Abstract
Climate change poses significant threats to agriculture globally, particularly in arid and semi-arid regions where drought stress (DS) is most severe, disrupting ecosystems and constraining progress in agriculture and horticulture. Roses, valued for their aesthetic appeal, are highly susceptible to abiotic stresses, especially DS, which markedly reduces flower quantity and quality. Under DS conditions, roses exhibit diverse morphological, physiological, biochemical, and molecular adaptations that vary across species. This review examines the effects of DS on rose growth, yield, and physiological traits, including gas exchange, photosynthesis, phytohormone dynamics, and water and nutrient relationships, alongside their biochemical and molecular responses. Furthermore, DS impacts the biosynthesis of secondary metabolites, notably reducing the yield and quality of essential oils in roses, which are critical for their commercial value in perfumery and aromatherapy. Additionally, the impact of DS on rose flower quality and post-harvest longevity is assessed. By elucidating these diverse responses, this review provides a framework for understanding DS effects on roses and offers insights to develop strategies for mitigating its adverse impacts.
1. Introduction
Plants thrive in dynamic environments that often impose challenging conditions on their growth and development. Environmental stressors, classified as biotic or abiotic, significantly impact plant life, with abiotic stressors such as drought and salt stress (Figure 1) impairing growth, development, and productivity [1,2]. DS, a critical stressor in horticulture and floriculture, reduces crop yield, limits growth, and disrupts the synthesis of secondary metabolites—an escalating concern amid evolving global climate patterns [3,4,5,6,7]. The Intergovernmental Panel on Climate Change (IPCC) projects that this trend will continue, with average temperatures expected to rise by 1.8–4.0 °C by 2100, exacerbating drought prevalence in many regions and posing a significant threat to agricultural productivity and efficiency [8]. This scenario presents a major challenge for the scientific community aiming to develop sustainable strategies to mitigate DS impacts and ensure global food and horticultural security.
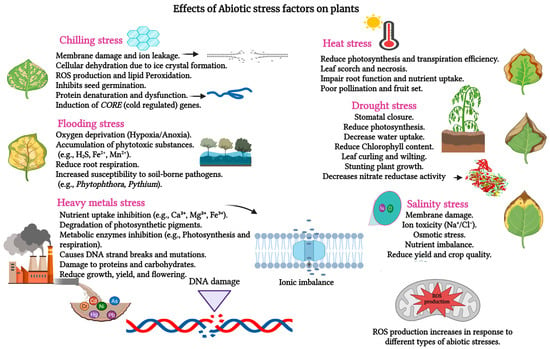
Figure 1.
Different kinds of abiotic stresses and their effects on plants. Macronutrients: Ca2+—Calcium. Micronutrients: Fe2+—Ferrous Iron, Fe3+—Ferric Iron, Mn2+—Manganese, Ni—Nickel, Na+—Sodium, Cl−—Chloride. Toxic Elements: Pb—Lead, As—Arsenic, Cd—Cadmium, Cr—Chromium, Hg—Mercury. Signaling Compound: H2S—Hydrogen Sulfide.
Roses (Rosa spp. L.), a key genus in the Rosaceae family, encompass nearly 200 species and over 30,000 modern cultivars distributed across diverse climatic zones globally [9,10,11,12]. Its value is due to its economic, cultural, and symbolic significance; roses dominate floriculture, with cut roses comprising 32% of the global cut flower market [13]. The global rose market, valued at USD 525.94 million in 2023, is projected to reach USD 1092.77 million by 2032, with a compound annual growth rate (CAGR) of 8.7% from 2024 to 2032 [14]. Europe held the largest market share in 2023, led by the Netherlands, the top exporter with a USD 939 million market share, followed by Ecuador (USD 850 million) and Kenya (USD 581 million), where rose cultivation supports millions of livelihoods [15]. Their adaptability to varied environments and climates makes them a preferred choice for cultivation in gardens, parks, public spaces, and floriculture [16,17]. For centuries, their diverse colors, shapes, and fragrances have made them a favored flower, widely used as cut flowers and perfume ingredients, and a valuable resource in the medical, cosmetic, and food industries, rendering them a profitable agricultural crop in many countries [17,18]. However, their wide geographical distribution and extensive cultivation increase their vulnerability to adverse environmental conditions, particularly DS, significant abiotic stress in horticulture and floriculture that threatens global rose production by impairing growth, yield, and the biosynthesis of economically critical compounds, thereby affecting both quantity and quality [3,4,19,20]. Water scarcity, caused by insufficient rainfall or uneven distribution of moisture, accounts for about 70% of global crop yield and productivity losses, posing a serious threat to agriculture and floriculture [21,22,23].
For roses, a valuable ornamental crop, developing effective DS management strategies and predicting plant responses requires a comprehensive understanding of their multifaceted responses to DS, encompassing morphological, physiological, biochemical, and molecular dimensions, as examined in this review. Insights into roses’ adaptive and survival mechanisms in arid environments will enhance our understanding of plant responses to abiotic stress, facilitating the development of targeted strategies to mitigate DS impacts.
2. Plant DS: Causes and Classification
2.1. Causes of Water Deficiency in Plants
DS arises when the soil water available to plants diminishes over time due to insufficient soil moisture, driven by climate change-related factors such as altered precipitation patterns, elevated atmospheric carbon dioxide levels, and rising air temperatures [24,25,26]. High-intensity light and dry tropical winds further exacerbate DS by accelerating soil water evaporation and dehydration [27]. Notably, plants can experience DS even when soil moisture is adequate, as environmental conditions like salinity, low soil temperatures, or waterlogging impair root water absorption, leading to a condition known as physiological drought or pseudo-drought [24,28].
2.2. Classification of DS
DS can be categorized into three severity levels based on relative water content (RWC), a reliable indicator of drought intensity: mild (RWC 60–70%), moderate (RWC 40–60%), and severe (RWC 0–40%). Severe DS significantly impairs plant function, reducing photosynthetic activity, inhibiting growth and development, and, with prolonged exposure, ultimately leading to plant death [29].
3. Effect of DS on the Morphology, Physiology, and Biochemistry of Roses
DS exerts complex effects on plants, impacting a wide range of morphological, physiological, biochemical, and molecular characteristics and processes, thereby impairing plant functions at any growth stage affected by DS [30,31]. Figure 2 illustrates the diverse effects of DS on rose plants.
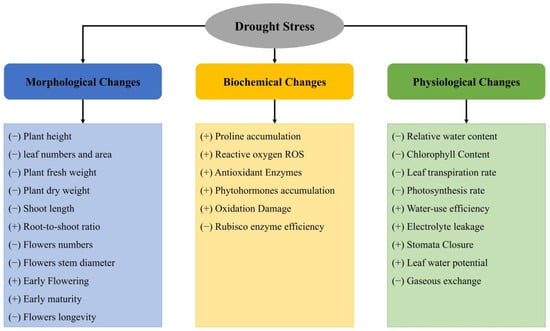
Figure 2.
Effects of DS on the morphology, physiology, and biochemistry of roses. (+) Induce; (−) Reduction. Partially adapted from Seleiman et al. [32].
3.1. Drought-Morphological Attributes in Roses
A lack of water induces significant morphological changes in plants, including reduced fresh and dry weight, decreased leaf area, increased leaf thickness, leaf curling, wilting, accelerated senescence, stomatal closure, and uneven leaf surface stratification [33,34,35,36]. Numerous studies have shown that increasing DS severity leads to a corresponding decline in rose growth parameters and biomass. For example, the Damask rose (R. damascena Mill.), a member of the Rosaceae family originating from Damascus and valued for its aromatic properties in the global cosmetics and pharmaceutical industries, exhibited significant reductions in leaf number, leaf area, and fresh and dry biomass under DS conditions [37,38]. Similarly, DS caused a notable decrease in fresh and dry leaf weight and biomass percentage in the Damask rose cultivars Maragheh and Kashan [39]. In another study, R. damascena var. Dieck trigintipetala was subjected to three water availability levels over 90 days: optimal conditions at full field capacity (FC), mild DS at 50% FC, and severe DS at 25% FC. The plants displayed reduced overall growth, with significant decreases in total fresh and dry weight, greater under severe DS than mild DS, although leaf number remained unaffected [40].
Damask roses subjected to varying levels of water deprivation (70%, 40%, and 10% water availability) exhibited significant declines in growth and flowering characteristics from June to October. DS increased the root-to-shoot ratio throughout the experiment while negatively impacting other growth parameters, including a reduction in main stem length (up to 49.7%), canopy diameter, number of leaves (up to 54%), individual leaf area (up to 64%), total plant leaf area (up to 83%), and overall shoot dry weight, severely limiting plant growth. DS also adversely affected all measured floral traits, such as time to flowering, number of flowers, flower diameter, individual flower dry weight, number of petals, receptacle diameter, and individual petal dry weight. Furthermore, reduced soil water availability accelerated the growth cycle, leading to earlier flowering by up to 7.4 days [41]. In addition, Shi et al. [42] determined that water scarcity contributed to the lower total fresh weight of R. hybrida cv. Charming Black plants in comparison with those exposed to natural irrigation. Williams et al. [43] further demonstrated that even a partial water supply (60–75% of requirements) significantly decreases the fresh-to-dry weight ratio in miniature rose cultivars. In their study, the authors confirmed that water restriction decreased the amount of water in plant tissues, thus reducing the fresh-to-dry weight ratio. A consistent association was established between water deficiency (WD) and a significant reduction in shoot length, shoot weight, and leaf area at different growth stages in the cut rose cultivar Charming Black [42]. R. hybrida cv. Club Nika was exposed to three irrigation schemes (100%, 50%, and 25% of the irrigation requirement), aiming to determine the impact of dehydration on their morphological and qualitative traits. Water-deficient plants showed significantly decreased fresh and dry flowering stem weight than well-irrigated seedlings. The decreases were 19% and 36% at 75% and 50% irrigation levels, respectively. The bud sprouting occurred faster (about 6.83 days) in well-irrigated plants than under stress conditions [44]. As a result of dehydration conditions, the fresh and dry weight of rose seedlings decreased. It is worth noting that the seedlings subjected to extreme water deprivation stress had significantly lower dry weights than those exposed to normal and mild drought stress treatments [45].
