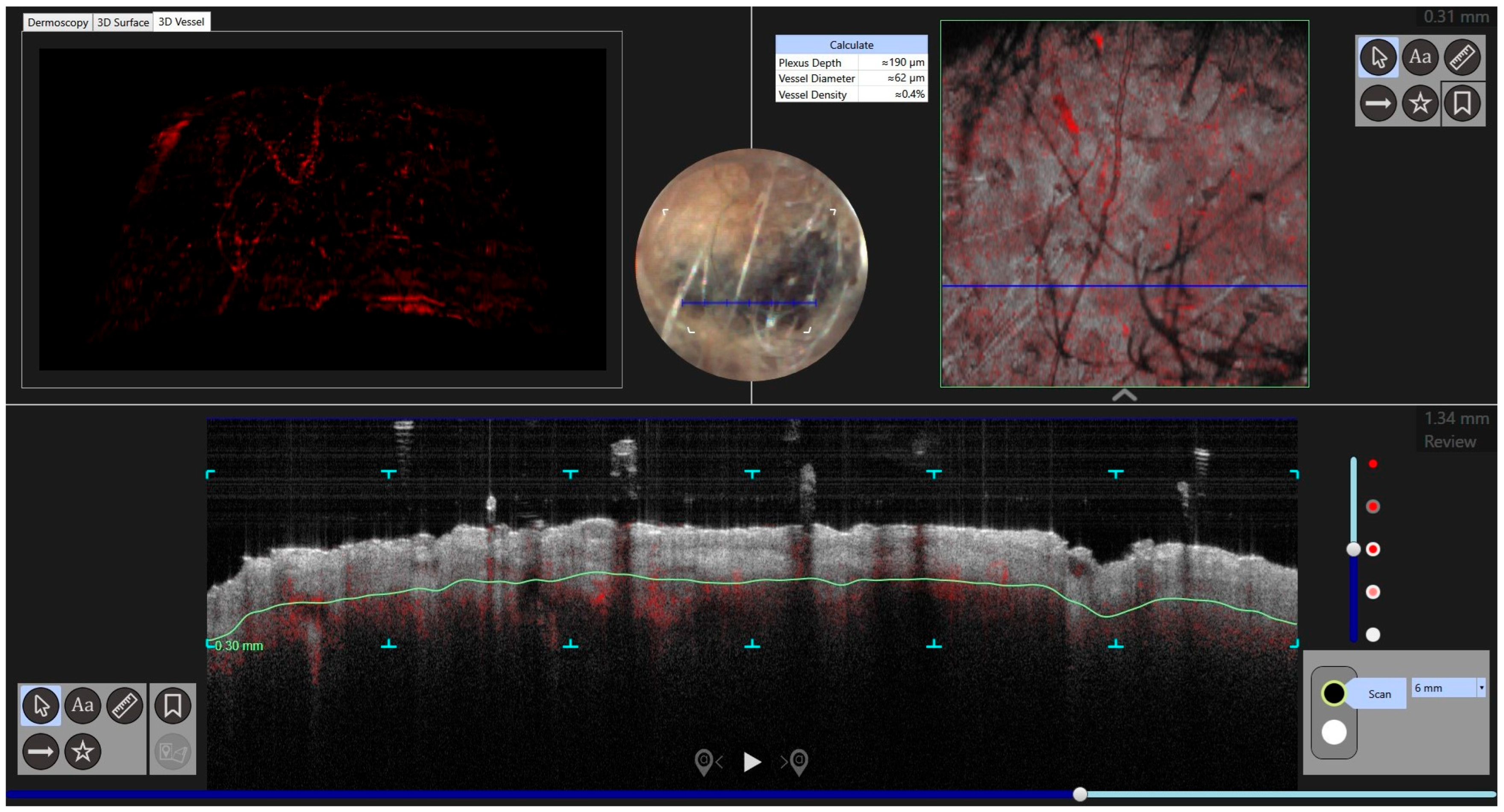
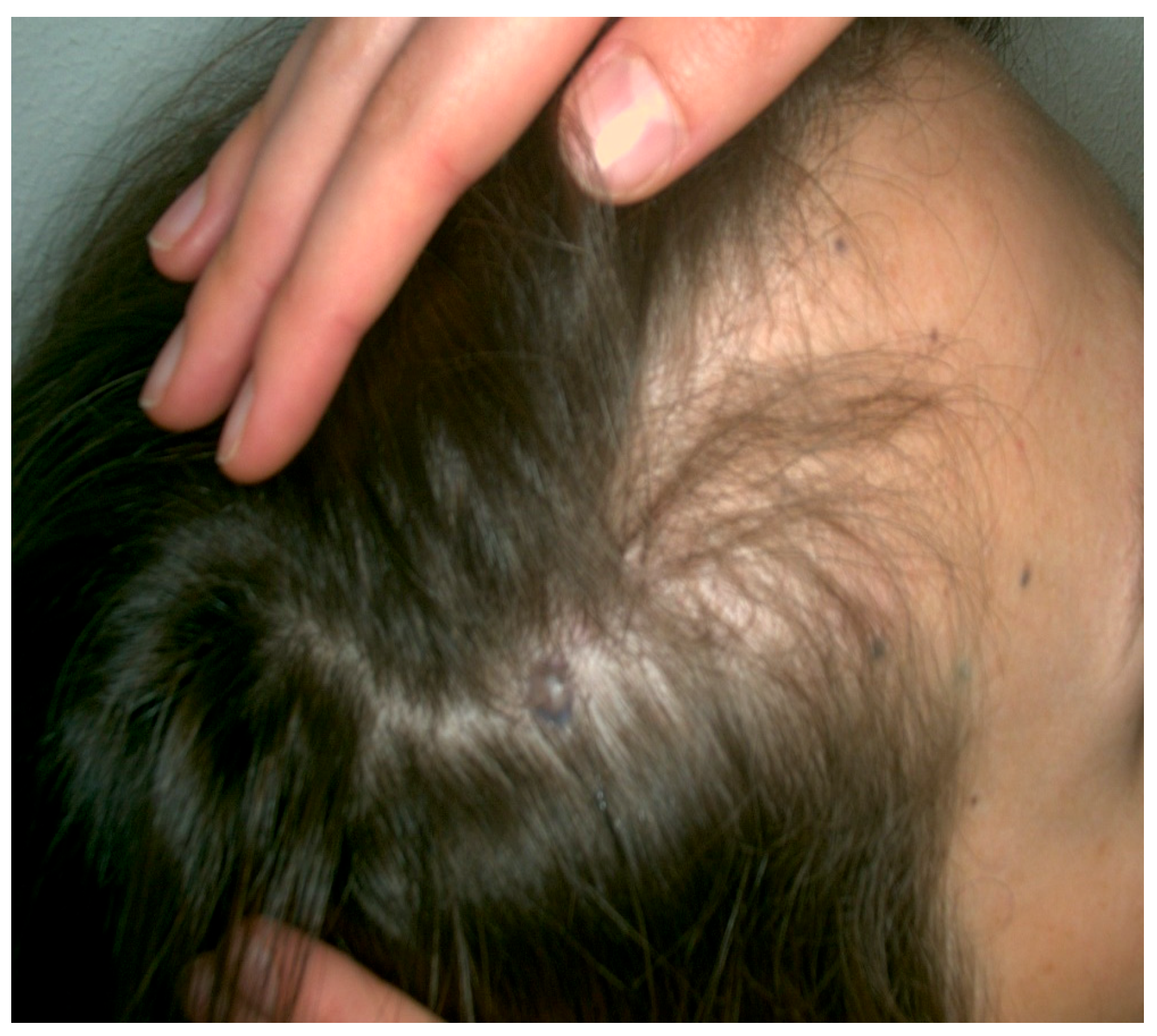

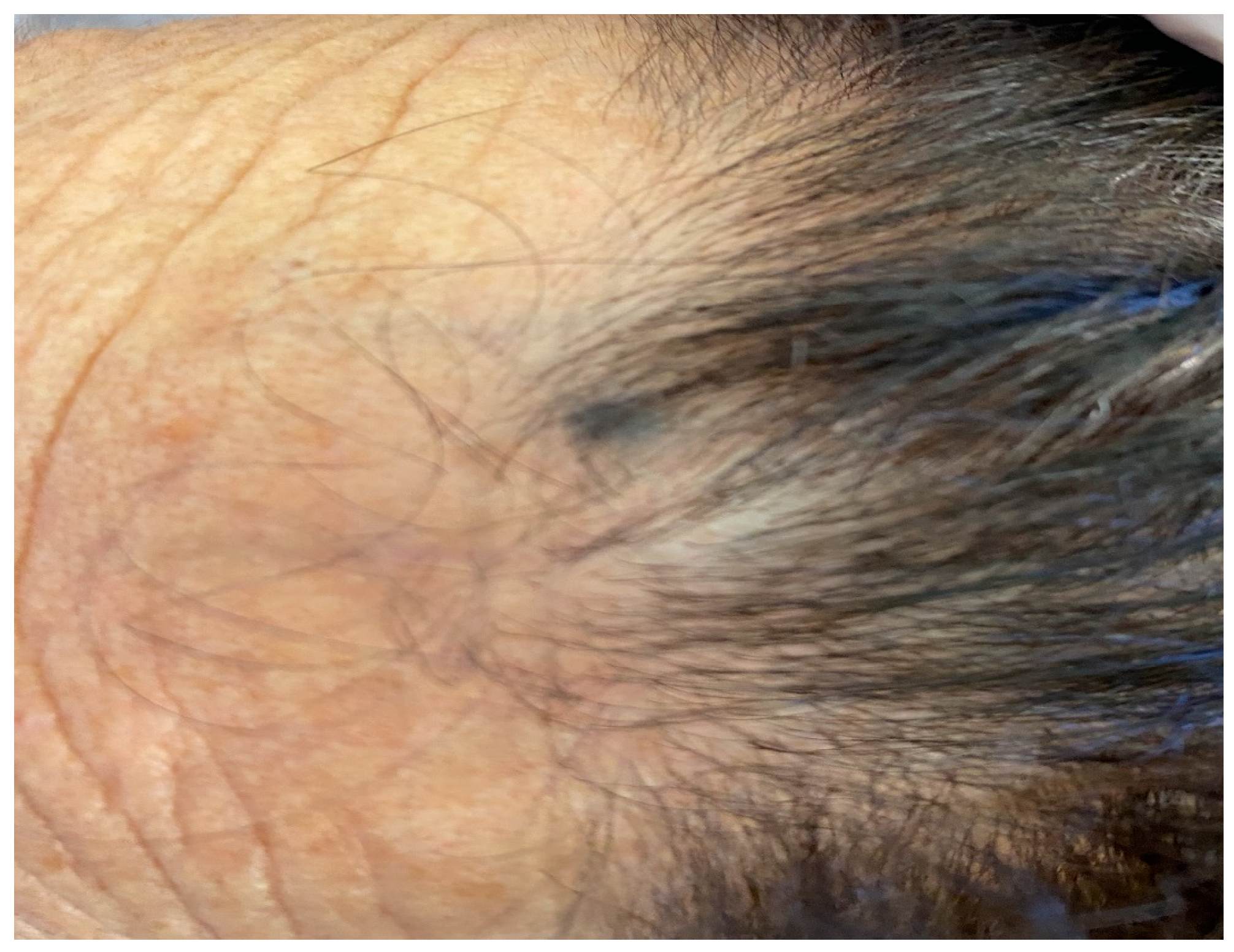
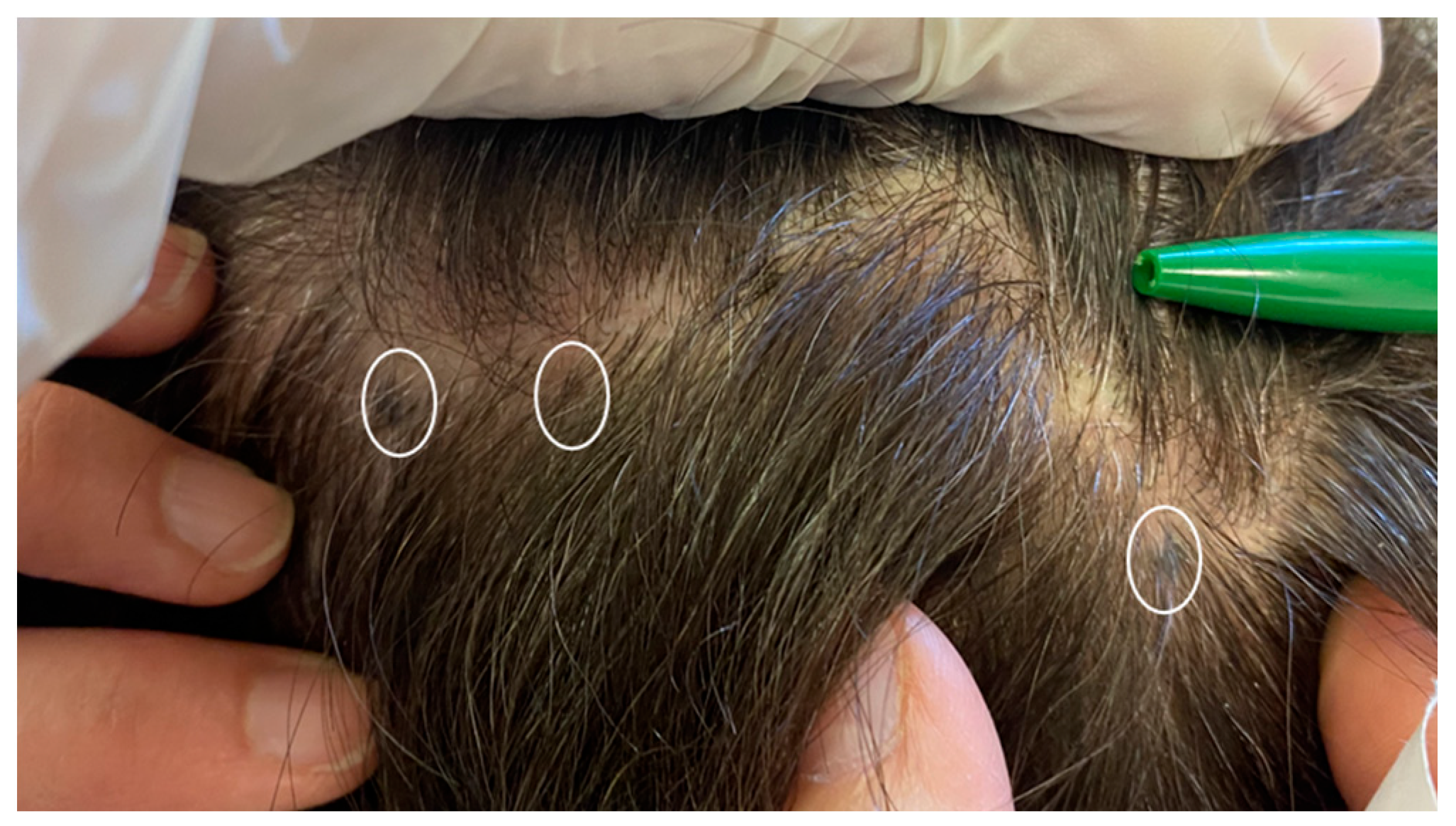
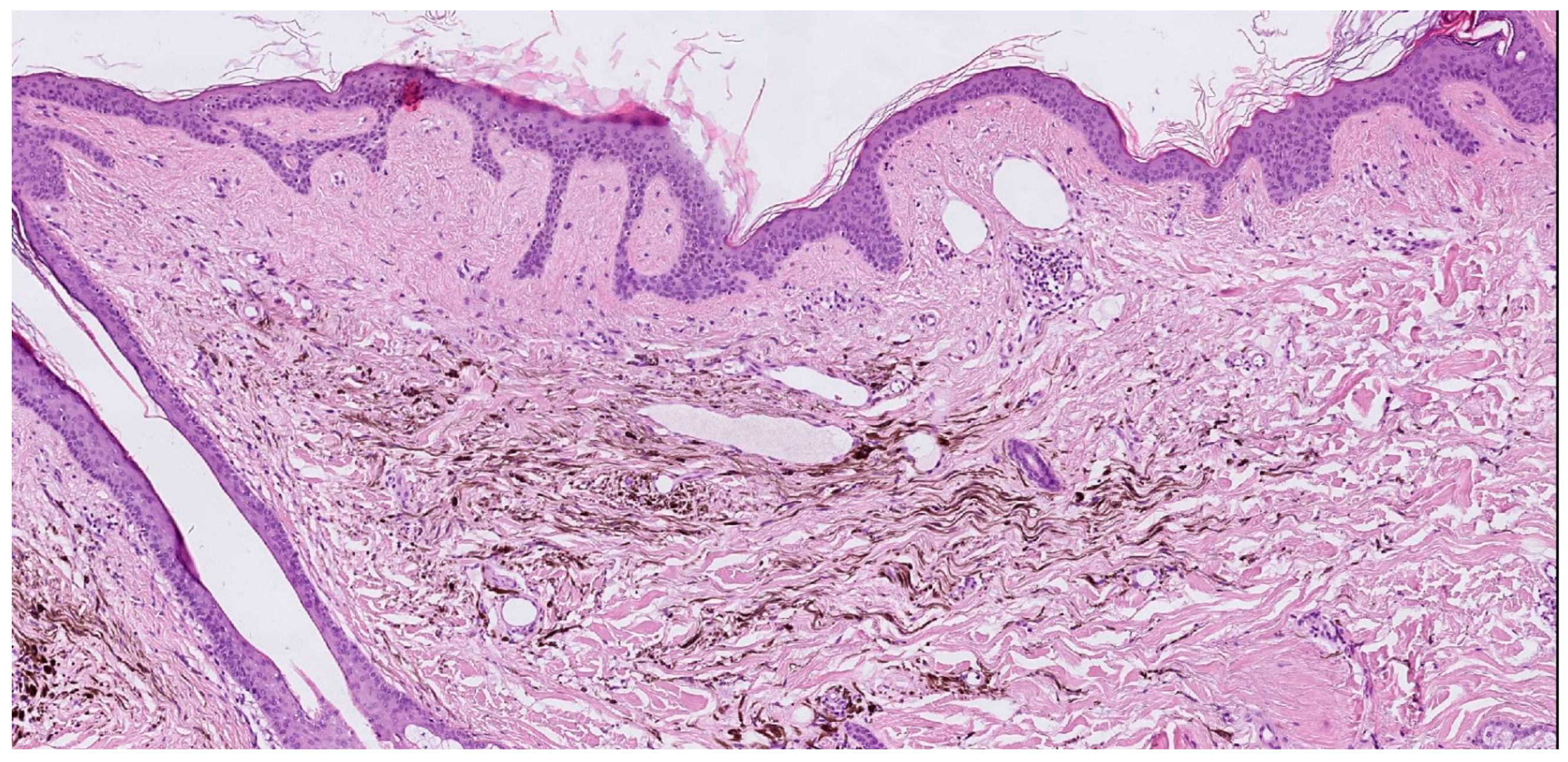
| Case | Age in Years/Gender | Clinical Morphology | Dermoscopic Features | Histology |
|---|---|---|---|---|
| 1 | 53/M | Blue–black plaque with multiple bluish papules and macules | Homogenous blue–gray pattern with yellow hue | Locally advanced melanoma |
| 2 | 86/M | Blue–black plaque with multiple blue satellitosis | Homogenous blue–gray pattern | Metastatic melanoma |
| 3 | 38/F | Blue nodule with agminated pigmentated macules | Homogenous violet-blue pattern with serpentine vessels | Desmoplastic blue nevus |
| 4 | 77/F | Blue–black macule and interspersed pigmentated lesions of scalp | Homogenous blue–brown pattern | Common blue nevus |
| Author | Year | Age | Sex | Site | Cases | Dermatoscopy | Histology |
|---|---|---|---|---|---|---|---|
| Upshaw [1] | 1947 | 9 | M | Trunk | 1 | NP | Common blue nevus |
| Dorsey [2] | 1954 | NP | NP | NP | 2 | NP | Cellular blue nevus |
