Ecosynthesis and Optimization of Nano rGO/Ag-Based Electrode Materials for Superior Supercapacitor Coin Cell Devices
Abstract
1. Introduction
2. Results and Discussion
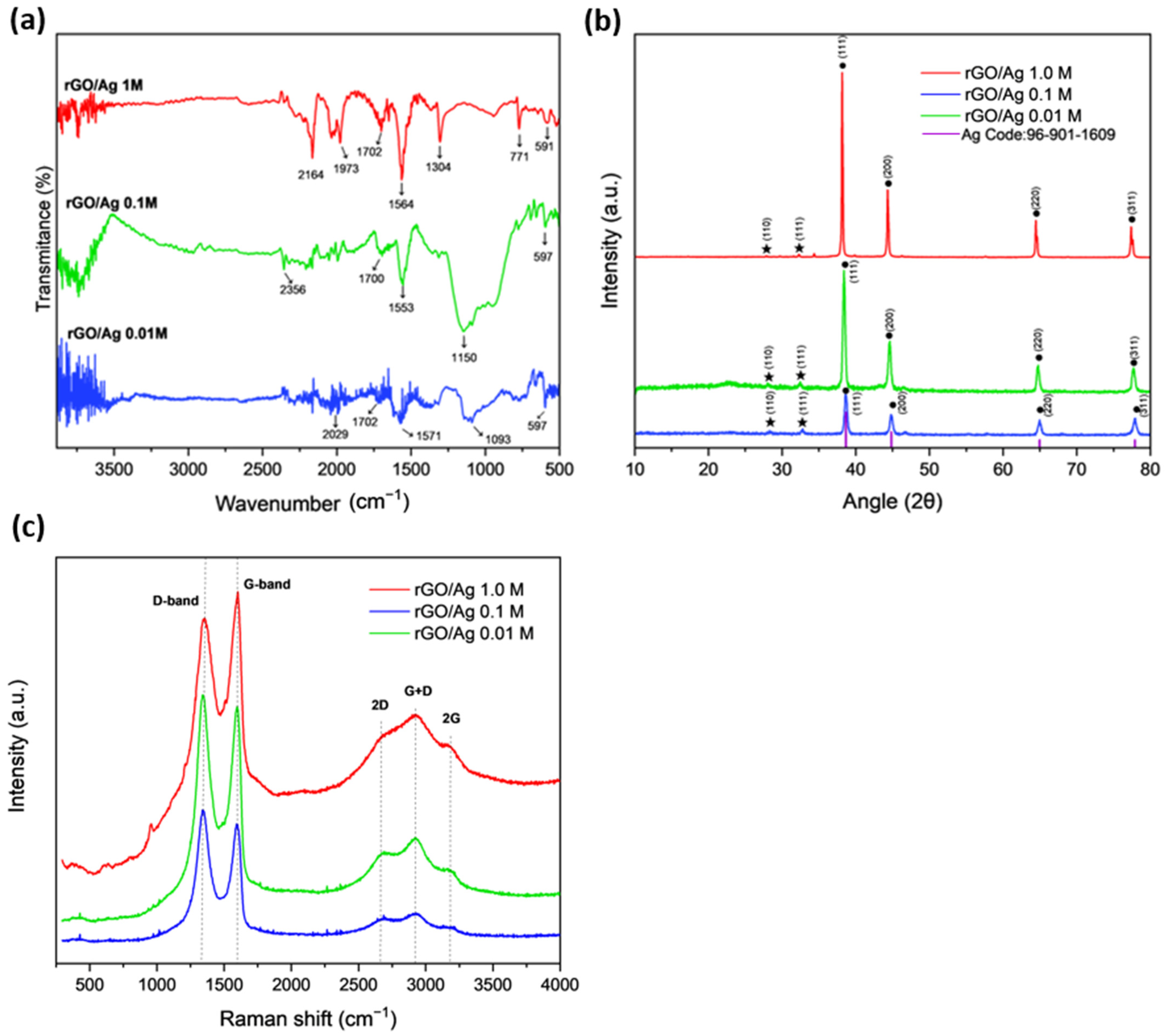
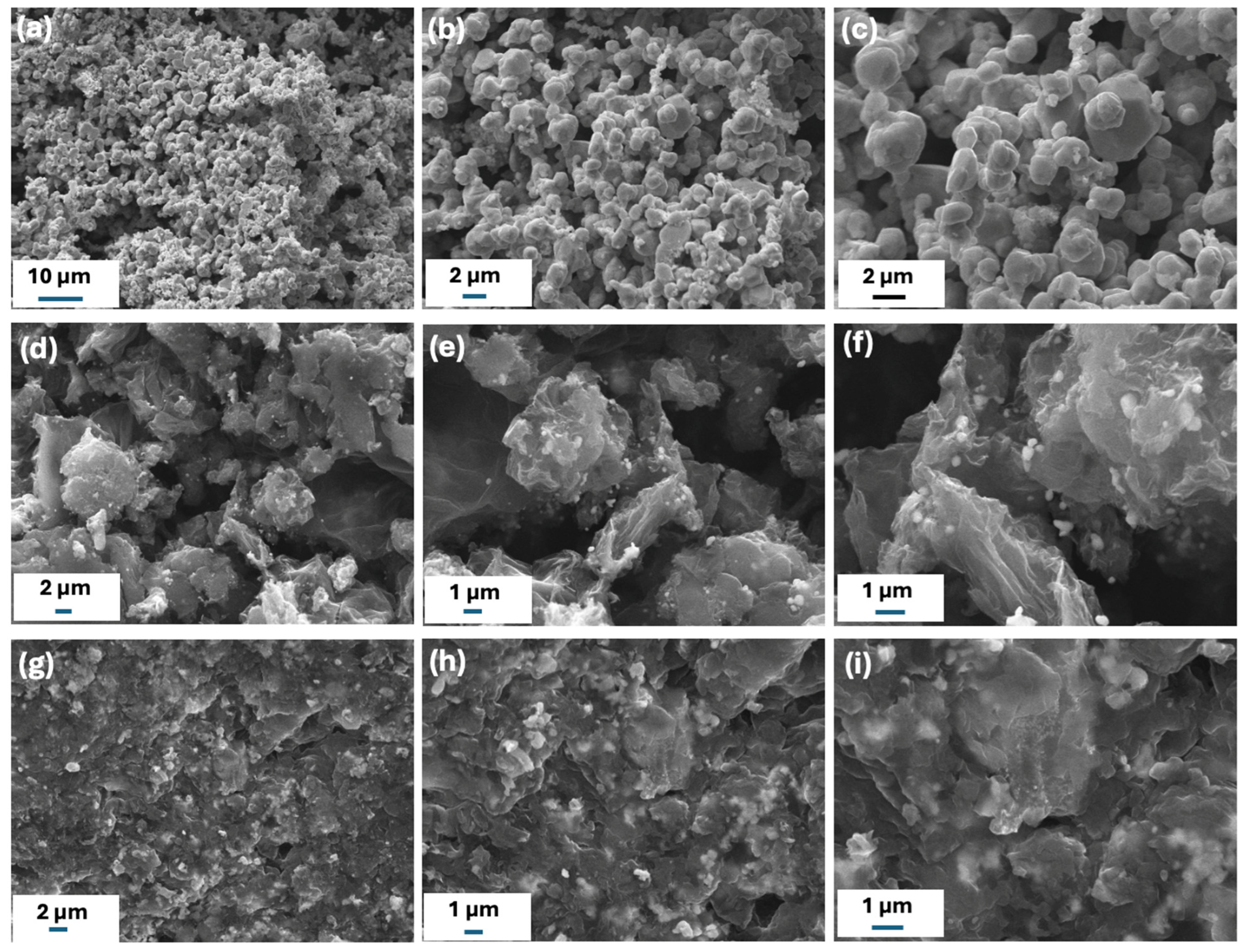
2.1. Structural and Spectral Characterization
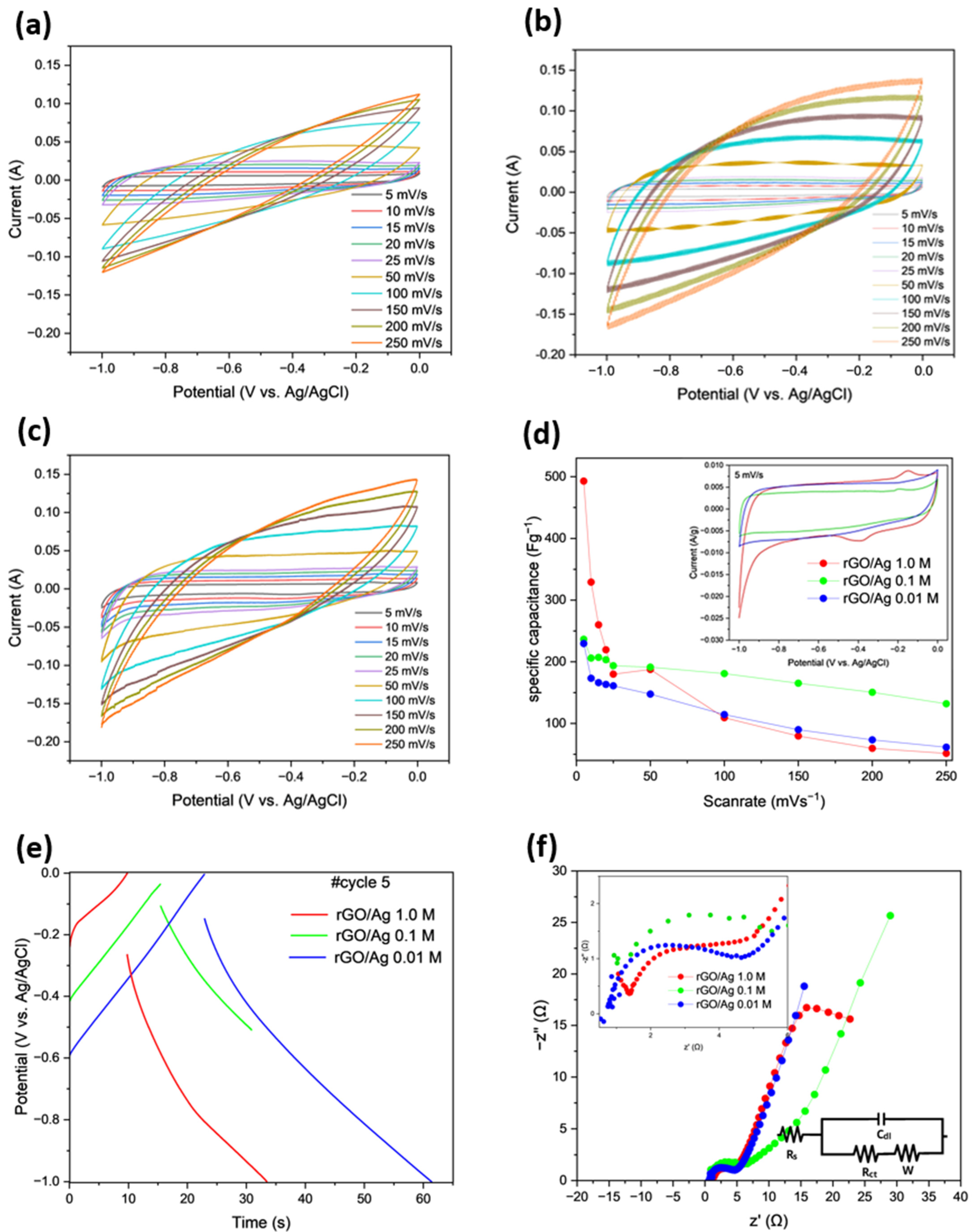
2.1.1. Electrochemical Measurements in the Three-Electrode Configuration
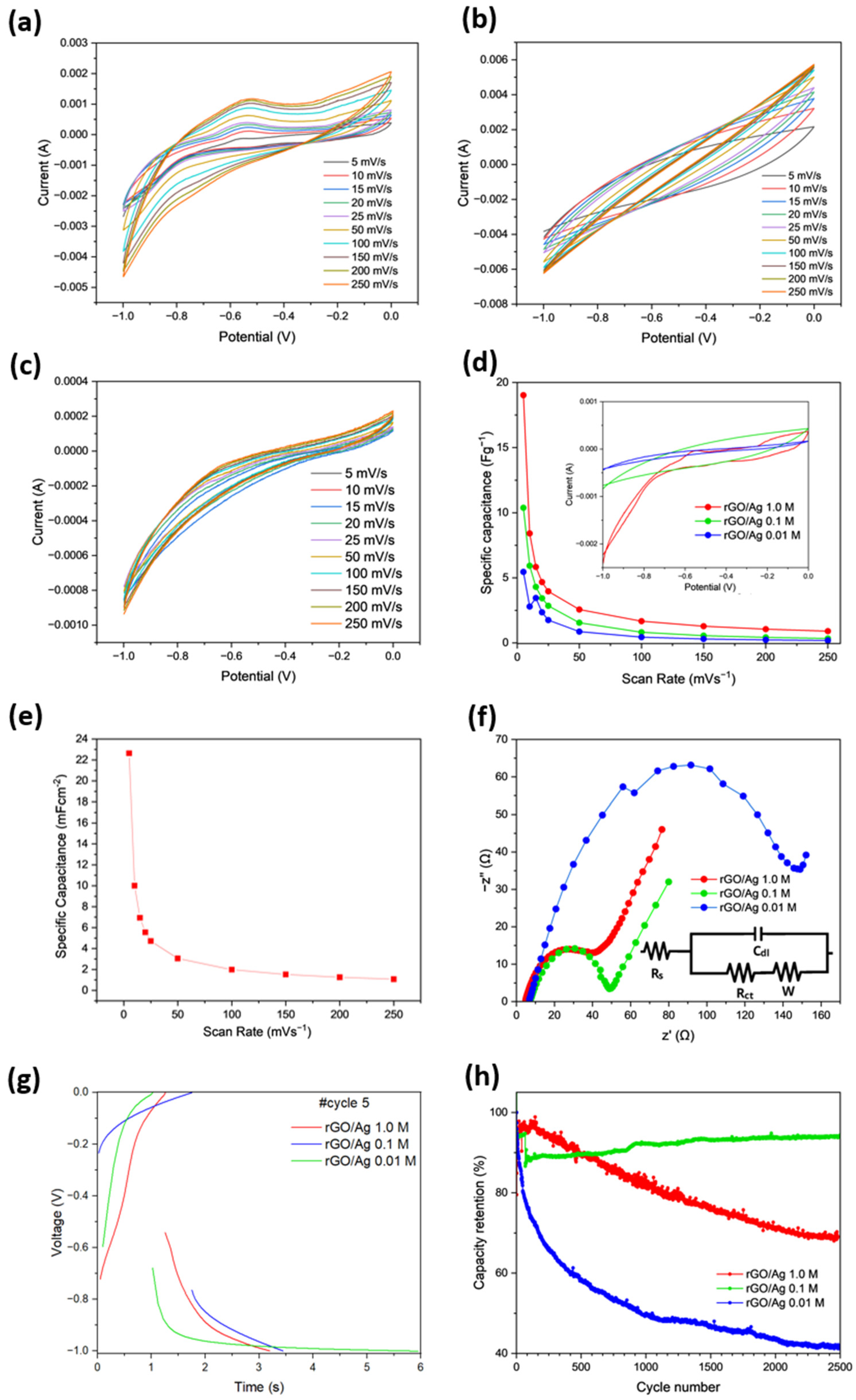
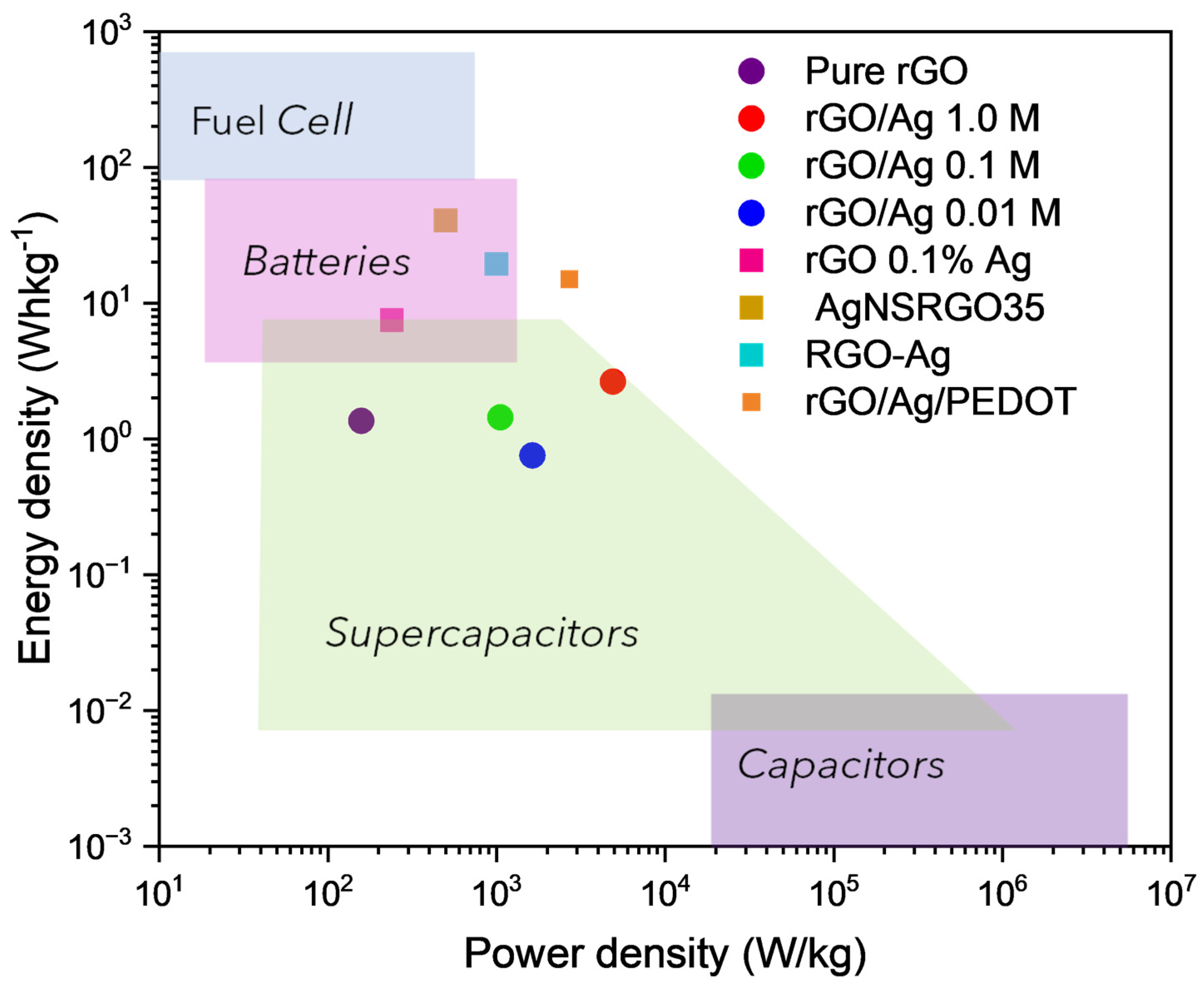
2.1.2. Electrochemical Measurements in Two-Electrode Configuration
3. Materials and Methods
3.1. Materials
3.2. Synthesis Methods
3.2.1. Synthesis of Graphene Oxide (GO)
3.2.2. Synthesis of rGO/Ag Composites
3.3. Structural, Microstructural, and Spectral Characterization
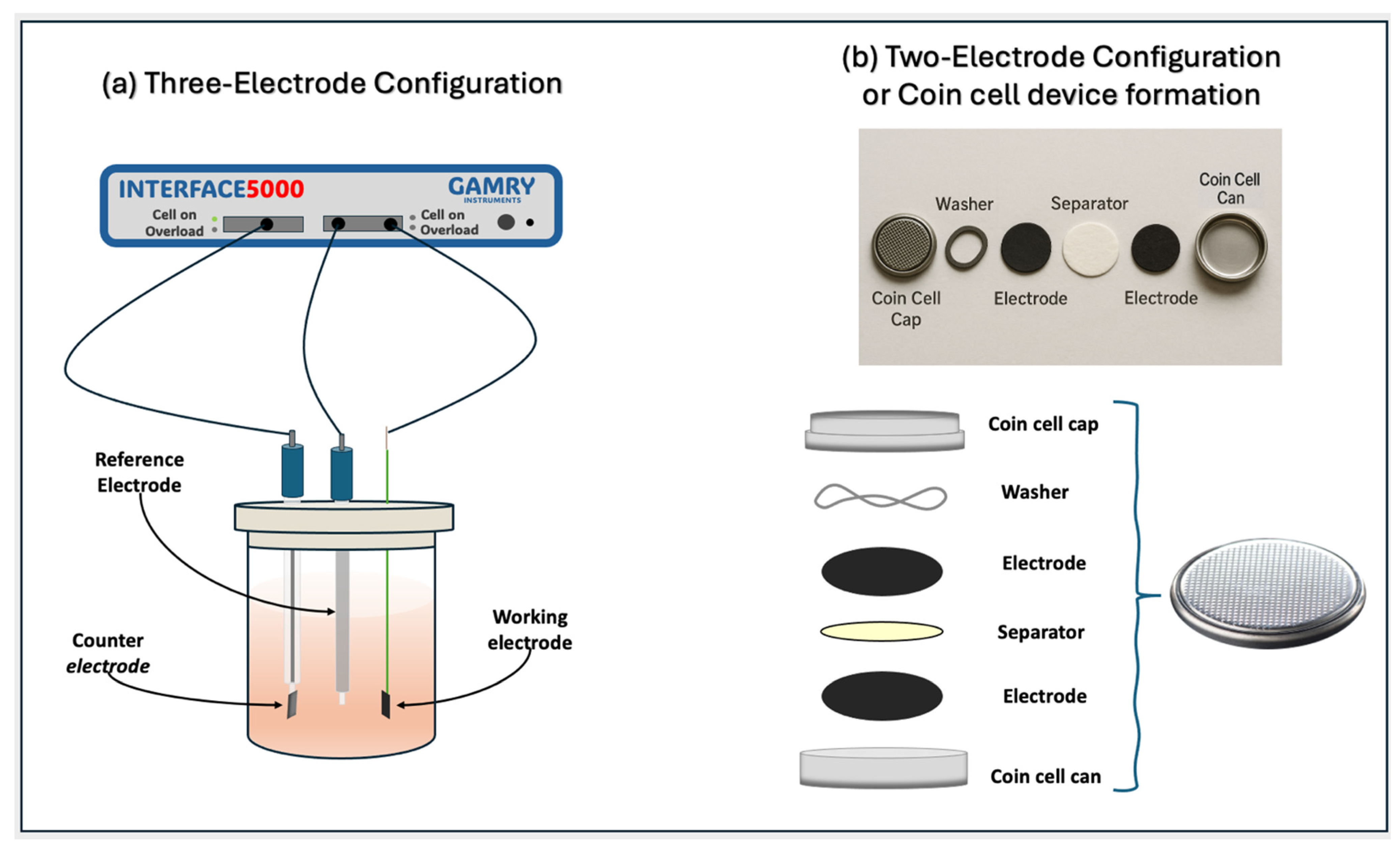
3.4. Electrochemical Characterization
3.4.1. Electrode Preparation
3.4.2. Standard Three-Electrode Configuration
3.4.3. Two-Electrode Configuration or Coin Cell Device Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Li, B.; Zheng, S.; Xu, Y.; Xue, H.; Pang, H. Syntheses and Energy Storage Applications of MxSy (M = Cu, Ag, Au) and Their Composites: Rechargeable Batteries and Supercapacitors. Adv. Funct. Mater. 2017, 27, 1703949. [Google Scholar] [CrossRef]
- Jeanmairet, G.; Rotenberg, B.; Salanne, M. Microscopic Simulations of Electrochemical Double-Layer Capacitors. Chem. Rev. 2022, 122, 10860–10898. [Google Scholar] [CrossRef]
- Hardianto, Y.P.; Shah, S.S.; Shuaibu, A.D.; Mohamed, M.; Sarker, S.; Alzahrani, A.S.; Aziz, A. Modeling supercapacitors with the simplified Randles circuit: Analyzing electrochemical behavior through cyclic voltammetry and Galvanostatic charge-discharge. Electrochim. Acta 2025, 513, 145552. [Google Scholar] [CrossRef]
- Takaloo, S.; Zand, M.M. Wearable electrochemical flexible biosensors: With the focus on affinity biosensors. Sens. Bio-Sens. Res. 2021, 32, 100403. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Yadav, R.M. Graphene-metal oxide hybrid materials with 2D and 3D morphologies for advanced supercapacitor electrodes: Status, challenges and prospects. Mater. Today Nano 2023, 24, 100399. [Google Scholar] [CrossRef]