In a parallel investigation on roses conducted by Farahani et al. [46], extreme WS led to a 40% decrease in leaf numbers and total leaf area. Conversely, mild stress did not affect leaf quantity but caused a 20% reduction in the dimensions of each leaflet. Katsoulas et al. [47] studied how irrigation frequency affects the dry and fresh weights of cut roses. They proved that high frequencies increased cut flower buds’ dry and fresh weights by 33% compared to low frequencies. Nevertheless, no significant differences in the number of flower stalks were recorded across three distinct irrigation levels [48]. Additionally, Raviv et al. [49] stated that DS induces stomatal closure, decreasing plant turgor and inhibiting expansion growth.
For instance, a decrease in soil moisture content in rose plants reduces shoot length. This finding is consistent with research conducted by Sotelo-Cuitiva et al. [50] on rose seedlings. Water scarcity negatively impacts the quality and amount of roses produced, causing a 20% drop in flower production compared to plants that receive full irrigation, as shown by Farahani et al. [51]; additionally, all water scarcity treatments reduce the number of flower buds. Similarly, it was noted that under fully irrigated conditions, the yield of R. alba L. fluctuated between 3930 and 7700 kg/ha. In contrast, in non-irrigated conditions, it exhibited the same range of 3930 to 7700 kg/ha [52]. Finally, fresh and dried flower weights, quantity, and width significantly declined in water-deficient rose plants relative to adequately irrigated ones [38].
The root system is crucial for plant growth, forming a root–soil interface that enables water and nutrient uptake, particularly under abiotic stresses like drought. Roots are the first to detect soil drying, triggering chemical, hydraulic, and molecular signals that modify plant morphology, phenology, and physiology [53,54,55]. Soil drying exacerbates soil impedance, alters texture, reduces water-holding capacity, and restricts root development [56], often triggering adaptive responses such as an increased root-to-shoot ratio to prioritize water acquisition [57]. In roses, water stress (WS) responses vary significantly across species and cultivars. For example, R. damascena and R. odorata exhibited higher root-to-shoot dry weight ratios under water deprivation, driven by a significant reduction in shoot growth compared to roots [58,59]. Similarly, R. multiflora seedlings under extreme drought (0–10% soil moisture) allocated 1.4 times more biomass to roots than under high water conditions, reflecting a drought tolerance strategy [60]. Conversely, DS reduced the root-to-shoot ratio in some rose cultivars. Al-Yasi et al. and Bolla et al. [40,61] reported significant dry-weight ratios and reduced stem biomass declines, while Cai et al. [62] observed impaired root growth in Belinda’s Dream and Marie Pavie’s garden roses. In contrast, R. hybrida cultivars showed increased root dry weight under WS compared to controls, highlighting genotypic variability in WS responses [63]. In R. hybrid Cv. Rouge Meiland, WS induced by polyethylene glycol (PEG 6000) stimulated root elongation but significantly reduced root biomass. Interestingly, the total root volume and surface area showed no significant differences across treatments [64]. These contrasting findings underscore the need for further research into the genetic and environmental factors that lead to morphological differences in rose root adaptations under DS.
Water shortage adaptive morphological changes in plants, often increasing the root-to-shoot ratio to enhance root system growth while limiting aerial parts in response to reduced water availability [57]. In R. damascena Mill., researchers observed an increase in the dry weight root-to-shoot ratio under DS [54], though Al-Yasi et al. and Bolla et al. [40,61] reported a significant decrease in this ratio in roses. DS also reduced the number of rose stems compared to well-irrigated plants. However, it did not affect the number of high-quality stems or the average length and weight of flower stems, indicating that while DS impacts overall stem production, it does not compromise individual flower stem quality.
Leaf morphology is notably affected by DS, as demonstrated by Jia et al. [65], where R. chinensis Jacq. cv. Old Blush plants subjected to DS for 15, 30, 45, 60, 75, and 90 days, followed by 7 days of rehydration, showed progressive changes: leaves remained intact after 15 days, developed slight wrinkling after 30 days, and exhibited severe wrinkling and wilting by 45 to 90 days, but fully recovered, turning green and thin, after rehydration. Rose species exhibit varying DS tolerance; for instance, cyclic DS testing on four rose rootstocks (R. fortuniana, R. multiflora, R. odorata, and R. hybrida cv. Dr. Huey) revealed that R. fortuniana displayed superior vegetative growth parameters and greater leaf area, while R. odorata experienced severe growth reduction, with R. multiflora and cv. Dr. Huey showed intermediate responses, R. fortuniana being the most drought-tolerant [59]. Blum et al. [66] identified mechanisms reducing leaf area under DS, including decreased cell proliferation and division, leaf curling, and necrosis of apical regions and entire leaves, contributing to the typical DS-induced reduction in leaf area. Additionally, a study on DS tolerance in R. rugosa genotypes (wild type [W] and five lines) showed that the W exhibited the highest tolerance due to structural adaptations such as sunken stomata, robust tissue, and low stomatal density, which minimize water loss and enhance DS resistance [67].
Collectively, the results highlight the negative impact of WD on the morphological traits of rose plants. WD affects bud development, growth, leaf structure, and overall growth, reducing yield and poor crop quality.
3.2. Physiological and Biochemical Responses
3.2.1. Gaseous Exchange
A significant consequence of dehydration in crops is the alteration of gas exchange processes, critical for photosynthesis and respiration, primarily through enhanced stomatal closure and reduced gas exchange due to limited water availability [68]. Key indices for assessing these changes include stomatal conductance (Gs), transpiration rate (E), intracellular CO2 content (Ci), and net photosynthesis (Pn) [69]. In R. damascena Mill., mild DS 50% FC increased Pn and Gs by 31% and 19%, respectively, compared to optimal irrigation, while severe DS 25% FC reduced them by 55% and 36%, respectively [40]. Similarly, Li et al. [45] reported significant declines in Pn, E, and Gs under DS in R. chinensis cv. Mutabilis. Miniature rose cultivars under DS exhibited 25% lower Pn and 63% lower Gs than well-irrigated plants. However, Gs and Pn increased during recovery but remained 35% and 8% lower than the control group [43]. Moreover, Sotelo-Cuitiva et al. [50] indicated that rose seedlings under DS had lower Gs, with leaf photosynthetic indices (Pn, Gs, E) significantly affected, though Ci remained constant; higher DS levels also reduced Pn by 27% compared to adequately irrigated plants [43]. Moreover, higher water-stressed plants showed a 27% decrease in Pn compared to adequately irrigated plants [44]. Additionally, a study on six R. rugosa genotypes under DS, induced by varying levels of PEG-6000, showed reductions in E, Pn, and Gs, with a 5% PEG treatment causing a 29.42% decrease in E; the wild-type genotype displayed superior drought tolerance, as evidenced by higher Pn and Gs values [67].
3.2.2. Photosynthesis Parameters: Chlorophyll Content and Photosynthesis
Photosynthesis is a fundamental metabolic process that profoundly affects plant growth and production. Drought can significantly affect this process by impeding plants’ typical photosynthesis rate and gas exchange [70]. Limited water availability causes stomatal closure, which restricts the delivery of carbon dioxide (CO2) to the leaves—an essential component for photosynthesis—and promotes the production of reactive oxygen species (ROS) [71], as illustrated in Figure 3. In addition, water deficiency (WD) increases leaf temperature, inhibiting the synthesis of photosynthetic pigments [72]. DS also diminishes the activity of key photosynthetic enzymes, such as Rubisco, slowing down photosynthesis and damaging its machinery [73]. According to Mishra et al. and Sher et al. [35,68], plant pigments, particularly chlorophyll, are vital for photosynthesis as they absorb light and generate reducing power. DS hampers the ability of mesophyll cells to utilize available CO2, leading to a reduction in chlorophyll content, as reported by Sarwar et al. [69]. A study on Damask roses demonstrated that increasing DS decreased total chlorophyll (T Chl), chlorophyll A, and chlorophyll B concentrations. Among these, chlorophyll B was most affected, exhibiting a 37% reduction under mild water stress (MWS) and a 54% reduction under severe water stress (SWS). Conversely, the chlorophyll A/B ratio increased by 29% under MWS and 46% under SWS conditions [37].
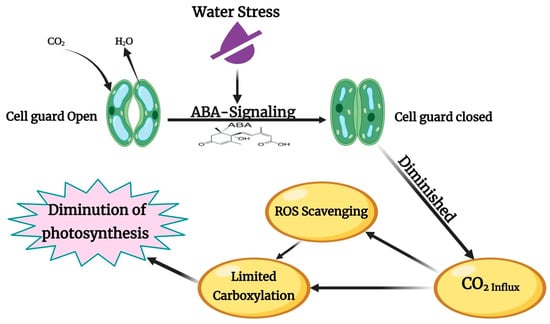
Figure 3.
