Decoding Macrophage Dynamics: A Pathway to Understanding and Treating Inflammatory Skin Diseases
Abstract
:1. Introduction
2. Macrophages Overview
2.1. Function and Characteristics of Macrophages
2.2. Polarization of Macrophages
3. The Role of Macrophages in Skin Injury
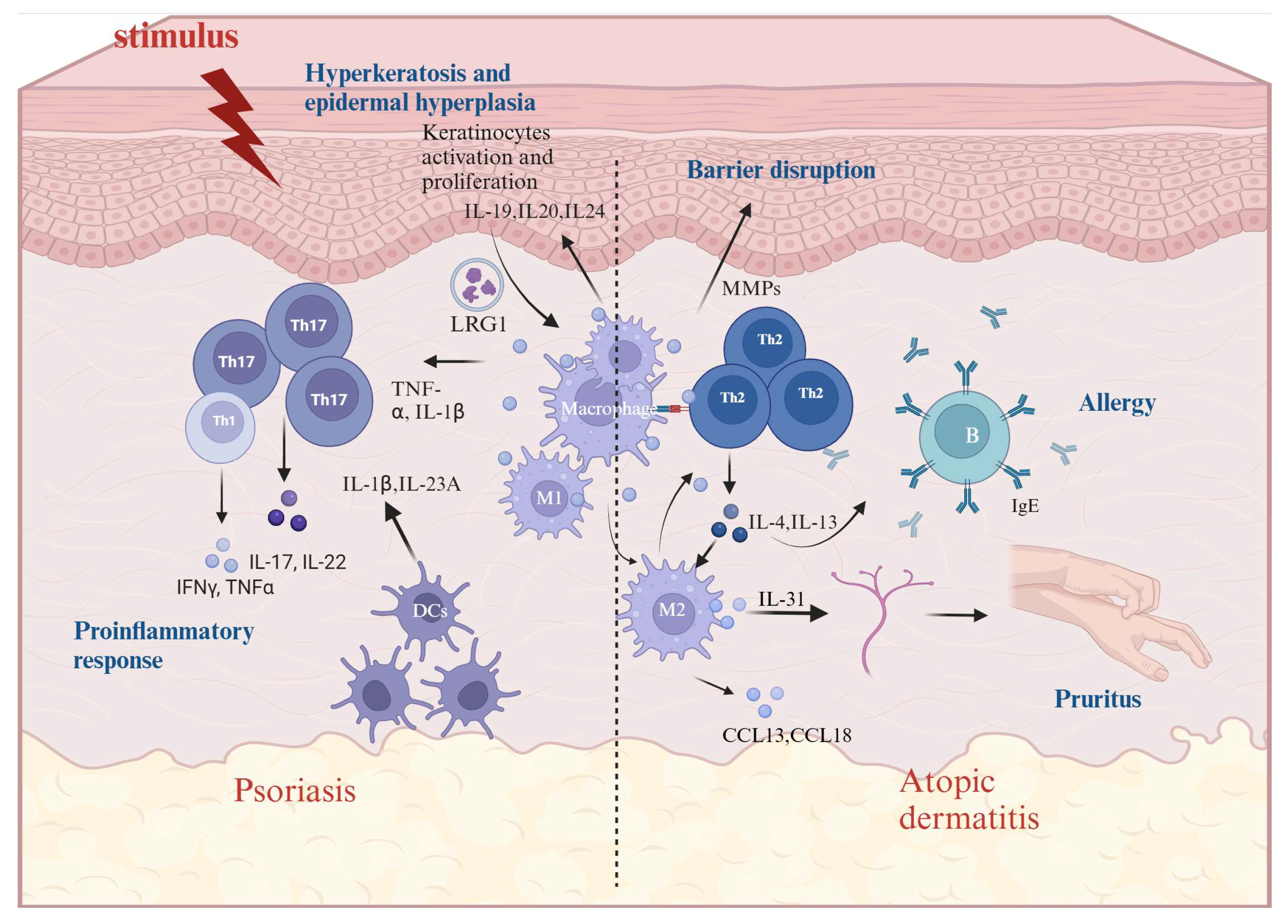
4. The Role of Macrophages in AD and Psoriasis
5. The Role of Macrophages in Inflammatory Pruritus
6. Factors Influencing Macrophage Polarization in AD and Psoriasis
6.1. Cell-to-Cell Cross-Talk
6.2. Epigenetic Regulation of Macrophages
6.2.1. DNA Methylation
6.2.2. miRNA
6.3. Other Factors
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| LPS | Lipopolysaccharide |
| IFN-γ | Interferon-gamma |
| iNOS | Inducible nitric oxide synthase |
| PAMPs | Pathogen-Associated Molecular Patterns |
| SOCS3 | Suppressors Of Cytokine Signaling 3 |
| ECM | Extracellular matrix |
| DNMT | DNA methyltransferase |
| MHC | Major histocompatibility complex |
| Arg1 | Arginase 1 |
| MMPs | Matrix metalloproteinases |
| CaMK4 | Calcium/calmodulin-dependent protein kinase IV |
| DAMPs | Damage-associated molecular patterns |
| TRP | Transient receptor potential |
| HMGB1 | High Mobility Group Box 1 |
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Laughter, M.R.; Maymone, M.B.C.; Mashayekhi, S.; Arents, B.W.M.; Karimkhani, C.; Langan, S.M.; Dellavalle, R.P.; Flohr, C. The global burden of atopic dermatitis: Lessons from the Global Burden of Disease Study 1990–2017. Br. J. Dermatol. 2021, 184, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.H.; Hwang, J.; Kwon, R.; Lee, S.W.; Kim, M.S.; Shin, J.I.; Yon, D.K. Global, regional, and national burden of allergic disorders and their risk factors in 204 countries and territories, from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Allergy 2023, 78, 2232–2254. [Google Scholar] [CrossRef]
- Dainichi, T.; Kitoh, A.; Otsuka, A.; Nakajima, S.; Nomura, T.; Kaplan, D.H.; Kabashima, K. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat. Immunol. 2018, 19, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, A.; Moradzad, M.; Ghalamkari, S.; Fadaei, M.; Cowin, A.J.; Hassanshahi, M. Macrophage-Mediated Inflammation in Skin Wound Healing. Cells 2022, 11, 2953. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Ross, E.A.; Devitt, A.; Johnson, J.R. Macrophages: The Good, the Bad, and the Gluttony. Front. Immunol. 2021, 12, 708186. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Xia, T.; Fu, S.; Yang, R.; Yang, K.; Lei, W.; Yang, Y.; Zhang, Q.; Zhao, Y.; Yu, J.; Yu, L.; et al. Advances in the study of macrophage polarization in inflammatory immune skin diseases. J. Inflamm.-Lond. 2023, 20, 33. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, J.; Yan, W.; Xu, L. Macrophage polarization in inflammatory bowel disease. Cell Commun. Signal. 2023, 21, 367. [Google Scholar] [CrossRef] [PubMed]
- Koelwyn, G.J.; Corr, E.M.; Erbay, E.; Moore, K.J. Regulation of macrophage immunometabolism in atherosclerosis. Nat. Immunol. 2018, 19, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, H.; Tang, B.; Luo, Y.; Yang, Y.; Zhong, X.; Chen, S.; Xu, X.; Huang, S.; Liu, C. Macrophages in cardiovascular diseases: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 130. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, G.; Li, H. Approaches for studying human macrophages. Trends Immunol. 2024, 45, 237–247. [Google Scholar] [CrossRef]
- Bian, Z.; Gong, Y.; Huang, T.; Lee, C.Z.W.; Bian, L.; Bai, Z.; Shi, H.; Zeng, Y.; Liu, C.; He, J.; et al. Deciphering human macrophage development at single-cell resolution. Nature 2020, 582, 571–576. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Miao, K.; Zheng, Y.; Deng, C.; Liu, T. Imaging of macrophage mitochondria dynamics in vivo reveals cellular activation phenotype for diagnosis. Theranostics 2020, 10, 2897–2917. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, Z.; Li, B.; Li, J.; Ou, Y.; Yu, X.; Zhang, Z.; Liu, S.; Fu, X.; Jin, H.; et al. Lipid-associated macrophages in the tumor-adipose microenvironment facilitate breast cancer progression. OncoImmunology 2022, 11, 2085432. [Google Scholar] [CrossRef]
- Wermuth, P.J.; Jimenez, S.A. The significance of macrophage polarization subtypes for animal models of tissue fibrosis and human fibrotic diseases. Clin. Transl. Med. 2015, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Mildner, A.; Yona, S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018, 49, 595–613. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Gentek, R.; Molawi, K.; Sieweke, M.H. Tissue macrophage identity and self-renewal. Immunol. Rev. 2014, 262, 56–73. [Google Scholar] [CrossRef]