| Pittman [3] | 1976 | 18 | M | Leg | 1 | NP | Common blue nevus |
| Shenfield [4] | 1980 | 56 | M | Epigastrium | 1 | NP | Common blue nevus |
| Atherton [5] | 1980 | 10 | M | Trunk, limbs | 1 | NP | Common blue nevus |
| Hedricks [6] | 1981 | 14 | M | Sternum | 1 | NP | Common blue nevus |
| Tuthill [7] | 1982 | 18 | F | Collarbone | 1 | NP | Combined blue nevus |
| Ishibashi [8] | 1990 | 25, 16, 15 | F, F, M | Chest, leg, shoulder | 3 | NP | Common blue nevus |
| Hofmann [9] | 1992 | 66, 36 | NP | Arm, back | 2 | NP | Common blue nevus |
| Misago [10] | 1993 | 67 | M | Hypocondrium | 1 | NP | Combined blue nevus |
| Velez [11] | 1993 | 57 | M | Shoulder | 1 | NP | Common blue nevus |
| Kiene [12] | 1995 | 29 | F | Nasolabial area | 1 | NP | Combined blue nevus |
| El-Ansary [13] | 2001 | 9 | M | Sole | 1 | NP | Common blue nevus |
| Pizzichetta [14] | 2007 | 59 | F | Leg | 1 | Homogeneous, linear pigmented structures. Darker-sulci. | Superficial blue nevus |
| Chen [15] | 2013 | 31 | F | Forearm | 1 | NP | Common blue nevus |
