Plasma Thallium Concentration, Kidney Function, Nephrotoxicity and Graft Failure in Kidney Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Study Population
2.2. Outcome Measurements
2.3. Laboratory Methods and Thallium Measurement
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Associations of Plasma Thallium with Clinical and Kidney Function Parameters
3.3. Associations of Plasma Thallium with Parameters of Nephrotoxicity and Tubular Damage
3.4. Associations with Outcome
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamb, K.E.; Lodhi, S.; Meier-Kriesche, H.-U. Long-Term Renal Allograft Survival in the United States: A Critical Reappraisal. Am. J. Transplant. 2011, 11, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Sotomayor, C.G.; Groothof, D.; Vodegel, J.J.; Gacitúa, T.A.; Gomes-Neto, A.W.; Osté, M.C.J.; Pol, R.A.; Ferreccio, C.; Berger, S.P.; Chong, G.; et al. Circulating Arsenic is Associated with Long-Term Risk of Graft Failure in Kidney Transplant Recipients: A Prospective Cohort Study. J. Clin. Med. 2020, 9, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotomayor, C.G.; Giubergia, F.; Groothof, D.; Ferreccio, C.; Nolte, I.M.; Navis, G.J.; Gomes-Neto, A.W.; Kremer, D.; Knobbe, T.J.; Eisenga, M.F.; et al. Plasma Lead Concentration and Risk of Late Kidney Allograft Failure: Findings From the TransplantLines Biobank and Cohort Studies. Am. J. Kidney Dis. 2021. [Google Scholar] [CrossRef]
- Sotomayor, C.G.; Groothof, D.; Vodegel, J.J.; Eisenga, M.F.; Knobbe, T.J.; Ijmker, J.; Lammerts, R.G.; de Borst, M.H.; Berger, S.P.; Nolte, I.M.; et al. Plasma cadmium is associated with increased risk of long-term kidney graft failure. Kidney Int. 2021, 99, 1213–1224. [Google Scholar] [CrossRef]
- Barbier, O.; Jacquillet, G.; Tauc, M.; Cougnon, M.; Poujeol, P. Effect of Heavy Metals on, and Handling by, the Kidney. Nephron Physiol. 2005, 99, 105–110. [Google Scholar] [CrossRef]
- Pefanis, A.; Ierino, F.L.; Murphy, J.M.; Cowan, P.J. Regulated necrosis in kidney ischemia-reperfusion injury. Kidney Int. 2019, 96, 291–301. [Google Scholar] [CrossRef]
- de Mattos, A.M.; Olyaei, A.J.; Bennett, W.M. Nephrotoxicity of immunosuppressive drugs: Long-term consequences and challenges for the future. Am. J. Kidney Dis. 2000, 35, 333–346. [Google Scholar] [CrossRef]
- Campanella, B.; Colombaioni, L.; Benedetti, E.; Di Ciaula, A.; Ghezzi, L.; Onor, M.; D’Orazio, M.; Giannecchini, R.; Petrini, R.; Bramanti, E. Toxicity of Thallium at Low Doses: A Review. Int. J. Environ. Res. Public Health 2019, 16, 4732. [Google Scholar] [CrossRef] [Green Version]
- Brockhaus, A.; Dolgner, R.; Ewers, U.; Soddemann, H.; Wiegand, H. Intake and health effects of thallium among a population living in the vicinity of a cement plant emitting thallium containing dust. Int. Arch. Occup. Environ. Health 1981, 48, 375–389. [Google Scholar] [CrossRef]
- Emers, U. Environmental exposure to thallium. Sci. Total Environ. 1988, 71, 285–292. [Google Scholar] [CrossRef]
