Comprehensive Evaluation and Construction of Drought Resistance Index System in Hulless Barley Seedlings
Abstract
:1. Introduction
2. Results
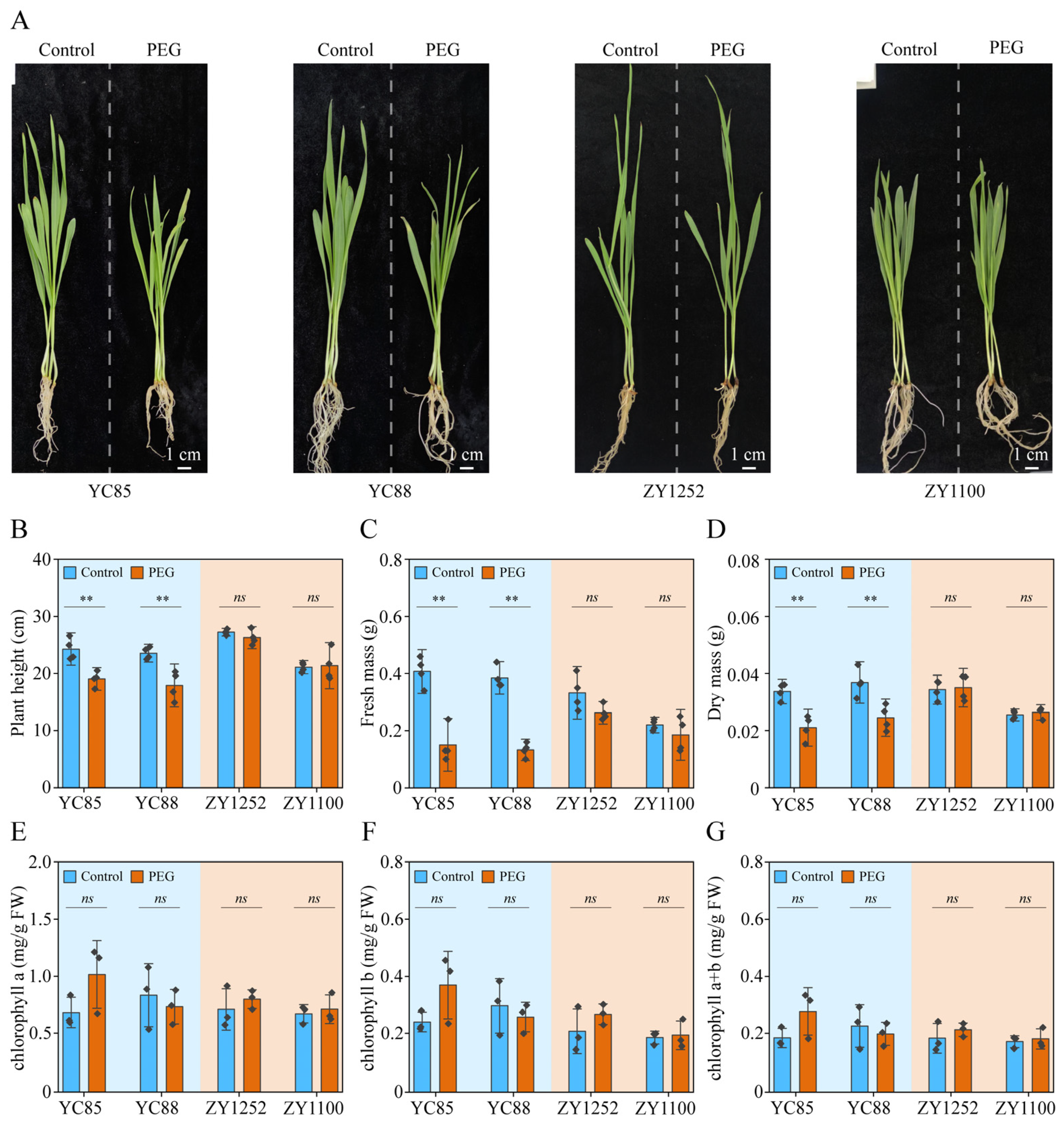
2.1. PEG Treatment Simulates Drought Stress in Hulless Barley Cultivars
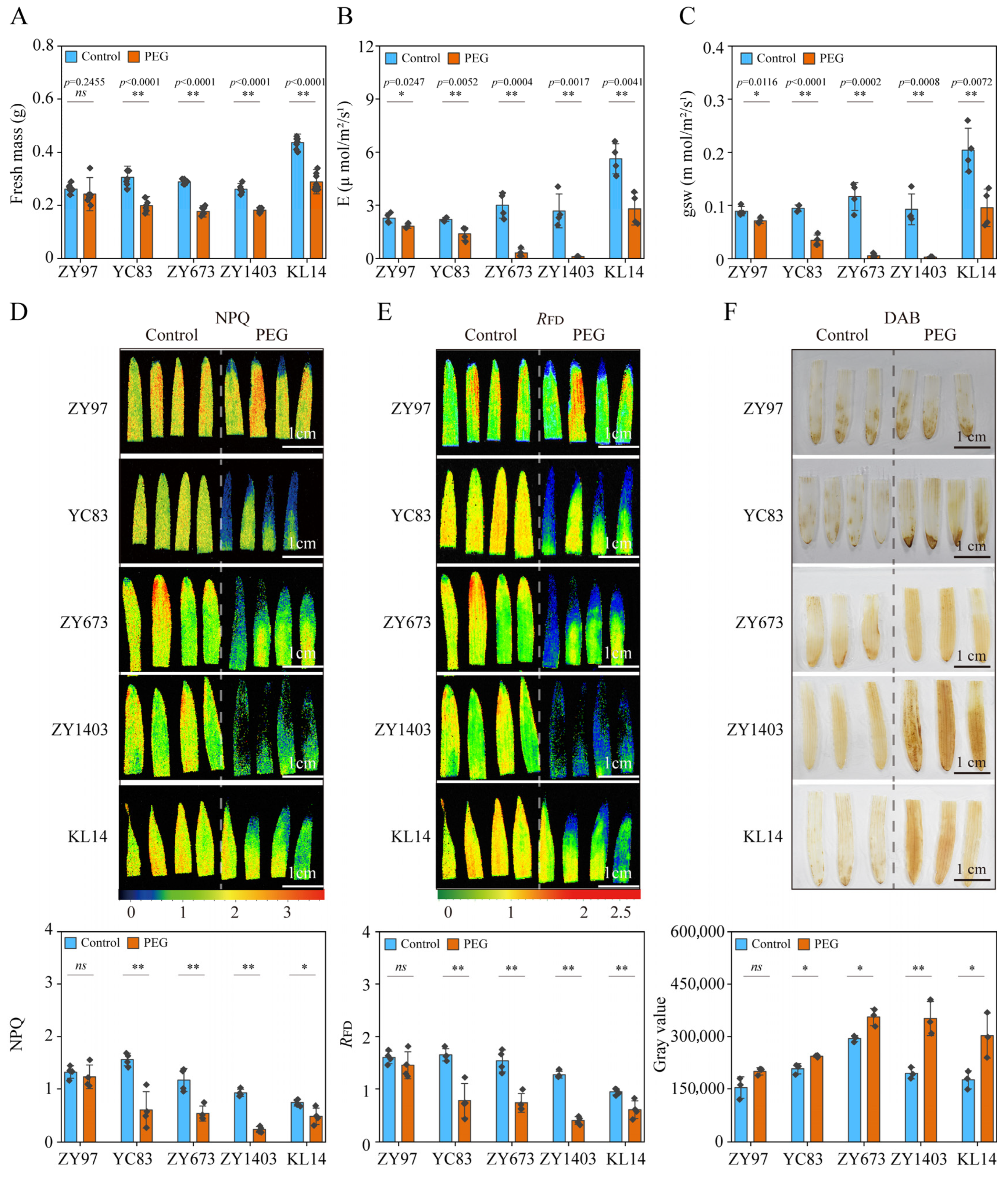
2.2. PEG-Simulated Drought Treatment Had a Significant Effect on Photosynthetic Parameters
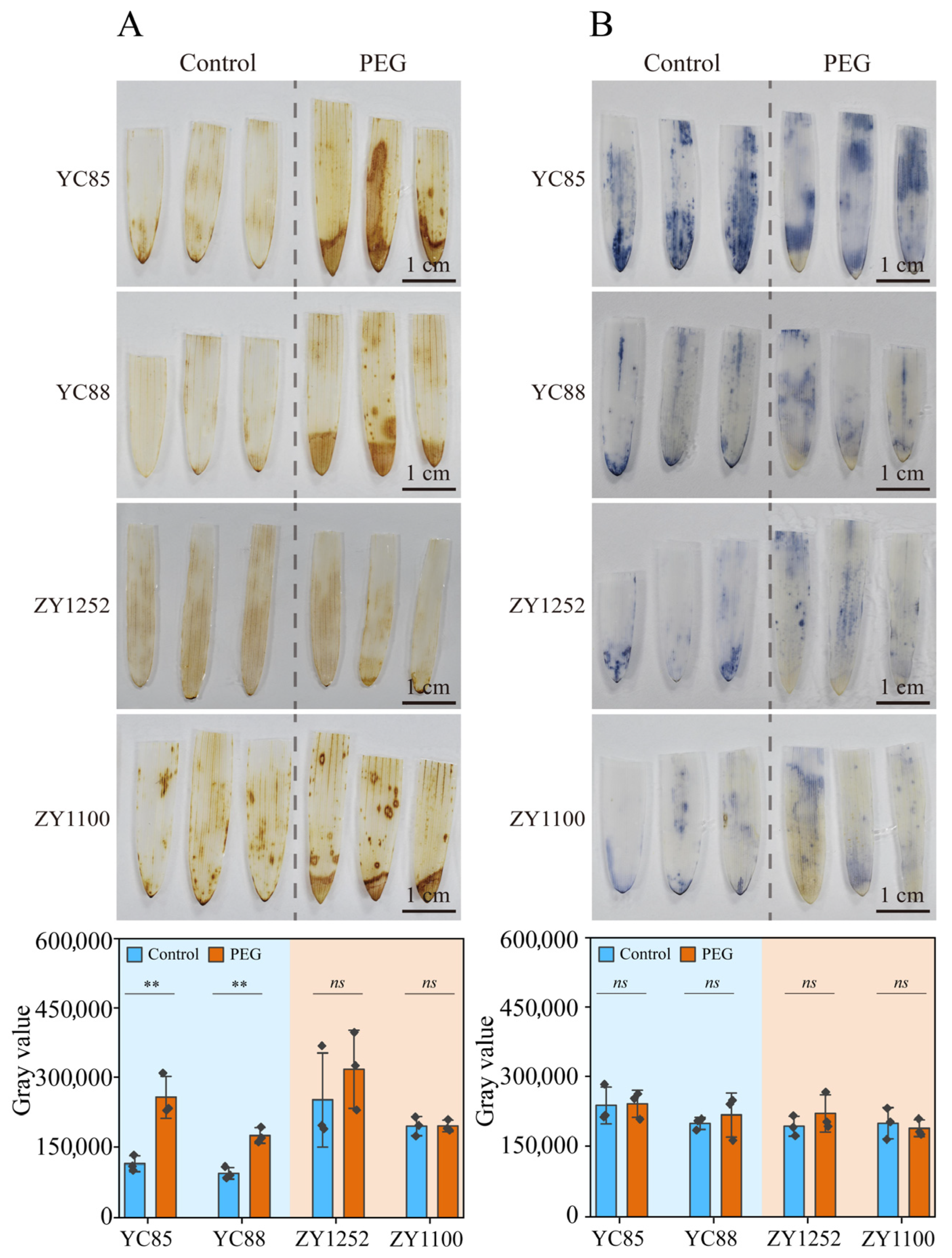
2.3. PEG-Simulated Drought Treatment on Reactive Oxygen Species (ROS) Levels
2.4. Verification of Drought Resistance Evaluation System
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Plant Growth and Drought Treatments
4.3. Morphological and Biomass Measurement
4.4. Assessment of Photosynthetic Parameters
4.5. Measurement of Chlorophyll Fluorescence Parameters
4.6. Measurement of Chlorophyll Content
4.7. Detection of Reactive Oxygen Species (ROS)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Bongaarts, J. IPCC, 2023: Climate Change 2023: Synthesis Report. Popul. Dev. Rev. 2024, 50, 577–580. [Google Scholar] [CrossRef]
- Matsuura, H. Further acceleration in fertility decline in 2023: Deviation of Recently published provisional fertility estimates in selected OECD countries from those in the 2022 revision of the world population prospects. Biodemogr. Soc. Biol. 2024, 69, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Hammer, G.L.; McLean, G.; Messina, C.; Roberts, M.J.; Schlenker, W. The critical role of extreme heat for maize production in the United States. Nat. Clim. Chang. 2013, 3, 497–501. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Chauhan, J.; Prathibha, M.D.; Singh, P.; Choyal, P.; Mishra, U.N.; Saha, D.; Kumar, R.; Anuragi, H.; Pandey, S.; Bose, B.; et al. Plant photosynthesis under abiotic stresses: Damages, adaptive, and signaling mechanisms. Plant Stress 2023, 10, 100296. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sust. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Karimi, M.; Venditti, A.; Zahra, N.; Siddique, K.H.M.; Farooq, M. Plant Adaptation to Drought Stress: The Role of Anatomical and Morphological Characteristics in Maintaining the Water Status. J. Soil Sci. Plant Nut. 2025, 25, 409–427. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Kooyers, N.J. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Sci. 2015, 234, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agr. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Tardieu, F.; Cabrera-Bosquet, L.; Pridmore, T.; Bennett, M. Plant Phenomics, From Sensors to Knowledge. Curr. Biol. 2017, 27, R770–R783. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Patel, M.K.; Kumar, N.; Bajpai, A.B.; Siddique, K.H.M. Metabolomics and Molecular Approaches Reveal Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 9108. [Google Scholar] [CrossRef]
- Maurel, C.; Nacry, P. Root architecture and hydraulics converge for acclimation to changing water availability. Nat. Plants 2020, 6, 744–749. [Google Scholar] [CrossRef]
- Panahi, B.; Hamid, R.; Jalaly, H.M.Z. Deciphering plant transcriptomes: Leveraging machine learning for deeper insights. Curr. Plant Biol. 2025, 41, 100432. [Google Scholar] [CrossRef]
- Varshney, R.K.; Bohra, A.; Yu, J.M.; Graner, A.; Zhang, Q.F.; Sorrells, M.E. Feature Review Designing Future Crops: Genomics-Assisted Breeding Comes of Age. Trends Plant Sci. 2021, 26, 631–649. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Flexas, J.; Carriquí, M.; Coopman, R.E.; Gago, J.; Galmés, J.; Martorell, S.; Morales, F.; Diaz-Espejo, A. Stomatal and mesophyll conductances to CO₂ in different plant groups: Underrated factors for predicting leaf photosynthesis responses to climate change? Plant Sci. 2014, 226, 41–48. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.M.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Kromdijk, J. Govindjee, Chlorophyll fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Y.C.; Sang, Y.; Li, Q.H.; Yang, H.Q. A role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening. Proc. Natl. Acad. Sci. USA 2005, 102, 12270–12275. [Google Scholar] [CrossRef]