Effect of drought on the photosynthesis process, modified from Farooq et al. [74]. ABA—Abscisic acid. CO2—carbon dioxide. H2O—water.
The findings align with Seyed Hajizadeh et al. [39], who reported that as the PEG concentration increased, Damask rose genotypes of the cultivars Maragheh and Kashan’s chlorophyll content decreased by up to 30% and 41%, respectively, suggesting that some rose genotypes may be more resistant to WS than others. In contrast, Adamipour et al. [75] found elevated chlorophyll content in R. canina L. and R. damascena Mill. under DS compared to well-watered conditions, suggesting adaptive traits like R. canina’s genetic capacity to retain green leaves during stress. However, studies by Dolatkhahi et al. and Li et al. [44,76] observed no significant impact of water deprivation on photosynthetic pigments in rose plants. On the other hand, R. hybrida cv. Charming Black [77], R. rubiginosa [78], and R. damascena cv. Cachan 93 [41] exhibited reduced ph-osynthetic pigments under water-deficit conditions, which was attributed to impaired photosynthetic mechanisms.
3.2.3. Water-Relations
WD significantly impacts plant-water interactions, affecting key parameters such as RWC, water use efficiency (WUE), transpiration rate (Tr), leaf water potential (ψ), stomatal resistance, membrane integrity index, and leaf temperature, all of which are critical for plant growth [74,79]. RWC is an essential indicator of plant hydration, reflecting the metabolic activity in tissues and providing a valuable measure for assessing plant resilience to (DS) [74]. Furthermore, Farooq et al. [31] suggest that leaf water potential (LWP) is a useful tool for evaluating the extent of soil WS experienced by plants, offering critical insights into the plant-water relationship. According to Noctor et al. [80], RWC is an early response to DS, followed by a decline in leaf water potential (LWP) and stomatal closure. DS reduces RWC and LWP in Damask roses, intensifying effects under severe conditions [37,40]. Under severe water stress (SWS), leaf water potential decreased by 72% compared to the control, while mild water stress (MWS) resulted in a 46% reduction; additionally, leaf water content (LWC) decreased significantly by 17% under SWS [40]. Adamipour et al. and Alavi et al. [75,81] observed consistent DS impacts in R. hybrida cv. Red, R. canina, and R. damascena, while De Dauw et al. [82] reported comparable results in tetraploid and diploid roses under water scarcity. Li et al. [76] found that the LWC of 2-month-old rose seedlings decreased significantly under MWS and SWS, reaching 41% and 15% of the control, respectively. A 90-day water deficit in R. chinensis cv. Old Blush led to the lowest recorded values for LWC and soil water content (SWC) [65], a trend also observed by Sotelo-Cuitiva et al. [50] in Charlotte roses grafted onto Natal Briar under DS. Additionally, rose cultivars Poul Happy Charming Parade and Polabian Bianca Parade exhibited higher WUE under DS compared to unstressed plants [43], while excessive irrigation led to lower WUE values [45,49,61,83]. This is consistent with findings by Shangguan et al. and Kapoor et al. [84,85], who noted that drought-stressed plants often have elevated WUE. However, Aalam et al. [41] reported that soil water deficits don’t consistently affect photosynthetic or shoot WUE in roses, highlighting variability in these responses.
3.2.4. Nutrient Relations
In addition to its detrimental effects on plant growth and productivity, DS impairs nutrient uptake by limiting water availability. Water shortage reduces nutrient absorption by roots and their subsequent translocation to shoots [86,87,88], primarily due to decreased root growth, reduced nutrient flow per unit root length, and lower root biomass under low soil moisture conditions [89]. DS negatively impacts the uptake of essential minerals, including nitrogen (N), phosphorus (P), calcium (Ca), silicate, and magnesium (Mg), often resulting in stunted growth and development in stressed plants [90]. A recent study on roses revealed that irrigation levels influence nutrient accumulation in leaves, with nutrients such as potassium (K+), P, Mg, and zinc (Zn) showing reduced accumulation in shoots under a subsurface drip irrigation (SDI) system operating at 50% FC [81]. Similarly, in R. damascena Mill., DS induced by PEG decreased leaf K+ and P contents by 56% and 52% in the Maragheh cultivar and by 47% and 52% in the Kashan cultivar, respectively, while iron (Fe) content dropped by up to 49% in Maragheh and 51% in Kashan with increasing PEG levels [39]. However, previous research on drought-stressed rose leaves reported variable accumulation of inorganic elements, with K+ levels increasing while Ca and chloride (Cl−) either decreased or remained unchanged [40]. Elevated K+ concentration plays a critical role in drought tolerance by regulating osmotic pressure, modulating membrane potential, facilitating CO2 fixation, supporting the translocation of photosynthetic products to sink organs, and reducing electron transfer to O2, thereby lowering reactive oxygen species (ROS) levels and promoting sustainable growth and yield under arid conditions [91,92]. Furthermore, Luo et al. [93] found that adequate water availability significantly reduced N and P levels in the leaves of two R. roxburghii cultivars (Gui 2 and Gui 7), while DS had no significant effect on leaf K+ levels.
3.2.5. Phytohormone Regulation
Plant hormones, or phytohormones, are essential regulators of plant growth, development, and responses to abiotic stresses, particularly drought and salinity [94,95,96]. They mitigate the adverse effects of DS through physiological and developmental adaptations, such as maintaining osmotic balance, inducing stomatal closure, and regulating root growth to enhance water uptake [95,97,98,99]. The mechanisms of action vary by hormone [100], with abscisic acid (ABA), gibberellins (GAs), ethylene (ET), jasmonates (JA), and salicylic acid (SA) enhancing DS tolerance by altering molecular processes through cell signaling pathways [101]. For instance, Li et al. [45] found that ABA, a primary drought-responsive hormone, increases in rose leaves and roots during DS adaptation, underscoring its role in stress response.
Additionally, auxins promote DS tolerance by stimulating lateral root formation to enhance water absorption and by upregulating stress-responsive genes, which increase ABA synthesis and modulate antioxidant enzyme activity, thereby reducing water loss [102,103]. Notably, higher ABA levels and lower GA concentrations are associated with improved plant resistance to DS [104]. Table 1 summarizes the roles of phytohormones in rose plants under DS conditions. Moreover, phytohormones regulate plant responses to DS by modulating transcription factors (TFs), a key step in cell signaling pathways. In R. hybrida, the DS-tolerance gene RhMED15a is upregulated by ABA treatment, enhancing its expression, whereas methyl jasmonate (MeJA) downregulates RhMED15a, suggesting that JA may reduce the gene’s sensitivity to DS [105]. Furthermore, changes in JA signalling pathways under DS indicate a broader role for JA in plant responses to drought stress [105].

Table 1.
Hormonal Changes in Rose Plants in Response to DS.
3.2.6. Oxidative Stress: Production of Reactive Oxygen Species and Adaptive Responses
Under abiotic stresses like DS, plants accumulate ROS, including hydrogen peroxide (H2O2), hydroxyl radicals (OH·), superoxide radicals (O2−), and singlet oxygen (1O2), which disrupt normal physiological functions [109,110,111]. At low concentrations, ROS serve as signaling molecules, activating stress-responsive pathways and enabling intercellular communication [112]. The interplay between ROS-producing and scavenging enzymes, along with the antioxidant system, maintains cellular redox homeostasis by regulating intracellular ROS concentrations in response to environmental stresses [112]. However, elevated ROS levels are phytotoxic, impairing plant growth and development by disrupting essential biological and physiological processes [113,114,115,116]. Excessive ROS cause oxidative damage through mechanisms such as lipid peroxidation, malondialdehyde (MDA) production, and cell death, targeting proteins, DNA, RNA, cellular membranes, and other biomolecules [109,117,118,119]. DS exacerbates this damage by increasing ROS production, leading to oxidative stress and amplifying the detrimental effects on plant cells [120]. ROS thus play a dual role as byproducts of oxygen metabolism, regulating cellular redox status and activating stress responses while contributing to oxidative damage under adverse conditions, a balance critical for plant survival [112].
Oxidative stress can be assessed by analyzing different substances, such as malondialdehyde (MDA), a byproduct of lipid peroxidation, hydrogen peroxide (H2O2) and electrolyte leakage (EL) [117,121,122]. In drought-stressed rose species, such as R. damascena and R. canina, elevated levels of MDA and H2O2 accumulate [75]. This is consistent with findings that R. canina exhibits higher H2O2 levels during DS [123]. Similarly, in R. chinensis, MDA concentrations peaked after 90 days of DS but decreased upon re-watering [65]. Several R. rugosa genotypes also showed increased MDA and reactive oxygen species (ROS) levels with increasing DS severity [67]. DS-induced rose petals exhibited higher H2O2 and MDA levels, which subsequently decreased after rehydration, mirroring observations in dehydrated and rehydrated cut roses [124]. Prolonged DS in cut roses led to O2− accumulation in petals, potentially aiding in repairing cell membrane damage, with levels decreasing upon rehydration [125]. Under adverse conditions like DS, plants activate robust antioxidant defense mechanisms to mitigate ROS-induced cellular damage [126,127]. These mechanisms involve enzymatic antioxidants, such as ascorbate peroxidase (APX), guaiacol peroxidase (GPX), superoxide dismutase (SOD), dehydroascorbate reductase (DHAR), and glutathione reductase (GR), as well as non-enz-ymatic antioxidants, including glutathione (GSH) and ascorbate (AsA) [109,126,128,129]. These components work synergistically across subcellular compartments to neutralize ROS or regenerate antioxidants, often using energy from sunlight [109,130,131,132,133]. Adequate water supply maintains a balance between ROS production and neutralization, limiting the formation of harmful oxygen metabolites. However, DS disrupts this balance, causing a rapid increase in intracellular ROS levels. The plant’s stress response can be assessed by the presence and activity of antioxidant molecules and enzymes, as detailed in Table 2.

