Comprehensive Review of Silver Nanoparticles in Food Packaging Applications
Abstract
1. Introduction
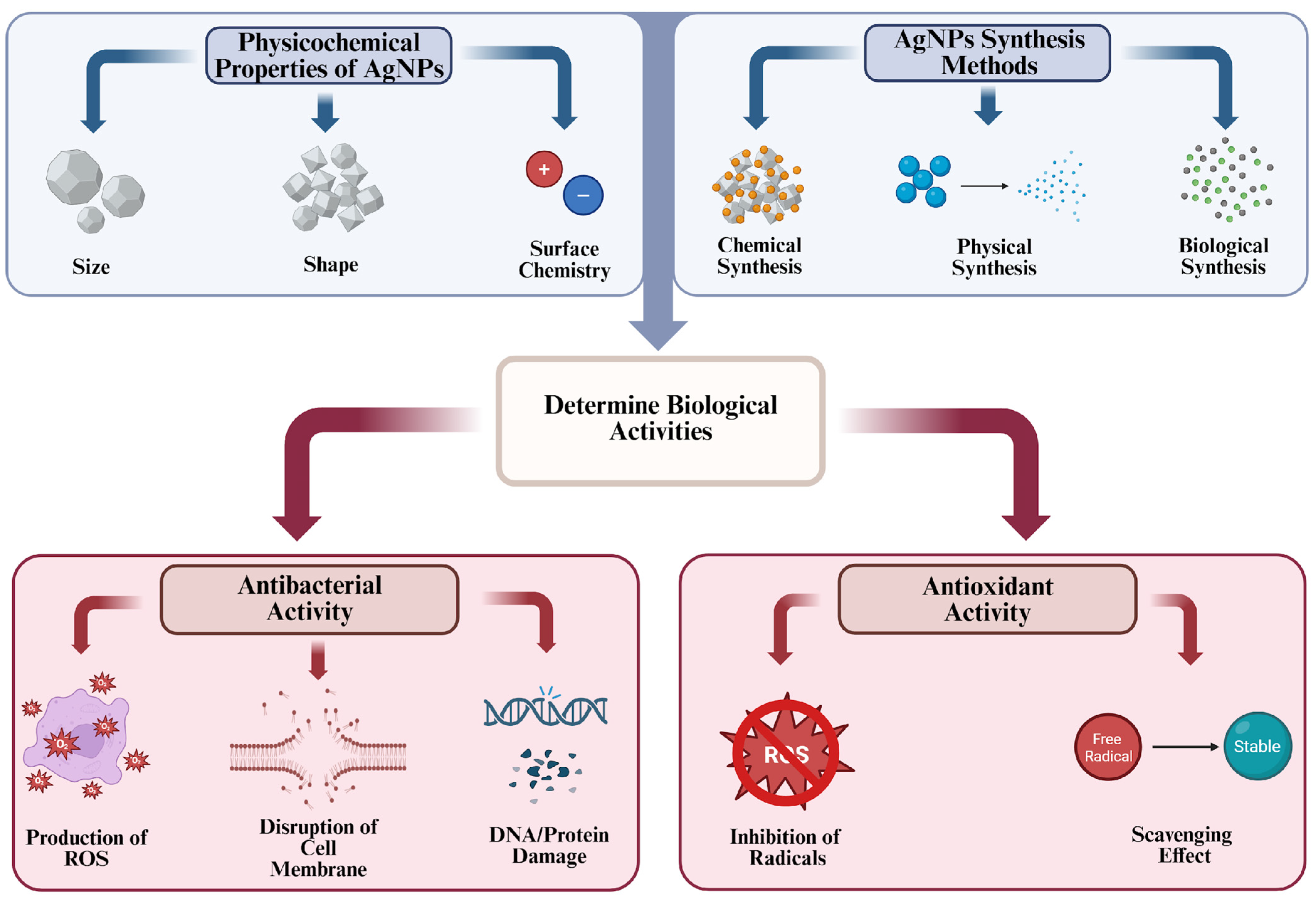
2. Protective Properties of AgNPs: Antibacterial and Antioxidant Activities
2.1. Antibacterial Properties
2.1.1. Mechanism Insight
2.1.2. Factors Affecting Antibacterial Activity of AgNPs
Size
Shape
Surface Chemistry
2.2. Antibiofilm Properties
2.3. Antioxidant Properties
3. Food Packaging Applications of AgNPs in Various Food Types
3.1. Fruit
3.2. Vegetable
3.3. Meat
3.4. Others
3.4.1. Dairy
3.4.2. Bakery
4. Toxicity Concerns and Biocompatibility of AgNPs-Based Nanocomposites
5. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, W.; Ouyang, Z.; Zhang, Y.; Lu, Y.; Wei, C.; Tu, Y.; He, B. Research Progress on the Artificial Intelligence Applications in Food Safety and Quality Management. Trends Food Sci. Technol. 2025, 156, 104855. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased Materials for Active Food Packaging: A Review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Ahari, H.; Soufiani, S.P. Smart and Active Food Packaging: Insights in Novel Food Packaging. Front. Microbiol. 2021, 12, 657233. [Google Scholar] [CrossRef]
- Kumar, Y.; Bist, Y.; Thakur, D.; Nagar, M.; Saxena, D.C. A Review on the Role of pH-Sensitive Natural Pigments in Biopolymers Based Intelligent Food Packaging Films. Int. J. Biol. Macromol. 2024, 276, 133869. [Google Scholar] [CrossRef]
- El Guerraf, A.; Jadi, S.B.; Ziani, I.; Dalli, M.; Sher, F.; Bazzaoui, M.; Bazzaoui, E.A. Multifunctional Smart Conducting Polymers–Silver Nanocomposites-Modified Biocellulose Fibers for Innovative Food Packaging Applications. Ind. Eng. Chem. Res. 2023, 62, 4540–4553. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Łopusiewicz, Ł.; Merino, D.; Sani, M.A.; Kalita, P.; Roy, S.; Yang, T.; Fei, T.; Zhang, W. High-Performance Ethyl Cellulose Composite Films: Innovations in Functional Enhancement for Sustainable Food Packaging. Carbohydr. Polym. 2025, 368, 124154. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Song, J.; Xu, F.; Yun, D.; Li, C.; Liu, J. Characterization and Application of Guar Gum/Polyvinyl Alcohol-Based Food Packaging Films Containing Betacyanins from Pokeweed (Phytolacca acinosa Roxb.) Berries and Silver Nanoparticles. Molecules 2023, 28, 6243. [Google Scholar] [CrossRef] [PubMed]
- Shavisi, N. Electrospun Fiber Mats Based on Chitosan-Carrageenan Containing Malva Sylvestris Anthocyanins: Physic-Mechanical, Thermal, and Barrier Properties along with Application as Intelligent Food Packaging Materials. Int. J. Biol. Macromol. 2024, 266, 131077. [Google Scholar] [CrossRef]
- Hu, Y.; Li, T. Chapter One—Smart Food Packaging: Recent Advancement and Trends. In Advances in Food and Nutrition Research; Lu, X., Ed.; Smart Food Safety; Academic Press: Cambridge, MA, USA, 2024; Volume 111, pp. 1–33. [Google Scholar]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- González-Fernández, S.; Blanco-Agudín, N.; Rodríguez, D.; Fernández-Vega, I.; Merayo-Lloves, J.; Quirós, L.M. Silver Nanoparticles: A Versatile Tool Against Infectious and Non-Infectious Diseases. Antibiotics 2025, 14, 289. [Google Scholar] [CrossRef]
- Istiqola, A.; Syafiuddin, A. A Review of Silver Nanoparticles in Food Packaging Technologies: Regulation, Methods, Properties, Migration, and Future Challenges. J. Chin. Chem. Soc. 2020, 67, 1942–1956. [Google Scholar] [CrossRef]
- Alavi, M.; Hamblin, M.R. Antibacterial Silver Nanoparticles: Effects on Bacterial Nucleic Acids. Cell. Mol. Biomed. Rep. 2023, 3, 35–40. [Google Scholar] [CrossRef]
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. J. Nanomater. 2021, 2021, 6687290. [Google Scholar] [CrossRef]
- Nguyen, N.P.U.; Dang, N.T.; Doan, L.; Nguyen, T.T.H. Synthesis of Silver Nanoparticles: From Conventional to ‘Modern’ Methods—A Review. Processes 2023, 11, 2617. [Google Scholar] [CrossRef]
- Prasad Panthi, K.; Panda, C.; Mohan Pandey, L.; Lal Sharma, M.; Kumar Joshi, M. Bio-interfacial insights of nanoparticles integrated plant protein-based films for sustainable food packaging applications. Food Rev. Int. 2025, 41, 1948–1980. [Google Scholar] [CrossRef]
- Hong, S.-I.; Cho, Y.; Rhim, J.-W. Effect of Agar/AgNP Composite Film Packaging on Refrigerated Beef Loin Quality. Membranes 2021, 11, 750. [Google Scholar] [CrossRef] [PubMed]
- Rievaj, M.; Culková, E.; Šandorová, D.; Durdiak, J.; Bellová, R.; Tomčík, P. A Review of Analytical Techniques for the Determination and Separation of Silver Ions and Its Nanoparticles. Nanomaterials 2023, 13, 1262. [Google Scholar] [CrossRef] [PubMed]
- Kedar, K.; Nayak, S.; Bhaskar, V.H. Synthesis of Silver Nanoparticles by Chemical Reduction Method. Int. J. Pharm. Pharm. Res. 2022, 25, 364–376. [Google Scholar]
- Harun-Ur-Rashid, M.; Foyez, T.; Naidu Krishna, S.B.; Poda, S.; Bin Imran, A. Recent Advances of Silver Nanoparticle-Based Polymer Nanocomposites for Biomedical Applications. RSC Adv. 2025, 15, 8480–8505. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.P.; Otoni, C.G.; Soares, N.F.F. Chapter 37—Zinc Oxide Nanoparticles for Food Packaging Applications. In Antimicrobial Food Packaging, 2nd ed.; Barros-Velázquez, J., Ed.; Academic Press: San Diego, CA, USA, 2025; pp. 603–610. ISBN 978-0-323-90747-7. [Google Scholar]
- Stuparu-Cretu, M.; Braniste, G.; Necula, G.-A.; Stanciu, S.; Stoica, D.; Stoica, M. Metal Oxide Nanoparticles in Food Packaging and Their Influence on Human Health. Foods 2023, 12, 1882. [Google Scholar] [CrossRef] [PubMed]
- Natsuki, J.; Natsuki, T.; Hashimoto, Y. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Appl. 2015, 4, 325–332. [Google Scholar] [CrossRef]
- Granja Alvear, A.; Pineda-Aguilar, N.; Lozano, P.; Lárez-Velázquez, C.; Suppan, G.; Galeas, S.; Debut, A.; Vizuete, K.; De Lima, L.; Saucedo-Vázquez, J.P.; et al. Synergistic Antibacterial Properties of Silver Nanoparticles and Its Reducing Agent from Cinnamon Bark Extract. Bioengineering 2024, 11, 517. [Google Scholar] [CrossRef]