- Bonnardel, J.; T’Jonck, W.; Gaublomme, D.; Browaeys, R.; Scott, C.L.; Martens, L.; Vanneste, B.; De Prijck, S.; Nedospasov, S.A.; Kremer, A.; et al. Stellate Cells, Hepatocytes, and Endothelial Cells Imprint the Kupffer Cell Identity on Monocytes Colonizing the Liver Macrophage Niche. Immunity 2019, 51, 638–654. [Google Scholar] [CrossRef] [PubMed]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and non-immune functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.D.; Jeong, D.; Chung, D.H. Development and Functions of Alveolar Macrophages. Mol. Cells 2021, 44, 292–300. [Google Scholar] [CrossRef]
- Wenes, M.; Shang, M.; Di Matteo, M.; Goveia, J.; Martin-Perez, R.; Serneels, J.; Prenen, H.; Ghesquiere, B.; Carmeliet, P.; Mazzone, M. Macrophage Metabolism Controls Tumor Blood Vessel Morphogenesis and Metastasis. Cell Metab. 2016, 24, 701–715. [Google Scholar] [CrossRef]
- Gunassekaran, G.R.; Poongkavithai Vadevoo, S.M.; Baek, M.; Lee, B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials 2021, 278, 121137. [Google Scholar] [CrossRef]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, S. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front. Immunol. 2018, 9, 1930. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, X.; Chai, Y.; Yao, Y. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sanchez-Rodriguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Amoupour, M.; Brouki Milan, P.; Barati, M.; Hivechi, A.; Rajabi Fomeshi, M.; Kiani Ghalesardi, O.; Ahmadvand, D.; Karkuki Osguei, N.; Samadikuchaksaraei, A. Suppression of SOCS3 expression in macrophage cells: Potential application in diabetic wound healing. Int. J. Biol. Macromol. 2024, 262, 129876. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.E.; Whyte, C.S.; Gordon, P.; Barker, R.N.; Rees, A.J.; Wilson, H.M. A critical role for suppressor of cytokine signalling 3 in promoting M1 macrophage activation and function in vitro and in vivo. Immunology 2014, 141, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Kadomoto, S.; Izumi, K.; Mizokami, A. Macrophage Polarity and Disease Control. Int. J. Mol. Sci. 2021, 23, 144. [Google Scholar] [CrossRef] [PubMed]
- Kashfi, K.; Kannikal, J.; Nath, N. Macrophage Reprogramming and Cancer Therapeutics: Role of iNOS-Derived NO. Cells 2021, 10, 3194. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.; Kim, Y.; Kwon, Y.; Park, Y.; Lee, H.; Jung, H.S.; Jeoung, D. Human Adipose Tissue-Derived Mesenchymal Stem Cells Attenuate Atopic Dermatitis by Regulating the Expression of MIP-2, miR-122a-SOCS1 Axis, and Th1/Th2 Responses. Front. Pharmacol. 2018, 9, 1175. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, Y.; Xing, C.; Huang, Y.; Sun, X.; Sun, G. Saponin from Periploca forrestii Schltr Mitigates Oxazolone-Induced Atopic Dermatitis via Modulating Macrophage Activation. Mediat. Inflamm. 2020, 2020, 4346367. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Abdelwahab, S.F.; Wan, J.; Cai, W.; Huixuan, W.; Jianjun, C.; Kumar, K.D.; Vasudevan, A.; Sadek, A.; Su, Z.; et al. Alternatively activated macrophages; a double-edged sword in allergic asthma. J. Transl. Med. 2020, 18, 58. [Google Scholar] [CrossRef]
- Vlk, A.M.; Prantner, D.; Shirey, K.A.; Perkins, D.J.; Buzza, M.S.; Thumbigere-Math, V.; Keegan, A.D.; Vogel, S.N. M2a macrophages facilitate resolution of chemically-induced colitis in TLR4-SNP mice. mBio 2023, 14, e120823. [Google Scholar] [CrossRef]
- Luo, L.; Wang, S.; Hu, Y.; Wang, L.; Jiang, X.; Zhang, J.; Liu, X.; Guo, X.; Luo, Z.; Zhu, C.; et al. Precisely Regulating M2 Subtype Macrophages for Renal Fibrosis Resolution. ACS Nano 2023, 17, 22508–22526. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Wu, H.; Rong, X.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef]
- Gigliotti, C.L.; Dianzani, C.; Stoppa, I.; Monge, C.; Sutti, S.; Sblattero, D.; Puricelli, C.; Rolla, R.; Dianzani, U.; Boggio, E. Differential Modulation of Human M1 and M2 Macrophage Activity by ICOS-Mediated ICOSL Triggering. Int. J. Mol. Sci. 2023, 24, 2953. [Google Scholar] [CrossRef] [PubMed]
- Ollewagen, T.; Myburgh, K.H.; van de Vyver, M.; Smith, C. Rheumatoid cachexia: The underappreciated role of myoblast, macrophage and fibroblast interplay in the skeletal muscle niche. J. Biomed. Sci. 2021, 28, 15. [Google Scholar] [CrossRef]
- Tseng, W.; Tsai, M.; Chen, N.; Tarng, D. Trichostatin A Alleviates Renal Interstitial Fibrosis Through Modulation of the M2 Macrophage Subpopulation. Int. J. Mol. Sci. 2020, 21, 5966. [Google Scholar] [CrossRef]