Table 2.
Antioxidant enzyme activity in response to DS.
3.2.7. Osmotic Adjustment (OA)
Adapting DS involves multiple morphological, physiological, and biochemical pathways [58,136]. Osmoregulation, a critical physiological mechanism, enables plants to mitigate the adverse effects of osmotic stress, such as DS and salinity, by synthesizing osmotically active compounds and ions, including organic acids, soluble sugars, proline, glycine betaine, phenolic compounds, calcium (Ca2+), potassium (K+), and chloride (Cl−) ions [137,138,139]. These osmoprotectants allow plants to sustain growth, photosynthesis, and cell division under water-scarce conditions by promoting intracellular water uptake and retention, maintaining cell turgor, protecting against dehydration-induced damage, and stabilizing subcellular structures like membranes and proteins [138,140,141,142,143]. Proline, in particular, plays a vital role in preventing protein degradation and stabilizing cellular structures [33,143]. For instance, R. damascena seedlings exposed to DS for 90 days exhibited elevated proline and soluble sugar levels compared to unstressed controls, with proline increasing by 9.9% and 34.6% and soluble carbohydrates by 22.8% and 33.6% under mild and severe DS, respectively [40]. Similarly, Adamipour et al. [123] reported a significant increase in proline levels in DS-stressed rose plants, rising from 14.5 mM under optimal conditions to 33.8 mM at 50% FC and 75.5 mM at 25% FC. Comparable trends were observed by Luo et al. [93] in R. roxburghii Tratt., though Dolatkhahi et al. [44] noted relatively stable proline levels in rose leaves under DS. Miniature roses (R. hybrida) subjected to repeated DS cycles demonstrated osmotic adaptation through a decreased osmotic potential, driven by the accumulation of solutes like proline, soluble carbohydrates, and K+ rather than Na+ [144]. Rose plants subjected to continuous deficit irrigation (SDI) exhibited a 23.71% increase in total soluble carbohydrates compared to those not receiving SDI. Additionally, leaves from SDI-treated plants displayed higher proline concentrations [81]. Additionally, in arid environments, R. rubiginosa displays elevated soluble carbohydrate levels and slower water loss compared to humid sites; however, under drought stress (DS), these carbohydrates primarily accumulate rather than being utilized for growth or secondary metabolism [78,106,145,146].
3.2.8. Influence of DS on Rose Petal Essential Oil Yield and Composition
Roses are highly valued as cut flowers for their aesthetic appeal and significant economic importance, primarily due to the essential oils in their petals [147]. Rose oil, one of the most expensive essential oils globally, requires approximately 3000 kg of rose petals to produce 1 kg, reflecting its low yield [148]. The global rose oil market, valued at USD 1.6 billion in 2022, is projected to grow from USD 1.67 billion in 2023 to USD 2.5 billion by 2032, with an expected CAGR of 4.56% from 2024 to 2032 [149]. The distinctive fragrance of rose oil arises from volatile aromatic compounds, including monoterpenes like linalool, beta-citronellol, and geraniol, as well as stress-related compounds like benzaldehyde [150]. Citronellol and geraniol are critical for rose oil’s quality, making it a key ingredient in perfumery, cosmetics, and pharmaceuticals [151,152,153]. Thus, understanding the factors that influence the production and quality of this essential oil is crucial, especially for producers [154].
Soil WD negatively impacts essential oil yield, intensifying effects as WS increases [41]. However, Farahani et al. [51] reported a 20% increase in crucial oil concentration in rose flowers under water deficit compared to well-watered conditions. This enhancement under drought is supported by Yousefi’s study of 49 Iranian damask rose landraces [155], which showed higher flower yields and essential oil content in landraces from temperate, warm, and arid regions compared to those from cooler, semi-arid, or humid areas. The composition of essential oils, critical to their quality and economic value, is influenced by environmental stressors [156]. Water shortage increases compounds like linalool, citronellol, geraniol, eugenol, methyl eugenol, and dodecanoic acid, likely protecting plant cells from oxidative damage [50]. Kiymaz et al. [157] found that R. damascena essential oil maintained quality under reduced irrigation (I1.00 to I0.50), with gas chromatography–mass spectrometry (GC-MS) showing minimal changes in key components like citronellol, nerol, and geraniol attributed to stable water use efficiency (WUE). However, lower water and nitrogen levels decreased essential oil yield, indicating a trade-off between stress adaptation and production during flowering. Similarly, Uçar et al. [158] demonstrated that drought (no irrigation) reduced flower production and oil yield in R. damascena but enhanced oil quality by increasing citronellol, geraniol, and nerol levels.
We compiled key findings across various rose species and cultivars to provide a comprehensive overview of roses’ morphological, physiological, and biochemical responses to DS. Table 3 summarizes these responses, focusing on the effects of differing DS severities, resistance means, and associated processes. This compilation underscores the complexity of rose adaptation to DS and establishes a foundation for developing targeted strategies to enhance their resilience.

Table 3.
Morphological, physiological, and biochemical responses of rose species and cultivars to DS: Effects of severity and resistance mechanisms.
4. Influence of Dehydration Stress on Rose Quality and Postharvest Longevity
Water relations are critical in regulating the lifespan of cut flowers, including cut roses, as transpiration-induced water loss significantly shortens vase life and impairs quality during the post-harvest stage [159]. WS occurs when water loss through stomata exceeds absorption, leading to wilting, premature senescence, impaired flower growth and development, and a reduced vase life [124,125,160]. For example, DS in cut roses caused abnormal flower opening and reduced flower size [124,125,161]. Stomatal responses to DS vary across cut rose cultivars; a study on cut roses subjected to long-term dry storage at low temperatures revealed differing stomatal opening and transpiration rates among cultivars [162]. Depending on the cultivar, this variability highlights a strong correlation between stomatal function, DS tolerance, and vase life maintenance [162].
Additionally, exposing cut R. hybrida cv. Wild Look to air for 1–3 h without stem submersion in water increased stomatal opening under dark conditions, elevating transpiration rates [163]. Thus, regulating stomatal closure is essential for enhancing DS tolerance and extending vase life in cut roses [164].
DS significantly reduces water availability, impairing the quality and longevity of rose flowers, as evidenced by numerous studies across various DS scenarios and growth stages (Table 4). Beyond its impact on water relations, DS also alters morphological and developmental traits, leading to bud deformation, shortened petal length, and petal deformities in roses, as reported by Shi et al. [42]. These findings align with observations in Damask roses, where DS affects multiple floral traits, including flowering time, flower quantity, size, dry weight, number of petals, receptacle diameter, and individual petal dry weight, confirming its adverse impact on rose quality [41].

Table 4.
Summarize the impact of WS on water relations, rose flower quality, and longevity.
5. Molecular Responses to Drought Stress
Under adverse conditions, plants have evolved complex adaptive mechanisms to tolerate low soil moisture [166,167]. At the molecular level, plants respond to DS through coordinated regulatory mechanisms involving cellular signalling pathways and transcriptional regulation, enabling specific gene responses to stress [168,169]. Generally, plants react to DS in three key steps: signal perception, signal transduction, and expression of stress responses, which trigger physiological and metabolic adaptations [5,170,171]. Plant cells detect DS stimuli via sensors or receptors primarily on the cell membrane, activating intracellular signaling molecules through second messengers like ROS, Ca2+, NO and H2O2, which then initiate corresponding signaling pathways [172]. Protein phosphorylation and dephosphorylation, mediated by protein kinases and phosphatases, respectively, are critical in these signal transduction pathways, with mitogen-activated protein kinases (MAPKs) and calcium-dependent protein kinases (CDPKs) playing key roles in DS signaling [173]. In the final step, kinases or phosphatases activate or deactivate TFs, which regulate downstream gene expression by binding to specific cis-elements in promoter regions [174]. TFs are further regulated transcriptionally by upstream components [175] and undergo post-transcriptional modifications, such as ubiquitination and sumoylation, for-ming a complex regulatory network that governs stress-responsive gene expression and orchestrates physiological and metabolic processes [176].
Figure 4 comprehensively summarizes the genes and signaling pathways involved in DS tolerance.
In plants, DS induces numerous signaling pathways involving many proteins, such as TFs, functional proteins, enzymes, molecular chaperones, and metabolites [177]. Based on genome-wide analysis, a wide range of transcription factor families relevant to DS responses have been identified in different plant species [178]. Plants use a complex system of TFs to control and mitigate drought damage at different stages of plant life during times of low water availability, which is essential for their growth and development [179].
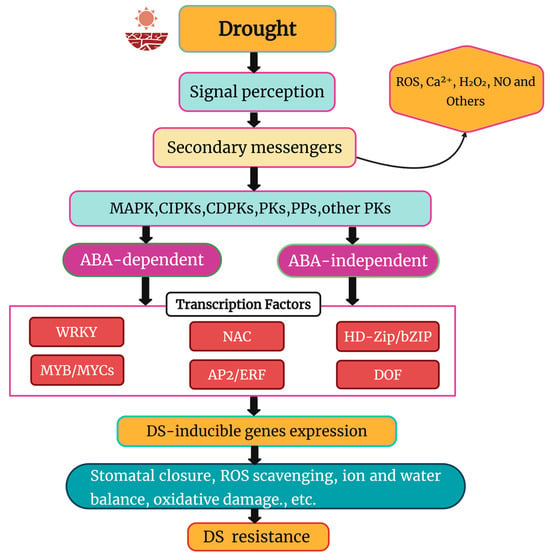
Figure 4.