| Milkova [16] | 2013 | 57 | F | Forehead | 1 | NP | Common blue nevus |
| Rocha [17] | 2013 | 9 | M | Thigh | 1 | Homogeneous pattern, diffuse brownish areas, regular network, numerous regularly distributed small dots. | Common blue nevus |
| Spring [18] | 2013 | 42 | F | Ear | 1 | NP | Fusiform and epithelioid cells |
| Koba [19] | 2014 | 11 | M | Sole | 1 | Homogeneous blue-grey pattern | Common blue nevus |
| Paolino [20] | 2016 | 60 | F | Forehead | 1 | Homogeneous blue-grey pattern | Agminated blue nevus |
| Paolino [20] | 2016 | 58 | M | Vertex | 1 | Homogeneous blue-grey pattern | Agminated blue nevus |
| Lisboa [21] | 2016 | 64 | M | Upper back | 1 | Homogeneous bluish or grayish pattern | Agminated blue naevus |
| Oliveira [22] | 2017 | 15 | F | Arm | 1 | Blue–gray in a homogenous pattern, with peripheral streaks and satellite lesions | Common blue nevus |
| Benson [23] | 2018 | 19 | M | Neck | 1 | NP | Eruptive Blue Nevus |
| Woo [24] | 2019 | 49 | M | Shoulder | 1 | Blue homogenous areas with no network pattern or structures | Agminated blue naevus and naevus spilus |
