Balanced Crystalloids versus Normal Saline in Adults with Sepsis: A Comprehensive Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Data Sources and Search Strategy
2.3. Data Extraction
2.4. Outcomes of Interest
2.5. Statistical Analysis
2.6. Bias Assessment
3. Results
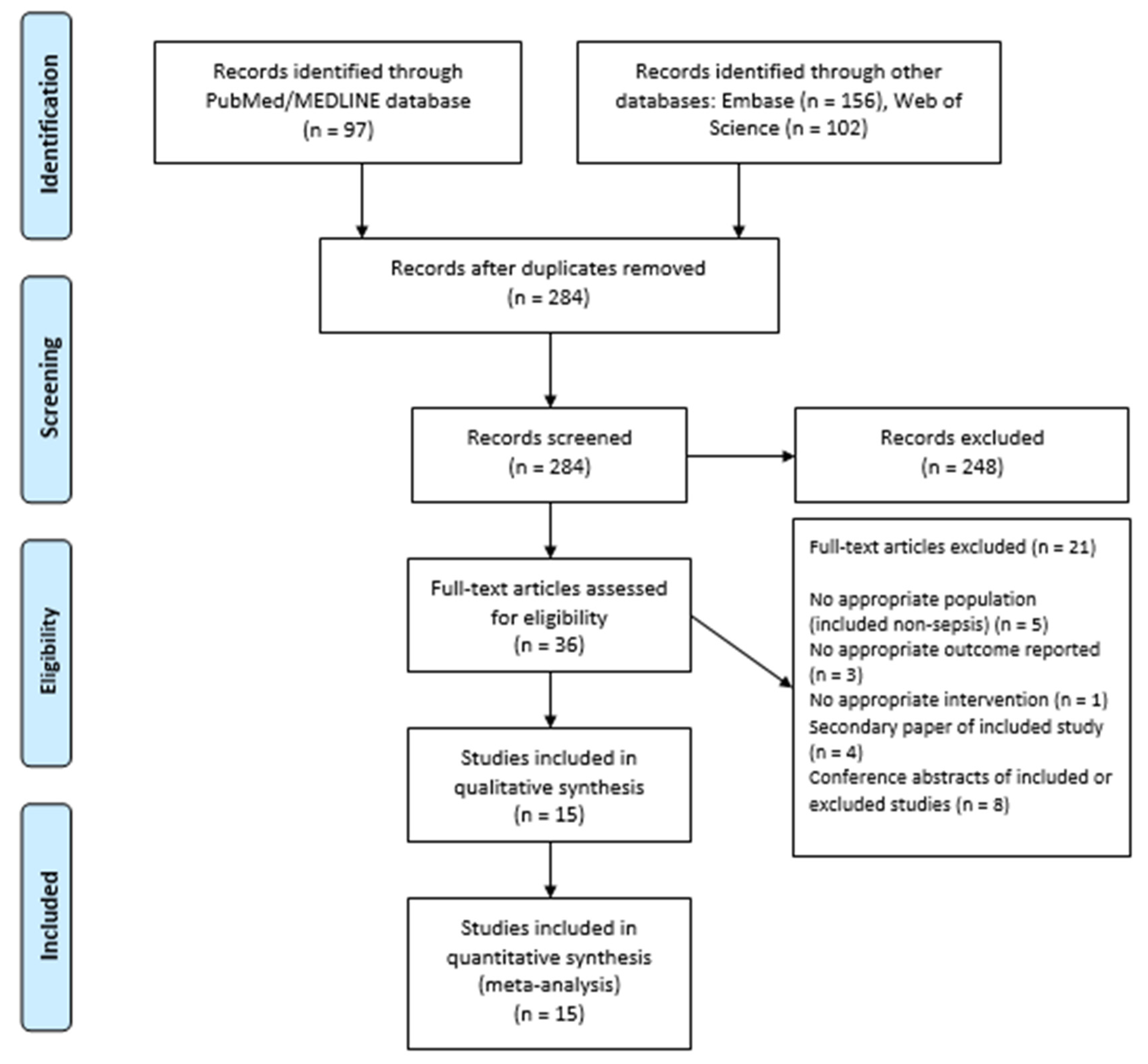
3.1. Study Selection
3.2. Study and Patients’ Characteristics
3.3. Outcomes of Interest
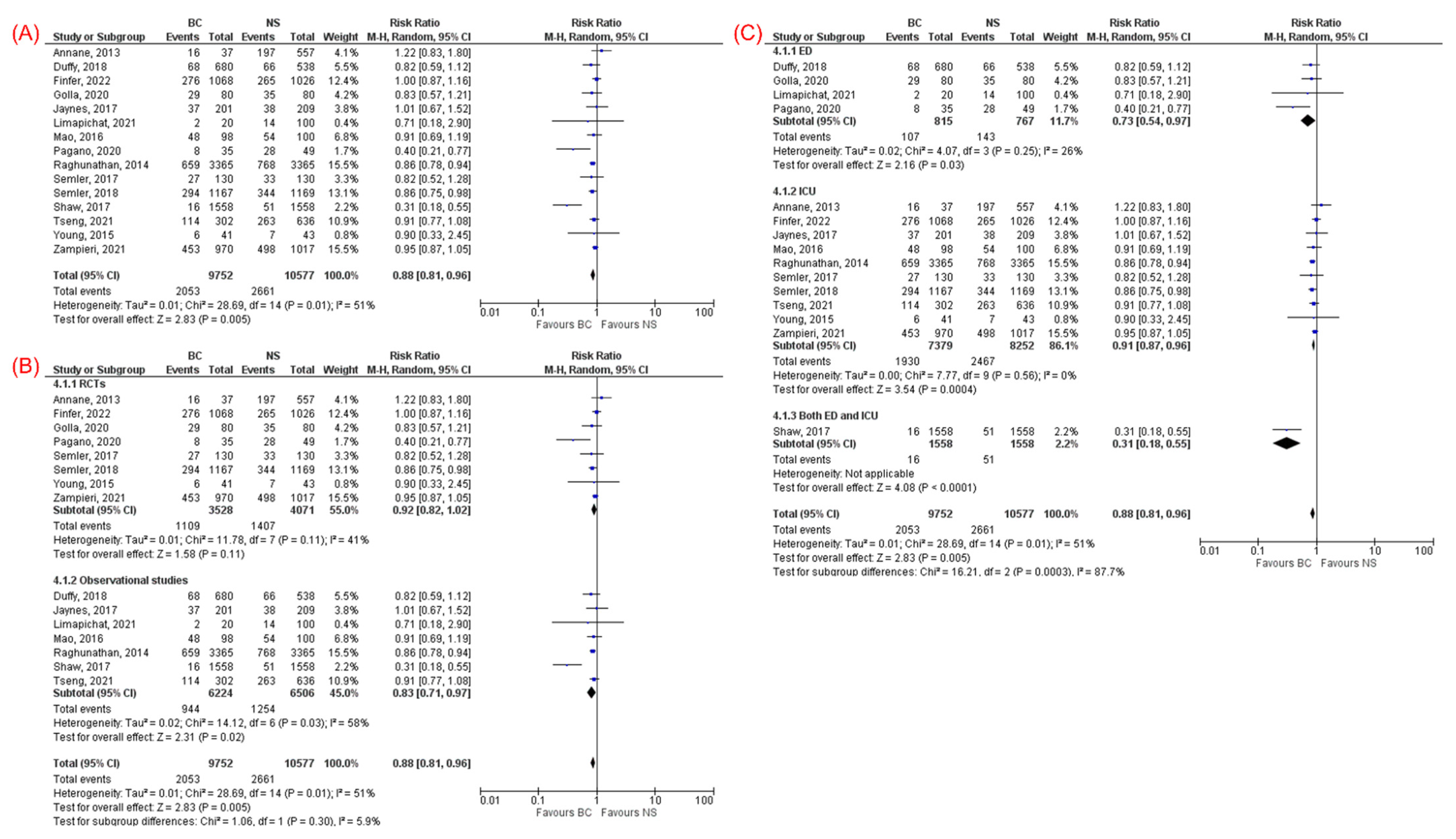
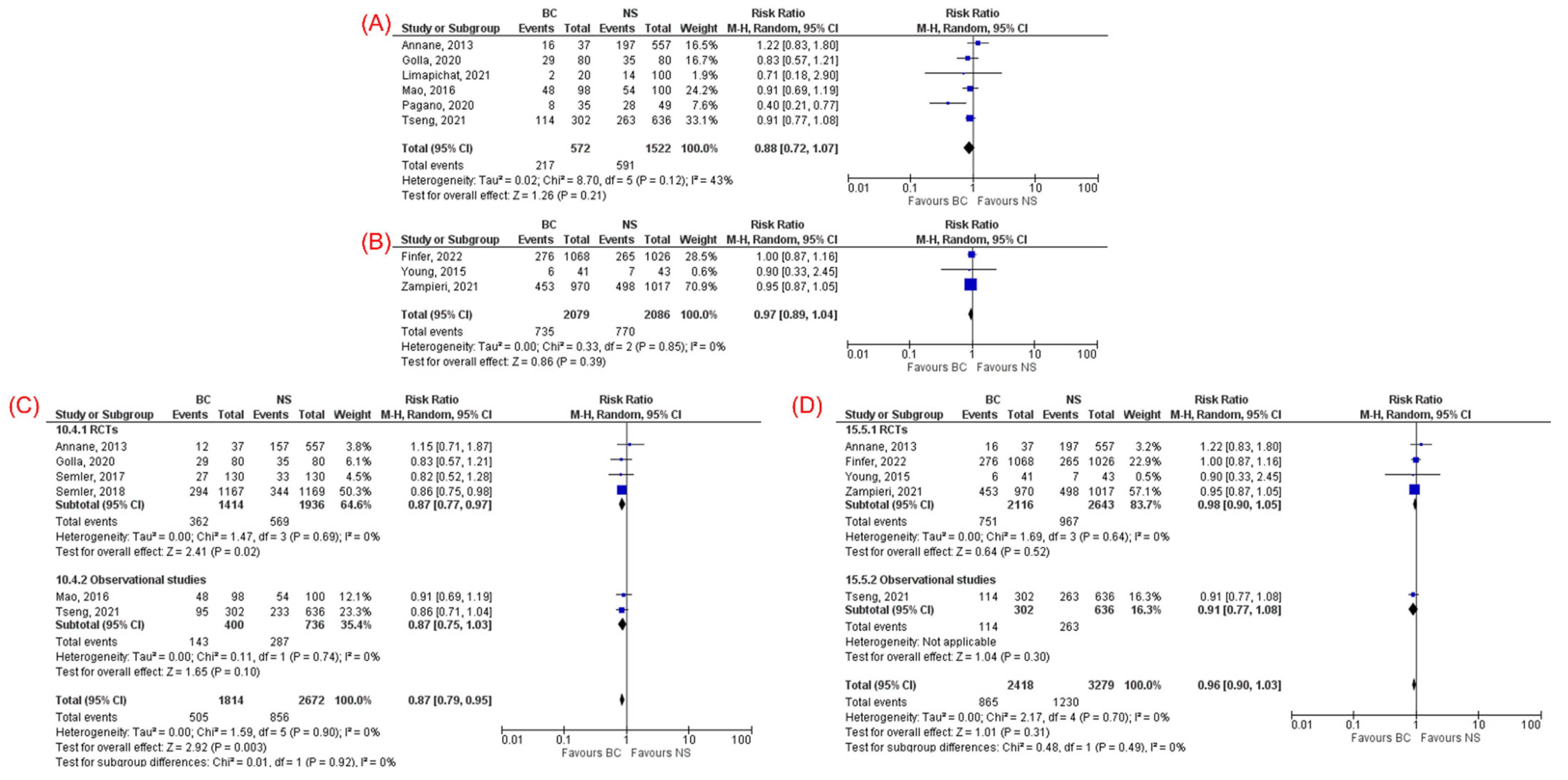
3.3.1. Mortality
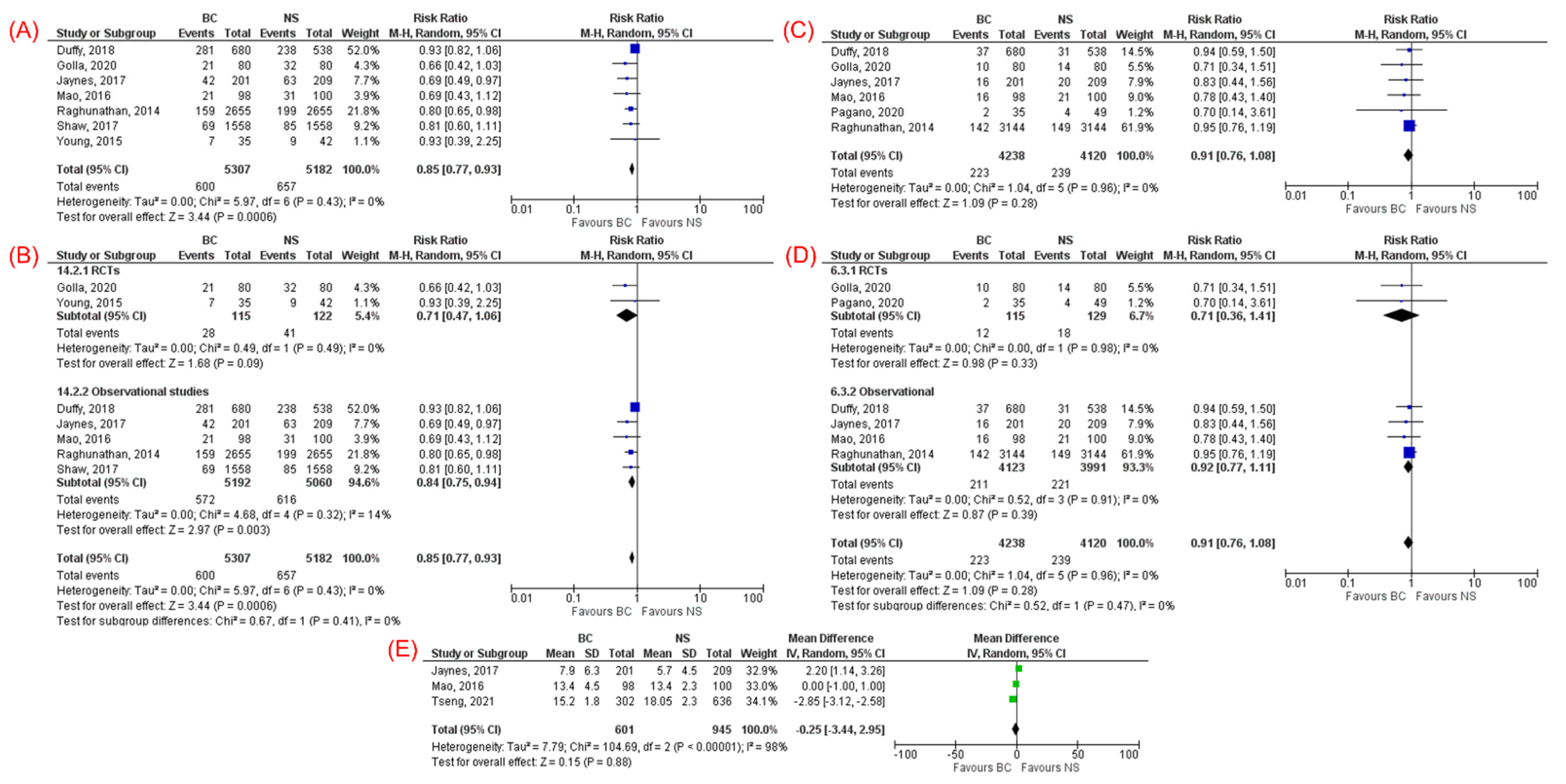
3.3.2. Acute Kidney Injury
3.3.3. Need for Renal Replacement Therapy
3.3.4. ICU Length of Stay
3.4. Sensitivity Analysis
3.5. Quality and Publication Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Lagu, T.; Rothberg, M.B.; Shieh, M.S.; Pekow, P.S.; Steingrub, J.S.; Lindenauer, P.K. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit. Care Med. 2012, 40, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Karakala, N.; Raghunathan, K.; Shaw, A.D. Intravenous fluids in sepsis: What to use and what to avoid. Curr. Opin. Crit. Care 2013, 19, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Holcomb, J.B. Choice of Fluid Therapy in the Initial Management of Sepsis, Severe Sepsis, and Septic Shock. Shock 2016, 46, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Chua, J.; Chiu, H.H.; Benedicto, J. Balanced Crystalloids versus Normal Saline as Intravenous Fluid Therapy among Critically Ill patients: A Meta-Analysis of Randomized Controlled Trials. Philipp. J. Intern. Med. 2019, 57, 115–119. [Google Scholar]
- Hammond, D.A.; Lam, S.W.; Rech, M.A.; Smith, M.N.; Westrick, J.; Trivedi, A.P.; Balk, R.A. Balanced Crystalloids Versus Saline in Critically Ill Adults: A Systematic Review and Meta-analysis. Ann. Pharmacother. 2020, 54, 5–13. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Rochwerg, B.; Alhazzani, W.; Sindi, A.; Heels-Ansdell, D.; Thabane, L.; Fox-Robichaud, A.; Mbuagbaw, L.; Szczeklik, W.; Alshamsi, F.; Altayyar, S.; et al. Fluid resuscitation in sepsis: A systematic review and network meta-analysis. Ann. Intern. Med. 2014, 161, 347–355. [Google Scholar] [CrossRef]