- Dong, H.; Qiao, Y.; Peng, S.; Li, Y.; Zhen, Y.; Tan, W.; Cheng, Q.; Wang, Y. 2D material/epoxy composite coatings, a perspective from the regulation of 2D materials. Prog. Org. Coat. 2023, 183, 107817. [Google Scholar] [CrossRef]
- Shubha, B.S.; Praveen, B.M.; Bhat, V. Graphene-Calculation of Specific Surface Area. Int. J. Appl. Eng. Manag. Lett. 2023, 7, 91–97. [Google Scholar] [CrossRef]
- Wu, Z.S.; Parvez, K.; Feng, X.; Müllen, K. Graphene-based in-plane micro-supercapacitors with high power and energy densities. Nat. Commun. 2013, 4, 2487. [Google Scholar] [CrossRef]
- Faiz, M.S.A.; Azurahanim, C.A.C.; Raba’ah, S.A.; Ruzniza, M.Z. Low cost and green approach in the reduction of graphene oxide (GO) using palm oil leaves extract for potential in industrial applications. Results Phys. 2020, 16, 102954. [Google Scholar] [CrossRef]
- Gutić, S.J.; Kozlica, D.K.; Korać, F.; Bajuk-Bogdanović, D.; Mitrić, M.; Mirsky, V.M.; Mentus, S.V.; Pašti, I.A. Electrochemical tuning of capacitive response of graphene oxide. Phys. Chem. Chem. Phys. 2018, 20, 22698–22709. [Google Scholar] [CrossRef]
- Peng, Q.; Tan, X.; Stempień, Z.; Venkataraman, M.; Militky, J.; Kejzlar, P.; Korzeniewska, E. Inkjet printing of silver/graphene flexible composite electrodes for high-performance supercapacitors. Mater. Charact. 2024, 218, 114505. [Google Scholar] [CrossRef]
- Ma, L.; Shen, X.; Ji, Z.; Zhu, G.; Zhou, H. Ag nanoparticles decorated MnO2/reduced graphene oxide as advanced electrode materials for supercapacitors. Chem. Eng. J. 2014, 252, 95–103. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, Y.; Chen, R.; Wang, X. High performance fully paper-based all-solid-state supercapacitor fabricated by a papermaking process with silver nanoparticles and reduced graphene oxide-modified pulp fibers. EcoMat 2021, 3, 12076. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Sarno, M.; Casa, M. Green and one-step synthesis for Ag/graphene hybrid supercapacitor with remarkable performance. J. Phys. Chem. Solids 2018, 120, 241–249. [Google Scholar] [CrossRef]