- Sousa, S.; Brion, R.; Lintunen, M.; Kronqvist, P.; Sandholm, J.; Monkkonen, J.; Kellokumpu-Lehtinen, P.; Lauttia, S.; Tynninen, O.; Joensuu, H.; et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015, 17, 101. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Liu, S.; Guo, J.; Lu, Y.; Cheng, J.; Liu, J. Macrophage-derived extracellular vesicles: Diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 2020, 11, 924. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Deng, W.; Zhang, Y.; Chang, Y.; Shelat, V.G.; Tsuchida, K.; Lino-Silva, L.S.; Wang, Z. NLRP3 activation in tumor-associated macrophages enhances lung metastasis of pancreatic ductal adenocarcinoma. Transl. Lung Cancer Res. 2022, 11, 858–868. [Google Scholar] [CrossRef]
- van Zuuren, E.J.; Arents, B.W.M.; van der Linden, M.M.D.; Vermeulen, S.; Fedorowicz, Z.; Tan, J. Rosacea: New Concepts in Classification and Treatment. Am. J. Clin. Dermatol. 2021, 22, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L. Contact Dermatitis: Classifications and Management. Clin. Rev. Allergy Immunol. 2021, 61, 245–281. [Google Scholar] [CrossRef]
- Schuler, C.F.T.; Billi, A.C.; Maverakis, E.; Tsoi, L.C.; Gudjonsson, J.E. Novel insights into atopic dermatitis. J. Allergy Clin. Immunol. 2023, 151, 1145–1154. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Sabat, R.; Wolk, K.; Loyal, L.; Docke, W.; Ghoreschi, K. T cell pathology in skin inflammation. Semin. Immunopathol. 2019, 41, 359–377. [Google Scholar] [CrossRef]
- Penzes, Z.; Horvath, D.; Molnar, P.; Fekete, T.; Pazmandi, K.; Bacsi, A.; Szollosi, A.G. Anandamide modulation of monocyte-derived Langerhans cells: Implications for immune homeostasis and skin inflammation. Front. Immunol. 2024, 15, 1423776. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, O.; Ayyangar, U.; Kurbet, A.S.; Lakshmanan, V.; Palakodeti, D.; Ginhoux, F.; Raghavan, S. Epithelial-Macrophage Crosstalk Initiates Sterile Inflammation in Embryonic Skin. Front. Immunol. 2021, 12, 718005. [Google Scholar] [CrossRef]
- Brazil, J.C.; Quiros, M.; Nusrat, A.; Parkos, C.A. Innate immune cell-epithelial crosstalk during wound repair. J. Clin. Investig. 2019, 129, 2983–2993. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhang, T.; Qiu, Y.; Liu, Q.; Chen, X.; Wang, Q.; Min, X.; Ouyang, L.; Jia, S.; Lu, Q.; et al. Keratinocyte-to-macrophage communication exacerbate psoriasiform dermatitis via LRG1-enriched extracellular vesicles. Theranostics 2024, 14, 1049–1064. [Google Scholar] [CrossRef] [PubMed]
- Leite Dantas, R.; Masemann, D.; Schied, T.; Bergmeier, V.; Vogl, T.; Loser, K.; Brachvogel, B.; Varga, G.; Ludwig, S.; Wixler, V. Macrophage-mediated psoriasis can be suppressed by regulatory T lymphocytes. J. Pathol. 2016, 240, 366–377. [Google Scholar] [CrossRef]
- Vu, R.; Jin, S.; Sun, P.; Haensel, D.; Nguyen, Q.H.; Dragan, M.; Kessenbrock, K.; Nie, Q.; Dai, X. Wound healing in aged skin exhibits systems-level alterations in cellular composition and cell-cell communication. Cell Rep. 2022, 40, 111155. [Google Scholar] [CrossRef]
- Sharifiaghdam, M.; Shaabani, E.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Macrophages as a therapeutic target to promote diabetic wound healing. Mol. Ther. 2022, 30, 2891–2908. [Google Scholar] [CrossRef]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Eming, S.A.; Murray, P.J.; Pearce, E.J. Metabolic orchestration of the wound healing response. Cell Metab. 2021, 33, 1726–1743. [Google Scholar] [CrossRef] [PubMed]
- Terziroli Beretta-Piccoli, B.; Mainetti, C.; Peeters, M.; Laffitte, E. Cutaneous Granulomatosis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2018, 54, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage polarization and diabetic wound healing. Transl. Res. 2021, 236, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Aitcheson, S.M.; Frentiu, F.D.; Hurn, S.E.; Edwards, K.; Murray, R.Z. Skin Wound Healing: Normal Macrophage Function and Macrophage Dysfunction in Diabetic Wounds. Molecules 2021, 26, 4917. [Google Scholar] [CrossRef]
- Global, regional, and national incidence of six major immune-mediated inflammatory diseases: Findings from the global burden of disease study 2019. EClinicalMedicine 2023, 64, 102193. [CrossRef]
- Nakamizo, S.; Dutertre, C.; Khalilnezhad, A.; Zhang, X.M.; Lim, S.; Lum, J.; Koh, G.; Foong, C.; Yong, P.J.A.; Tan, K.J.; et al. Single-cell analysis of human skin identifies CD14+ type 3 dendritic cells co-producing IL1B and IL23A in psoriasis. J. Exp. Med. 2021, 218, e20202345. [Google Scholar] [CrossRef]