- Tripathi, N.; Goshisht, M.K. Recent Advances and Mechanistic Insights into Antibacterial Activity, Antibiofilm Activity, and Cytotoxicity of Silver Nanoparticles. ACS Appl. Bio Mater. 2022, 5, 1391–1463. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial Activity of Silver Nanoparticles: A Surface Science Insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Crisan, C.M.; Mocan, T.; Manolea, M.; Lasca, L.I.; Tăbăran, F.-A.; Mocan, L. Review on Silver Nanoparticles as a Novel Class of Antibacterial Solutions. Appl. Sci. 2021, 11, 1120. [Google Scholar] [CrossRef]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver Nanoparticles and Silver Ions as Potential Antibacterial Agents. J. Inorg. Organomet. Polym. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, G.C.; Schito, A.M.; Zuccari, G. Reactive Oxygen Species (ROS)-Mediated Antibacterial Oxidative Therapies: Available Methods to Generate ROS and a Novel Option Proposal. Int. J. Mol. Sci. 2024, 25, 7182. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-Induced Lipid Peroxidation Modulates Cell Death Outcome: Mechanisms behind Apoptosis, Autophagy, and Ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Urnukhsaikhan, E.; Bold, B.-E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial Activity and Characteristics of Silver Nanoparticles Biosynthesized from Carduus Crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Du, J.; Tang, J.; Xu, S.; Ge, J.; Dong, Y.; Li, H.; Jin, M. A Review on Silver Nanoparticles-Induced Ecotoxicity and the Underlying Toxicity Mechanisms. Regul. Toxicol. Pharmacol. 2018, 98, 231–239. [Google Scholar] [CrossRef]
- Kong, I.C.; Ko, K.-S.; Koh, D.-C. Evaluation of the Effects of Particle Sizes of Silver Nanoparticles on Various Biological Systems. Int. J. Mol. Sci. 2020, 21, 8465. [Google Scholar] [CrossRef]
- Martínez-Castañón, G.A.; Niño-Martínez, N.; Martínez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and Antibacterial Activity of Silver Nanoparticles with Different Sizes. J. Nanopart. Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- Agrawal, N.; Mishra, P.; Ranjan, R.; Awasthi, P.; Srivastava, A.; Prasad, D.; Kohli, E. Nano-Cubes over Nano-Spheres: Shape Dependent Study of Silver Nanomaterial for Biological Applications. Bull. Mater. Sci. 2021, 44, 191. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-Dependent Antimicrobial Activities of Silver Nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef]
- Samal, D.; Khandayataray, P.; Sravani, M.; Murthy, M.K. Silver Nanoparticle Ecotoxicity and Phytoremediation: A Critical Review of Current Research and Future Prospects. Envrion. Sci. Pollut. Res. 2024, 31, 8400–8428. [Google Scholar] [CrossRef]
- Haidari, H.; Bright, R.; Kopecki, Z.; Zilm, P.S.; Garg, S.; Cowin, A.J.; Vasilev, K.; Goswami, N. Polycationic Silver Nanoclusters Comprising Nanoreservoirs of Ag+ Ions with High Antimicrobial and Antibiofilm Activity. ACS Appl. Mater. Interfaces 2022, 14, 390–403. [Google Scholar] [CrossRef]
- Soni, J.F.; Ribeiro, V.S.T.; Cieslinski, J.; de Andrade, A.P.; Dantas, L.R.; Pereira, B.Z.; Almeida, B.M.R.C.; de Suss, P.H.; Tuon, F.F. Evaluation of Silver Nanoparticle-Impregnated PMMA Loaded with Vancomycin or Gentamicin against Bacterial Biofilm Formation. Injury 2023, 54, 110649. [Google Scholar] [CrossRef]
- Porter, G.C.; Tompkins, G.R.; Schwass, D.R.; Li, K.C.; Waddell, J.N.; Meledandri, C.J. Anti-Biofilm Activity of Silver Nanoparticle-Containing Glass Ionomer Cements. Dent. Mater. 2020, 36, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Ballal, A.; Rath, D.; Rath, A. Novel Silver Nanoparticle-Antibiotic Combinations as Promising Antibacterial and Anti-Biofilm Candidates against Multiple-Antibiotic Resistant ESKAPE Microorganisms. Colloids Surf. B Biointerfaces 2024, 236, 113826. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Wang, H.; Zhu, M.; Feng, W.; Liang, G. Enhanced Antibacterial and Anti-Biofilm Activities of Antimicrobial Peptides Modified Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 4831–4846. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Prasad, A.S.; Kumar, R. Anti-Inflammatory and Antioxidant Activity of Neem and Kirata-Induced Silver Nanoparticles Against Oral Biofilm: An In Vitro Study. Cureus 2024, 16, e67708. [Google Scholar] [CrossRef] [PubMed]
- Dube, E.; Okuthe, G.E. Silver Nanoparticle-Based Antimicrobial Coatings: Sustainable Strategies for Microbial Contamination Control. Microbiol. Res. 2025, 16, 110. [Google Scholar] [CrossRef]
- Naskar, A.; Khan, H.; Sarkar, R.; Kumar, S.; Halder, D.; Jana, S. Anti-Biofilm Activity and Food Packaging Application of Room Temperature Solution Process Based Polyethylene Glycol Capped Ag-ZnO-Graphene Nanocomposite. Mater. Sci. Eng. C 2018, 91, 743–753. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Alharthi, M.A.; Alotaibi, A.S.; Alenzi, A.M.; Albalawi, D.A.; Makharita, R.R. Biogenic Nanoparticles Silver and Copper and Their Composites Derived from Marine Alga Ulva Lactuca: Insight into the Characterizations, Antibacterial Activity, and Anti-Biofilm Formation. Molecules 2023, 28, 6324. [Google Scholar] [CrossRef]
- Gecer, E.N. Green Synthesis of Silver Nanoparticles from Salvia aethiopis L. and Their Antioxidant Activity. J. Inorg. Organomet. Polym. 2021, 31, 4402–4409. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Mehwish, H.M.; Zhang, H.; Ashraf, M.; Fang, H.; Zeng, X.; Wu, Y.; Khurshid, M.; Zhao, L.; He, Z. Antibacterial and Antioxidant Activity of Exopolysaccharide Mediated Silver Nanoparticle Synthesized by Lactobacillus brevis Isolated from Chinese Koumiss. Colloids Surf. B Biointerfaces 2020, 186, 110734. [Google Scholar] [CrossRef] [PubMed]
- Redolfi-Bristol, D.; Yamamoto, K.; Marin, E.; Zhu, W.; Mazda, O.; Riello, P.; Pezzotti, G. Exploring the Cellular Antioxidant Mechanism against Cytotoxic Silver Nanoparticles: A Raman Spectroscopic Analysis. Nanoscale 2024, 16, 9985–9997. [Google Scholar] [CrossRef]
- Sritong, N.; Chumsook, S.; Siri, S. Light Emitting Diode Irradiation Induced Shape Conversion of DNA-Capped Silver Nanoparticles and Their Antioxidant and Antibacterial Activities. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 955–963. [Google Scholar] [CrossRef]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and Antibacterial Activity of Silver Nanoparticles Synthesized by Cestrum Nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef]