- Grieco, M.; Roustan, V.; Dermendjiev, G.; Rantala, S.; Jain, A.; Leonardelli, M.; Neumann, K.; Berger, V.; Engelmeier, D.; Bachmann, G.; et al. Adjustment of photosynthetic activity to drought and fluctuating light in wheat. Plant Cell Environ. 2020, 43, 1484–1500. [Google Scholar] [CrossRef]
- Li, X.P.; Bjorkman, O.; Shih, C.; Grossman, A.R.; Rosenquist, M.; Jansson, S.; Niyogi, K.K. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 2000, 403, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Nosalewicz, A.; Okon, K.; Skorupka, M. Non-Photochemical Quenching under Drought and Fluctuating Light. Int. J. Mol. Sci. 2022, 23, 5182. [Google Scholar] [CrossRef]
- Li, L.J.; Gu, W.R.; Li, J.; Li, C.F.; Xie, T.L.; Qu, D.Y.; Meng, Y.; Li, C.F.; Wei, S. Exogenously applied spermidine alleviates pho-tosynthetic inhibition under drought stress in maize (L.) seedlings associated with changes in endogenous polyamines and phyto-hormones. Plant Physiol. Biochem. 2018, 129, 35–55. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Liu, M.X.; Gu, W.; Chen, Z.Y.; Gu, Y.C.; Pei, L.F.; Tian, R. Effect of drought on photosynthesis, total antioxidant capacity, bioactive component accumulation, and the transcriptome of. BMC Plant Biol. 2021, 21, 293. [Google Scholar] [CrossRef]
- Ruban, A.V.; Wilson, S. The Mechanism of Non-Photochemical Quenching in Plants: Localization and Driving Forces. Plant Cell Physiol. 2021, 62, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.P.; Ruban, A.V. Photoprotective Energy Dissipation in Higher Plants Involves Alteration of the Excited State Energy of the Emitting Chlorophyll(s) in the Light Harvesting Antenna II (LHCII). J. Biol. Chem. 2009, 284, 23592–23601. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Neukermans, J.; Queval, G.; Noctor, G.; Harbinson, J. Photosynthetic control of electron transport and the regulation of gene expression. J. Exp. Bot. 2012, 63, 1637–1661. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Roychoudhury, A.; Saha, P.P.; Sengupta, D.N. Differential antioxidative responses of indica rice cultivars to drought stress. Plant Growth Regul. 2010, 60, 51–59. [Google Scholar] [CrossRef]