Schematic representation of DS signaling in plants. Modified from Khan et al. [180]. DS triggers ABA-dependent and ABA-independent pathways, activating transcription factors such as MYB/MYCs, WRKYs, HD-Zip/bZIP, NACs, DOF, and AP2/ERF. ROS—Reactive Oxygen Species, Ca2+—Calcium ions, H2O2—Water, NO—Nitric Oxide, MAPK—Mitogen-Activated Protein Kinase, CIPKs—CBL-Interacting Protein Kinases, CDPKs—Calcium-Dependent Protein Kinases, PKs—Protein Kinases, PPs—Protein Phosphatases, ABA—Abscisic Acid.
TFs regulate a diverse array of genes across various gene families, including NAC, WRKY, HD-Zip/bZIP, AP2/ERF, and MYB, among others, with their activity influenced by factors such as plant species, cultivar, growth stage, and DS severity [181]. Approximately 7% of coding sequences in plant genomes encode TFs, many of which are involved in the immediate early response to environmental stresses [182]. This section explores the prominent TF families contributing to abiotic stress tolerance in rose species, focusing on their roles in coordinating responses to DS in rose plants.
The NAC family, a prominent group of plant-specific TFs, contains approximately 110 genes in Arabidopsis and 150 genes in rice [183]. NAC TFs have been known for their essential role in plant growth and their ability to modulate plant responses to abiotic stresses, including drought, salt and cold stress [183,184,185,186]. RcNAC72 is an important TF gene that responds to WD in rose plants [65,187]. Overexpression of NAC72 in Arabidopsis thaliana has been shown to enhance plant resistance to DS, revealing its potential function in regulating ABA-responsive gene expression [187]. In R. chinensis cv. Old Blush. RcNAC72 expression is significantly elevated in response to ABA, water and salt stress, and cold conditions, while its overexpression in Arabidopsis thaliana enhances dehydration tolerance; conversely, RcNAC72 suppression increases DS resistance and reduces water loss in roses [65]. Similarly, RhNAC2 and RhNAC3, two NAC TFs, enhance DS tolerance in rose flowers by regulating genes involved in osmoregulation and cell wall synthesis, respectively [165,188]. In a related mechanism, RhNAC31 upregulates gene expression to improve tolerance to multiple abiotic stresses in genetically modified plants [189]. Additionally, RcNAC091 enhances DS response in roses via the ABA-dependent pathways [190]. The NAC protein RD26, expressed under DS conditions, aids plants in regulating ABA-induced gene transcription during abiotic stress [191]. Furthermore, Fu et al. [192] demonstrated that DS activates the RD22 (RcBURP4) gene in roses, underscoring its critical role in DS resistance.
The AP2/ERF family of TFs is critical in regulating plant growth, development, and responses to environmental stresses, including drought and salt stress [193,194,195]. In roses, RcDREB2B, an AP2/ERF gene, exhibits reduced expression under DS, though plants with higher expression are more sensitive to PEG, ABA, and salinity [45]. A subsequent study indicates that ethylene response factor 109 (ERF109) is critical for the DS resilience of roses and significantly contributes to WS regulation [65]. Another AP2/ERF TF, RcTINY2, identified in roses, mediates multiple processes and enhances abiotic stress resistance via the ABA pathway [20]. ABA treatment upregulates RcTINY2 transcription in rose leaves but inhibits it in roots, where NaCl and PEG also suppress its expression; while overexpression of RcTINY2 increases sensitivity to ABA, NaCl, and PEG in Arabidopsis, its suppression in roses has minimal impact on drought or salinity tolerance [65]. These findings highlight the diverse roles of the AP2/ERF TF family in modulating rose responses to DS. Internal signals driving osmotic changes have been extensively studied, revealing that RhNAP (involved in ABA responses) and RhCKX6 (involved in cytokinin degradation) confer DS tolerance in immature flowers of R. hybrida cv. Samantha, though this interaction accelerates petal wilting in mature flowers [196]. DS also activates RhABF2, a TF in the ABA signaling pathway, which directly induces RhFer1, a ferritin gene regulating iron levels during DS; this RhABF2/RhFer1 activation enhances DS resistance and supports flower reopening after drought [196]. Additionally, P5CS, encoding pyrroline-5-carbox-ylate synthetase—a key enzyme in proline synthesis—is highly expressed during DS, further aiding stress tolerance [123].
Several studies have confirmed the critical role of Dof TFs in regulating plant responses to abiotic stress, particularly DS [76,197,198]. Studies in maize involving CRISPR/Cas9 and overexpression demonstrated that ZmDOF22 enhances DS resistance by modulating the ABA pathway, promoting stomatal closure, and reducing water loss [199]. Similarly, Md-DOF54 in apples positively regulates drought resistance [198]. In kiwifruit, different abiotic stresses induce varying expression levels of six AcDOF TFs, with drought-resistant cultivars exhibiting significantly higher AcDOF22 expression [200]. After analyzing the rose genome, Nan et al. [201] identified 24 Dof genes (from RchDof1 to RchDof24). In response to drought and salinity, most of these genes showed elevated expression levels, indicating the crucial function of Dof genes in the ability of rose plants to tolerate abiotic stress.
MYBs are a core family of TFs regulating secondary metabolism, interacting with hormones and environmental signals, regulating gene expression and cell differentiation, and increasing the plant’s resistance to abiotic stresses such as water deprivation [202]. According to Jia et al. [65], MYB family members and basic helix-loop-helix (bHLH) TFs, especially bHLH162 and bHLH35, are essential for adapting R. chinensis to cold and DS. In their research, Shang et al. [203] proposed that RcMYB8 modulates the action of two proteins, RcPR5/1 and RcP5CS1. RcP5CS1 promotes proline synthesis, enhancing tolerance to osmotic stress, while RcPR5/1 strengthens plant defense mechanisms. Rose plants overexpressing RcMYB8 showed improved growth, higher survival rates, and enhanced physiological parameters under stress, indicating increased resilience to salinity and DS.
In R. chinensis Jacq., researchers studied RcTCP genes during growth stages under DS using a public RNA-seq dataset, finding that without DS, RcTCP genes are significantly increased, highlighting their essential role in protecting against DS [204]. Furthermore, the SOD2 gene, which helps produce superoxide dismutase two in Escherichia coli, dramatically improves the rose’s ability to handle DS [205]. On the other hand, reducing the activity of RhHB1—a type of protein that helps control gene activity in rose flowers—raises the levels of jasmonate-isoleucine (JA-Ile) and lowers the plant’s ability to tolerate DS; RhHB1 attaches to the RhLOX4 gene’s control region, inhabiting the production of lipoxygenase 4 (RhLOX4) [206].
Mediator’s multiprotein complex acts as an intermediary between RNA polymerase II (Pol II) and DNA-binding transcription factors. It is essential for transcription initiation, elongation, and splicing, among other steps of transcription [207,208]. Moreover, plants’ ability to adapt to various abiotic stresses, including dehydration, cold, and salinity, depends on the mediator modules [209,210]. Recent studies have investigated the function of some mediators in DS tolerance. DS rapidly activates RhMED15a, a mediator in roses, which ABA and MeJA modulate. Inhibition of RhMED15a led to a significant decline in DS tolerance [203].
Furthermore, the scientists identified a novel mediator, RhMED15a-like, in hybrid rose plants. WS and ABA/MeJA treatment decreased its expression. Silencing RhMED15a-like in roses decreased DS tolerance while overexpressing it in Arabidopsis increased osmotic stress tolerance. Moreover, genes of the JA and ABA signalling pathways were more expressed when RhMED15a-like was silenced, while genes that interact with stress were less expressed. Indicating that RhMED15a-like enhances the drought resistance of roses by regulating genes that interact with stress hormones and drought signaling pathways [105]. A genome scan of R. chinensis identified 41 genes encoding CCHC-type zinc finger proteins (RcCCHC-ZFPs), with RcCCHC25 showing significant overexpression in response to lack of water conditions [211]. The qRT-PCR test showed that RcHSP90-1, RcHSP90-5, and RcHSP90-6 are crucial for controlling how cells react to water and salt stress. The findings show that CCHC-type zinc finger proteins and HSP90 genes play a part in R. chinensis’s ability to survive under WS [212].
A study on the WRKY gene family in roses identified eight RcWRKY genes that respond to heat, salinity, and DS conditions, exhibiting both positive and negative regulatory effects on plant stress responses; the rapid activation of RcWRKY14 and RcWRKY16 highlights their potential as candidate genes for further exploring stress resistance pathways in roses [213]. DS significantly impacts cut rose lifespan, particularly in ethylene-sensitive varieties, by increasing ethylene production through the upregulation of biosynthetic genes RhACS1 and RhACS2 (encoding ethylene-1-aminocyclopropane-1-carboxylate synthase), especially in sepals, and by modifying the ethylene receptor gene RhETR3 [161]. Ethylene-sensitive roses under DS sustainably produce ethylene, which impairs flower development and reduces vase life, as reported by Sukpitak et al. and Dar et al. [164,214]. In rose petals, five superoxide dismutase (SOD) genes were analyzed, with four—RhMnSOD1, RhCu/ZnSOD2, RhCu/ZnSOD1, and RhCu/ZnSOD3—showing significant functional decline after cell dehydration, though their expression levels normalized post-rehydration [125]. In Arabidopsis, DS activates MAPK cascade components like MPK4 and MPK6, underscoring their role in DS signaling [215,216,217]. Similarly, RhMKK9, a MAPK cascade component in cut roses, exhibits rapid, high expression in the gynoecium upon desiccation; silencing RhMKK9 significantly reduces ethylene production during rehydration, thereby affecting flower growth and longevity [218]. Indicating that the MAPK cascade is critically essential in plant responses to DS and rehydration, emphasizing its importance in controlling responses to water availability.