- Brown, R.M.; Wang, L.; Coston, T.D.; Krishnan, N.I.; Casey, J.D.; Wanderer, J.P.; Ehrenfeld, J.M.; Byrne, D.W.; Stollings, J.L.; Siew, E.D.; et al. Balanced Crystalloids versus Saline in Sepsis. A Secondary Analysis of the SMART Clinical Trial. Am. J. Respir. Crit. Care Med. 2019, 200, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Finfer, S.; Micallef, S.; Hammond, N.; Navarra, L.; Bellomo, R.; Billot, L.; Delaney, A.; Gallagher, M.; Gattas, D.; Li, Q.; et al. Balanced Multielectrolyte Solution versus Saline in Critically Ill Adults. N. Engl. J. Med. 2022, 386, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, F.G.; Machado, F.R.; Biondi, R.S.; Freitas, F.G.R.; Veiga, V.C.; Figueiredo, R.C.; Lovato, W.J.; Amêndola, C.P.; Serpa-Neto, A.; Paranhos, J.L.R.; et al. Effect of Intravenous Fluid Treatment With a Balanced Solution vs. 0.9% Saline Solution on Mortality in Critically Ill Patients: The BaSICS Randomized Clinical Trial. JAMA 2021, 326, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Chen, T.T.; Chan, M.C.; Chen, K.Y.; Wu, S.M.; Shih, M.C.; Tu, Y.K. Impact of Comorbidities on Beneficial Effect of Lactated Ringers vs. Saline in Sepsis Patients. Front. Med. 2021, 8, 758902. [Google Scholar] [CrossRef] [PubMed]
- Pagano, A.; Porta, G.; Bosso, G.; Rosato, V.; Allegorico, E.; Serra, C.; Sforza, A.; Paladino, F.; Numis, F.G. Ringer lactate versus saline solution for resuscitation of sepsis and septic shock. Ital. J. Emerg. Med. 2020, 9, 29–34. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, N.; Song, M.; Xia, F.; Wu, Y.; Wang, X.; Chen, T.; Yang, Z.; Yang, S.; Zhang, Y.; et al. Balanced crystalloids versus saline in critically ill patients: The PRISMA study of a meta-analysis. Medicine 2021, 100, e27203. [Google Scholar] [CrossRef]
- Hammond, N.E.; Zampieri, F.G.; di Tanna, G.L.; Garside, T.; Adigbli, D.; Cavalcanti, A.B.; Machado, F.R.; Micallef, S.; Myburgh, J.; Ramanan, M.; et al. Balanced Crystalloids versus Saline in Critically Ill Adults—A Systematic Review with Meta-Analysis. NEJM Evid. 2022, 1, EVIDoa2100010. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fohner, A.E.; Greene, J.D.; Lawson, B.L.; Chen, J.H.; Kipnis, P.; Escobar, G.J.; Liu, V.X. Assessing clinical heterogeneity in sepsis through treatment patterns and machine learning. J. Am. Med. Inform. Assoc. 