| Cantisani [25] | 2021 | 31 | F | Scalp | 1 | Homogenous blue–gray pattern, with two grey satellite lesions | Blue nevus with satellitosis |
| Rodríguez-Jiménez [26] | 2021 | 52 | M | Jaw | 1 | Homogenous blue pattern | Agminated blue nevus |
| Sławińska [27] | 2022 | 52, 40, 37, 69 | M, M, F, F | Scalp, Nose, Shoulder, Hand | 4 | Structureless blue pattern or brownish lesions | Agminated blue naevus |
| Hassab-El-Naby [28] | 2023 | NP | NP | NP | 2 | Central pigmented part surrounded by a well-defined hypopigmented area | Agminated halo nevus |
| Allen [29] | 2023 | 67 | M | Trunk | 1 | NP | Common blue nevus |
| Palazzo [30] | 1988 | NP | F | Scalp | 1 | NP | Spitz nevus |
| Renfro [31] | 1989 | 5 | M | Face | 1 | NP | Spitz nevus |
| Abramovits [32] | 1993 | 12 | F | Sole | 1 | NP | Spitz nevus |
| Herd [33] | 1994 | 2, 6, 10 | F, F, M | Arm, arm, arm | 3 | NP | Spitz nevus |
| Bullen [34] | 1995 | 4, | F, F | Cheek, back | 2 | NP | Spitz nevus |
| Krasovec [35] | 1995 | 11 | M | Shoulder | 1 | NP | Spitz nevus |