- Peter, A.L.J.; Viraraghavan, T. Thallium: A review of public health and environmental concerns. Environ. Int. 2005, 31, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Yuan, L.; Peng, X.; Long, J.; Wang, C.; Bai, L.; Lu, X.; Dong, J.; Liu, Y.; Wang, Y.; et al. Clinical characteristics and treatment of thallium poisoning in patients with delayed admission in China. Medicine 2019, 98, e16471. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.-W.; Xu, Q.-Y.; Zhang, X.-J.; Wu, Q.; Liu, Z.-S.; Kan, Q.-C.; Sun, C.-Y.; Wang, L. Management of thallium poisoning in patients with delayed hospital admission. Clin. Toxicol. 2012, 50, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Al Hammouri, F.; Darwazeh, G.; Said, A.; Abu Ghosh, R. Acute Thallium Poisoning: Series of Ten Cases. J. Med. Toxicol. 2011, 7, 306–311. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, B.; Long, J.; Zhang, X.; Chen, G. Thallium pollution associated with mining of thallium deposits. Sci. China Ser. D Earth Sci. 1998, 41, 75–81. [Google Scholar] [CrossRef]
- Fleck, C.; Appenroth, D. Renal amino acid transport in immature and adult rats during thallium-induced nephrotoxicity. Toxicology 1996, 106, 229–236. [Google Scholar] [CrossRef]
- Appenroth, D.; Gambaryan, S.; Winnefeld, K.; Leiterer, M.; Fleck, C.; Bräunlich, H. Functional and morphological aspects of thallium-induced nephrotoxicity in rats. Toxicology 1995, 96, 203–215. [Google Scholar] [CrossRef]
- Van den Berg, E.; Geleijnse, J.M.; Brink, E.J.; Van Baak, M.A.; van der Heide, J.J.H.; Gans, R.O.; Navis, G.; Bakker, S.J. Sodium intake and blood pressure in renal transplant recipients. Nephrol. Dial. Transplant. 2012, 27, 3352–3359. [Google Scholar] [CrossRef] [Green Version]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and Cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Standards of Medical Care in Diabetes. Diabetes Care 2017, 40, S11–S24. [Google Scholar]
- Rehkämper, M.; Nielsen, S.G. The mass balance of dissolved thallium in the oceans. Mar. Chem. 2004, 85, 125–139. [Google Scholar] [CrossRef]
- World Health Organization; International Programme for Chemical Safety. Thallium; World Health Organization: Genève, Switserland, 1996; Available online: https://apps.who.int/iris/handle/10665/37751 (accessed on 28 December 2021).
- Minoia, C.; Sabbioni, E.; Apostoli, P.; Pietra, R.; Pozzoli, L.; Gallorini, M.; Nicolaou, G.; Alessio, L.; Capodaglio, E. Trace element reference values in tissues from inhabitants of the European community I. A study of 46 elements in urine, blood and serum of Italian subjects. Sci. Total Environ. 1990, 95, 89–105. [Google Scholar] [CrossRef]
- Hologgitas, J.; Ullucci, P.; Driscoll, J.; Grauerholz, J.; Martin, H. Thallium Elimination Kinetics in Acute Thallotoxicosis. J. Anal. Toxicol. 1980, 4, 68–73. [Google Scholar] [CrossRef]
- Léonard, A.; Gerber, G.B. Mutagenicity, carcinogenicity and teratogenicity of thallium compounds. Mutat. Res. Rev. Mutat. Res. 1997, 387, 47–53. [Google Scholar] [CrossRef]
