Prevalence of Arrhythmia in Adults after Fontan Operation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Echocardiography
2.3. Ambulatory 24-h Electrocardiogram
2.4. Arrhythmia
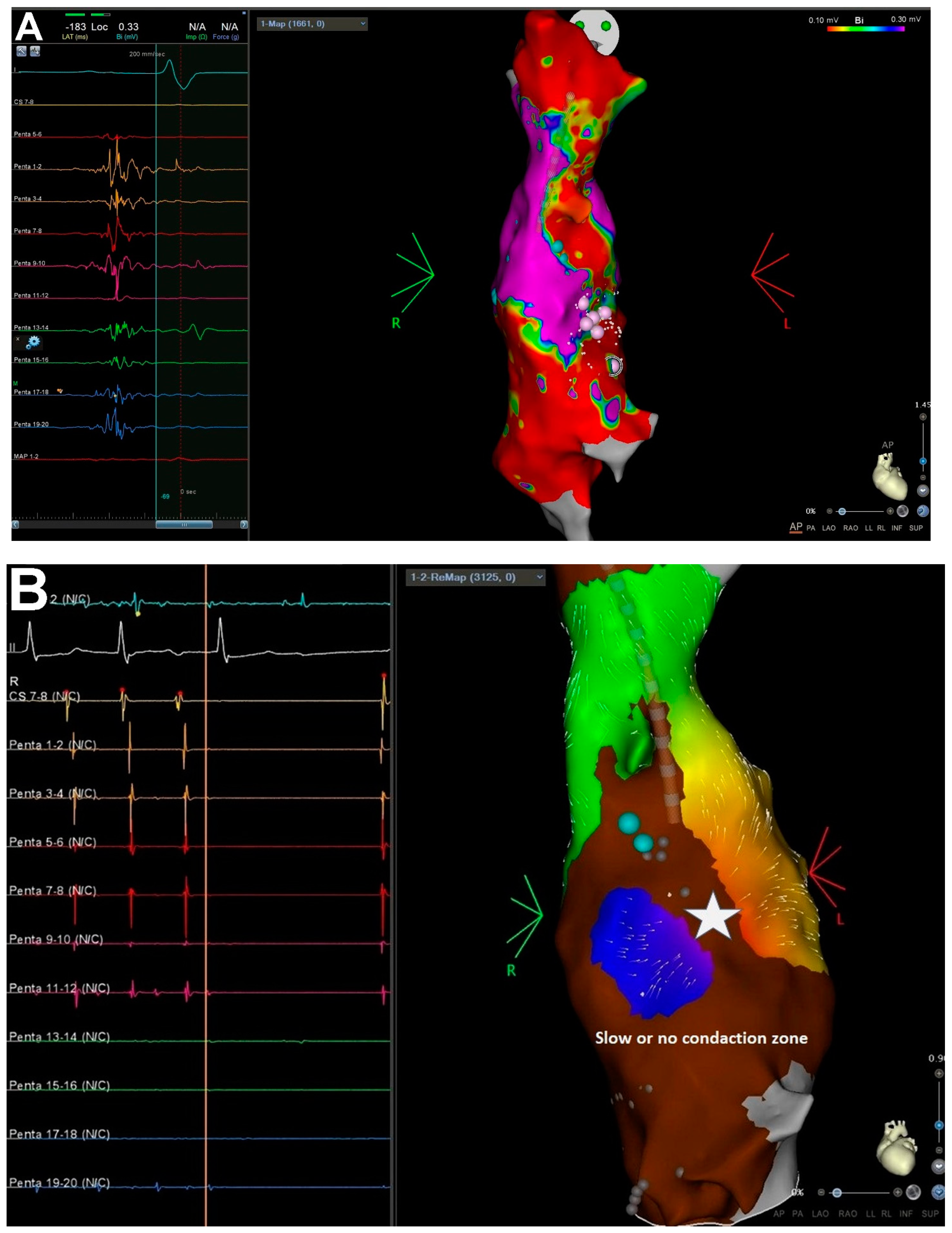
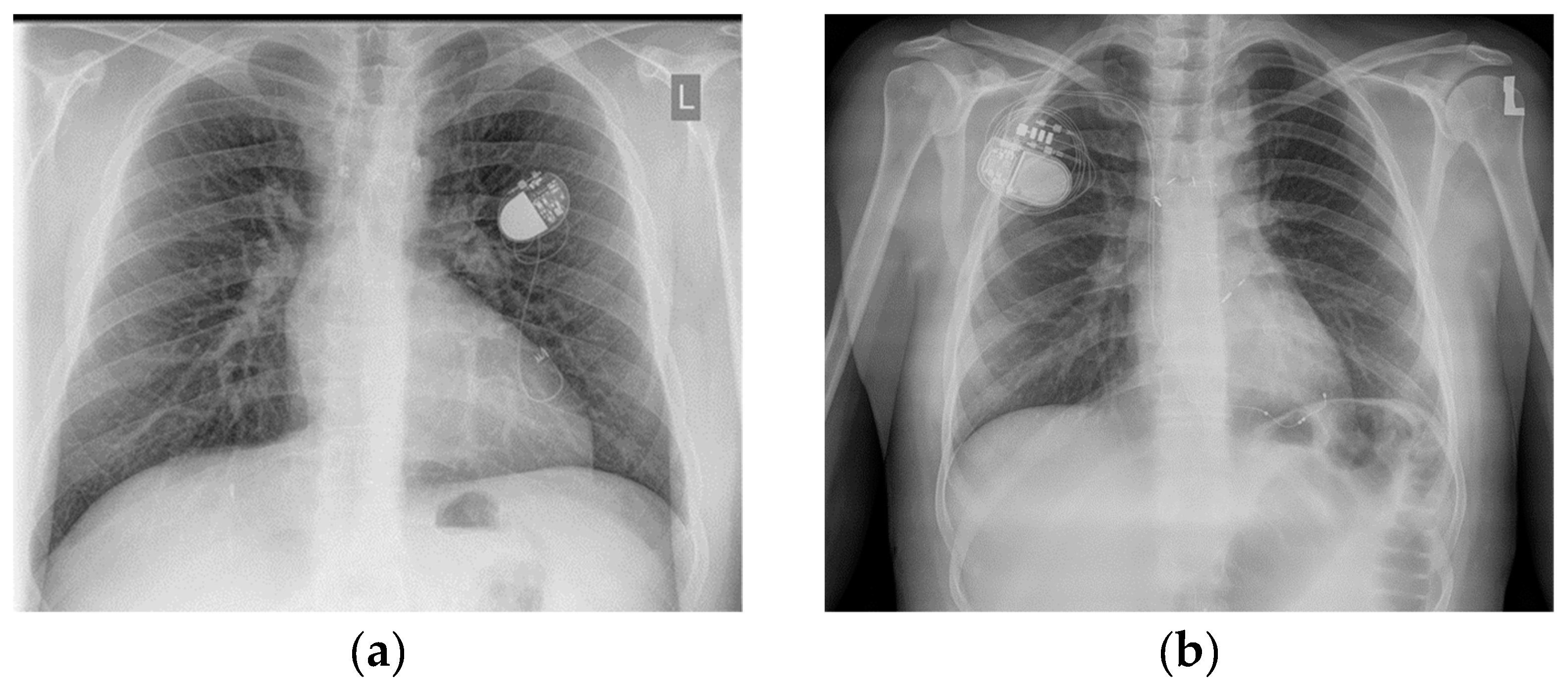
2.5. Mapping and Ablation Procedure
2.6. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Arrhythmia
3.3. Catheter Ablation
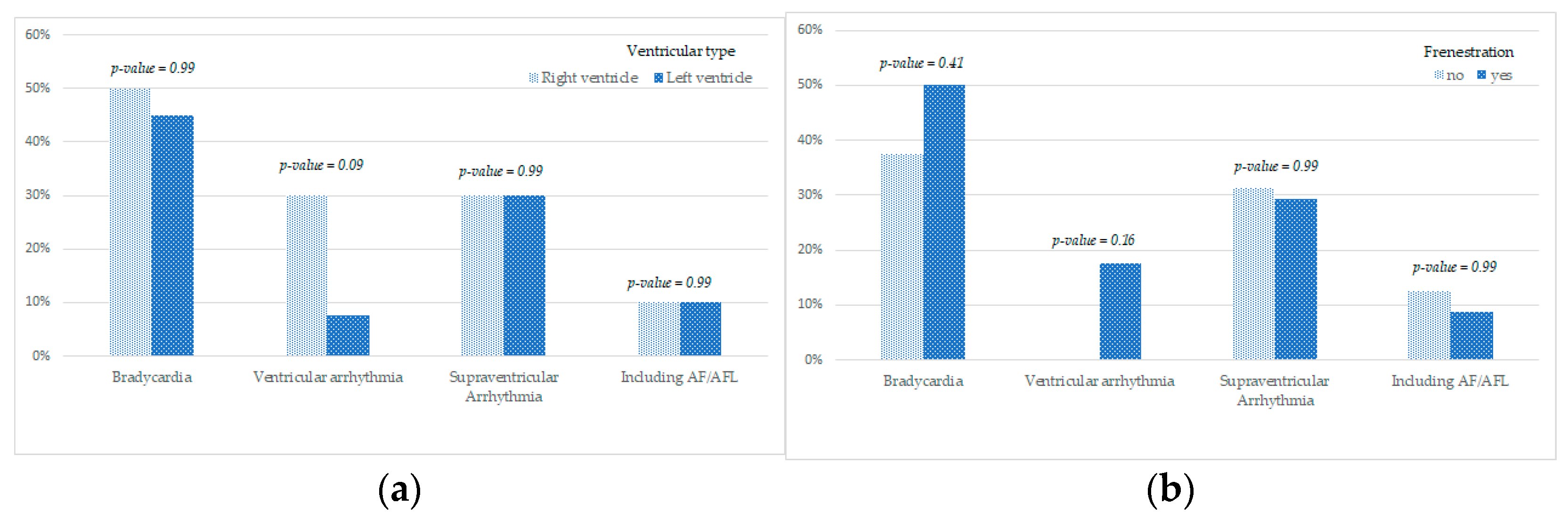
3.4. Influence of Systemic Ventricle Morphology and Fenestration on the Incidence of Rhythm Abnormalities
3.5. The Survival Assessment of Enrolled Patients
4. Discussion
5. Conclusions
Limitation of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumagartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- de Leval, M.R.; Kilner, P.; Gewillig, M.; Bull, C. Total cavopulmonary connection: A logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J. Thorac. Cardiovasc. Surg. 1988, 96, 682–695. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, W.H.; Lim, H.G.; Lee, J.Y. Outcome of 200 patients after an extracardiac Fontan procedure. J. Thorac. Cardiovasc. Surg. 2008, 136, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Malec, E.; Zając, A.; Pająk, J. The results of one-stage and two-stage Fontan operation in children with single ventricle. Kardiol. Pol. 1998, 48, 23–30. [Google Scholar]
- Bae, E.J.; Lee, J.Y.; Noh, C.I.; Kim, W.H.; Kim, Y.J. Sinus node dysfunction after Fontan modifications—Influence of surgical method. Int. J. Cardiol. 2003, 88, 285–291. [Google Scholar] [CrossRef]
- Blaufox, A.D.; Sleeper, L.A.; Bradley, D.J.; Breitbart, R.E.; Hordof, A.; Kanter, R.J.; Stephenson, E.A.; Vetter, V.L.; Saul, J.P.; Pediatric Heart Network Investigators; et al. Functional status, heart rate, and rhythm abnormalities in 521 Fontan patients 6 to 18 years of age. J. Thorac. Cardiovasc. Surg. 2008, 136, 100–107.e1. [Google Scholar] [CrossRef] [PubMed]