2019, 26, 1466–1477. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Moher, D.; Pham, B.; Jones, A.; Cook, D.J.; Jadad, A.R.; Moher, M.; Tugwell, P.; Klassen, T.P. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998, 352, 609–613. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [Green Version]
- Annane, D.; Siami, S.; Jaber, S.; Martin, C.; Elatrous, S.; Declère, A.D.; Preiser, J.C.; Outin, H.; Troché, G.; Charpentier, C.; et al. Effects of fluid resuscitation with colloids vs. crystalloids on mortality in critically ill patients presenting with hypovolemic shock: The CRISTAL randomized trial. JAMA 2013, 310, 1809–1817. [Google Scholar] [CrossRef] [Green Version]
- Duffy, R.A.; Foroozesh, M.B.; Loflin, R.D.; Ie, S.R.; Icard, B.L.; Tegge, A.N.; Nogueira, J.R.; Kuehl, D.R.; Smith, D.C.; Loschner, A.L. Normal saline versus Normosol™-R in sepsis resuscitation: A retrospective cohort study. J. Intensive Care Soc. 2019, 20, 223–230. [Google Scholar] [CrossRef]
- Golla, R.; Kumar, S.; Dhibhar, D.P.; Bhalla, A.; Sharma, N. 0.9% saline V/S Ringer’s lactate for fluid resuscitation in adult sepsis patients in emergency medical services: An open-label randomized controlled trial. Hong Kong J. Emerg. Med. 2020, 1024907920948983. [Google Scholar] [CrossRef]
- Limapichat, T.; Pattanapong, K. Normal Saline Solution or Lactated Ringer’s Solution to Enhance Lactate Clearance in Septic Patients After Initial Resuscitation in the ED: A Retrospective Cohort Trial. Open Access Emerg. Med. 2021, 13, 511–519. [Google Scholar] [CrossRef]
- Raghunathan, K.; Shaw, A.; Nathanson, B.; Stürmer, T.; Brookhart, A.; Stefan, M.S.; Setoguchi, S.; Beadles, C.; Lindenauer, P.K. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit. Care Med. 2014, 42, 1585–1591. [Google Scholar] [CrossRef]
- Semler, M.W.; Self, W.H.; Wanderer, J.P.; Ehrenfeld, J.M.; Wang, L.; Byrne, D.W.; Stollings, J.L.; Kumar, A.B.; Hughes, C.G.; Hernandez, A.; et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N. Engl. J. Med. 2018, 378, 829–839. [Google Scholar] [CrossRef]