- Elsaffany, A.H.; Abdelaziz, A.E.M.; Zahra, A.A.; Mekky, A.E. Green Synthesis of Silver Nanoparticles Using Cocoon Extract of Bombyx mori L.: Therapeutic Potential in Antibacterial, Antioxidant, Anti-Inflammatory, and Anti-Tumor Applications. BMC Biotechnol. 2025, 25, 38. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.A.; Bhatia, S.; Al-Harrasi, A.; Afzaal, M.; Saeed, F.; Anwer, M.K.; Khan, M.R.; Jawad, M.; Akram, N.; Faisal, Z. Mechanical Properties of Protein-Based Food Packaging Materials. Polymers 2023, 15, 1724. [Google Scholar] [CrossRef]
- Pandey, V.; Kumar, M. Development of a Simple Model for Size and Shape-Dependent Young’s Modulus for Nanomaterials. Pramana—J. Phys. 2023, 97, 88. [Google Scholar] [CrossRef]
- Ezati, P.; Khan, A.; Priyadarshi, R.; Bhattacharya, T.; Tammina, S.K.; Rhim, J.-W. Biopolymer-Based UV Protection Functional Films for Food Packaging. Food Hydrocoll. 2023, 142, 108771. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Physical and Mechanical Characteristics of Gelatin-Based Films as a Potential Food Packaging Material: A Review. Membranes 2022, 12, 442. [Google Scholar] [CrossRef]
- Ciftci, F. Bioadhesion, Antimicrobial Activity, and Biocompatibility Evaluation Bacterial Cellulose Based Silver Nanoparticle Bioactive Composite Films. Process Biochem. 2024, 137, 99–110. [Google Scholar] [CrossRef]
- Hasan, K.F.; Xiaoyi, L.; Shaoqin, Z.; Horváth, P.G.; Bak, M.; Bejó, L.; Sipos, G.; Alpár, T. Functional Silver Nanoparticles Synthesis from Sustainable Point of View: 2000 to 2023—A Review on Game Changing Materials. Heliyon 2020, 8, e12322. [Google Scholar] [CrossRef]
- Fu, Y.; Dudley, E.G. Antimicrobial-Coated Films as Food Packaging: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3404–3437. [Google Scholar] [CrossRef]
- Shankar, S.; Bang, Y.-J.; Rhim, J.-W. Antibacterial LDPE/GSE/Mel/ZnONP Composite Film-Coated Wrapping Paper for Convenience Food Packaging Application. Food Packag. Shelf Life 2019, 22, 100421. [Google Scholar] [CrossRef]
- Yazdi, J.S.; Salari, M.; Ehrampoush, M.H.; Bakouei, M. Development of Active Chitosan Film Containing Bacterial Cellulose Nanofibers and Silver Nanoparticles for Bread Packaging. Food Sci. Nutr. 2024, 12, 8186–8199. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fang, C.; Wei, N.; Wei, J.; Feng, T.; Liu, F.; Liu, X.; Wu, B. Antimicrobial, Antioxidative, and UV-Blocking Pectin/Gelatin Food Packaging Films Incorporated with Tannic Acid and Silver Nanoparticles for Strawberry Preservation. Int. J. Biol. Macromol. 2025, 308, 142445. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tian, R.; Zhou, J.; Liu, Y. Multifunctional Chitosan/Grape Seed Extract/Silver Nanoparticle Composite for Food Packaging Application. Int. J. Biol. Macromol. 2022, 207, 152–160. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, N.; Kaushal, N. Utilization of Novel Bacteriocin Synthesized Silver Nanoparticles (AgNPs) for Their Application in Antimicrobial Packaging for Preservation of Tomato Fruit. Front. Sustain. Food Syst. 2023, 7, 1072738. [Google Scholar] [CrossRef]
- Mao, M.; Wang, J.; Hu, S.; Zou, M.; Meng, Y.; Bao, W.; Zhang, Q.; Peng, C. Lipopeptide of Bacillus Velezensis Incorporated with Silver Nanoparticles into a Functional Coating for Sweet Cherry Preservation. Food Packag. Shelf Life 2025, 49, 101537. [Google Scholar] [CrossRef]
- Yang, Z.; Wen, T.; Peng, F. Integrating Green Synthesized Lichenan-Silver Nanoparticles into Lichenan/Chitosan Matrix for Photo-Responsive Multifunctional Food Packaging Application. Chem. Eng. J. 2025, 514, 163197. [Google Scholar] [CrossRef]
- Kowsalya, E.; MosaChristas, K.; Balashanmugam, P.; Manivasagan, V.; Devasena, T.; Jaquline, C.R.I. Sustainable Use of Biowaste for Synthesis of Silver Nanoparticles and Its Incorporation into Gelatin-Based Nanocomposite Films for Antimicrobial Food Packaging Applications. J. Food Process Eng. 2021, 44, e13641. [Google Scholar] [CrossRef]
- Ediyilyam, S.; George, B.; Shankar, S.S.; Dennis, T.T.; Wacławek, S.; Černík, M.; Padil, V.V.T. Chitosan/Gelatin/Silver Nanoparticles Composites Films for Biodegradable Food Packaging Applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef] [PubMed]
- Pandian, H.; Senthilkumar, K.; Naveenkumar, M.; Samraj, S. Azadirachta indica Leaf Extract Mediated Silver Nanoparticles Impregnated Nano Composite Film (AgNP/MCC/Starch/Whey Protein) for Food Packaging Applications. Environ. Res. 2023, 216, 114641. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Hu, X.; Chelliah, R.; Oh, D.-H.; Kathiresan, K.; Wang, M.-H. Biogenic Silver Nanoparticles-Polyvinylpyrrolidone Based Glycerosomes Coating to Expand the Shelf Life of Fresh-Cut Bell Pepper (Capsicum annuum L. Var. Grossum (L.) Sendt). Postharvest Biol. Technol. 2020, 160, 111039. [Google Scholar] [CrossRef]
- Singh, M.; Sahareen, T. Investigation of Cellulosic Packets Impregnated with Silver Nanoparticles for Enhancing Shelf-Life of Vegetables. LWT 2017, 86, 116–122. [Google Scholar] [CrossRef]
- Rasheed, S.; Amin, A.; Sarwar, A.; Saleem, H.G.M.; Hassan, A. Evaluation of Antimicrobial Effect of Silver Nanoparticle Based Whey Emulsions and Edible Films for the Extension of Shelf Life of Fruits and Vegetables. Curr. Microbiol. 2023, 80, 158. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hashim, S.B.H.; Arslan, M.; Zhang, K.; Siman, L.; Mukhtar, A.; Zhihua, L.; Tahir, H.E.; Zhai, X.; Shishir, M.R.I.; et al. Development of an Active Biogenic Silver Nanoparticles Composite Film Based on Berry Wax and Chitosan for Rabbit Meat Preservation. Int. J. Biol. Macromol. 2024, 275, 133128. [Google Scholar] [CrossRef]
- Gasti, T.; Dixit, S.; Shastri, L.A.; Mudigoudra, B.S.; Chougale, R.B.; Masti, S.P. One-Pot Fabrication of Chitosan/Poly(Vinyl Alcohol) Films with Spondias Pinnata Fruit Extract-Mediated Silver Nanoparticles for Meat Preservation. ACS Food Sci. Technol. 2025, 5, 2430–2443. [Google Scholar] [CrossRef]