Generally, studying TFs and how they contribute to WS tolerance in roses has important implications for improving drought resistance in this valuable ornamental crop.
6. Different Strategies to Mitigate the Adverse Effects of Drought Stress on Rose Plants
To mitigate the adverse effects of DS on plants, effective strategies such as genetic control, soil management, water delivery, foliar spraying, and mulching should be implemented [219], as illustrated in Figure 5. These strategies are discussed in detail below.
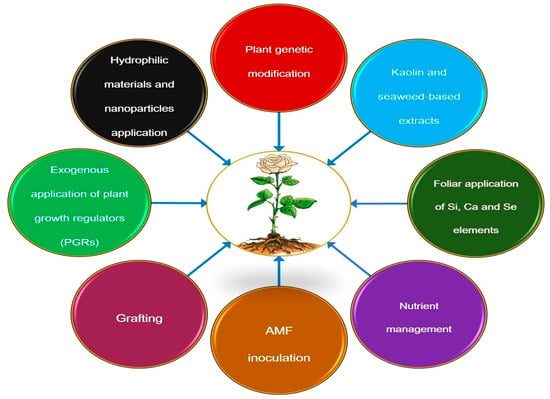
Figure 5.
Schematic representation of an integrated management strategy to improve plant resistance to DS.
Arbuscular mycorrhizal fungi (AMF) are common soil microorganisms that form symbiotic relationships with the roots of approximately 90% of plant species, including angiosperms [220,221]. This symbiosis improves soil structure and enhances plant resist-ance to DS through various physiological, physical, nutritional, and cellular processes [38,222,223,224]. AMF supports seedling survival, facilitates water absorption and transport, modifies root architecture, enhances the effects of plant-produced hormones, and accelerates the removal of ROS from plant cells [108,225,226,227,228].
Multiple studies demonstrate that AMF mitigates DS in rose crops. For instance, AMF-treated Damask roses exhibited reduced susceptibility to DS due to enhanced physiological, biochemical, and water retention factors, leading to increased flower production [38]. Similarly, Augé et al. [229] reported that R. hybrida cv. Love, colonized by Glomus intraradices Schenk and Smith showed greater DS resistance attributed to elevated levels of free amino acids and carbohydrates in the roots. Additionally, Glomus intraradical significantly improved photosynthesis in R. hybrida cv. New Dawn under DS conditions [230]. Inoculation with either Glomus deserticola Trappe, Bloss, and Menge or Glomus intraradices Schenk and Smith also alleviated DS effects in R. hybrida cv. Samantha [231,232]. Green et al. [233] observed that leaves of Glomus intraradices-treated roses exhibited reduced transpiration, suggesting that harvested flowering stems may have an extended vase life.
Plant growth regulators (PGRs), including ABA, salicylic acid (SA), and JA, as well as cytokinins, are effective in promoting plant growth and productivity under water limitation conditions [228,234]. ABA enhances DS resistance by reducing transpiration and sustaining photosynthesis [97,235,236]; its application in spring or summer further improves water retention in R. hybrida flowers, extending their lifespan [237].
Applying nanoparticles offers a novel approach to alleviate DS, as they induce morphological, biochemical, and physiological changes in plants, enhancing DS tolerance by increasing hydraulic conductance, improving root water uptake, modulating proteins involved in hormone pathways, stress signa, redox processes, and ROS scavenging [238,239]. Foliar application of metal oxide nanoparticles, such as titanium dioxide (TiO2), iron oxide (Fe3O4), and zinc oxide (ZnO), has been shown to improve metabolic and physiological processes in plants under DS [240]. Several studies, such as those by Siddiqui et al. and Seleiman et al. [32,241], have shown that silicon nanoparticles (Si-NPs) can help plants cope with the adverse effects of abiotic stresses such as water and salt. Research conducted by Seyed Hajizadeh et al. and Alavi et al. [39,81] clarified that Si-NPs and TiO2 nanoparticle applications enhanced the growth traits, WUE, and mineral uptake of rose plants during WS.
Exogenous application of nutrients and chemicals offers an effective strategy to mitigate the adverse effects of DS on plants. Calcium (Ca2+), a critical nutrient in plant responses to environmental stresses like DS, reduces lipid peroxidation and enhances antioxidant enzyme activity, thereby alleviating DS-induced damage [242,243]. For example, Zhao et al. [64] demonstrated that exogenous Ca2+ application significantly improved DS resistance in roses. Similarly, silicon (Si) helps plants withstand abiotic stresses, including heavy metals, salinity, and DS, by supporting growth and survival [244,245,246,247]. Foliar silicon and potassium silicate application on Damask rose seedlings, under both optimal and DS conditions, enhances flower growth and increases essential oil production [46,51]. These findings suggest that exogenous Ca2+ and Si applications effectively mitigate DS impacts on roses and other crops. Additionally, polyamine treatment alleviates DS-induced stress, with spermine (Spm) or spermidine (Spd) at 0.5 mmol, improving plant growth and physiological traits in Damask roses under DS [248]. Moftah et al. [249] also revealed that kaolin application to the ornamental plant Polianthes tuberosa L. improves its water status, water use efficiency (WUE), and photosynthetic activity under DS conditions.
In line with these findings, Sukpitak et al. [164] noted that floricultural products can be assisted in coping with WS through the exogenous application of chemical treatments, including ethylene inhibitors, select phytohormones, and other substances, as shown in Figure 6.
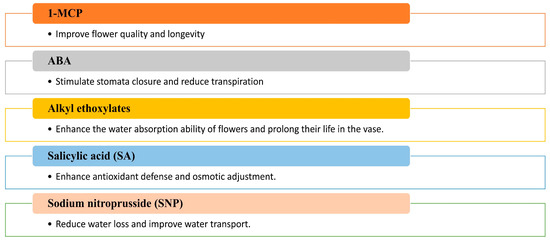
Figure 6.
Effects of exogenous application of synthetic compounds (1-MCP, alkyl ethoxylate, SNP) and phytohormones (ABA, SA) on the drought tolerance of rose flowers.
Pre-treatment with ethylene inhibitors before DS can mitigate its adverse effects on cut flower longevity, as observed by Sukpitak et al. [164]. For instance, 1-methylcyclopropene (1-MCP), an ethylene action inhibitor, can be applied to cut flowers of R. hybrida cv. Samantha, before dehydration, prevents DS-induced impairments in flower opening and petal cell growth [161]. Similarly, ABA minimizes water loss in flowers by rapidly inducing stomatal closure in chrysanthemum petals during DS, thereby reducing transpiration [162,164]. ABA treatment also maintains the marketable quality of flowers post-rehydration under harsh conditions and, in the Akito cultivar of cut roses, soaking in 0.1 mM ABA enhances stomatal closure, further reducing transpiration [162]. Based on these investigations, using ABA can improve the quality and longevity of post-harvest floral products and water consumption efficiency.
Apart from ABA, antitranspirant compounds like sodium nitroprusside (SNP), a nitric oxide (NO) donor [250], and acetylsalicylic acid [251] decrease water loss from leaf tissue by reducing the size and number of stomata, thus promoting a balanced water supply [252]. These compounds are expected to extend the vase life of partially dehydrated cut flowers [253]. A case in point is that the cut flowers of three rose cultivars, Testarossa, Bordeaux, and Lenny, were placed in vase water containing 15 mM SNP (an NO inducer) or 15 mM acetylsalicylic acid (known by Aspirin) under mild desiccation (12% weight loss). The data showed that treatments that slow the transpiration rate by closing the stomata can significantly reduce the adverse effect of mild desiccation on vase life. This study was conducted by Fanourakis et al. [253]. In addition, conditions that facilitate water absorption, such as adding surfactants to the vase water [254,255], can reduce the adverse effects of partial dehydration on vase life. Van Doorn et al. [255] reported that certain alkyl ethoxylate mixtures can significantly improve the ability of cut roses and Bouvadia flowers to uptake water and last longer in the vase.
Mulching, an effective agronomic practice to mitigate DS, enhances soil moisture retention, regulates soil temperature, and improves fertility [256]. Horo et al. [257], mulching suppresses weed growth and increases moisture retention, improving agronomic and floral characteristics in rose plants. Comparable findings were reported by Sardar et al. [258] in R. centifolia and by Tripathy et al. [234] in rose cv. ‘Mainu Parle’, who demonstrated that black polythene mulch (300 micron) significantly increased flowering duration and flower number, supporting its role in DS mitigation by potentially in alleviating water retention and reducing different physiological stress indicators.