- Semler, M.W.; Wanderer, J.P.; Ehrenfeld, J.M.; Stollings, J.L.; Self, W.H.; Siew, E.D.; Wang, L.; Byrne, D.W.; Shaw, A.D.; Bernard, G.R.; et al. Balanced Crystalloids versus Saline in the Intensive Care Unit. The SALT Randomized Trial. Am. J. Respir. Crit. Care Med. 2017, 195, 1362–1372. [Google Scholar] [CrossRef]
- Shaw, A.D.; Schermer, C.R.; Lobo, D.N.; Munson, S.H.; Khangulov, V.; Hayashida, D.K.; Kellum, J.A. Impact of intravenous fluid composition on outcomes in patients with systemic inflammatory response syndrome. Crit. Care 2015, 19, 334. [Google Scholar] [CrossRef] [Green Version]
- Young, P.; Bailey, M.; Beasley, R.; Henderson, S.; Mackle, D.; McArthur, C.; McGuinness, S.; Mehrtens, J.; Myburgh, J.; Psirides, A.; et al. Effect of a Buffered Crystalloid Solution vs Saline on Acute Kidney Injury Among Patients in the Intensive Care Unit: The SPLIT Randomized Clinical Trial. JAMA 2015, 314, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Q.; Lou, B.H.; Wu, D.J. Efficacy of Lactated Ringer’s versus Normal Saline in Treating Patients with Septic Shock. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2018, 40, 349–355. [Google Scholar]
- Jaynes, M.P.; Murphy, C.V.; Ali, N.; Krautwater, A.; Lehman, A.; Doepker, B.A. Association between chloride content of intravenous fluids and acute kidney injury in critically ill medical patients with sepsis. J. Crit. Care 2018, 44, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lu, G.; Wang, D.; Lei, Y.; Mao, Z.; Hu, P.; Hu, J.; Liu, R.; Han, D.; Zhou, F. Balanced crystalloids versus normal saline for fluid resuscitation in critically ill patients: A systematic review and meta-analysis with trial sequential analysis. Am. J. Emerg. Med. 2019, 37, 2072–2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayed, Y.Z.M.; Aburahma, A.M.Y.; Barbarawi, M.O.; Hamid, K.; Banifadel, M.R.N.; Rashdan, L.; Bachuwa, G.I. Balanced crystalloids versus isotonic saline in critically ill patients: Systematic review and meta-analysis. J. Intensive Care 2018, 6, 51. [Google Scholar] [CrossRef]
- Guidet, B.; Soni, N.; della Rocca, G.; Kozek, S.; Vallet, B.; Annane, D.; James, M. A balanced view of balanced solutions. Crit. Care 2010, 14, 325. [Google Scholar] [CrossRef] [Green Version]