- Wang, G.Y.; Ahmad, S.; Wang, Y.; Wang, B.W.; Huang, J.H.; Jahan, M.S.; Zhou, X.B.; Shi, C.Q. Multivariate analysis compares and evaluates drought and flooding tolerances of maize germplasm. Plant Physiol. 2023, 193, 339–355. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.N.; Hussain, S.; Zhang, Q.W.; Ashraf, U.; Anjum, S.A.; Ali, I.; Wang, L.C. Maize Tolerance against Drought and Chilling Stresses Varied with Root Morphology and Antioxidative Defense System. Plants 2020, 9, 720. [Google Scholar] [CrossRef]
- Furlan, A.L.; Bianucci, E.; Giordano, W.; Castro, S.; Becker, D.F. Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol. Biochem. 2020, 151, 566–578. [Google Scholar] [CrossRef]
- Xu, Z.S.; Ni, Z.Y.; Li, Z.Y.; Li, L.C.; Chen, M.; Gao, D.Y.; Yu, X.D.; Liu, P.; Ma, Y.Z. Isolation and functional characterization of HvDREB1—A gene encoding a dehydration-responsive element binding protein in Hordeum vulgare. J. Plant Res. 2009, 122, 121–130. [Google Scholar] [CrossRef]
- Pan, R.; Buitrago, S.; Feng, Z.B.; Abou-Elwafa, S.F.; Xu, L.; Li, C.D.; Zhang, W.Y. HvbZIP21, a Novel Transcription Factor from Wild Barley Confers Drought Tolerance by Modulating ROS Scavenging. Front Plant Sci. 2022, 13, 878459. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, W.X.; Cao, F.B.; Wang, Y.Z.; Zhang, G.P.; Chen, Z.H.; Wu, F.B. Overexpression of improves drought tolerance in barley by regulating root ion homeostasis and ROS and NO signaling. J. Exp. Bot. 2020, 71, 6587–6600. [Google Scholar] [CrossRef]
- Shi, S.H.; Zeeshan, M.; Shan, W.N.; Qiu, C.W.; Chen, Z.H.; Wu, F.B. Transcriptome and molecular evidence of HvMORF8 con-ferring drought-tolerance in barley. Plant Physiol. Biochem. 2024, 217, 109289. [Google Scholar] [CrossRef]
- Meng, L.H.; Yang, J.; Guo, W.; Tian, B.; Chen, G.J.; Yang, Y.P.; Duan, Y.W. Differentiation in drought tolerance mirrors the geographic distributions of alpine plants on the Qinghai-Tibet Plateau and adjacent highlands. Sci. Rep. 2017, 7, 42466. [Google Scholar] [CrossRef]