7. Conclusions and Future Perspectives
DS continues to pose a significant challenge to the global ornamental and cut flower industries, with rose production particularly affected due to declines in ornamental quality and economic value. This review has demonstrated that DS triggers a wide range of morphological, physiological, biochemical, and molecular responses in rose plants. A comprehensive understanding of these mechanisms is essential to formulate innovative approaches to mitigate the adverse effects of water shortages. Current strategies focus on developing drought-resistant varieties, genetic modification, and implementing natural drought-tolerant genotypes. Additionally, emerging treatments involving plant hormones such as ABA and GA, combined with nanomaterials like TiO2and Si-NPs, offer promising prospects for enhancing drought resistance. However, significant research gaps remain, especially in the genetic regulation of drought responses in roses, which may reveal valuable genetic stress memory mechanisms for breeding programs.
Furthermore, the effect of drought on the biosynthesis of secondary metabolites, including essential oil synthesis, requires further study to improve yield and quality under water-limited conditions. Root system architecture’s role in improving water absorption efficiency remains insufficiently studied. Expanding the study to include multiple commercial and wild rose cultivars may reveal distinct adaptive characteristics while examining the interactions of various stresses (e.g., drought with heat or salinity), which will improve breeding approaches based on cross-tolerance. Future research should focus on identifying genetic and transcriptional networks associated with drought adaptation, enhancing precision irrigation and soil moisture management tailored to rose cultivation, and evaluating the synergistic effects of hormone and nanotechnology applications under field conditions. Furthermore, incorporating genomic selection and gene editing techniques, such as CRISPR, into breeding programs may accelerate the development of drought-resistant rose varieties. Addressing these deficiencies is crucial to sustaining rose production under increasing water scarcity problems, thus ensuring this critical horticultural crop’s environmental sustainability and economic viability.
Author Contributions
C.W. and H.Z. designed and prepared the review paper. Y.C., J.S. and C.F. draw some figures. G.Y., R.Z., J.L. (Jun Lu) and J.L (Jinyi Liu). were involved in manuscript editing and reference collection. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the ‘JBGS’ Project of Seed Industry Revitalization in Jiangsu Province: JBGS (2021)020 and supported by NSFC (32372744, 32302594, 32172615, and 32102418).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fedoroff, N.V.; Battisti, D.S.; Beachy, R.N.; Cooper, P.J.; Fischhoff, D.A.; Hodges, C.; Knauf, V.C.; Lobell, D.; Mazur, B.J.; Molden, D.; et al. Radically rethinking agriculture for the 21st century. Science 2010, 327, 833–834. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Ghosh, A.; Li, Z.-G.; Siddiqui, M.N.; Fujita, M.; Tran, L.-S.P. Methylglyoxal—A signaling molecule in plant abiotic stress responses. Free. Radic. Biol. Med. 2018, 122, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Amiri, R.; Nikbakht, A.; Etemadi, N. Alleviation of drought stress on rose geranium [Pelargonium graveolens (L.) Herit.] in terms of antioxidant activity and secondary metabolites by mycorrhizal inoculation. Sci. Hortic. 2015, 197, 373–380. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Ma, Z.; Boken, V.K.; Zeng, H.; Shang, J.; Igor, S.; Wang, J.; Yan, N. Regional differences in the performance of drought mitigation measures in 12 major wheat-growing regions of the world. Agric. Water Manag. 2022, 273, 107888. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- De Vries, D.; Dubois, L.A. Rose breeding: Past, present, prospects. Acta Hortic. 1996, 424, 241–248. [Google Scholar] [CrossRef]
- Ercisli, S. Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem. 2007, 104, 1379–1384. [Google Scholar] [CrossRef]
- Leus, L.; Van Laere, K.; De Riek, J.; Van Huylenbroeck, J. Ornamental crops. In Handbook of Plant Breeding; Huylenbroeck, J.V., Ed.; Springer: Cham, Switzerland, 2018; pp. 719–767. [Google Scholar]
- Che, D.; Zhang, X.; Zhang, J.; Yang, T.; Zhang, W.; Xiong, Y. Research progress on locus location of quantitative traits in Rosa plants. J. Acta Hortic. Sin. 2016, 43, 1765–1775. [Google Scholar]
- Mordor Intelligence. Netherlands Floriculture Market—Growth, Trends, COVID-19 Impact, and Forecasts (2021–2026); Mordor Intelligence: Hyderabad, India, 2020. [Google Scholar]
- DataIntelo. Rose Market Report: Global Forecast From 2025 To 2033. Available online: https://dataintelo.com/report/global-rose-market (accessed on 18 April 2025).
- OEC. Roses (HS: 060311). Available online: https://oec.world/en/profile/hs/roses (accessed on 18 April 2025).
- Cai, X.; Niu, G.; Starman, T.; Hall, C. Response of six garden roses (Rosa × hybrida L.) to salt stress. Sci. Hortic. 2014, 168, 27–32. [Google Scholar] [CrossRef]
- Erbas, S.; Baydar, H. Variation in scent compounds of oil-bearing rose (Rosa damascena Mill.) produced by headspace solid phase microextraction, hydrodistillation and solvent extraction. Rec. Nat. Prod. 2016, 10, 555. [Google Scholar]
- Raymond, O. Domestication et Sélection Dirigée chez le Rosier: Analyse Historique via les Phénotypes Morphologique, Chimique et Biochimique. Ph.D. Thesis, Université Claude Bernard Lyon 1, Lyon, France, 1999. [Google Scholar]
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Su, L.; Wang, Y.; Geng, Z.; Lin, S.; Zhang, Y.; Yu, S.; Fu, L.; Liu, Q.; Cheng, C.; et al. Role of RcTINY2 in the Regulation of Drought and Salt Stress Response in Arabidopsis and Rose. Horticulturae 2022, 8, 747. [Google Scholar] [CrossRef]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of abiotic stress on crops. In Sustainable Crop Production; IntechOpen: London, UK, 2020; Volume 3, pp. 5–16. [Google Scholar]
- Saini, H.S.; Westgate, M.E. Reproductive development in grain crops during drought. Adv. Agron. 1999, 68, 59–96. [Google Scholar]
- Arbona, V.; Manzi, M.; de Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Change 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought tolerance in wheat. Sci. World J. 2013, 2013, 610721. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Sytar, O. Osmotic Adjustment and Plant Adaptation to Drought Stress. In Drought Stress Tolerance in Plants, Vol 1: Physiology and Biochemistry; Hossain, M.A., Wani, S.H., Bhattacharjee, S., Burritt, D.J., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 105–143. [Google Scholar]
- Malinowska, M.; Donnison, I.; Robson, P. Morphological and physiological traits that explain yield response to drought stress in Miscanthus. Agronomy 2020, 10, 1194. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, N.; Armada, E.; Duque, E.; Roldán, A.; Azcón, R. Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: Effectiveness of autochthonous or allochthonous strains. J. Plant Physiol. 2015, 174, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought Stress in Plants: An Overview. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-fertilization as an emerging fertilization technique: Why can modern agriculture benefit from its use? Plants 2020, 10, 2. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Mishra, S.S.; Behera, P.K.; Panda, D. Genotypic variability for drought tolerance-related morpho-physiological traits among indigenous rice landraces of Jeypore tract of Odisha, India. J. Crop Improv. 2019, 33, 254–278. [Google Scholar] [CrossRef]
- Panda, D.; Mishra, S.S.; Behera, P.K. Drought tolerance in rice: Focus on recent mechanisms and approaches. Rice Sci. 2021, 28, 119–132. [Google Scholar] [CrossRef]
- Hessini, K.; Wasli, H.; Al-Yasi, H.M.; Ali, E.F.; Issa, A.A.; Hassan, F.A.S.; Siddique, K.H.M. Graded Moisture Deficit Effect on Secondary Metabolites, Antioxidant, and Inhibitory Enzyme Activities in Leaf Extracts of Rosa damascena Mill. var. trigentipetala. Horticulturae 2022, 8, 177. [Google Scholar] [CrossRef]
- Abdel-Salam, E.; Alatar, A.; El-Sheikh, M.A. Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J. Biol. Sci. 2018, 25, 1772–1780. [Google Scholar] [CrossRef]
- Seyed Hajizadeh, H.; Azizi, S.; Rasouli, F.; Kaya, O. Evaluation of nano-silicon efficiency on compatible solutes and nutrient status of Damask rose affected by in vitro simulated drought stress. Chem. Biol. Technol. Agric. 2023, 10, 22. [Google Scholar] [CrossRef]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Ali, E.; Elshazly, S.; Siddique, K.H.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef]