- Neyra, J.A.; Canepa-Escaro, F.; Li, X.; Manllo, J.; Adams-Huet, B.; Yee, J.; Yessayan, L.; Acute Kidney Injury in Critical Illness Study Group. Association of Hyperchloremia With Hospital Mortality in Critically Ill Septic Patients. Crit. Care Med. 2015, 43, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Medina-Lombo, R.A.; Sánchez-García, V.L.; Gómez-Gómez, L.F.; Vidal-Bonilla, S.A.; Castro-Castro, J.J.; Sánchez-Vanegas, G. Mortality and hyperchloremia in the intensive care unit. Colomb. J. Anesthesiol. 2018, 46, 216–221. [Google Scholar] [CrossRef]
- Kim, S.Y.; Huh, K.H.; Lee, J.R.; Kim, S.H.; Jeong, S.H.; Choi, Y.S. Comparison of the effects of normal saline versus Plasmalyte on acid-base balance during living donor kidney transplantation using the Stewart and base excess methods. Transpl. Proc. 2013, 45, 2191–2196. [Google Scholar] [CrossRef]
- Hansen, P.B.; Jensen, B.L.; Skott, O. Chloride regulates afferent arteriolar contraction in response to depolarization. Hypertension 1998, 32, 1066–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005, 16, 3365–3370. [Google Scholar] [CrossRef] [Green Version]
- Mandelbaum, T.; Lee, J.; Scott, D.J.; Mark, R.G.; Malhotra, A.; Howell, M.D.; Talmor, D. Empirical relationships among oliguria, creatinine, mortality, and renal replacement therapy in the critically ill. Intensive Care Med. 2013, 39, 414–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marttinen, M.; Wilkman, E.; Petäjä, L.; Suojaranta-Ylinen, R.; Pettilä, V.; Vaara, S.T. Association of plasma chloride values with acute kidney injury in the critically ill–A prospective observational study. Acta Anaesthesiol. Scand. 2016, 60, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, X.; Fan, H.; Li, D.; Deng, H. Higher serum chloride concentrations are associated with acute kidney injury in unselected critically ill patients. BMC Nephrol. 2013, 14, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zampieri, F.G.; Ranzani, O.T.; Azevedo, L.C.; Martins, I.D.; Kellum, J.A.; Libório, A.B. Lactated Ringer Is Associated With Reduced Mortality and Less Acute Kidney Injury in Critically Ill Patients: A Retrospective Cohort Analysis. Crit. Care Med. 2016, 44, 2163–2170. [Google Scholar] [CrossRef]