- Dilawar, M.; Bradley, S.M.; Saul, J.P.; Stroud, M.R.; Balaji, S. Sinus node dysfunction after intraatrial lateral tunnel and extracardiac conduit Fontan procedures. Pediatr. Cardiol. 2003, 24, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.; Rubinstein, C.S.; Simsic, J.M.; Taylor, A.B.; Saul, J.P.; Bradley, S.M. Lateral tunnel versus extracardiac conduit Fontan procedure: A concurrent comparison. Ann. Thorac. Surg. 2003, 76, 1389–1396, discussion 96–97. [Google Scholar] [CrossRef]
- Nurnberg, J.H.; Ovroutski, S.; Alexi-Meskishvili, V.; Ewert, P.; Hetzer, R.; Lange, P.E. New onset arrhythmias after the extracardiac conduit Fontan operation compared with the intraatrial lateral tunnel procedure: Early and midterm results. Ann. Thorac. Surg. 2004, 78, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Ovroutski, S.; Dahnert, I.; Alexi-Meskishvili, V.; Nurnberg, J.H.; Hetzer, R.; Lange, P.E. Preliminary analysis of arrhythmias after the Fontan operation with extracardiac conduit compared with intra-atrial lateral tunnel. J. Thorac. Cardiovasc. Surg. 2001, 49, 334–337. [Google Scholar] [CrossRef]
- Stephenson, E.A.; Lu, M.; Berul, C.I.; Etheridge, S.P.; Idriss, S.F.; Margossian, R.; Sleeper, L.A.; Vetter, V.L.; Blaufox, A.D.; Pediatric Heart Network Investigators; et al. Arrhythmias in a contemporary Fontan cohort: Prevalence and clinical associations in a multicenter cross sectional study. J. Am. Coll. Cardiol. 2010, 56, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Idorn, L.; Juul, K.; Jensen, A.S.; Hanel, B.; Nielsen, K.G.; Andersen, H.; Reimers, J.I.; Sørensen, K.E.; Søndergaard, L. Arrhythmia and exercise intolerance in Fontan patients: Current status and future burden. Int. J. Cardiol. 2013, 168, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.; Anderson, R.; Nisbet, A.; Kalla, M.; du Plessis, K.; d’Udekem, Y. Ablation of Atrial Arrhythmias After the Atriopulmonary Fontan Procedure. JACC Clin. Electrophysiol. 2018, 10, 1338–1346. [Google Scholar] [CrossRef]