- Sun, P.C.; Hao, R.R.; Fan, F.J.; Wang, Y.; Zhu, F.Y. Adaptation of High-Altitude Plants to Plateau Abiotic Stresses: A Case Study of the Qinghai-Tibet Plateau. Int. J. Mol. Sci. 2025, 26, 2292. [Google Scholar] [CrossRef]
- Dondup, D.; Yang, Y.; Xu, D.D.; Namgyal, L.; Wang, Z.H.; Shen, X.; Dorji, T.; Kyi, N.; Drolma, L.; Gao, L.Y.; et al. Genome diversity and highland-adaptative variation in Tibet barley landrace population of China. Front. Plant Sci. 2023, 14, 1189642. [Google Scholar] [CrossRef]
- Chang, Y.X.; Zhang, J.T.; Bao, G.Z.; Yan, B.R.; Qu, Y.; Zhang, M.Y.; Tang, W.Y. Physiological Responses of Highland Barley Seedlings to NaCl, Drought, and Freeze-Thaw Stress. J. Plant Growth Regul. 2021, 40, 154–161. [Google Scholar] [CrossRef]
- Gopal, J.; Iwama, K. In vitro screening of potato against water-stress mediated through sorbitol and polyethylene glycol. Plant Cell Rep. 2007, 26, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Zahra, N.; Hafeez, M.B.; Kausar, A.; Al Zeidi, M.; Asekova, S.; Siddique, K.H.M.; Farooq, M. Plant photosynthetic responses under drought stress: Effects and management. J. Agron. Crop Sci. 2023, 209, 651–672. [Google Scholar] [CrossRef]
- Hu, C.; Elias, E.; Nawrocki, W.J.; Croce, R. Drought affects both photosystems in Arabidopsis thaliana. New Phytol. 2023, 240, 663–675. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Bae, H. ROS interplay between plant growth and stress biology: Challenges and future perspectives. Plant Physiol. Biochem. 2023, 203, 108032. [Google Scholar] [CrossRef]
- Ahmad, Z.; Waraich, E.A.; Akhtar, S.; Anjum, S.; Ahmad, T.; Mahboob, W.; Hafeez, O.B.; Tapera, T.; Labuschagne, M.; Rizwan, M. Physiological responses of wheat to drought stress and its mitigation approaches. Acta Physiol. Plant 2018, 40, 80. [Google Scholar] [CrossRef]
- Sun, X.M.; Xiong, H.Y.; Jiang, C.H.; Zhang, D.M.; Yang, Z.L.; Huang, Y.P.; Zhu, W.B.; Ma, S.S.; Duan, J.Z.; Wang, X.; et al. Natural variation of confers drought adaptation in upland rice. Nat. Commun. 2022, 13, 4265. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, T.S.R.S.; Godi, S. Drought stress tolerance in rice: Advances in physiology and genetics research. Plant Physiol. Rep. 2023, 28, 349–361. [Google Scholar] [CrossRef]