- Shelley, R.; Kim, N.-S.; Parsons, P.; Lee, B.-K.; Jaar, B.; Fadrowski, J.; Agnew, J.; Matanoski, G.M.; Schwartz, B.S.; Steuerwald, A.J.; et al. Associations of multiple metals with kidney outcomes in lead workers. Occup. Environ. Med. 2012, 69, 727–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, V.M.; Vargas, G.G.; Silbergeld, E.K.; Rothenberg, S.J.; Fadrowski, J.J.; Rubio-Andrade, M.; Parsons, P.; Steuerwald, A.J.; Navas-Acien, A.; Guallar, E. Impact of urine concentration adjustment method on associations between urine metals and estimated glomerular filtration rates (eGFR) in adolescents. Environ. Res. 2014, 132, 226–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Zhang, K.; Jiang, S.; Liu, D.; Zhou, H.; Zhong, R.; Zeng, Q.; Cheng, L.; Miao, X.; Tong, Y.; et al. Association of co-exposure to heavy metals with renal function in a hypertensive population. Environ. Int. 2018, 112, 198–206. [Google Scholar] [CrossRef]
- Jin, R.; Zhu, X.; Shrubsole, M.J.; Yu, C.; Xia, Z.; Dai, Q. Associations of renal function with urinary excretion of metals: Evidence from NHANES 2003–2012. Environ. Int. 2018, 121, 1355–1362. [Google Scholar] [CrossRef]
- Eneh, O.C. Toxic effects of selected trace elements contained in make-ups on female university students in Nigeria. Environ. Monit. Assess. 2021, 193, 412. [Google Scholar] [CrossRef]
- Lu, M.; Wang, H.; Li, X.-F.; Lu, X.; Cullen, W.R.; Arnold, L.L.; Cohen, S.M.; Le, X.C. Evidence of Hemoglobin Binding to Arsenic as a Basis for the Accumulation of Arsenic in Rat Blood. Chem. Res. Toxicol. 2004, 17, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Simons, T.J. Passive transport and binding of lead by human red blood cells. J. Physiol. 1986, 378, 267–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Świergosz-Kowalewska, R. Cadmium distribution and toxicity in tissues of small rodents. Microsc. Res. Technol. 2001, 55, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Post, A.; Gomes-Neto, A.W.; Groothof, D.; Kunutsor, S.K.; Nilsen, T.; Hidden, C.; Sundrehagen, E.; Eisenga, M.F.; Navis, G.; et al. Plasma neutrophil gelatinase-associated lipocalin and kidney graft outcome. Clin. Kidney J. 2021, 15, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Yepes-Calderón, M.; Sotomayor, C.G.; Kretzler, M.; Gans, R.O.B.; Berger, S.P.; Navis, G.J.; Ju, W.; Bakker, S.J.L. Urinary Epidermal Growth Factor/Creatinine Ratio and Graft Failure in Renal Transplant Recipients: A Prospective Cohort Study. J. Clin. Med. 2019, 8, 1673. [Google Scholar] [CrossRef] [Green Version]
- Yepes-Calderón, M.; Sotomayor, C.G.; Pena, M.; Eisenga, M.F.; Gans, R.O.B.; Berger, S.P.; Moers, C.; Sugaya, T.; Doekharan, D.; Navis, G.J.; et al. Urinary liver-type fatty-acid binding protein is independently associated with graft failure in outpatient kidney transplant recipients. Am. J. Transplant. 2021, 21, 1535–1544. [Google Scholar] [CrossRef]
- Xu, X.; Wang, G.; Chen, N.; Lu, T.; Nie, S.; Xu, G.; Zhang, P.; Luo, Y.; Wang, Y.; Wang, X.; et al. Long-Term Exposure to Air Pollution and Increased Risk of Membranous Nephropathy in China. J. Am. Soc. Nephrol. 2016, 27, 3739–3746. [Google Scholar] [CrossRef] [Green Version]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Thallium; U.S. Department of Health and Human Services: Atlanta, GA, USA, 1992.