- Aouay, M.; Haddar, A.; Sellami, E.; Magnin, A.; Putaux, J.-L.; Boufi, S. Cellulose Nanocrystal-Supported Silver Nanoparticles as an Antibacterial Additive for PVA and PLLA Matrices in Meat Packaging. RSC Adv. 2025, 15, 15893–15903. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wei, N.; Wei, J.; Fang, C.; Feng, T.; Liu, F.; Liu, X.; Wu, B. Curcumin and Silver Nanoparticles Loaded Antibacterial Multifunctional Pectin/Gelatin Films for Food Packaging Applications. Int. J. Biol. Macromol. 2024, 266, 131248. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, W.; Pang, C.; Deng, W.; Xu, C.; Wang, X. Multifunctional Chitosan-Based Coating with Liposomes Containing Laurel Essential Oils and Nanosilver for Pork Preservation. Food Chem. 2019, 295, 16–25. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Shi, J.; Huang, X.; Sun, Z.; Zhang, D.; Zou, X.; Sun, Y.; Zhang, J.; Holmes, M.; et al. A Colorimetric Hydrogen Sulfide Sensor Based on Gellan Gum-Silver Nanoparticles Bionanocomposite for Monitoring of Meat Spoilage in Intelligent Packaging. Food Chem. 2019, 290, 135–143. [Google Scholar] [CrossRef]
- Cao, Y.; Song, Z.; Dong, C.; Zhang, L.; Yu, Q.; Han, L. Potato Oxidized Hydroxypropyl Starch/Pectin-Based Indicator Film with Clitoria Ternatea Anthocyanin and Silver Nanoparticles for Monitoring Chilled Beef Freshness. Int. J. Biol. Macromol. 2024, 273, 133106. [Google Scholar] [CrossRef]
- Kumari, S.; Kumari, A.; Sharma, R. Safe and Sustainable Food Packaging: Argemone albiflora Mediated Green Synthesized Silver-Carrageenan Nanocomposite Films. Int. J. Biol. Macromol. 2024, 264, 130626. [Google Scholar] [CrossRef]
- Pouyamanesh, M.; Ahari, H.; Anvar, A.A.; Karim, G. Packaging Based on Ag-Low Density Polyethylene for Shelf-Life Extension of Pasteurized and Traditional Butters at Refrigerated Temperature. Food Sci. Technol. 2021, 42, e67020. [Google Scholar] [CrossRef]
- Braun, S.; Ilberg, V.; Blum, U.; Langowski, H.-C. Nanosilver in Dairy Applications—Antimicrobial Effects on Streptococcus Thermophilus and Chemical Interactions. Int. J. Dairy Technol. 2020, 73, 376–383. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Chandel, M.; Gupta, V.; Kaur, K.; Patel, A.; Kaur, K.; Kishore, A.; Prabhakar, P.K.; Singh, A.; Shankar Prasad, J.; et al. Valorisation of Fruit Peel Bioactive into Green Synthesized Silver Nanoparticles to Modify Cellulose Wrapper for Shelf-Life Extension of Packaged Bread. Food Res. Int. 2023, 164, 112321. [Google Scholar] [CrossRef]
- Nguyen, C.T.K.; Tran, K.Q.; Nguyen, A.T.L.; Dang, L.L.P.; Le, H.N.; Do, K.D.; Duong, Q.X.; Do, H.H.; Nguyen, V.D.; Ngo, T.H.A. Nanoarchitectonics of Antibacterial Bio-Packaging Film from Grapefruit Peel-Derived Low Methoxy Pectin Integrated with Silver Nanoparticles. J. Polym. Res. 2025, 32, 90. [Google Scholar] [CrossRef]
- Kumari, S.C.; Padma, P.N.; Anuradha, K. Green Silver Nanoparticles Embedded in Cellulosic Network for Fresh Food Packaging. J. Pure Appl. Microbiol. 2021, 15, 1236–1244. [Google Scholar] [CrossRef]
- Shankar, S.; Khodaei, D.; Lacroix, M. Effect of Chitosan/Essential Oils/Silver Nanoparticles Composite Films Packaging and Gamma Irradiation on Shelf Life of Strawberries. Food Hydrocoll. 2021, 117, 106750. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Yang, J.-X.; Liu, E.-J.; Hu, R.-Z.; Yao, X.-H.; Chen, T.; Zhao, W.-G.; Liu, L.; Fu, Y.-J. Soft and Elastic Silver Nanoparticle-Cellulose Sponge as Fresh-Keeping Packaging to Protect Strawberries from Physical Damage and Microbial Invasion. Int. J. Biol. Macromol. 2022, 211, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Begum, T.; Follett, P.A.; Shankar, S.; Moskovchenko, L.; Salmieri, S.; Lacroix, M. Evaluation of Bioactive Low-Density Polyethylene (LDPE) Nanocomposite Films in Combined Treatment with Irradiation on Strawberry Shelf-Life Extension. J. Food Sci. 2023, 88, 2141–2161. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.-C.; Ficai, A.; Ene, V.-L.; Vasile, B.-S.; Andronescu, E.; Holban, A.-M. Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil–Innovative Packaging for Cheese. Nanomaterials 2021, 11, 2377. [Google Scholar] [CrossRef] [PubMed]
- Nayab, D.E.; Akhtar, S. Green Synthesized Silver Nanoparticles from Eucalyptus Leaves Can Enhance Shelf Life of Banana without Penetrating in Pulp. PLoS ONE 2023, 18, e0281675. [Google Scholar] [CrossRef]
- Mohammed Ali Eesa, A.; Bazargani-Gilani, B.; Obaid Hasson, S. Comparison of Green and Synthetic Silver Nanoparticles in Zein-Based Edible Films: Shelf-Life Study of Cold-Stored Turkey Breasts. Food Sci. Nutr. 2023, 11, 7352–7363. [Google Scholar] [CrossRef]
- Vieira, A.C.F.; de Matos Fonseca, J.; Menezes, N.M.C.; Monteiro, A.R.; Valencia, G.A. Active Coatings Based on Hydroxypropyl Methylcellulose and Silver Nanoparticles to Extend the Papaya (Carica papaya L.) Shelf Life. Int. J. Biol. Macromol. 2020, 164, 489–498. [Google Scholar] [CrossRef]
- Cheng, J.; Lin, X.; Wu, X.; Liu, Q.; Wan, S.; Zhang, Y. Preparation of a Multifunctional Silver Nanoparticles Polylactic Acid Food Packaging Film Using Mango Peel Extract. Int. J. Biol. Macromol. 2021, 188, 678–688. [Google Scholar] [CrossRef]
- Feng, Q.; Fan, B.; He, Y.-C.; Ma, C. Antibacterial, Antioxidant and Fruit Packaging Ability of Biochar-Based Silver Nanoparticles-Polyvinyl Alcohol-Chitosan Composite Film. Int. J. Biol. Macromol. 2024, 256, 128297. [Google Scholar] [CrossRef]
- Yang, X.; Niu, Y.; Fan, Y.; Zheng, T.; Fan, J. Green Synthesis of Poria Cocos Polysaccharides-Silver Nanoparticles and Their Applications in Food Packaging. Int. J. Biol. Macromol. 2024, 269, 131928. [Google Scholar] [CrossRef]
- das Neves, M.d.S.; Scandorieiro, S.; Pereira, G.N.; Ribeiro, J.M.; Seabra, A.B.; Dias, A.P.; Yamashita, F.; Martinez, C.B.d.R.; Kobayashi, R.K.T.; Nakazato, G. Antibacterial Activity of Biodegradable Films Incorporated with Biologically-Synthesized Silver Nanoparticles and the Evaluation of Their Migration to Chicken Meat. Antibiotics 2023, 12, 178. [Google Scholar] [CrossRef]
- Yang, D.; Fan, B.; Sun, G.; He, Y.-C.; Ma, C. Ultraviolet Blocking Ability, Antioxidant and Antibacterial Properties of Newly Prepared Polyvinyl Alcohol-Nanocellulose-silver Nanoparticles-ChunJian Peel Extract Composite Film. Int. J. Biol. Macromol. 2023, 252, 126427. [Google Scholar] [CrossRef]