- Tomkiewicz-Pajak, L.; Podolec, P.; Drabik, L.; Pajak, J.; Kolcz, J.; Plazak, W. Single ventricle function and exercise tolerance in adult patients after Fontan operation. Acta Cardiol. 2014, 69, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Brugada, J.; Katritsis, D.G.; Arbelo, E.; Arribas, F.; Bax, J.J.; Blomström-Lundqvist, C.; Calkins, H.; Corrado, D.; Deftereos, S.G.; Diller, G.P.; et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia: The Task Force for the Management of Patients with Supraventricular Tachycardia of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 655–720, Erratum in Eur. Heart J. 2020, 41, 4258. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2018, 72, e91–e220, Erratum in J. Am. Coll. Cardiol. 2018, 72, 1760. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, A.; Bieganowska, K.; Kozłowski, D.; Kukla, M.; Kurpesa, M.; Lelakowski, J.; Maciejewska, M.; Miszczak-Knecht, M.; Pierścińska, M. Zalecenia dotyczące stosowania rozpoznań elektrokardiograficznych Dokument opracowany przez Grup’ Roboczà powołanà przez Zarzàd Sekcji Elektrokardiologii Nieinwazyjnej i Telemedycyny Polskiego Towarzystwa Kardiologicznego Pod patronatem Polskiego Towarzystwa Kardiologicznego. Kardiol. Pol. 2010, 68, 336–389. [Google Scholar]
- Cohen, M.; Wernovsky, G.; Vetter, V.L.; Wieand, T.S.; Gaynor, J.W.; Jacobs, M.L.; Spray, T.L.; A Rhodes, L. Sinus node function after a systematically staged Fontan procedure. Circulation 1998, 98, II352–II359. [Google Scholar]
- Lasa, J.J.; Glatz, A.C.; Daga, A.; Shah, M. Prevalence of arrhythmias late after the Fontan operation. Am. J. Cardiol. 2014, 113, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Fan, Q.; Hirata, Y.; Ono, M.; An, Q. Arrhythmias after Fontan operation with intra-atrial lateral tunnel versus extra-cardiac conduit: A systematic review and meta-analysis. Pediatr. Cardiol. 2017, 38, 873–880. [Google Scholar] [CrossRef]
- Okólska, M.; Łach, J.; Matusik, P.T.; Pająk, J.; Mroczek, T.; Podolec, P.; Tomkiewicz-Pająk, L. Heart rate variability and its associations with organ complications in adults after Fontan operation. J. Clin. Med. 2021, 10, 4492. [Google Scholar] [CrossRef] [PubMed]