- Blain, R.; Kazantzis, G. Chapter 55—Thallium. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1229–1240. ISBN 9780444594532. [Google Scholar]


| N = 672 | Linear Regression with Plasma Thallium as Dependent Variable | ||
|---|---|---|---|
| Plasma thallium (µg/L) | 0.12 (0.06) | St. β (95% CI) | p-Value |
| Clinical characteristics | |||
| Female sex, n (%) | 285 (42%) | −0.08 (−0.23 to 0.07) | 0.3 |
| Age, y | 53 (13) | −0.12 (−0.20 to −0.04) | 0.002 |
| Primary renal disease, n (%) | |||
| Unknown | 103 (15%) | Ref. | |
| Glomerulonephritis | 176 (26%) | 0.08 (−0.17 to 0.32) | 0.5 |
| Interstitial nephritis | 82 (12%) | 0.12 (−0.18 to 0.41) | 0.4 |
| Cystic kidney disease | 139 (20%) | 0.02 (−0.27 to 0.24) | 0.9 |
| Other congenital/hereditary disease | 39 (6%) | 0.01 (−0.38 to 0.36) | 0.9 |
| Renal vascular disease | 37 (6%) | −0.09 (−0.47 to 0.29) | 0.6 |
| Diabetes mellitus | 32 (5%) | −0.14 (−0.54 to 0.29) | 0.5 |
| Other multisystem diseases | 45 (7%) | 0.06 (−0.30 to 0.41) | 0.8 |
| Other | 19 (3%) | −0.01 (−0.48 to 0.51) | 0.9 |
| Height, cm | 174 (10) | 0.02 (−0.06 to 0.09) | 0.7 |
| Weight, kg | 80.7 (16.5) | 0.02 (−0.06 to 0.09) | 0.6 |
| Body surface area, m2 | 1.95 (0.22) | 0.02 (−0.06 to 0.10) | 0.6 |
| Body mass index, kg/m2 | 26.7 (4.8) | 0.01 (−0.06 to 0.09) | 0.8 |
| Systolic blood pressure, mmHg | 136 (17) | −0.07 (−0.15 to 0.01) | 0.07 |
| Diabetes, n (%) | 161 (24%) | −0.01 (−0.19 to 0.17) | 0.9 |
| History of cardiovascular disease, n (%) | 162 (24%) | 0.00 (−0.18 to 0.18) | 0.9 |
| Smoking status, n (%) | |||
| Never | 267 (42%) | Ref. | |
| History of smoking | 285 (45%) | −0.02 (−0.19 to 0.15) | 0.8 |
| Current smoking | 80 (13%) | 0.11 (−0.15 to 0.36) | 0.4 |
| Pre-emptive transplantation, n (%) | 107 (16%) | −0.06 (−0.27 to 0.15) | 0.6 |
| Duration of dialysis, months # | 23 [5 to 47] | 0.02 (−0.05 to 0.10) | 0.6 |
| Time after transplantation, y # | 5.4 [1.9 to 12.8] | 0.03 (−0.05 to 0.10) | 0.5 |