- Kabiri, B.; Heidari, H. Synthesis and catalytic activity of silver- reduced graphene oxide and silver- magnetite- reduced graphene oxide nanocomposites in the reduction of 4-nitrophenol. Sci. Rep. 2025, 15, 14539. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Tian, C.; Yu, H.; He, J.; Song, K.; Guo, J.; Zhou, X.; Zhuo, O.; Liu, S. In Situ Green Synthesis of Graphene Oxide-Silver Nanoparticles Composite with Using Gallic Acid. Front. Chem. 2022, 10, 905781. [Google Scholar] [CrossRef]
- Ansari, A.R.; Ansari, S.A.; Parveen, N.; Ansari, M.O.; Osman, Z. Silver Nanoparticle Decorated on Reduced Graphene Oxide-Wrapped Manganese Oxide Nanorods as Electrode Materials for High-Performance Electrochemical Devices. Crystals 2022, 12, 389. [Google Scholar] [CrossRef]
- Mallouka, G.; Karkoutly, O.; Karzoun, O.; AlHannan, F.; Akhtar, S.; Henari, F.; Deen, G.R. Room temperature green synthesis and time resolved kinetics of formation of xanthan gum stabilised silver nanoparticles with catalytic and antibacterial potential. Sci. Rep. 2025, 15, 31626. [Google Scholar] [CrossRef]
- Shaban, M.; Mohamed, A.; Kordy, M.G.M.; AlMohamadi, H.; Eissa, M.F.; Hamdy, H. Design and Performance of CuNi-rGO and Ag-CuNi-rGO Composite Electrodes for Use in Fuel Cells. Catalysts 2024, 14, 551. [Google Scholar] [CrossRef]
- Fumoto, E.; Sato, S.; Kawamata, Y.; Koyama, Y.; Yoshikawa, T.; Nakasaka, Y.; Tago, T.; Masuda, T. Determination of carbonyl functional groups in lignin-derived fraction using infrared spectroscopy. Fuel 2022, 318, 123530. [Google Scholar] [CrossRef]