- Aldosary, S.K.; El-Rahman, S.N.A.; Al-Jameel, S.S.; Alromihi, N.M. Antioxidant and Antimicrobial Activities of Thymus Vulgaris Essential Oil Contained and Synthesis Thymus (Vulgaris) Silver Nanoparticles. Braz. J. Biol. 2021, 83, e244675. [Google Scholar] [CrossRef]
- Zhuo, M.; Liu, C.; Wang, Q.; Wang, Z.; Wang, Y.; Yu, F.; Zhang, Y. Catharanthus roseus Extract-Assisted Silver Nanoparticles Chitosan Films with High Antioxidant and Antimicrobial Properties for Fresh Food Preservation. Int. J. Biol. Macromol. 2025, 309, 142771. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Fei, X.; Peng, L. Carboxymethyl Cellulose/Cellulose Nanocrystals Immobilized Silver Nanoparticles as an Effective Coating to Improve Barrier and Antibacterial Properties of Paper for Food Packaging Applications. Carbohydr. Polym. 2021, 252, 117156. [Google Scholar] [CrossRef] [PubMed]
- Gumber, S.; Kanwar, S.; Mazumder, K. Properties and Antimicrobial Activity of Wheat-Straw Nanocellulose-Arabinoxylan Acetate Composite Films Incorporated with Silver Nanoparticles. Int. J. Biol. Macromol. 2023, 246, 125480. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Jiang, Q.; Luo, B.; Liu, C.; Ren, J.; Wang, X.; Wang, X. A Sandwich-like Chitosan-Based Antibacterial Nanocomposite Film with Reduced Graphene Oxide Immobilized Silver Nanoparticles. Carbohydr. Polym. 2021, 260, 117835. [Google Scholar] [CrossRef]
- Qiu, M.; Tian, Y.; Qu, W.; Ma, Y.; Zhao, F.; Jiang, Y.; Zhao, Q.; Man, C. Postbiotic-Biosynthesized Silver Nanoparticles Anchored on Covalent Organic Frameworks Integrated into Carboxymethyl Chitosan-Based Film for Enhancing Antibacterial Packaging. Int. J. Biol. Macromol. 2025, 291, 139143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, L.; Xu, M.; Wang, H.; Gao, X.; Niu, B.; Li, W. Gallic Acid Functionalized Chitosan Immobilized Nanosilver for Modified Chitosan/Poly (Vinyl Alcohol) Composite Film. Int. J. Biol. Macromol. 2022, 222, 2987–3000. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Tran, T.T.; Nguyen, T.-M.T.; Le, H.V.; Nguyen, K.-P.L.; Vu, A.N. Fabrication of a Ternary Biocomposite Film Based on Polyvinyl Alcohol, Cellulose Nanocrystals, and Silver Nanoparticles for Food Packaging. RSC Adv. 2024, 14, 18671–18684. [Google Scholar] [CrossRef] [PubMed]
- Sari Gencag, B.; Kahraman, K.; Ekici, L. Green Synthesis of Silver Nanoparticles from Pomegranate Peel and Their Application in PVA-Based Nanofibers for Coating Minced Meat. Sci. Rep. 2025, 15, 17083. [Google Scholar] [CrossRef]
- Trotta, F.; Da Silva, S.; Massironi, A.; Mirpoor, S.F.; Lignou, S.; Ghawi, S.K.; Charalampopoulos, D. Advancing Food Preservation: Sustainable Green-AgNPs Bionanocomposites in Paper-Starch Flexible Packaging for Prolonged Shelf Life. Polymers 2024, 16, 941. [Google Scholar] [CrossRef] [PubMed]
- Pluta-Kubica, A.; Jamróz, E.; Khachatryan, G.; Florkiewicz, A.; Kopel, P. Application of Furcellaran Nanocomposite Film as Packaging of Cheese. Polymers 2021, 13, 1428. [Google Scholar] [CrossRef]
- Pandey, V.K.; Upadhyay, S.N.; Niranjan, K.; Mishra, P.K. Antimicrobial Biodegradable Chitosan-Based Composite Nano-Layers for Food Packaging. Int. J. Biol. Macromol. 2020, 157, 212–219. [Google Scholar] [CrossRef]
- Plaeyao, K.; Talodthaisong, C.; Yingyuen, W.; Kaewbundit, R.; Tun, W.S.T.; Saenchoopa, A.; Kayunkid, N.; Wiwattananukul, R.; Sakulsombat, M.; Kulchat, S. Biodegradable Antibacterial Food Packaging Based on Carboxymethyl Cellulose from Sugarcane Bagasse/Cassava Starch/Chitosan/Gingerol Extract Stabilized Silver Nanoparticles (Gin-AgNPs) and Vanillin as Cross-Linking Agent. Food Chem. 2025, 466, 142102. [Google Scholar] [CrossRef]
- Shaw, S.; Mondal, R.; Dam, P.; Mandal, A.; Acharya, R.; Manna, S.; Gangopadhyay, D.; Mandal, A.K. Synthesis, Characterization and Application of Silk Sericin-Based Silver Nanocomposites for Antibacterial and Food Coating Solutions. RSC Adv. 2024, 14, 33068–33079. [Google Scholar] [CrossRef]
- Das, U.; Saikia, S.; Biswas, R. Highly Sensitive Biofunctionalized Nanostructures for Paper-Based Colorimetric Sensing of Hydrogen Peroxide in Raw Milk. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 316, 124290. [Google Scholar] [CrossRef]
- Grzebieniarz, W.; Tkaczewska, J.; Juszczak, L.; Krzyściak, P.; Cholewa-Wójcik, A.; Nowak, N.; Guzik, P.; Szuwarzyński, M.; Mazur, T.; Jamróz, E. Improving the Quality of Multi-Layer Films Based on Furcellaran by Immobilising Active Ingredients and Impact Assessment of the Use of a New Packaging Material. Food Chem. 2023, 428, 136759. [Google Scholar] [CrossRef]
- Pandey, S.; Sekar, H.; Gundabala, V. Development and Characterization of Bilayer Chitosan/Alginate Cling Film Reinforced with Essential Oil Based Nanocomposite for Red Meat Preservation. Int. J. Biol. Macromol. 2024, 279, 135524. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Feng, H.; Wang, S.; Zhuang, D.; Wen, Y.; Zhu, J. A Double-Layer Film Based on the Strategy of Tannic Acid-Anthocyanin Co-Pigmentation and Tannic-Crosslinked-Gelatin/−reduced Ag Nanoparticles for Beef Preservation and Monitoring. Food Chem. 2024, 460, 140642. [Google Scholar] [CrossRef]
- Ghanbar Soleiman Abadi, F.; Bazargani-Gilani, B.; Emamifar, A.; Nourian, A. Beet Root Peel Extract as a Natural Cost-Effective pH Indicator and Food Preservative in Edible Film: Shelf Life Improvement of Cold-Stored Trout Fillet. Food Sci. Nutr. 2024, 12, 10561–10575. [Google Scholar] [CrossRef]
- Strużyńska, L. Dual Implications of Nanosilver-Induced Autophagy: Nanotoxicity and Anti-Cancer Effects. Int. J. Mol. Sci. 2023, 24, 15386. [Google Scholar] [CrossRef]
- Do, H.T.T.; Nguyen, N.P.U.; Saeed, S.I.; Dang, N.T.; Doan, L.; Nguyen, T.T.H. Advances in Silver Nanoparticles: Unraveling Biological Activities, Mechanisms of Action, and Toxicity. Appl. Nanosci. 2024, 15, 1. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, W.; Qiao, K.; Feng, J.; Zhu, L.; Zhu, X. Bioavailability and Toxicity of Silver Nanoparticles: Determination Based on Toxicokinetic–Toxicodynamic Processes. Water Res. 2021, 204, 117603. [Google Scholar] [CrossRef] [PubMed]
- Kaabipour, S.; Hemmati, S. A Review on the Green and Sustainable Synthesis of Silver Nanoparticles and One-Dimensional Silver Nanostructures. Beilstein J. Nanotechnol. 2021, 12, 102–136. [Google Scholar] [CrossRef]