- Durongpisitkul, K.; Porter, C.J.; Cetta, F.; Offord, K.P.; Slezak, J.M.; Puga, F.J.; Schaff, H.V.; Danielson, G.K.; Driscoll, D.J. Predictors of early- and late-onset supraventricular tachyarrhythmias after Fontan operation. Circulation 1998, 98, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Tomkiewicz-Pająk, L.; Hoffman, P.; Trojnarska, O.; Bednarek, J.; Płazak, W.; Pająk, J.W.; Olszowska, M.; Komar, M.; Podolec, P.S. Long-term follow-up in adult patients after Fontan operation. Kardiochir. Torakochirurgia Pol. 2013, 10, 357–363. [Google Scholar]
- Tomkiewicz-Pająk, L.; Lelakowski, J.; Pająk, J.; Kopeć, G.; Podolec, P.; Bednarek, J. Ablation of arrhythmias in adult patients after Fontan operation. Pol. Arch. Med. Wewn. 2013, 123, 723–725. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kibos, A.; Chang, S.L.; Lee, P.C.; Chen, S.A. Catheter ablation of an intra-atrial reentrant tachycardia in a young adult Fontan patient with complex palliated congenital heart disease. Circ. J. 2012, 76, 2494–2495. [Google Scholar] [CrossRef] [PubMed]
- Rychik, J.; Atz, A.M.; Celermajer, D.S.; Deal, B.J.; Gatzoulis, M.A.; Gewillig, M.H.; Hsia, T.Y.; Hsu, D.T.; Kovacs, A.H.; McCrindle, B.W.; et al. Evaluation and management of the child and adult with Fontan circulation: A scientific statement from the American Heart Association. Circulation 2019, 140, e234–e284. [Google Scholar] [CrossRef]
- Karkowski, G.; Kuniewicz, M.; Badacz, R.; Rajs, T.; Lelakowski, J.; Legutko, J. The CARTOPRIME module with the Coherent Mapping algorithm for ablation of complex (scar-related) atrial tachycardia. Kardiol. Pol. 2020, 78, 1180–1182. [Google Scholar] [CrossRef] [PubMed]
- Khairy, P.; Fernandes, S.M.; Mayer, J.E., Jr.; Triedman, J.K.; Walsh, E.P.; Lock, J.E.; Landzberg, M.J. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008, 117, 85–92. [Google Scholar] [CrossRef]
- Pundi, K.N.; Pundi, K.N.; Johnson, J.N.; Dearani, J.A.; Li, Z.; Driscoll, D.J.; Wackel, P.L.; McLeod, C.J.; Cetta, F.; Cannon, B.C. Sudden cardiac death and late arrhythmias after the Fontan operation. Congenit. Heart Dis. 2017, 12, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Agir, A.A.; Celikyurt, U.; Karauzum, K.; Yilmaz, I.; Ozbudak, E.; Bozyel, S.; Kanko, M.; Vural, A.; Ural, D. Clinical ventricular tachycardia and surgical epicardial ICD implantation in a patient with a Fontan operation for double-inlet left ventricle. Cardiovasc. J. Afr. 2014, 25, e6–e10. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, N.A.; Patris, V.; Samiotis, I.; Koutouzis, M.; Koutouzi, G.; Argiriou, M. Epicardial cardioverter defibrillator implantation due to post-Fontan ventricular tachycardia. Ann. Cardiac Anaesth. 2020, 23, 235–236. [Google Scholar] [CrossRef] [PubMed]
- Padanilam, M.S.; Ahmed, A.S.; Clark, B.A.; Mozes, J.I.; Steinberg, L.A. Novel approach to intracardiac defibrillator placement in patients with atriopulmonary Fontan: Ventricular defibrillation with an atrial positioned ICD lead. J. Cardiovasc. Electrophysiol. 2021, 32, 3275–3278. [Google Scholar] [CrossRef] [PubMed]
| Variables | Fontan Patients (n = 50) |
|---|---|
| Age, years | 24 (5.7) |
| Female sex, n (%) | 22 (44) |
| Height, cm | 170 (8.1) |
| Body mass index, kg/m2 | 22.6 (3.2) |
| Anatomic diagnosis, n (%) | |
| Tricuspid atresia | 8 (16) |
| Pulmonary stenosis/TGA | 15 (30) |
| Right ventricular hypoplasia | 13 (26) |
| Hypoplastic left heart syndrome | 6 (12) |
| Double-outlet right ventricle with left ventricular hypoplasia | 6 (12) |
| Double-inflow left ventricle | 1 (2) |
| Common atrioventricular canal | 1 (2) |
| Systemic ventricle type, n (%) | |
| Left ventricle | 30 (60) |
| Right ventricle | 20 (40) |
| NYHA functional class, n (%) | |
| I | 5 (10) |
| II | 41 (82) |
| III | 4 (8) |
| IV | 0 (0) |
| Types of FO, n (%) | |
| Total cavopulmonary connection, 48 (96) | |
| Lateral tunnel | 47 |
| Extracardiac conduit | 1 |
| Atriopulmonary connection | 2 (4) |
| Arrythmia Type, Catheter Ablation, Device Implanted | Fontan Patients (n = 50) |
|---|---|
| Dominant SSS with bradycardia | 25 (55%) |
| 6 |
| 5 |
| 5 |
| 2 |
| 0 |
| 6 |
| Supraventricular tachyarrhythmias | 14 (28%) |
| 3 (6%) |
| 8 (16%) |
| 2 (4%) |
| VAs (in the form of nsVT and PVC) | 6 (12%) |
| Catheter ablation | 3 (6%) |
| 2 (4%) |
| 1 (2%) |
| Device implanted (VVI/DDD) | 6 (12%) |
| VVI—5, DDD—1, 2 devices removed because of cardiac device-related infective endocarditis |