| Aloi [36] | 1995 | 40 | F | Thigh | 1 | NP | Spitz nevus within a congenital speckled lentiginous nevus |
| Sabroe [37] | 1996 | 21 | F | Arm | 1 | NP | Spitz nevus |
| Hulshof [38] | 1998 | 16 | F | Shoulder, arm and hand | 1 | NP | Spitz nevus |
| Akyürek [39] | 1999 | 19 | M | Scalp | 1 | NP | Spitz nevus |
| Böer [40] | 2001 | 16 | F | Thigh | 1 | NP | Spitz nevus |
| Glasgow [41] | 2005 | 2 | M | Arm | 1 | NP | Spitz nevus |
| Aida [42] | 2010 | 3 | M | Face | 1 | NP | Nevus spilus with agminated Spitz nevus |
| Hassanein [43] | 2011 | 5 | F | Face | 1 | NP | Atypical spitz nevus |
| Zeng [44] | 2012 | 2 | M | Face | 1 | NP | Agminated spitz nevus |
| Gupta [45] | 2015 | 60 | F | Sole | 1 | NP | Spitz nevus |
| Adachi [46] | 2016 | 23 | M | Penis | 1 | NP | Atypical spitz nevus |
| Porubsky [47] | 2017 | 14 | F | Shoulder, arm | 1 | NP | Desmoplastic spitz nevus, nevus spilus, compound nevus |
| Pontoizeau [48] | 2017 | 4 | M | Arm | 1 | NP | Agminated spitz nevus |
| Van Kester [49] | 2018 | 4 | F | Foot | 1 | NP | Spitzoid melanoma |
| Adler [50] | 2018 | 31 | M | Thigh | 1 | NP | Spitz nevus |