- Lu, C.; Lai, C.; Yeh, D.; Liu, Y.; Su, Y.; Hsu, L.; Chang, C.; Catherine Jin, S.; Chuang, T. Involvement of M1 Macrophage Polarization in Endosomal Toll-Like Receptors Activated Psoriatic Inflammation. Mediat. Inflamm. 2018, 2018, 3523642. [Google Scholar] [CrossRef]
- Vicic, M.; Kastelan, M.; Brajac, I.; Sotosek, V.; Massari, L.P. Current Concepts of Psoriasis Immunopathogenesis. Int. J. Mol. Sci. 2021, 22, 1574. [Google Scholar] [CrossRef]
- Gaire, B.P.; Lee, C.; Kim, W.; Sapkota, A.; Lee, D.Y.; Choi, J.W. Lysophosphatidic Acid Receptor 5 Contributes to Imiquimod-Induced Psoriasis-Like Lesions through NLRP3 Inflammasome Activation in Macrophages. Cells 2020, 9, 1753. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Israel, A.; Zhang, N.; Leonard, A.; Wen, H.; Huynh, T.; Tran, G.; Lyon, S.; Rodriguez, G.; Immaneni, S.; et al. Early-onset pediatric atopic dermatitis is characterized by T(H)2/T(H)17/T(H)22-centered inflammation and lipid alterations. J. Allergy Clin. Immunol. 2018, 141, 2094–2106. [Google Scholar] [CrossRef]
- Mitamura, Y.; Reiger, M.; Kim, J.; Xiao, Y.; Zhakparov, D.; Tan, G.; Ruckert, B.; Rinaldi, A.O.; Baerenfaller, K.; Akdis, M.; et al. Spatial transcriptomics combined with single-cell RNA-sequencing unravels the complex inflammatory cell network in atopic dermatitis. Allergy 2023, 78, 2215–2231. [Google Scholar] [CrossRef] [PubMed]
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef]
- Goh, M.S.; Yun, J.S.; Su, J.C. Management of atopic dermatitis: A narrative review. Med. J. Aust. 2022, 216, 587–593. [Google Scholar] [CrossRef]
- Pidwill, G.R.; Gibson, J.F.; Cole, J.; Renshaw, S.A.; Foster, S.J. The Role of Macrophages in Staphylococcus aureus Infection. Front. Immunol. 2020, 11, 620339. [Google Scholar] [CrossRef] [PubMed]
- Bishayi, B.; Bandyopadhyay, D.; Majhi, A.; Adhikary, R. Possible role of Toll-like receptor-2 in the intracellular survival of Staphylococcus aureus in murine peritoneal macrophages: Involvement of cytokines and anti-oxidant enzymes. Scand. J. Immunol. 2014, 80, 127–143. [Google Scholar] [CrossRef]
- Mantegazza, A.R.; Magalhaes, J.G.; Amigorena, S.; Marks, M.S. Presentation of phagocytosed antigens by MHC class I and II. Traffic 2013, 14, 135–152. [Google Scholar] [CrossRef]
- Liu, B.; Chen, R.; Wang, J.; Li, Y.; Yin, C.; Tai, Y.; Nie, H.; Zeng, D.; Fang, J.; Du, J.; et al. Exploring neuronal mechanisms involved in the scratching behavior of a mouse model of allergic contact dermatitis by transcriptomics. Cell. Mol. Biol. Lett. 2022, 27, 16. [Google Scholar] [CrossRef]
- Sutaria, N.; Adawi, W.; Goldberg, R.; Roh, Y.S.; Choi, J.; Kwatra, S.G. Itch: Pathogenesis and treatment. J. Am. Acad. Dermatol. 2022, 86, 17–34. [Google Scholar] [CrossRef]
- Stander, S.; Luger, T.; Kim, B.; Lerner, E.; Metz, M.; Adiri, R.; Canosa, J.M.; Cha, A.; Yosipovitch, G. Cutaneous Components Leading to Pruritus, Pain, and Neurosensitivity in Atopic Dermatitis: A Narrative Review. Dermatol. Ther. 2024, 14, 45–57. [Google Scholar] [CrossRef]
- Misery, L.; Pierre, O.; Le Gall-Ianotto, C.; Lebonvallet, N.; Chernyshov, P.V.; Le Garrec, R.; Talagas, M. Basic mechanisms of itch. J. Allergy Clin. Immunol. 2023, 152, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Kim, B.; Luger, T.; Lerner, E.; Metz, M.; Adiri, R.; Canosa, J.M.; Cha, A.; Stander, S. Similarities and differences in peripheral itch and pain pathways in atopic dermatitis. J. Allergy Clin. Immunol. 2024, 153, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Ochiai, S.; Jin, J.; Takahashi, N.; Toshima, S.; Ishigame, H.; Kabashima, K.; Kubo, M.; Nakayama, M.; Shiroguchi, K.; et al. Sensory neuronal STAT3 is critical for IL-31 receptor expression and inflammatory itch. Cell Rep. 2023, 42, 113433. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Yokozeki, H.; Karasuyama, H.; Satoh, T. IL-31-generating network in atopic dermatitis comprising macrophages, basophils, thymic stromal lymphopoietin, and periostin. J. Allergy Clin. Immunol. 2023, 151, 737–746. [Google Scholar] [CrossRef]
- Grace, M.S.; Bonvini, S.J.; Belvisi, M.G.; McIntyre, P. Modulation of the TRPV4 ion channel as a therapeutic target for disease. Pharmacol. Ther. 2017, 177, 9–22. [Google Scholar] [CrossRef]
- Lan, Z.; Chen, L.; Feng, J.; Xie, Z.; Liu, Z.; Wang, F.; Liu, P.; Yue, X.; Du, L.; Zhao, Y.; et al. Mechanosensitive TRPV4 is required for crystal-induced inflammation. Ann. Rheum. Dis. 2021, 80, 1604–1614. [Google Scholar] [CrossRef]
- Shibasaki, K. TRPV4 activation by thermal and mechanical stimuli in disease progression. Lab. Investig. 2020, 100, 218–223. [Google Scholar] [CrossRef]