- Tiong, P.; Lintang, H.O.; Endud, S.; Yuliati, L. Improved interfacial charge transfer and visible light activity of reduced graphene oxide-graphitic carbon nitride photocatalysts. RSC Adv. 2015, 5, 94029–94039. [Google Scholar] [CrossRef]
- Fan, B.; Guo, H.; Shi, J.; Shi, C.; Jia, Y.; Wang, H.; Chen, D.; Yang, Y.; Lu, H.; Xu, H.; et al. Facile one-pot preparation of silver/reduced graphene oxide nanocomposite for cancer photodynamic and photothermal therapy. J. Nanosci. Nanotechnol. 2016, 16, 7049–7054. [Google Scholar] [CrossRef]
- Pai, A.; Vidyapeetham, A.V.; Pai, A.R.; Nair, B. Synthesis of Reduced Graphene Oxide Using Novel Exfoliation Technique and its Characterizations. J. Nano- Electron. Phys. 2013, 5, 02032-1. [Google Scholar]
- Kim, K.S.; Park, S.J. Influence of multi-walled carbon nanotubes on the electrochemical performance of graphene nanocomposites for supercapacitor electrodes. Electrochim. Acta 2011, 56, 1629–1635. [Google Scholar] [CrossRef]
- Dhoondia, Z.H.; Chakraborty, H. Lactobacillus Mediated Synthesis of Silver Oxide Nanoparticles Regular Paper. Nanomater. Nanotechnol. 2012, 2, 15. [Google Scholar] [CrossRef]
- Zikalala, N.E.; Azizi, S.; Mpeta, L.S.; Ahmed, R.; Dube, A.; Mketo, N.; Zinatizadeh, A.; Mokrani, T.; Maaza, M.M. Green synthesis of reduced graphene oxide using Persea americana mill. extract: Characterization, oxygen reduction reaction and antibacterial application. Diam. Relat. Mater. 2024, 149, 111560. [Google Scholar] [CrossRef]
- Jaworski, S.; Wierzbicki, M.; Sawosz, E.; Jung, A.; Gielerak, G.; Biernat, J.; Jaremek, H.; Łojkowski, W.; Woźniak, B.; Wojnarowicz, J.; et al. Graphene oxide-based nanocomposites decorated with silver nanoparticles as an antibacterial agent. Nanoscale Res. Lett. 2018, 13, 116. [Google Scholar] [CrossRef]
- Dai, Z.; Ren, P.; Guo, Z.; Hou, X.; He, W.; Ren, F.; Jin, Y. Silver nanoparticles as a conductive bridge for high-performance flexible all-solid-state asymmetric supercapacitor. Int. J. Energy Res. 2022, 46, 1813–1825. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.A.; Park, W.; Cao, H.; Chen, Y.P. Raman Spectroscopy of Graphene and Related Materials. In New Developments in Photon and Materials Research; NOVA Science Publishers: Hauppauge, NY, USA, 2013; pp. 403–418. [Google Scholar]