- Suetrong, B.; Pisitsak, C.; Boyd, J.H.; Russell, J.A.; Walley, K.R. Hyperchloremia and moderate increase in serum chloride are associated with acute kidney injury in severe sepsis and septic shock patients. Crit. Care 2016, 20, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Zhang, J.; Zhu, Y.; Su, L.; Li, Y.; He, L.; Yu, L.; Peng, Z. Acetate Ringer’s solution versus 0.9% saline for septic patients: Study protocol for a multi-center parallel controlled trial. Trials 2021, 22, 89. [Google Scholar] [CrossRef] [PubMed]
| Study, Year | Study Design | Country | Total n (BC/NS) | Male, n | Age, Mean ± SD or Median (IQR), Years | Severity of Sepsis (BC/NS) | Enrollment Location | Type of BC | Fluid Volume (BC/NS), Mean ± SD or Median (IQR) mL | Follow-Up Duration |
|---|---|---|---|---|---|---|---|---|---|---|
| Annane, 2013 | RCT | France | 594 (37/557) | NR | NR | NR | ICU | LR | NR | 90 days |
| Duffy, 2018 | RC | USA | 1218 (680/538) | 581 | 60.6 (18.7)/60.6 (18.7) | qSOFA: 0.68 (0.76)/0.68 (0.76) | ED | Normosol-R | Total: 6000 ± 4600/6500 ± 4800 | NR |
| Finfer, 2022 | RCT | Australia and New Zealand | 2094 (1068/1026) | NR | NR | NR | ICU | Plasma-Lyte 148 | NR | 90 days |
| Golla, 2020 | RCT | India | 160 (80/80) | 85 | 43.46 ± 17.99/42.44 ± 19.37 | SOFA: 7.64 ± 2.56/7.63 ± 2.49 | ED | LR | NR | 30 days |
| Jaynes, 2017 | RC | USA | 410 (201/209) | 220 | 61 ± 14.1/58 ± 14.7 | APACHE II: 16.7 ± 6.1/17.3 ± 5.9 | ICU | LR and Electrolyte-A | Total: 6750 (4013–10,000)/6500 (4550–12,000) | NR |
| Limapichat, 2021 | RC | Thailand | 120 (20/100) | 75 | 69 (59.8–80)/68 (57–82.2) | NEWS: 9 (7, 10.2)/10 (8, 12) | ED | LR | NR | 2 days |
| Mao, 2018 | RC | China | 198 (98/100) | 105 | 72 ± 9/73 ± 10 | NR | ICU | LR | First 72 h: 5092 ± 929/5470 ± 1078 | NR |
| Pagano, 2020 | RCT | Italy | 84 (35/49) | 51 | 75.9 (12.3)/75.8 (12.1) | SOFA: 5.9 (2.9)/6 (2.8) | ED | LR | First 1 h: 1410/2130 | NR |
| Raghunathan, 2014 | RC | USA | 6730 (3365/3365) | NR | NR | NR | ICU | NR | NR | 2 days |