- Luo, J.; Feng, J.; Yu, G.; Yang, P.; Mack, M.R.; Du, J.; Yu, W.; Qian, A.; Zhang, Y.; Liu, S.; et al. Transient receptor potential vanilloid 4-expressing macrophages and keratinocytes contribute differentially to allergic and nonallergic chronic itch. J. Allergy Clin. Immunol. 2018, 141, 608–619. [Google Scholar] [CrossRef]
- Goswami, R.; Merth, M.; Sharma, S.; Alharbi, M.O.; Aranda-Espinoza, H.; Zhu, X.; Rahaman, S.O. TRPV4 calcium-permeable channel is a novel regulator of oxidized LDL-induced macrophage foam cell formation. Free. Radic. Biol. Med. 2017, 110, 142–150. [Google Scholar] [CrossRef]
- Dutta, B.; Arya, R.K.; Goswami, R.; Alharbi, M.O.; Sharma, S.; Rahaman, S.O. Role of macrophage TRPV4 in inflammation. Lab. Investig. 2020, 100, 178–185. [Google Scholar] [CrossRef]
- Sanjel, B.; Kim, B.; Song, M.; Carstens, E.; Shim, W. Glucosylsphingosine evokes pruritus via activation of 5-HT(2A) receptor and TRPV4 in sensory neurons. Br. J. Pharmacol. 2022, 179, 2193–2207. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yu, L.; Cheng, Z.; Peng, Y.; Cao, Z.; Chen, B.; Duan, Y.; Wang, Y. SHED-derived exosomes promote LPS-induced wound healing with less itching by stimulating macrophage autophagy. J. Nanobiotechnol. 2022, 20, 239. [Google Scholar] [CrossRef]
- Van Raemdonck, K.; Umar, S.; Palasiewicz, K.; Romay, B.; Volkov, S.; Arami, S.; Sweiss, N.; Shahrara, S. TLR7 endogenous ligands remodel glycolytic macrophages and trigger skin-to-joint crosstalk in psoriatic arthritis. Eur. J. Immunol. 2021, 51, 714–720. [Google Scholar] [CrossRef]
- Liu, T.; Deng, Z.; Xie, H.; Chen, M.; Xu, S.; Peng, Q.; Sha, K.; Xiao, W.; Zhao, Z.; Li, J. ADAMDEC1 promotes skin inflammation in rosacea via modulating the polarization of M1 macrophages. Biochem. Biophys. Res. Commun. 2020, 521, 64–71. [Google Scholar] [CrossRef]
- Hou, Y.; Zhu, L.; Tian, H.; Sun, H.; Wang, R.; Zhang, L.; Zhao, Y. IL-23-induced macrophage polarization and its pathological roles in mice with imiquimod-induced psoriasis. Protein Cell 2018, 9, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhao, B.; Zhang, D.; Rui, X.; Hou, X.; Chen, X.; Zhang, B.; Yuan, Y.; Deng, H.; Ge, G. Viola yedoensis Makino formula alleviates DNCB-induced atopic dermatitis by activating JAK2/STAT3 signaling pathway and promoting M2 macrophages polarization. Phytomedicine 2022, 103, 154228. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Xian, D.; Liu, J.; Pan, S.; Tang, R.; Zhong, J. Regulating the Polarization of Macrophages: A Promising Approach to Vascular Dermatosis. J. Immunol. Res. 2020, 2020, 8148272. [Google Scholar] [CrossRef]
- Chen, J.; Fu, Y.; Xiong, S. Keratinocyte derived HMGB1 aggravates psoriasis dermatitis via facilitating inflammatory polarization of macrophages and hyperproliferation of keratinocyte. Mol. Immunol. 2023, 163, 1–12. [Google Scholar] [CrossRef]
- Huang, J.; Lin, Y.; Wang, L.; Chiang, B. M2-like macrophages polarized by Foxp3(-) Treg-of-B cells ameliorate imiquimod-induced psoriasis. J. Cell. Mol. Med. 2023, 27, 1477–1492. [Google Scholar] [CrossRef]
- Tugal, D.; Liao, X.; Jain, M.K. Transcriptional control of macrophage polarization. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1135–1144. [Google Scholar] [CrossRef]
- Mommert, S.; Schaper, J.T.; Schaper-Gerhardt, K.; Gutzmer, R.; Werfel, T. Histamine Increases Th2 Cytokine-Induced CCL18 Expression in Human M2 Macrophages. Int. J. Mol. Sci. 2021, 22, 1648. [Google Scholar] [CrossRef] [PubMed]
- Pereira Da Fonseca, A.; Traidl, S.; Gutzmer, R.; Schaper-Gerhardt, K.; Werfel, T.; Mommert, S. Histamine and Th2 cytokines independently and synergistically upregulate MMP12 expression in human M2 macrophages. Front. Immunol. 2024, 15, 1429009. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Hsu, C.; Aljuffali, I.A.; Alalaiwe, A.; Lai, W.; Gu, P.; Tseng, C.; Fang, J. Skin delivery of synthetic benzoyl pterostilbenes suppresses atopic dermatitis-like inflammation through the inhibition of keratinocyte and macrophage activation. Biomed. Pharmacother. 2024, 170, 116073. [Google Scholar] [CrossRef]
- Li, Y.; Takaki, E.; Ouchi, Y.; Tamai, K. Guided monocyte fate to FRbeta/CD163(+) S1 macrophage antagonises atopic dermatitis via fibroblastic matrices in mouse hypodermis. Cell. Mol. Life Sci. 2024, 82, 14. [Google Scholar] [CrossRef] [PubMed]
- Gibson, F.; Hanly, A.; Grbic, N.; Grunberg, N.; Wu, M.; Collard, M.; Alani, R.M. Epigenetic Dysregulation in Autoimmune and Inflammatory Skin Diseases. Clin. Rev. Allergy Immunol. 2022, 63, 447–471. [Google Scholar] [CrossRef]
- Mervis, J.S.; McGee, J.S. DNA methylation and inflammatory skin diseases. Arch. Dermatol. Res. 2020, 312, 461–466. [Google Scholar] [CrossRef]