- Campos, J.L.E.; Miranda, H.; Rabelo, C.; Sandoz-Rosado, E.; Pandey, S.; Riikonen, J.; Cano-Marquez, A.G.; Jorio, A. Applications of Raman spectroscopy in graphene-related materials and the development of parameterized PCA for large-scale data analysis. J. Raman Spectrosc. 2018, 49, 54–65. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Kaur, R.; Avti, P.; Kumar, V.; Kumar, R. Effect of various synthesis parameters on the stability of size controlled green synthesis of silver nanoparticles. Nano Express 2021, 2, 020005. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Selvan, R.K.; Lee, Y.S.; Melo, J.S. Electric double layer capacitor and its improved specific capacitance using redox additive electrolyte. J. Mater. Chem. A 2013, 1, 1086–1095. [Google Scholar] [CrossRef]
- Bhujel, R.; Rai, S.; Baruah, K.; Deka, U.; Biswas, J.; Swain, B.P. Capacitive and Sensing Responses of Biomass Derived Silver Decorated Graphene. Sci. Rep. 2019, 9, 19725. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Zhai, L. Multiwall carbon nanotube supported poly(3,4-ethylenedioxythiophene)/manganese oxide nano-composite electrode for super-capacitors. Electrochim. Acta 2009, 54, 7148–7155. [Google Scholar] [CrossRef]
- Strimaitis, J.; Danquah, S.A.; Denize, C.F.; Pradhan, S.K.; Bahoura, M. The Effects of Graphene Oxide and Reduced Graphene Oxide Conductive Additives on Activated Carbon Supercapacitors. Processes 2022, 10, 2190. [Google Scholar] [CrossRef]
- Ray, A.; Roth, J.; Saruhan, B. Laser-Induced Interdigital Structured Graphene Electrodes Based Flexible Micro-Supercapacitor for Efficient Peak Energy Storage. Molecules 2022, 27, 329. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Zhao, X.; Qiao, Z.; Jung, J.; Zhu, Y.; Lu, Y.; Zhang, L.L.; MacDonald, A.H.; Ruoff, R.S. Capacitance of carbon-based electrical double-layer capacitors. Nat. Commun. 2014, 5, 3317. [Google Scholar] [CrossRef]