| Semler, 2017 | RCT | USA | 260 (130/130) | NR | NR | NR | ICU | LR or Plasmalyte | NR | 30 days |
| Semler, 2018 | RCT | USA | 2336 (1167/1169) | NR | NR | NR | ICU | LR or Plasmalyte | NR | 30 days |
| Shaw, 2017 | RC | USA | 3116 (1558/1558) | 1333 | NR | NR | ED, ICU and ward | Plasma-Lyte or Normosol | NR | NR |
| Tseng, 2021 | RC | Taiwan | 938 (302/636) | 707 | 71.3 ± 15.6 | APACHE II: 29 (6.4)/29 (6.4) | ICU | LR | First 24 h: 3172 (2442)/4587 (3776) | 90 days |
| Young, 2015 | RCT | Australia and New Zealand | 84 (41/43) | NR | NR | APACHE II: 14.1 (6.9)/14.1 (6.9) | ICU | Plasma-Lyte 148 | First 24 h: 1200 (0–3000)/1000 (0–3000) | 90 days |
| Zampieri, 2021 | RCT | Brazil | 1987 (970/1017) | NR | NR | NR | ICU | Plasma-Lyte 148 | NR | 90 days |
| Study, Year | Overall Mortality, n (BC/NS) | 28/30-Day Mortality, n (BC/NS) | 90-Day Mortality, n (BC/NS) | AKI, n (BC/NS) | Need for RRT, n (BC/NS) | ICU LOS, Mean ± SD, Days (BC/NS) |
|---|---|---|---|---|---|---|
| Annane, 2013 | 16/197 | 12/157 | 16/197 | NR | NR | NR |
| Duffy, 2018 | 68/66 | NR | NR | 281/238 | 37/31 | 4.6/5.6 |
| Finfer, 2022 | 276/265 | NR | 276/265 | NR | NR | NR |
| Golla, 2020 | 29/35 | 29/35 | NR | 21/32 | 10/14 | NR |
| Jaynes, 2017 | 37/38 | NR | NR | 42/63 | 16/20 | 7 (4–12.5)/5 (3–9) |
| Limapichat, 2021 | 2/14 | NR | NR | NR | NR | NR |
| Mao, 2018 | 48/54 | 48/54 | NR | 21/31 | 16/21 | 12 (11–17)/13 (12–15) |
| Pagano, 2020 | 8/28 | NR | NR | NR | 2/4 | NR |
| Raghunathan, 2014 | 659/768 | NR | NR | (159/2655)/(199/2655) | (142/3144)/(149/3144) | 5.50/5.50 |
| Semler, 2017 | 27/33 | 27/33 | NR | NR | NR | NR |
| Semler, 2018 | 294/344 | 294/344 | NR | NR | NR | NR |
| Shaw, 2017 | 16/51 | NR | NR | 69/85 | NR | NR |
| Tseng, 2021 | 114/263 | 95/233 | 114/263 | NR | NR | 15.9 (13.7–16.1)/17.8 (16.6–19.7) |
| Young, 2015 | 6/7 | NR | 6/7 | (7/35)/(9/42) | NR | NR |
| Zampieri, 2021 | 453/498 | NR | 453/498 | NR | NR | NR |
| Outcomes (Number of Studies) | RR (95% CI) | p-Value | I2 | Subgroup Analysis Based on the Study Design | |||
|---|---|---|---|---|---|---|---|
| Study Design (Number of Studies) | RR (95% CI) | p-Value | I2 | ||||
| Overall mortality (15) | 0.88 (0.81–0.96) | 0.005 | 51% | RCT (8) | 0.92 (0.82–1.02) | 0.11 | 41% |