- Olejnik-Wojciechowska, J.; Boboryko, D.; Bratborska, A.W.; Rusinska, K.; Ostrowski, P.; Baranowska, M.; Pawlik, A. The Role of Epigenetic Factors in the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2024, 25, 3831. [Google Scholar] [CrossRef]
- Zhang, K.; Jagannath, C. Crosstalk between metabolism and epigenetics during macrophage polarization. Epigenet. Chromatin 2025, 18, 16. [Google Scholar] [CrossRef]
- Gao, L.; Lu, Q. The critical importance of epigenetics in autoimmune-related skin diseases. Front. Med. 2023, 17, 43–57. [Google Scholar] [CrossRef]
- Mattei, A.L.; Bailly, N.; Meissner, A. DNA methylation: A historical perspective. Trends Genet. 2022, 38, 676–707. [Google Scholar] [CrossRef] [PubMed]
- Skvortsova, K.; Stirzaker, C.; Taberlay, P. The DNA methylation landscape in cancer. Essays Biochem. 2019, 63, 797–811. [Google Scholar] [CrossRef]
- Angeloni, A.; Bogdanovic, O. Enhancer DNA methylation: Implications for gene regulation. Essays Biochem. 2019, 63, 707–715. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y. Role of Mammalian DNA Methyltransferases in Development. Annu. Rev. Biochem. 2020, 89, 135–158. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Cao, Y.; Qin, J.; Song, X.; Zhang, Q.; Shi, Y.; Cao, L. DNA methylation, its mediators and genome integrity. Int. J. Biol. Sci. 2015, 11, 604–617. [Google Scholar] [CrossRef]
- Lopez, M.; Gilbert, J.; Contreras, J.; Halby, L.; Arimondo, P.B. Inhibitors of DNA Methylation. Adv. Exp. Med. Biol. 2022, 1389, 471–513. [Google Scholar] [CrossRef]
- Lyko, F. The DNA methyltransferase family: A versatile toolkit for epigenetic regulation. Nat. Rev. Genet. 2018, 19, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Hayakawa, K.; Fujishiro, M.; Ikeda, K.; Tsushima, H.; Hirai, T.; Kawasaki, M.; Tominaga, M.; Suga, Y.; Takamori, K.; et al. Social defeat stress exacerbates atopic dermatitis through downregulation of DNA methyltransferase 1 and upregulation of C-C motif chemokine receptor 7 in skin dendritic cells. Biochem. Biophys. Res. Commun. 2020, 529, 1073–1079. [Google Scholar] [CrossRef]
- Cui, Q.; Du, H.; Ma, Y.; Wang, T.; Zhu, H.; Zhu, L.; Pan, S.; Min, N.; Wang, X.; Liu, Z. Matrine inhibits advanced glycation end products-induced macrophage M1 polarization by reducing DNMT3a/b-mediated DNA methylation of GPX1 promoter. Eur. J. Pharmacol. 2022, 926, 175039. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Q.; Yu, L.; Shi, H.; Xue, B.; Shi, H. Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity. JCI Insight 2016, 1, e87748. [Google Scholar] [CrossRef]
- Lu, Q.; Wu, R.; Zhao, M.; Garcia-Gomez, A.; Ballestar, E. miRNAs as Therapeutic Targets in Inflammatory Disease. Trends Pharmacol. Sci. 2019, 40, 853–865. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, eaav1741. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in action: Biogenesis, function and regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef] [PubMed]
- Cocks, A.; Del Vecchio, F.; Martinez-Rodriguez, V.; Schukking, M.; Fabbri, M. Pro-tumoral functions of tumor-associated macrophage EV-miRNA. Semin. Cancer Biol. 2022, 86, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kishore, A.; Petrek, M. Roles of Macrophage Polarization and Macrophage-Derived miRNAs in Pulmonary Fibrosis. Front. Immunol. 2021, 12, 678457. [Google Scholar] [CrossRef]
- Saradna, A.; Do, D.C.; Kumar, S.; Fu, Q.; Gao, P. Macrophage polarization and allergic asthma. Transl. Res. 2018, 191, 1–14. [Google Scholar] [CrossRef]
- Qiu, S.; Xie, L.; Lu, C.; Gu, C.; Xia, Y.; Lv, J.; Xuan, Z.; Fang, L.; Yang, J.; Zhang, L.; et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J. Exp. Clin. Cancer Res. 2022, 41, 296. [Google Scholar] [CrossRef]
- Dopytalska, K.; Czaplicka, A.; Szymanska, E.; Walecka, I. The Essential Role of microRNAs in Inflammatory and Autoimmune Skin Diseases-A Review. Int. J. Mol. Sci. 2023, 24, 9130. [Google Scholar] [CrossRef]
- Yan, F.; Meng, W.; Ye, S.; Zhang, X.; Mo, X.; Liu, J.; Chen, D.; Lin, Y. MicroRNA-146a as a potential regulator involved in the pathogenesis of atopic dermatitis. Mol. Med. Rep. 2019, 20, 4645–4653. [Google Scholar] [CrossRef]
- Huang, C.; Liu, X.; QunZhou; Xie, J.; Ma, T.; Meng, X.; Li, J. MiR-146a modulates macrophage polarization by inhibiting Notch1 pathway in RAW264.7 macrophages. Int. Immunopharmacol. 2016, 32, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Taganov, K.D.; Boldin, M.P.; Chang, K.; Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Nikamo, P.; Lohcharoenkal, W.; Li, D.; Meisgen, F.; Xu Landen, N.; Stahle, M.; Pivarcsi, A.; Sonkoly, E. MicroRNA-146a suppresses IL-17-mediated skin inflammation and is genetically associated with psoriasis. J. Allergy Clin. Immunol. 2017, 139, 550–561. [Google Scholar] [CrossRef]