- Anagbonu, P.; Ghali, M.; Allam, A. Low-temperature green synthesis of few-layered graphene sheets from pomegranate peels for supercapacitor applications. Sci. Rep. 2023, 13, 15627. [Google Scholar] [CrossRef]
- Johra, F.T.; Jung, W.G. Hydrothermally reduced graphene oxide as a supercapacitor. Appl. Surf. Sci. 2015, 357, 1911–1914. [Google Scholar] [CrossRef]
- Ariyoshi, K.; Siroma, Z.; Mineshige, A.; Takeno, M.; Fukutsuka, T.; Abe, T.; Uchida, S. Electrochemical Impedance Spectroscopy Part 1: Fundamentals. Electrochemistry 2022, 90, 102007. [Google Scholar] [CrossRef]
- Laschuk, N.O.; Easton, E.B.; Zenkina, O.V. Reducing the resistance for the use of electrochemical impedance spectroscopy analysis in materials chemistry. R. Soc. Chem. 2021, 11, 27925–27936. [Google Scholar] [CrossRef]
- Wang, J.; Han, X.; Zhang, S.; Hou, H.; Wei, C.; Liu, C.; Cao, L.; Zhang, J.; Wang, L.; Zhang, S. Cu-Ion Hybrid Porous Carbon with Nanoarchitectonics Derived from Heavy-Metal-Contaminated Biomass as Ultrahigh-Performance Supercapacitor. Int. J. Mol. Sci. 2025, 26, 569. [Google Scholar] [CrossRef] [PubMed]
- Pototskaya, V.V.; Gichan, O.I. On the origin of phase angle in warburg finite length diffusion impedance. Int. J. Electrochem. Sci. 2019, 14, 8195–8205. [Google Scholar] [CrossRef]
- Bissett, M.A.; Kinloch, I.A.; Dryfe, R.A.W. Characterization of MoS2-Graphene Composites for High-Performance Coin Cell Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 17388–17398. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors; Springer: New York, NY, USA, 1999. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy─A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193, Erratum in ACS Meas. Sci. Au 2025, 5, 156. [Google Scholar] [CrossRef]