- Tian, G.; Wang, S.B.; Wu, J.H.; Wang, Y.X.; Wang, X.T.; Liu, S.W.; Han, D.J.; Xia, G.M.; Wang, M.C. Allelic variation of TaWD40-4B.1 contributes to drought tolerance by modulating catalase activity in wheat. Nat. Commun. 2023, 14, 1200. [Google Scholar] [CrossRef]
- Marchin, R.M.; Backes, D.; Ossola, A.; Leishman, M.R.; Tjoelker, M.G.; Ellsworth, D.S. Extreme heat increases stomatal conductance and drought-induced mortality risk in vulnerable plant species. Glob. Change Biol. 2022, 28, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zhao, X.H.; Xia, L.; Jiang, C.J.; Wang, X.G.; Han, Y.; Wang, J.; Yu, H.Q. Effects of potassium deficiency on photosynthesis, chloroplast ultrastructure, ROS, and antioxidant activities in maize (Zea mays L.). J. Integr. Agr. 2019, 18, 395–406. [Google Scholar] [CrossRef]
- Khatri, K.; Rathore, M.S. Salt and osmotic stress-induced changes in physio-chemical responses, PSII photochemistry and chlorophyll a fluorescence in peanut. Plant Stress 2022, 3, 100063. [Google Scholar] [CrossRef]
- Zeng, X.Q.; Yuan, H.J.; Dong, X.K.; Peng, M.; Jing, X.Y.; Xu, Q.J.; Tang, T.; Wang, Y.L.; Zha, S.; Gao, M.; et al. Genome-wide Dissection of Co-selected UV-B Responsive Pathways in the UV-B Adaptation of Qingke. Mol. Plant 2020, 13, 112–127. [Google Scholar] [CrossRef]
- Hubbart, S.; Smillie, I.R.A.; Heatley, M.; Swarup, R.; Foo, C.C.; Zhao, L.; Murchie, E.H. Enhanced thylakoid photoprotection can increase yield and canopy radiation use efficiency in rice. Commun. Biol. 2018, 1, 22. [Google Scholar] [CrossRef]
- Bano, H.; Athar, H.U.R.; Zafar, Z.U.; Ogbaga, C.C.; Ashraf, M. Peroxidase activity and operation of photo-protective component of NPQ play key roles in drought tolerance of mung bean [Vigna radiata (L.) Wilcziek]. Physiol. Plant. 2021, 172, 603–614. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chen, G.; Zeng, F.R.; Han, Z.G.; Qiu, C.W.; Zeng, M.; Yang, Z.J.; Xu, F.; Wu, D.Z.; Deng, F.L.; et al. Molecular evidence for adaptive evolution of drought tolerance in wild cereals. New Phytol. 2023, 237, 497–514. [Google Scholar] [CrossRef]