- Abegunde, S.M.; Afolayan, B.O.; Ilesanmi, T.M. Ensuring Sustainable Plant-Assisted Nanoparticles Synthesis through Process Standardization and Reproducibility: Challenges and Future Directions—A Review. Sustain. Chem. One World 2024, 3, 100014. [Google Scholar] [CrossRef]
- Kulkarni, A.G.; De Britto, S.; Jogaiah, S. 18—Economic Considerations and Limitations of Green Synthesis vs. Chemical Synthesis of Nanomaterials. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Jogaiah, S., Singh, H.B., Fraceto, L.F., de Lima, R., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Amsterdam, The Netherlands, 2021; pp. 459–468. ISBN 978-0-12-820092-6. [Google Scholar]
- Dubey, R.K.; Shukla, S.; Hussain, Z. Green Synthesis of Silver Nanoparticles; A Sustainable Approach with Diverse Applications. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2023, 39, e20230007. [Google Scholar] [CrossRef]
- Sati, A.; Ranade, T.N.; Mali, S.N.; Ahmad Yasin, H.K.; Pratap, A. Silver Nanoparticles (AgNPs): Comprehensive Insights into Bio/Synthesis, Key Influencing Factors, Multifaceted Applications, and Toxicity—A 2024 Update. ACS Omega 2025, 10, 7549–7582. [Google Scholar] [CrossRef]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green Synthesis of Nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef]
- Castro, V.L.; Jonsson, C.M.; Silva, M.S.G.M.; Castanha, R.; Vallim, J.H.; da Silva, L.A.G.; de Oliveira, R.M.D.; Correa, D.S.; Ferreira, M.D. Estimates of AgNP Toxicity Thresholds in Support of Environmental Safety Policies. J. Nanopart. Res. 2022, 24, 9. [Google Scholar] [CrossRef]
- Haghi, P.B.; Mokarram, R.R.; Khiabani, M.S.; Hamishekar, H.; Kafil, H.S.; Paryad, P.; Abedi-Firoozjah, R.; Tavassoli, M. Green Synthesis of Silver Nanoparticles Using Chamomile Extract for Xanthan/Agar and Bacterial Nanocellulose Antimicrobial Nanobiocomposite. Food Meas. 2025, 19, 1567–1585. [Google Scholar] [CrossRef]
- Amin, H.H. Safe Ulvan Silver Nanoparticles Composite Films for Active Food Packaging. Am. J. Biochem. Biotechnol. 2021, 17, 28–39. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green Synthesis of Silver Nanoparticles Using Plant Extracts and Their Antimicrobial Activities: A Review of Recent Literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, S.; Jia, X.; Huang, Y.; Ji, R.; Zhao, L. Comparation of the Phytotoxicity between Chemically and Green Synthesized Silver Nanoparticles. Sci. Total Environ. 2021, 752, 142264. [Google Scholar] [CrossRef]
- Thavamurugan, S.; Annamalai, A.; Narayanan, M.; Devan, M.; Manoharan, N.; Prabha, A.L. Green Synthesis of Silver Nanoparticles Using Osbeckia leschenaultiana DC Extract: Optimization of Synthesis, Biological Activities, Larvicidal Activity and Toxicity Analysis. Inorg. Chem. Commun. 2024, 169, 113011. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface Charge-Dependent Toxicity of Silver Nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar]
- Liu, H.; Wang, X.; Wu, Y.; Hou, J.; Zhang, S.; Zhou, N.; Wang, X. Toxicity Responses of Different Organs of Zebrafish (Danio rerio) to Silver Nanoparticles with Different Particle Sizes and Surface Coatings. Environ. Pollut. 2019, 246, 414–422. [Google Scholar] [CrossRef]
- Gorka, D.E.; Osterberg, J.S.; Gwin, C.A.; Colman, B.P.; Meyer, J.N.; Bernhardt, E.S.; Gunsch, C.K.; DiGulio, R.T.; Liu, J. Reducing Environmental Toxicity of Silver Nanoparticles through Shape Control. Environ. Sci. Technol. 2015, 49, 10093–10098. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. Npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Nguyen, Q.L.; Le, D.V.; Phan, A.N.; Nguyen, V.D. Synthesis of Biodegradable and Antimicrobial Nanocomposite Films Reinforced for Coffee and Agri-Food Product Preservation. ACS Omega 2023, 8, 42177–42185. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Snigdha, S.; Mathew, J.; Radhakrishnan, E.K. Biodegradable and Active Nanocomposite Pouches Reinforced with Silver Nanoparticles for Improved Packaging of Chicken Sausages. Food Packag. Shelf Life 2019, 19, 155–166. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, Applications, Toxicity and Toxicity Mechanisms of Silver Nanoparticles: A Review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Noga, M.; Milan, J.; Frydrych, A.; Jurowski, K. Toxicological Aspects, Safety Assessment, and Green Toxicology of Silver Nanoparticles (AgNPs)—Critical Review: State of the Art. Int. J. Mol. Sci. 2023, 24, 5133. [Google Scholar] [CrossRef]
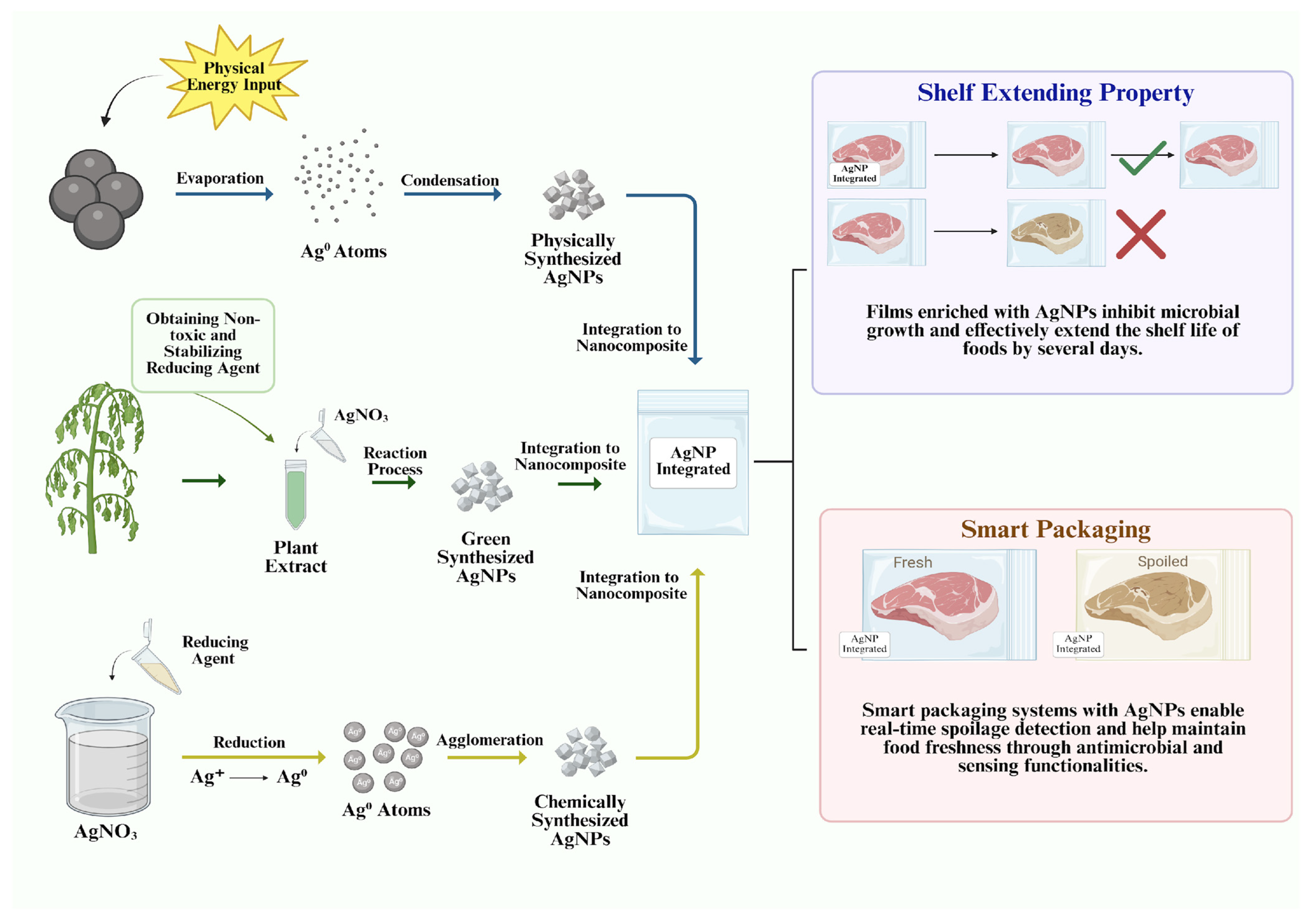

| Food Type | Packaging Form | AgNP Integration Method | Physicochemical Properties of AgNPs/Material | Observed Effects | Reference |
|---|---|---|---|---|---|
| Cherry tomato | Pectin/AgNPs biofilm | AgNPs synthesized with NaBH4, blended into pectin solution and cast. | AgNP: 7–10 nm. Low methoxy pectin; strong gel network; AgNPs enhanced antibacterial, mechanical, and WVTR properties. | Shelf life extended to 10 days; preserved weight and sensory quality; antibacterial against various bacteria. | [92] |
| Tomato, coriander leaf | Blotting paper coated with green-synthesized AgNPs | AgNO3 + plant extracts on blotting paper; in situ green synthesis. | AgNP: 27–31 nm. Biodegradable paper; porous matrix; SEM/TEM verified AgNPs presence; antimicrobial coating. | Shelf life extended to ~30 days for tomato and ~15 days for coriander; effective antibacterial action against various bacteria strains. | [93] |
| Strawberry | CH + EOs (essential oils) + AgNPs films ± γ-irradiation | AgNPs, blended with CH-EO matrix via solution casting. | Smooth surface; improved tensile strength; stable WVP; FTIR showed matrix interaction. | Reduced weight loss and decay; improved firmness and phenolic content after 12 days storage; strong antimicrobial activity against various bacteria. | [94] |
| Strawberry | AgNPs@CS | In situ reduction in AgNO3 on cellulose using hydroxyl groups; sponge form. | AgNPs: 6.84–54.78 nm. AgNPs uniformly distributed; sponge elastic, 16.8% unrecoverable deformation after 1000 cycles; high biocompatibility. | Extended shelf life to 12 days; protection from both microbial invasion and physical damage under vibration stress. | [95] |