| History of rejection, n (%) | 177 (26%) | 0.05 (−0.12 to 0.22) | 0.6 |
| History of delayed graft function, n (%) | 50 (7%) | −0.06 (−0.35 to 0.23) | 0.7 |
| Living donor, n (%) | 232 (35%) | 0.04 (−0.12 to 0.20) | 0.6 |
| Routine laboratory measurements | |||
| Sodium mmol/L | 140.9 (2.8) | 0.00 (−0.08 to 0.08) | 0.9 |
| Potassium, mmol/L | 4.0 (0.5) | −0.03 (−0.05 to 0.10) | 0.5 |
| HbA1c, % # | 5.8 [5.5 to 6.2] | 0.02 (−0.06 to 0.10) | 0.6 |
| Hemoglobin, mmol/L | 8.2 (1.1) | 0.15 (0.07 to 0.22) | <0.001 |
| Leukocyte count, 109/L | 8.1 (2.6) | 0.05 (−0.02 to 0.13) | 0.2 |
| hs-CRP, mg/L # | 1.6 [0.7 to 4.6] | −0.01 (−0.08 to 0.07) | 0.9 |
| Kidney function parameters | |||
| Cystatin C, mg/L | 1.88 (0.77) | −0.12 (−0.19 to −0.04) | 0.002 |
| Creatinine, µmol/L # | 124 [99 to 159] | −0.07 (−0.15 to 0.00) | 0.058 |
| eGFR, mL/min/1.73 m2 | 45 (19) | 0.13 (0.05 to 0.21) | 0.001 |
| Creatinine clearance, mL/min | 66 (26) | 0.13 (0.05 to 0.20) | 0.001 |
| Urea, mmol/L # | 9.5 [7.2, 13.3] | −0.10 (−0.18 to −0.03) | 0.007 |
| Markers of tubular damage | |||
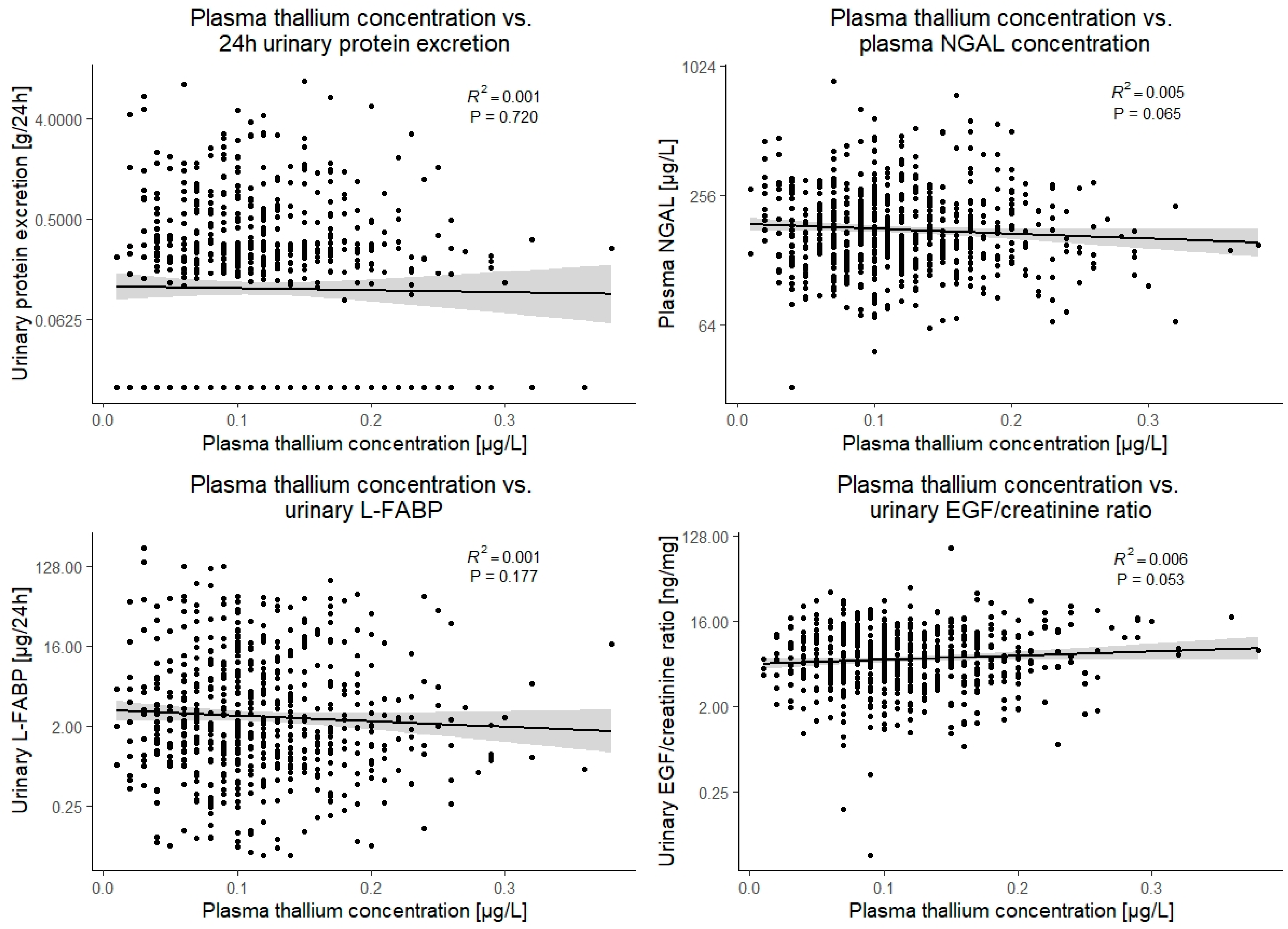
| Urinary protein excretion, g/24 h # | 0.2 [0.0 to 0.5] | −0.01 (−0.09 to 0.06) | 0.7 |
| Urinary liver-type fatty acid-binding protein, µg/24 h # | 2.06 [0.91 to 7.04] | −0.06 (−0.14 to 0.03) | 0.2 |
| Urinary endothelial growth factor/creatinine ratio, ng/mg # | 6.5 [4.1 to 10.8] | 0.08 (−0.00 to 0.16) | 0.053 |
| Plasma neutrophil-gelatinase associated lipocalin, µg/L # | 170 [133 to 232] | −0.07 (−0.15 to 0.00) | 0.065 |
| Other heavy metals in plasma | |||
| Cadmium, µg/L | 0.06 [0.04 to 0.07] | −0.14 (−0.21 to −0.06) | <0.001 |
| Lead, µg/L | 0.31 [0.22 to 0.44] | 0.02 (−0.06 to 0.09) | 0.6 |
| Arsenic, µg/L | 1.26 [1.04 to 2.04] | 0.01 (−0.07 to 0.08) | 0.8 |
| Mercury, µg/L | 0.29 [0.14 to 0.63] | 0.07 (−0.01 to 0.14) | 0.088 |
| Medication | |||
| Antihypertensive drugs, n (%) | 592 (88.1) | −0.10 (−0.34 to 0.13) | 0.4 |
| Prednisolone, n (%) | 665 (99.0) | −0.14 (−0.89 to 0.60) | 0.7 |