| Nevares-Pomales [51] | 2018 | 0 | F | Face | 1 | NP | Agminated spitz nevus |
| Nemeth [52] | 2018 | 1 | F | Trunk | 1 | NP | Nevus spilus with agminated Spitz nevi |
| Robertson [53] | 2021 | 3 | F | Face | 1 | NP | Agminated spitz nevus |
| De Giorgi [54] | 2021 | 6 | M | Buttock | Irregularreticular structures, regression areas, and blue-white veil. | Spitz nevus | |
| Wang [55] | 2022 | 21 | F | Ear | 1 | NP | Agminated spitz nevus |
| Fumero-Velázquez [56] | 2023 | 6, 0, 42 | M, F, F | Ear, foot, shin | 3 | NP | Atypical Spitz tumor, PRKCA fusion melanocytic tumor pigmented epithelioid melanocytoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantisani, C.; Paolino, G.; Di Guardo, A.; Gomes, V.; Carugno, A.; Greco, M.E.; Musolff, N.; Azzella, G.; Rossi, G.; Soda, G.; et al. Diagnostic Imaging of Agminated Blue Lesions and Blue Lesions with Satellitosis: Case Series with a Concise Review of the Current Literature. J. Clin. Med. 2024, 13, 894. https://doi.org/10.3390/jcm13030894