| Strawberry | LDPE + CNC + Glycerol + Active Formulation (EO + AgNPs) ± γ-irradiation | AgNPs (AGPPH) encapsulated with cinnamon EO; hot-pressed into LDPE blend. | AgNPs: 3–35 nm; CNC-reinforced LDPE; color and mechanical properties altered; stable WVP. | Decay and weight loss reduced by 94% at 12 days; total phenols (952→1711 mg/kg), anthocyanin (185→287 mg/kg); firmness and microbial load preserved. | [96] |
| Telemea cheese | Alginate film with AgNPs and lemongrass essential oil | Chemical reduction with AgNO3 and PVP. | AgNPs: 5–25 nm, spherical, poly/mono-crystalline, and enhanced opacity, thermal, and water vapor properties. | 14-day preservation of the cheese, reduced microbial load, maintained softness and surface texture. | [97] |
| Banana | Green synthesis AgNPs from eucalyptus leaf extract (ELE) | Mixing ELE with AgNO3. | AgNP: <100 nm, spherical, high stability. | Extended shelf life of the banana up to 32 days, reduced ethylene production, decay, and weight loss. | [98] |
| Turkey breast | Z-gAgNPs (Zein + green fabricated AgNPs) | Mixing of green AgNPs synthesized from green tea extract with zein and chemically synthesized AgNPs with NaBH4. | Size of chemically synthesized AgNPs: 43.4 ± 21.1 nm. Size of green-synthesized AgNPs: 60.78 nm. Green AgNPs: smaller, spherical, higher stability; Chem. AgNPs: larger, less uniform. | Z-gAgNPs film best preserved quality over 12 days (TVB-N, pH, microbial load); superior to synthetic AgNPs film. | [99] |
| Papaya | HPMC-based coating | HPMC-AgNP films were prepared by casting and air drying. | AgNPs: 20–100 nm, influenced thickness, moisture content, crystalline structure. | Film inhibited fungal growth, maintained fruit quality, and delayed ripening, extending papaya shelf life significantly. | [100] |
| Strawberry | PLA film with MPE/AgNPs | PLA solution was cast with MPE/AgNPs after ultrasonication-assisted mixing. | AgNPs: 2.5–6.5 nm, spherical, high stability, improved barrier and mechanical properties. | Strawberries stayed fresh for seven days, extending shelf life four days, and showed high antibacterial activity. | [101] |
| Blueberry | C-Ag@PVA/CS | Reduction with trisodium citrate onto biochar; added to PVA/CS matrix | Thermally stable, hydrophobic, effective at 3% concentration. | Reduced weight loss and acid degradation, delayed spoilage, effective for blueberry preservation. | [102] |
| Strawberry | PCP-AgNPs/CS | PCP-AgNPs were incorporated into CS film via solvent casting. | Spherical AgNPs (6.79 nm) stabilized by PCP; film showed improved mechanical strength, thermal stability, and uniform morphology. | Extended strawberry shelf life by 6 days; strong antibacterial activity against various bacteria strains. | [103] |
| Chicken meat | Starch + PBAT + Glycerol + Lyophilized Bio-AgNPs | Lyophilized bio-AgNPs were manually mixed into film matrix and processed via extrusion into films. | Bio-AgNPs were spherical (81.25 nm, −36.4 mV); films were biodegradable, flexible, thermoplastic, and extrusion-molded. | Films inhibited various bacteria species, extending chicken shelf life up to 10 days. | [104] |
| Fresh milk | PVA-CNC-AgNPs-CJPE | AgNO3 reduced by CJPE, incorporated into PVA-CNC via solvent casting. | Uniform spherical AgNPs; improved UV-blocking, antioxidant, thermal, barrier and mechanical properties. | Extended milk shelf life for 14 days; strong antibacterial activity against various pathogens. | [105] |
| - | Thymus vulgaris extract + AgNPs (TSNPs) | AgNP was obtained by biosynthesis by mixing the AgNO3 solution with the plant extract and heating. | AgNPs are 20–40 nm, spherical, brown; show high antioxidant and antimicrobial stability with plant-based surface functionalization. | TSNPs have antibacterial activity against various microorganisms. Also provided DPPH radical scavenging activity. | [106] |
| Strawberries and chicken breast | CS-Ca-Ag | AgNPs were biosynthesized using C. roseus extract and mixed into CS film via solvent casting. | Uniform AgNPs, improved Young’s modulus, WVP, UV blocking, and good thermal stability. | For strawberries: Delayed dehydration and mold growth; preserved firmness, acidity, vitamin C, and color. | [107] |
| For chicken breast: Reduced pH rise and MetMb formation; maintained weight and color; slowed microbial spoilage. | |||||
| Strawberry | CMC-CNC@AgNPs | AgNPs were immobilized onto CNC and dispersed in the CMC matrix. | AgNPs: 10–20 nm, spherical, well dispersed on CNC. Coated paper showed higher tensile strength, good thermal stability. | Delayed microbial growth, reduced weight loss, retained vitamin C and acidity, slowed TSS degradation; shelf life extended to 7 days. | [108] |
| - | AgNP incorporated nanocellulose (NC)-Arabinoxylan Acetate (AXAc). | Synthesized AgNPs blended into film-forming emulsions. | AgNPs: 40–70 nm; NC: −34.5 mV zeta; films showed good thermal and barrier traits. | Films showed strong antimicrobial activity against various bacteria, enhancing shelf life. | [109] |
| - | CS/rGO@AgNPs (Graphene Oxide: GO) | AgNPs immobilized on rGO, then embedded between CH layers forming a film. | Uniform AgNPs (15 ± 5 nm) on rGO; films showed high tensile strength and UV-blocking ability. | Demonstrated excellent antibacterial activity against various bacteria. Exhibited low cytotoxicity. | [110] |
| Citrus | CMCS@COF-AgNP | AgNPs immobilized into (Covalent Organic Frameworks) COFs via in situ reduction, and then incorporated into carboxymethyl CS (CMCS) films via casting. | AgNP: 46.22 ± 0.97, spherical, uniform dispersion; improved film tensile strength, opacity, WVP, solubility, and swelling. | Reduced weight loss, pH shift, and vitamin C degradation; extended citrus shelf life; excellent antibacterial activity against various bacteria. | [111] |
| Apple | CG/PVA/AgNPs | AgNPs incorporated into the CG/PVA film via aqueous blending. | AgNPs dispersed in water with CG and PVA, and cast into films via solution casting. | Reduced weight loss, delayed browning, lower PPO activity, and microbial load in samples. | [112] |