| Calcineurin inhibitor, n (%) | 384 (57.1) | −0.07 (−0.22 to 0.09) | 0.4 |
| Proliferation inhibitor, n (%) | 560 (83.3) | 0.01 (−0.19 to 0.22) | 0.9 |
| mTOR inhibitor, n (%) | 23 (3.4) | 0.13 (−0.29 to 0.55) | 0.5 |
| Graft Failure | All-Cause Mortality | |||
|---|---|---|---|---|
| Model | HR per SD (95% CI) | p-Value | HR per SD (95% CI) | p-Value |
| Crude | 0.89 (0.72 to 1.10) | 0.3 | 0.87 (0.74 to 1.01) | 0.073 |
| Model 1 | 0.90 (0.72 to 1.11) | 0.6 | 1.02 (0.86 to 1.20) | 0.8 |
| Model 2 | 0.90 (0.72 to 1.11) | 0.3 | 1.00 (0.85 to 1.19) | 0.9 |
| Model 3 | 0.90 (0.72 to 1.13) | 0.4 | 1.02 (0.87 to 1.21) | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kremer, D.; Riemersma, N.L.; Groothof, D.; Sotomayor, C.G.; Eisenga, M.F.; Post, A.; Knobbe, T.J.; Touw, D.J.; Bakker, S.J.L. Plasma Thallium Concentration, Kidney Function, Nephrotoxicity and Graft Failure in Kidney Transplant Recipients. J. Clin. Med. 2022, 11, 1970. https://doi.org/10.3390/jcm11071970
Kremer D, Riemersma NL, Groothof D, Sotomayor CG, Eisenga MF, Post A, Knobbe TJ, Touw DJ, Bakker SJL. Plasma Thallium Concentration, Kidney Function, Nephrotoxicity and Graft Failure in Kidney Transplant Recipients. Journal of Clinical Medicine. 2022; 11(7):1970. https://doi.org/10.3390/jcm11071970
Chicago/Turabian StyleKremer, Daan, Niels L. Riemersma, Dion Groothof, Camilo G. Sotomayor, Michele F. Eisenga, Adrian Post, Tim J. Knobbe, Daan J. Touw, and Stephan J. L. Bakker. 2022. "Plasma Thallium Concentration, Kidney Function, Nephrotoxicity and Graft Failure in Kidney Transplant Recipients" Journal of Clinical Medicine 11, no. 7: 1970. https://doi.org/10.3390/jcm11071970
APA StyleKremer, D., Riemersma, N. L., Groothof, D., Sotomayor, C. G., Eisenga, M. F., Post, A., Knobbe, T. J., Touw, D. J., & Bakker, S. J. L. (2022). Plasma Thallium Concentration, Kidney Function, Nephrotoxicity and Graft Failure in Kidney Transplant Recipients. Journal of Clinical Medicine, 11(7), 1970. https://doi.org/10.3390/jcm11071970