Cantisani C, Paolino G, Di Guardo A, Gomes V, Carugno A, Greco ME, Musolff N, Azzella G, Rossi G, Soda G, et al. Diagnostic Imaging of Agminated Blue Lesions and Blue Lesions with Satellitosis: Case Series with a Concise Review of the Current Literature. Journal of Clinical Medicine. 2024; 13(3):894. https://doi.org/10.3390/jcm13030894
Chicago/Turabian StyleCantisani, Carmen, Giovanni Paolino, Antonio Di Guardo, Vito Gomes, Andrea Carugno, Maria Elisabetta Greco, Noah Musolff, Giulia Azzella, Giovanni Rossi, Giuseppe Soda, and et al. 2024. "Diagnostic Imaging of Agminated Blue Lesions and Blue Lesions with Satellitosis: Case Series with a Concise Review of the Current Literature" Journal of Clinical Medicine 13, no. 3: 894. https://doi.org/10.3390/jcm13030894
APA StyleCantisani, C., Paolino, G., Di Guardo, A., Gomes, V., Carugno, A., Greco, M. E., Musolff, N., Azzella, G., Rossi, G., Soda, G., Longo, C., & Pellacani, G. (2024). Diagnostic Imaging of Agminated Blue Lesions and Blue Lesions with Satellitosis: Case Series with a Concise Review of the Current Literature. Journal of Clinical Medicine, 13(3), 894. https://doi.org/10.3390/jcm13030894