| Bananas | PVA/CNCd/Ag | AgNPs incorporated into the PVA matrix through solution casting. | AgNPs were spherical (~100 nm), uniformly distributed; film showed improved mechanical strength, thermal stability, and UV barrier properties. | PVA/CNCd/Ag film extended banana shelf life to 14 days, reducing decay and maintaining appearance. | [113] |
| Minced beef | AgPVA nanofibers | AgNPs were blended into a PVA solution and electrospun into nanofibers. | AgNPs were spherical, showed face-centered cubic (FCC) structure; nanofibers were 166–186 nm in diameter with smooth surface and improved crystallinity, enhancing structural stability. | Reduced lipid oxidation, better color retention, and lower microbial growth during 9-day cold storage. | [114] |
| Chilled beef | POHS/P/BA/AgNPs | Commercial AgNPs were directly mixed with butterfly pea (BA) and polymer solution, then ultrasonicated. | AgNPs were spherical (15–90 nm); films showed enhanced tensile strength, UV-blocking, and thermal stability. | Films visually indicated beef freshness and extended shelf life by ~3 days by inhibiting microbial growth and oxidation. | [87] |
| Green grape, cherry tomato and mushroom | G-AgNP | AgNPs were incorporated into starch coatings applied on paper surfaces. | AgNPs were spherical (5–20 nm, average 19 nm); improved elongation at break (8.34%), tensile strength, and WVP. | Reduced weight loss in grapes (6.77%) and tomatoes (8.59%); shelf-life extension observed also for mushrooms; strong antimicrobial activity against E. coli; low Ag+ migration. | [115] |
| Gouda (rennet-curd) and quark (acid-curd) cheese | Furcelleran (FUR) + AgNPs | AgNO3 reduced with xylose in furcellaran solution, dried into film. | AgNPs: 5–20 nm; film transparent; and showed high water vapor permeability and moderate tensile strength. | Reduced yeast and mold in both cheeses, improving microbial stability during 2-week (quark) and 4-week (gouda) storage; silver migrated more in quark cheese and organoleptic quality slightly declined. | [116] |
| Meat | P70-CH30-AgNLs | AgNPs were blended into the PVA/CH electrospinning solution. | AgNPs averaged 80 ± 11 nm; nanofibers ~196 nm diameter; hydrophobicity improved; nanolayers were thin. | Extended meat shelf life by 7 days; inhibited E. coli and L. monocytogenes; reduced microbial growth and odor. | [117] |
| Red grape | CT/CV/V/CMC/Gin-AgNPs | Gin-AgNPs were mixed into the film-forming solution. | AgNPs enhanced UV-blocking, thermal stability, and mechanical strength, producing flexible, moderately thick films with improved durability. | Maintained grape freshness for 21 days; reduced dehydration and microbial spoilage; >50% biodegradation. | [118] |
| Tomato | SS-AgNP | SS-AgNPs agar solution to create a homogeneous coating material. | AgNPs were 6–34 nm, mostly polydispersed; films showed good thermal and colloidal stability, and high crystallinity. | SS-AgNP coating reduced tomato weight loss to 15.5% and decay to 6% after 18 days, significantly enhancing shelf life compared to controls. | [119] |
| Raw cow milk | Paper pad coated with CT-AgNPs | AgNPs directly drop-cast onto paper substrates to form the sensor. | AgNPs were spherical, ~16 nm in size; they showed strong LSPR and good stability against aggregation. | The sensor detected H2O2 in milk as low as 1.5 ppm, with a detection limit of 8.46 ppm and 92% recovery. | [120] |
| Salmon fillet | FUR-HGEL/FUR + CAPS-CHIT + AgNPs-YM/FUR + CUR + MMT/FUR (Four layer films tested) | AgNPs incorporated into the second furcellaran layer of the multilayer film during casting. | AgNPs were nanoscale; films showed improved antioxidant activity but slightly reduced mechanical and thermal stability. | Despite no in vitro antimicrobial effect, due to limited release, the films reduced microbial growth in salmon and extended shelf life by 3 days, but failed to prevent lipid oxidation. | [121] |
| Goat meat | Chi + Alg + NC | AgNPs incorporated into lemongrass nanoemulsion by sonication. | AgNPs were approximately 10 nm in size and spherical in shape. The film was 22.5 ± 1.44 μm thick, smooth, and thermally stable with improved mechanical strength and hydrophilicity. | The film maintained meat color and reduced microbial count below 7 log CFU/g for 7 days, with 54% reduced weight loss compared to control. | [122] |
| Beef | AG-PAE-TA//C-OPE/TGNP-TA-AgNPs | AgNPs were incorporated into the film via mixing with carrageenan before casting into the agar layer. | AgNPs were spherical, ~50 nm in size; films showed UV-blocking, hydrophobicity, and enhanced tensile strength. | The film extended beef shelf life for 1 day, delayed microbial growth, and enabled freshness monitoring via pH-sensitive color change and smartphone RGB analysis app. | [123] |
| Rainbow trout fillet | CP-BRPE-gnAg | AgNPs were incorporated into film-forming solutions before casting. | AgNPs were spherical with a diameter of ~51 nm; films showed improved tensile strength, flexibility, and reduced water vapor permeability. | The composite film reduced microbial growth and preserved trout fillets for 12 days, with reduced pH, TVB-N, TBARS, and visible spoilage indicators. | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okur, E.E.; Eker, F.; Akdaşçi, E.; Bechelany, M.; Karav, S. Comprehensive Review of Silver Nanoparticles in Food Packaging Applications. Int. J. Mol. Sci. 2025, 26, 9842. https://doi.org/10.3390/ijms26209842
Okur EE, Eker F, Akdaşçi E, Bechelany M, Karav S. Comprehensive Review of Silver Nanoparticles in Food Packaging Applications. International Journal of Molecular Sciences. 2025; 26(20):9842. https://doi.org/10.3390/ijms26209842
Chicago/Turabian StyleOkur, Erkan Efe, Furkan Eker, Emir Akdaşçi, Mikhael Bechelany, and Sercan Karav. 2025. "Comprehensive Review of Silver Nanoparticles in Food Packaging Applications" International Journal of Molecular Sciences 26, no. 20: 9842. https://doi.org/10.3390/ijms26209842
APA StyleOkur, E. E., Eker, F., Akdaşçi, E., Bechelany, M., & Karav, S. (2025). Comprehensive Review of Silver Nanoparticles in Food Packaging Applications. International Journal of Molecular Sciences, 26(20), 9842. https://doi.org/10.3390/ijms26209842