- Lau, K.S.; Tan, S.T.; Ginting, R.T.; Khiew, P.S.; Chin, S.X.; Chia, C.H. A mechanistic study of silver nanostructure incorporating reduced graphene oxide: Via a flow synthesis approach. New J. Chem. 2020, 44, 1439–1445. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Yin, Z.; Zhao, J.; Song, M.; Wu, Z.; Li, H.; Wang, X. Ag nanoparticles decorated N/S dual-doped graphene nanohybrids for high-performance asymmetric supercapacitors. Carbon 2020, 161, 726–735. [Google Scholar] [CrossRef]
- Elsalam, A.A.; Selim, A.M.; Tash, M.; Khalifa, W.; El-Mahallawi, I. Silver assisted PANI-RGO: A Cutting-Edge approach to supercapacitor applications. Micro Nanostructures 2024, 189, 207821. [Google Scholar] [CrossRef]
- Belessi, V.; Koutsioukis, A.; Giasafaki, D.; Philippakopoulou, T.; Panagiotopoulou, V.; Mitzithra, C.; Kripotou, S.; Manolis, G.; Steriotis, T.; Charalambopoulou, G.; et al. One-Pot Synthesis of Functionalised rGO/AgNPs Hybrids as Pigments for Highly Conductive Printing Inks. Nanomaterials 2024, 14, 859. [Google Scholar] [CrossRef]
- Farbod, M.; Shojaeenezhad, S.S. A three-dimensional Ag nanoparticle/graphene hydrogel composite and its application as an improved supercapacitor’s electrode. J. Solid State Electrochem. 2019, 23, 3009–3017. [Google Scholar] [CrossRef]
- Ates, M.; Caliskan, S.; Ozten, E. Preparation of rGO/Ag/PEDOT nanocomposites for supercapacitors. Mater. Technol. 2018, 33, 872–883. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, J.; Shinya, N.; Qin, L.C. Hybrid graphene electrodes for supercapacitors of high energy density. Chem. Phys. Lett. 2013, 584, 124–129. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]


| Composite | Rs [Ω] | Rct [Ω] | Cdl [µF] | Leakage Current [μA] |
|---|---|---|---|---|
| rGO/Ag 1.0 M | 1.057 | 3.187 | 49.940 | 313.70 |
| rGO/Ag 0.1 M | 0.930 | 3.734 | 42.620 | 267.80 |
| rGO/Ag 0.01 M | 0.626 | 4.516 | 35.240 | 221.40 |
| Composite | Rs [Ω] | Rct [Ω] | Cdl [µF] | Leakage Current [μA] |
|---|---|---|---|---|
| rGO/Ag 1.0 M | 4.900 | 27.40 | 250.5 | 36.50 |