- Rebane, A.; Runnel, T.; Aab, A.; Maslovskaja, J.; Ruckert, B.; Zimmermann, M.; Plaas, M.; Karner, J.; Treis, A.; Pihlap, M.; et al. MicroRNA-146a alleviates chronic skin inflammation in atopic dermatitis through suppression of innate immune responses in keratinocytes. J. Allergy Clin. Immunol. 2014, 134, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Timis, T.L.; Orasan, R.I. Understanding psoriasis: Role of miRNAs. Biomed. Rep. 2018, 9, 367–374. [Google Scholar] [CrossRef]
- Shams, K.; Kurowska-Stolarska, M.; Schutte, F.; Burden, A.D.; McKimmie, C.S.; Graham, G.J. MicroRNA-146 and cell trauma down-regulate expression of the psoriasis-associated atypical chemokine receptor ACKR2. J. Biol. Chem. 2018, 293, 3003–3012. [Google Scholar] [CrossRef]
- Yang, L.; Liu, L.; Ying, H.; Yu, Y.; Zhang, D.; Deng, H.; Zhang, H.; Chai, J. Acute downregulation of miR-155 leads to a reduced collagen synthesis through attenuating macrophages inflammatory factor secretion by targeting SHIP1. J. Mol. Histol. 2018, 49, 165–174. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Z.; Zhang, X.; Wang, J.; Tian, W.; Ren, Y.; Liu, Y.; Wang, T.; Li, Y.; Liu, Y.; et al. Butyrate alleviates alcoholic liver disease-associated inflammation through macrophage regulation and polarization via the HDAC1/miR-155 axis. Int. Immunopharmacol. 2024, 131, 111852. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.; Cai, D.; Zhu, R.; Cao, Y. Exosomal miR-155-5p drives widespread macrophage M1 polarization in hypervirulent Klebsiella pneumoniae-induced acute lung injury via the MSK1/p38-MAPK axis. Cell. Mol. Biol. Lett. 2023, 28, 92. [Google Scholar] [CrossRef]
- Lu, Z.; Wu, J.; Jiang, W.; Xiao, J.; Tao, K.; Ma, L.; Zheng, P.; Wan, R.; Wang, X. MicroRNA-155 promotes the pathogenesis of experimental colitis by repressing SHIP-1 expression. World J. Gastroenterol. 2017, 23, 976–985. [Google Scholar] [CrossRef]
- Arranz, A.; Doxaki, C.; Vergadi, E.; Martinez De La Torre, Y.; Vaporidi, K.; Lagoudaki, E.D.; Ieronymaki, E.; Androulidaki, A.; Venihaki, M.; Margioris, A.N.; et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc. Natl. Acad. Sci. USA 2012, 109, 9517–9522. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Janson, P.; Majuri, M.; Savinko, T.; Fyhrquist, N.; Eidsmo, L.; Xu, N.; Meisgen, F.; Wei, T.; Bradley, M.; et al. MiR-155 is overexpressed in patients with atopic dermatitis and modulates T-cell proliferative responses by targeting cytotoxic T lymphocyte-associated antigen 4. J. Allergy Clin. Immunol. 2010, 126, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Leng, H.; Shi, X.; Ji, J.; Fu, J.; Leng, H. MiR-155 promotes cell proliferation and inhibits apoptosis by PTEN signaling pathway in the psoriasis. Biomed. Pharmacother. 2017, 90, 524–530. [Google Scholar] [CrossRef]
- Beer, L.; Kalinina, P.; Kocher, M.; Laggner, M.; Jeitler, M.; Abbas Zadeh, S.; Copic, D.; Tschachler, E.; Mildner, M. miR-155 Contributes to Normal Keratinocyte Differentiation and Is Upregulated in the Epidermis of Psoriatic Skin Lesions. Int. J. Mol. Sci. 2020, 21, 9288. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Eghtedarian, R.; Taheri, M.; Rakhshan, A. The eminent roles of ncRNAs in the pathogenesis of psoriasis. Non-Coding RNA Res. 2020, 5, 99–108. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, S.; Arias-Santiago, S.; Blasco-Morente, G.; Orgaz-Molina, J.; Rosal-Vela, A.; Navarro, P.; Magro-Checa, C.; Martinez-Lopez, A.; Ruiz, J.; Raya, E.; et al. Increased expression of microRNA-155 in peripheral blood mononuclear cells from psoriasis patients is related to disease activity. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 312–322. [Google Scholar] [CrossRef]
- Meisgen, F.; Xu, N.; Wei, T.; Janson, P.C.; Obad, S.; Broom, O.; Nagy, N.; Kauppinen, S.; Kemeny, L.; Stahle, M.; et al. MiR-21 is up-regulated in psoriasis and suppresses T cell apoptosis. Exp. Dermatol. 2012, 21, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, F.; Henriet, E.; Suet, A.; Arar, A.; Clemencon, R.; Malinge, J.; Lecellier, G.; Baril, P.; Pichon, C. miR-21-3p/IL-22 Axes Are Major Drivers of Psoriasis Pathogenesis by Modulating Keratinocytes Proliferation-Survival Balance and Inflammatory Response. Cells 2021, 10, 2547. [Google Scholar] [CrossRef]
- Lu, T.X.; Munitz, A.; Rothenberg, M.E. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J. Immunol. 2009, 182, 4994–5002. [Google Scholar] [CrossRef]