- Wingler, A.; Quick, W.P.; Bungard, R.A.; Bailey, K.J.; Lea, P.J.; Leegood, R.C. The role of photorespiration during drought stress: An analysis utilizing barley mutants with reduced activities of photorespiratory enzymes. Plant Cell Environ. 1999, 22, 361–373. [Google Scholar] [CrossRef]
- Hu, H.C.; He, B.B.; Ma, L.; Chen, X.; Han, P.L.; Luo, Y.L.; Liu, Y.H.; Fei, X.T.; Wei, A.Z. Physiological and transcriptome analyses reveal the photosynthetic response to drought stress in drought-sensitive (Fengjiao) and drought-tolerant (Hanjiao) Zanthoxylum bungeanum cultivars. Front. Plant Sci. 2022, 13, 968714. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Liu, H.L.; Yu, F.L.; Hu, B.W.; Jia, Y.; Sha, H.J.; Zhao, H.W. Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci. Rep. 2019, 9, 8543. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Jiang, C.K.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G.X. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef]
- Shirvani, H.; Mehrabi, A.A.; Farshadfar, M.; Safari, H.; Arminian, A.; Fatehi, F.; Pouraboughadareh, A.; Poczai, P. Investigation of the morphological, physiological, biochemical, and catabolic characteristics and gene expression under drought stress in tolerant and sensitive genotypes of wild barley [Hordeum vulgare subsp. spontaneum (K. Koch) Asch. & Graebn.]. BMC Plant Biol. 2024, 24, 214. [Google Scholar]
- Miller, G.; Suzuki, N.; Rizhsky, L.; Hegie, A.; Koussevitzky, S.; Mittler, R. Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol. 2007, 144, 1777–1785. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Fahlgren, N.; Feldman, M.; Gehan, M.A.; Wilson, M.S.; Shyu, C.; Bryant, D.W.; Hill, S.T.; McEntee, C.J.; Warnasooriya, S.N.; Kumar, I.; et al. A Versatile Phenotyping System and Analytics Platform Reveals Diverse Temporal Responses to Water Availability in. Mol. Plant 2015, 8, 1520–1535. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Niu, L.P.; Bo, L.; Qin, Z.M.Y.; Dawa, D.; Lhundrup, N.; Hou, X. Transcriptome analysis of the drought response in hulless barley. J. Biol. 2025, 41, 9–15. [Google Scholar]