| rGO/Ag 0.1 M | 7.630 | 39.50 | 29.00 | 25.32 |
| rGO/Ag 0.01 M | 7.060 | 128.5 | 700.8 | 7.78 |
| Materials | Cs | Electrolyte | Potential Window | Reference |
|---|---|---|---|---|
| rGO 0.01% Ag 0.1% Ag | 209.32 Fg−1 215.62 Fg−1 at 1 Ag−1 | 6 M KOH | [−1.0–0.0] | [51] |
| AgNSrGO35 | 923.3 Fg−1 at 1 Ag−1 | 6 M KOH | [−1.2–0.0] | [52] |
| rGO-Ag PANI rGO.Ag | 296.7 Fg−1 at 5 mVs−1 140 Fg−1 at 0.5 Ag−1 385.4 Fg−1 at 5 mVs−1 194.4 Fg−1 0.5 Ag−1 | 6 M KOH | [0.0–1.0] | [53] |
| rGO/Ag 1.0 M | 392 Fg−1 at 5 mVs−1 19 Fg−1 coin cell at 5 mVs−1 At 1 Ag−1 | 2 M KOH | [−1.0–0.0] | This work |
| rGO/Ag 0.1 M | 236 Fg−1 at 5 mVs−1 10 Fg−1 coin cell at 5 mVs−1 At 1 Ag−1 | 2 M KOH | [−1.0–0.0] | This work |
| rGO/Ag 0.01 M | 229 Fg−1 5.4 Fg−1 coin cell at 5 mVs−1 At 1 Ag−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orellana, B.; Vivas, L.; Manquian, C.; Brito, T.P.; Singh, D.P. Ecosynthesis and Optimization of Nano rGO/Ag-Based Electrode Materials for Superior Supercapacitor Coin Cell Devices. Int. J. Mol. Sci. 2025, 26, 9578. https://doi.org/10.3390/ijms26199578
Orellana B, Vivas L, Manquian C, Brito TP, Singh DP. Ecosynthesis and Optimization of Nano rGO/Ag-Based Electrode Materials for Superior Supercapacitor Coin Cell Devices. International Journal of Molecular Sciences. 2025; 26(19):9578. https://doi.org/10.3390/ijms26199578
Chicago/Turabian StyleOrellana, Belen, Leonardo Vivas, Carolina Manquian, Tania P. Brito, and Dinesh P. Singh. 2025. "Ecosynthesis and Optimization of Nano rGO/Ag-Based Electrode Materials for Superior Supercapacitor Coin Cell Devices" International Journal of Molecular Sciences 26, no. 19: 9578. https://doi.org/10.3390/ijms26199578
APA StyleOrellana, B., Vivas, L., Manquian, C., Brito, T. P., & Singh, D. P. (2025). Ecosynthesis and Optimization of Nano rGO/Ag-Based Electrode Materials for Superior Supercapacitor Coin Cell Devices. International Journal of Molecular Sciences, 26(19), 9578. https://doi.org/10.3390/ijms26199578