| Bradycardia | Ventricular Arrhythmia | Supraventricular Arrhythmia | Including AF/AFL | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no | yes | no | yes | no | yes | no | yes | |||||||||
| Fenestration | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
| no | 10 | 62.50% | 6 | 37.50% | 16 | 100% | 0 | 0.0% | 11 | 68.8% | 5 | 31.3% | 14 | 87.5% | 2 | 12.5% |
| yes | 17 | 50.00% | 17 | 50.00% | 18 | 82.4% | 6 | 17.6% | 24 | 70.6% | 10 | 29.4% | 31 | 91.2% | 3 | 8.8% |
| p-value | 0.41 | 0.16 | 0.99 | 0.99 | ||||||||||||
| no | yes | no | yes | no | yes | no | yes | |||||||||
| Ventricular type | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
| Right ventricle | 5 | 50.0% | 5 | 50.0% | 7 | 70.0% | 3 | 30.0% | 7 | 70.0% | 3 | 30.0% | 9 | 90.0% | 1 | 10.0% |
| Left ventricle | 22 | 55.0% | 18 | 45.0% | 37 | 92.5% | 3 | 7.5% | 28 | 70.0% | 12 | 30.0% | 36 | 90.0% | 4 | 10.0% |
| p-value | 0.99 | 0.09 | 0.990 | 0.99 | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okólska, M.; Karkowski, G.; Kuniewicz, M.; Bednarek, J.; Pająk, J.; Róg, B.; Łach, J.; Legutko, J.; Tomkiewicz-Pająk, L. Prevalence of Arrhythmia in Adults after Fontan Operation. J. Clin. Med. 2022, 11, 1968. https://doi.org/10.3390/jcm11071968
Okólska M, Karkowski G, Kuniewicz M, Bednarek J, Pająk J, Róg B, Łach J, Legutko J, Tomkiewicz-Pająk L. Prevalence of Arrhythmia in Adults after Fontan Operation. Journal of Clinical Medicine. 2022; 11(7):1968. https://doi.org/10.3390/jcm11071968
Chicago/Turabian StyleOkólska, Magdalena, Grzegorz Karkowski, Marcin Kuniewicz, Jacek Bednarek, Jacek Pająk, Beata Róg, Jacek Łach, Jacek Legutko, and Lidia Tomkiewicz-Pająk. 2022. "Prevalence of Arrhythmia in Adults after Fontan Operation" Journal of Clinical Medicine 11, no. 7: 1968. https://doi.org/10.3390/jcm11071968
APA StyleOkólska, M., Karkowski, G., Kuniewicz, M., Bednarek, J., Pająk, J., Róg, B., Łach, J., Legutko, J., & Tomkiewicz-Pająk, L. (2022). Prevalence of Arrhythmia in Adults after Fontan Operation. Journal of Clinical Medicine, 11(7), 1968. https://doi.org/10.3390/jcm11071968