- Wang, Y.T.; Zheng, Y.T.; Shi, Y.F.; Jiang, D.Y.; Kuang, Q.; Ke, X.S.; Li, M.; Wang, Y.K.; Yue, X.H.; Lu, Q.; et al. YELLOW, SERRATED LEAF is essential for cotyledon vein patterning in Arabidopsis. Plant Physiol. 2024, 196, 2504–2516. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Che, Y.F.; Garcia, V.J.; Shen, J.Q.; Zheng, Y.T.; Su, Z.Z.; Zhu, L.; Luan, S.; Hou, X. Cyclophilin 37 maintains electron transport via the cytochrome b6/f complex under high light in Arabidopsis. Plant Physiol. 2023, 192, 2803–2821. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, L.; Bo, L.; Chen, S.; Qin, Z.; Dondup, D.; Namgyal, L.; Quzong, X.; Ga, Z.; Zhang, Y.; Shi, Y.; et al. Comprehensive Evaluation and Construction of Drought Resistance Index System in Hulless Barley Seedlings. Int. J. Mol. Sci. 2025, 26, 3799. https://doi.org/10.3390/ijms26083799
Niu L, Bo L, Chen S, Qin Z, Dondup D, Namgyal L, Quzong X, Ga Z, Zhang Y, Shi Y, et al. Comprehensive Evaluation and Construction of Drought Resistance Index System in Hulless Barley Seedlings. International Journal of Molecular Sciences. 2025; 26(8):3799. https://doi.org/10.3390/ijms26083799
Chicago/Turabian StyleNiu, Liping, La Bo, Shuaihao Chen, Zhongmengyi Qin, Dawa Dondup, Lhundrup Namgyal, Xiruo Quzong, Zhuo Ga, Yanming Zhang, Yafei Shi, and et al. 2025. "Comprehensive Evaluation and Construction of Drought Resistance Index System in Hulless Barley Seedlings" International Journal of Molecular Sciences 26, no. 8: 3799. https://doi.org/10.3390/ijms26083799
APA StyleNiu, L., Bo, L., Chen, S., Qin, Z., Dondup, D., Namgyal, L., Quzong, X., Ga, Z., Zhang, Y., Shi, Y., & Hou, X. (2025). Comprehensive Evaluation and Construction of Drought Resistance Index System in Hulless Barley Seedlings. International Journal of Molecular Sciences, 26(8), 3799. https://doi.org/10.3390/ijms26083799