| Cohort (7) | 0.83 (0.71–0.97) | 0.02 | 58% | ||||
| 28/30-day mortality (6) | 0.87 (0.79, 0.95) | 0.003 | 0% | RCT (4) | 0.87 (0.77–0.97) | 0.02 | 0% |
| Cohort (2) | N/A | N//A | N/A | ||||
| 90-day mortality (5) | 0.96 (0.90–1.03) | 0.31 | 0% | RCT (4) | 0.98 (0.90–1.05) | 0.52 | 0% |
| Cohort (1) | N/A | N/A | N/A | ||||
| AKI (7) | 0.85 (0.77, 0.93) | 0.0006 | 0% | RCT (2) | 0.71 (0.47–1.06) | 0.09 | 0% |
| Cohort (5) | 0.84 (0.75–0.94) | 0.003 | 14% | ||||
| Need for RRT (6) | 0.91 (0.76, 1.08) | 0.28 | 0% | RCT (2) | 0.71 (0.36–1.41) | 0.33 | 0% |
| Cohort (4) | 0.92 (0.77–1.11) | 0.39 | 0% | ||||
| ICU LOS (3) | −0.25 (−3.44, 2.95) | 0.88 | 98% | RCTs (0) | N/A | N/A | N/A |
| Cohort (3) | −0.25 (−3.44, 2.95) | 0.88 | 98% | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beran, A.; Altorok, N.; Srour, O.; Malhas, S.-E.; Khokher, W.; Mhanna, M.; Ayesh, H.; Aladamat, N.; Abuhelwa, Z.; Srour, K.; et al. Balanced Crystalloids versus Normal Saline in Adults with Sepsis: A Comprehensive Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1971. https://doi.org/10.3390/jcm11071971
Beran A, Altorok N, Srour O, Malhas S-E, Khokher W, Mhanna M, Ayesh H, Aladamat N, Abuhelwa Z, Srour K, et al. Balanced Crystalloids versus Normal Saline in Adults with Sepsis: A Comprehensive Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(7):1971. https://doi.org/10.3390/jcm11071971
Chicago/Turabian StyleBeran, Azizullah, Nehaya Altorok, Omar Srour, Saif-Eddin Malhas, Waleed Khokher, Mohammed Mhanna, Hazem Ayesh, Nameer Aladamat, Ziad Abuhelwa, Khaled Srour, and et al. 2022. "Balanced Crystalloids versus Normal Saline in Adults with Sepsis: A Comprehensive Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 7: 1971. https://doi.org/10.3390/jcm11071971
APA StyleBeran, A., Altorok, N., Srour, O., Malhas, S.-E., Khokher, W., Mhanna, M., Ayesh, H., Aladamat, N., Abuhelwa, Z., Srour, K., Mahmood, A., Altorok, N., Taleb, M., & Assaly, R. (2022). Balanced Crystalloids versus Normal Saline in Adults with Sepsis: A Comprehensive Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(7), 1971. https://doi.org/10.3390/jcm11071971