- Bunnemann, R. New ways for the dental care of the young. Zahnarztl. Mitt. 1974, 64, 1170–1173. [Google Scholar]
- Caescu, C.I.; Guo, X.; Tesfa, L.; Bhagat, T.D.; Verma, A.; Zheng, D.; Stanley, E.R. Colony stimulating factor-1 receptor signaling networks inhibit mouse macrophage inflammatory responses by induction of microRNA-21. Blood 2015, 125, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Yong, L.; Yu, Y.; Li, B.; Ge, H.; Zhen, Q.; Mao, Y.; Yu, Y.; Cao, L.; Zhang, R.; Li, Z.; et al. Calcium/calmodulin-dependent protein kinase IV promotes imiquimod-induced psoriatic inflammation via macrophages and keratinocytes in mice. Nat. Commun. 2022, 13, 4255. [Google Scholar] [CrossRef]
- Yu, D.; Zhao, J.; Lee, E.E.; Kim, D.; Mahapatra, R.; Rose, E.K.; Zhou, Z.; Hosler, C.; El Kurdi, A.; Choe, J.; et al. GLUT3 promotes macrophage signaling and function via RAS-mediated endocytosis in atopic dermatitis and wound healing. J. Clin. Investig. 2023, 133, e170706. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Ma, Y.; Chen, L.; Huang, H.; Huang, S.; Zhang, H.; He, Y.; Tan, C.; He, Y.; et al. Macrophage autophagy deficiency-induced CEBPB accumulation alleviates atopic dermatitis via impairing M2 polarization. Cell Rep. 2023, 42, 113430. [Google Scholar] [CrossRef]
- Yang, L.; Fu, J.; Han, X.; Zhang, C.; Xia, L.; Zhu, R.; Huang, S.; Xiao, W.; Yu, H.; Gao, Y.; et al. Hsa_circ_0004287 inhibits macrophage-mediated inflammation in an N(6)-methyladenosine-dependent manner in atopic dermatitis and psoriasis. J. Allergy Clin. Immunol. 2022, 149, 2021–2033. [Google Scholar] [CrossRef]
- Gopee, N.H.; Winheim, E.; Olabi, B.; Admane, C.; Foster, A.R.; Huang, N.; Botting, R.A.; Torabi, F.; Sumanaweera, D.; Le, A.P.; et al. A prenatal skin atlas reveals immune regulation of human skin morphogenesis. Nature 2024, 635, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K. Multiple roles of macrophage in skin. J. Dermatol. Sci. 2021, 104, 2–10. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, E.; Mao, T.; Zhu, Y.; Ni, S.; Li, Y.; Liu, C.; Fang, Y.; Ni, K.; Lu, Y.; et al. Macrophage P2Y(6)R activation aggravates psoriatic inflammation through IL-27-mediated Th1 responses. Acta Pharm. Sin. B 2024, 14, 4360–4377. [Google Scholar] [CrossRef]
- Liebold, I.; Al Jawazneh, A.; Casar, C.; Lanzloth, C.; Leyk, S.; Hamley, M.; Wong, M.N.; Kylies, D.; Grafe, S.K.; Edenhofer, I.; et al. Apoptotic cell identity induces distinct functional responses to IL-4 in efferocytic macrophages. Science 2024, 384, eabo7027. [Google Scholar] [CrossRef]
- Wang, P.; Lin, T.; Yang, P.; Fang, J.; Li, W.; Pan, T. Therapeutic efficacy of Scutellaria baicalensis Georgi against psoriasis-like lesions via regulating the responses of keratinocyte and macrophage. Biomed. Pharmacother. 2022, 155, 113798. [Google Scholar] [CrossRef]
- Kwon, Y.; Kang, Y.J.; Kwon, J.; Cho, S.; Kim, J.; Le, T.T.; Hwang, H.; Deshar, B.; Kim, M.; Kim, J.Y.; et al. Forsythia velutina Nakai extract: A promising therapeutic option for atopic dermatitis through multiple cell type modulation. Allergy 2024, 79, 1242–1257. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suarez-Farinas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- Thaci, D.; Simpson, E.L.; Beck, L.A.; Bieber, T.; Blauvelt, A.; Papp, K.; Soong, W.; Worm, M.; Szepietowski, J.C.; Sofen, H.; et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: A randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet 2016, 387, 40–52. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, S.; Xu, L.; Huang, B.; Xiong, K.; Yang, X.; Ye, J. Decoding Macrophage Dynamics: A Pathway to Understanding and Treating Inflammatory Skin Diseases. Int. J. Mol. Sci. 2025, 26, 4287. https://doi.org/10.3390/ijms26094287
Gu S, Xu L, Huang B, Xiong K, Yang X, Ye J. Decoding Macrophage Dynamics: A Pathway to Understanding and Treating Inflammatory Skin Diseases. International Journal of Molecular Sciences. 2025; 26(9):4287. https://doi.org/10.3390/ijms26094287
Chicago/Turabian StyleGu, Shengliang, Lei Xu, Bin Huang, Kai Xiong, Xuesong Yang, and Jianzhou Ye. 2025. "Decoding Macrophage Dynamics: A Pathway to Understanding and Treating Inflammatory Skin Diseases" International Journal of Molecular Sciences 26, no. 9: 4287. https://doi.org/10.3390/ijms26094287
APA StyleGu, S., Xu, L., Huang, B., Xiong, K., Yang, X., & Ye, J. (2025). Decoding Macrophage Dynamics: A Pathway to Understanding and Treating Inflammatory Skin Diseases. International Journal of Molecular Sciences, 26(9), 4287. https://doi.org/10.3390/ijms26094287