- Aalam, F.; Rezaei Nejad, A.; Mousavi-Fard, S.; Raji, M.; Nikoloudakis, N.; Goumenaki, E.; Fanourakis, D. Water Deficit Severity during the Preceding Year Determines Plant Tolerance to Subsequent Year Drought Stress Challenges: A Case Study in Damask Rose. Horticulturae 2024, 10, 462. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Z.; Kim, W. Effect of drought stress on shoot growth and physiological response in the cut rose ‘Charming Black’at different developmental stages. Hortic. Environ. Biotechnol. 2019, 60, 1–8. [Google Scholar] [CrossRef]
- Williams, M.H.; Rosenqvist, E.; Buchhave, M. Response of potted miniature roses (Rosa × hybrida) to reduced water availability during production. J. Hortic. Sci. Biotechnol. 1999, 74, 301–308. [Google Scholar] [CrossRef]
- Dolatkhahi, A.; Shoor, M.; Bannayan, M.; Tehranifar, A.; Alizadeh, A. Water deficit decreases gas exchange parameters and marketable quality of Rosa hybrida ‘Club-Nika’irrespective of training systems. J. Agric. Sci. Technol. 2020, 22, 837–849. [Google Scholar]
- Li, W.; Fu, L.; Geng, Z.; Zhao, X.; Liu, Q.; Jiang, X. Physiological characteristic changes and full-length transcriptome of rose (Rosa chinensis) roots and leaves in response to drought stress. Plant Cell Physiol. 2020, 61, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Farahani, H.; Sajedi, N.A.; Madani, H.; Changizi, M.; Naeini, M.R. Effect of foliar-applied silicon on flower yield and essential oil composition of damask rose (Rosa damascena Miller) under water deficit stress. Silicon 2021, 13, 4463–4472. [Google Scholar] [CrossRef]
- Katsoulas, N.; Kittas, C.; Dimokas, G.; Lykas, C. Effect of irrigation frequency on rose flower production and quality. Biosyst. Eng. 2006, 93, 237–244. [Google Scholar] [CrossRef]
- Fascella, G.; Gugliuzza, G.; Mammano, M.; Maggiore, P. Effect of different irrigation regimes on yield and quality of hydroponic cut roses. In Proceedings of the VI International Symposium on Rose Research and Cultivation, Hannover, Germany, 25 August 2013; Volume 1064, pp. 259–263. [Google Scholar]
- Raviv, M.; Blom, T.J. The effect of water availability and quality on photosynthesis and productivity of soilless-grown cut roses. Sci. Hortic. 2001, 88, 257–276. [Google Scholar] [CrossRef]
- Sotelo-Cuitiva, Y.M.; Restrepo-Díaz, H.; García-Castro, A.; Ramírez-Godoy, A.; Flórez-Roncancio, V.J. Effect of kaolin film particle applications (Surround WP®) and water deficit on physiological characteristics in rose cut plants (Rose spp L.). Am. J. Plant Sci 2011, 2, 354–358. [Google Scholar] [CrossRef]
- Farahani, H.; Sajedi, N.; Madani, H.; Changizi, M.; Naeini, M.R. Effect of potassium silicate on water use efficiency, quantitative traits and essential oil yield of damask rose (Rosa damascena Miller) under water deficit stress. Iran. J. Hortic. Sci. 2021, 52, 171–182. [Google Scholar]
- Nedkov, N.; Matev, A.; Ovcharova, A. “Additional yield–irrigation depth” relationship for white bearing rose (Rosa alba L.). Ovidius Univ. Ann. Ser. Civ. Eng. 2014, 16, 91–104. [Google Scholar]
- Ober, E.S.; Alahmad, S.; Cockram, J.; Forestan, C.; Hickey, L.T.; Kant, J.; Maccaferri, M.; Marr, E.; Milner, M.; Pinto, F.; et al. Wheat root systems as a breeding target for climate resilience. Theor. Appl. Genet. 2021, 134, 1645–1662. [Google Scholar] [CrossRef]
- Amtmann, A.; Bennett, M.J.; Henry, A. Root phenotypes for the future. Plant Cell Environ. 2022, 45, 595–601. [Google Scholar] [CrossRef]
- Meister, A.; Finger, S.; Hause, G.; Blume, A. Morphological changes of bacterial model membrane vesicles. Eur. J. Lipid Sci. Technol. 2014, 116, 1228–1233. [Google Scholar] [CrossRef]
- Ashfaq, W.; Brodie, G.; Fuentes, S.; Pang, A.; Gupta, D. Silicon improves root system and canopy physiology in wheat under drought stress. Plant Soil 2024, 502, 279–296. [Google Scholar] [CrossRef]
- McWilliams, D. Drought Strategies for Cotton; Cooperative Extension Service Circular 582; College of Agriculture and Home Economics; New Mexico State University: Las Cruces, NM, USA, 2003; pp. 1–5. [Google Scholar]
- Hessini, K.; Kronzucker, H.J.; Abdelly, C.; Cruz, C. Drought stress obliterates the preference for ammonium as an N source in the C4 plant Spartina alterniflora. J. Plant Physiol. 2017, 213, 98–107. [Google Scholar] [CrossRef]
- Niu, G.; Rodriguez, D.S. Growth and physiological responses of four rose rootstocks to drought stress. J. Am. Soc. Hortic. Sci. 2009, 134, 202–209. [Google Scholar] [CrossRef]
- Murphy, J.E.; Burns, J.H. Rosa multiflora’s performance under water stress: The role of positive and negative density-dependent intraspecific interactions. Plant Ecol. 2019, 220, 951–963. [Google Scholar] [CrossRef]
- Bolla, A.; Voyiatzis, D.; Koukourikou-Petridou, M.; Chimonidou, D. Photosynthetic parameters and cut-flower yield of rose ‘Eurored’(HT) are adversely affected by mild water stress irrespective of substrate composition. Sci. Hortic. 2010, 126, 390–394. [Google Scholar] [CrossRef]
- Cai, X.; Starman, T.; Niu, G.; Hall, C.; Lombardini, L. Response of Selected Garden Roses to Drought Stress. HortScience 2012, 47, 1050–1055. [Google Scholar] [CrossRef]
- Harp, D.A.; Kay, K.; Zlesak, D.C.; George, S. The Effect of Rose Root Size on Drought Stress Tolerance and Landscape Plant Performance. Tex. J. Agric. Nat. Resour. 2015, 28, 82–88. [Google Scholar]
- Zhao, X.; Lin, S.; Yu, S.; Zhang, Y.; Su, L.; Geng, L.; Cheng, C.; Jiang, X. Exogenous calcium enhances the physiological status and photosynthetic capacity of rose under drought stress. Hortic. Plant J. 2024, 10, 853–865. [Google Scholar] [CrossRef]
- Jia, X.; Feng, H.; Bu, Y.; Ji, N.; Lyu, Y.; Zhao, S. Comparative transcriptome and weighted gene co-expression network analysis identify key transcription factors of Rosa chinensis ‘Old Blush’after exposure to a gradual drought stress followed by recovery. Front. Genet. 2021, 12, 690264. [Google Scholar] [CrossRef]
- Blum, A. Crop responses to drought and the interpretation of adaptation. Plant Growth Regul. 1996, 20, 135–148. [Google Scholar] [CrossRef]
- Li, L.; Zhu, H.; Ju, Y.; Lv, Z.; Qian, C.; Zhang, C.; Lu, Y.; Wang, J.; Li, W. Comparison of microstructure and physiological response of the leaves of six Rosa rugosa genotypes under drought stress. Ornam. Plant Res. 2024, 4, e016. [Google Scholar] [CrossRef]
- Sher, A.; Khan, A.; Hussain, S.; Cai, L.J.; Ahmad, M.I.; Jamro, S.A.; Rashid, A. Significance of chemical priming on yield and yield components of wheat under drought stress. Am. J. Plant Sci. 2017, 8, 1339–1344. [Google Scholar] [CrossRef]
- Sarwar, J.M.; Nozulaidi, B.N.M.; Khairi, B.C.L.M.; Mohd, K.Y. Effects of water stress on rice production: Bioavailability of potassium in soil. J. Stress Physiol. Biochem. 2013, 9, 97–107. [Google Scholar]
- Zhu, R.; Wu, F.; Zhou, S.; Hu, T.; Huang, J.; Gao, Y. Cumulative effects of drought–flood abrupt alternation on the photosynthetic characteristics of rice. Environ. Exp. Bot. 2020, 169, 103–901. [Google Scholar] [CrossRef]
- Yokota, A.; Kawasaki, S.; Iwano, M.; Nakamura, C.; Miyake, C.; Akashi, K. Citrulline and DRIP-1 protein (ArgE homologue) in drought tolerance of wild watermelon. Ann. Bot. 2002, 89, 825–832. [Google Scholar] [CrossRef]
- Liu, B.; Cheng, L.; Ma, F.; Zou, Y.; Liang, D. Growth, biomass allocation, and water use efficiency of 31 apple cultivars grown under two water regimes. Agrofor. Syst. 2012, 84, 117–129. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M. Plant drought stress: Effects, mechanisms and management. Sustain. Agric. 2009, 23, 153–188. [Google Scholar]
- Adamipour, N.; Khosh-Khui, M.; Salehi, H. Comparison of selected biochemical characteristics of damask rose and dog rose under deficit irrigation conditions. Italus Hortus 2022, 29, 138–155. [Google Scholar] [CrossRef]
- Li, G.; Xu, W.; Jing, P.; Hou, X.; Fan, X. Overexpression of VyDOF8, a Chinese wild grapevine transcription factor gene, enhances drought tolerance in transgenic tobacco. Environ. Exp. Bot. 2021, 190, 104592. [Google Scholar] [CrossRef]
- Shi, L.; Kim, W.S. Shoot growth and physiological disorder of cut rose ‘Charming Black’as affected by drought stress during nocturnal supplemental lighting. Hortic. Environ. Biotechnol. 2014, 55, 91–96. [Google Scholar] [CrossRef]
- Gadzinowska, J.; Hura, K.; Ostrowska, A.; Hura, T. Activity of the photosynthetic apparatus in dehydrated leaves of a perennial shrub Rosa rubiginosa L. with different levels of drought memory. Environ. Exp. Bot. 2021, 187, 104493. [Google Scholar] [CrossRef]
- Kirkham, M.B. Principles of Soil and Plant Water Relations, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef]
- Alavi, S.M.; Kamali, M.; Selahvarzi, Y.; Ansari, S. Deficit irrigation strategies (PRD, SDI) and titanium nanoparticles improve water use efficiency and flower quality in greenhouse-grown cut roses. Sci. Rep. 2023, 13, 18019. [Google Scholar] [CrossRef]
- De Dauw, K.; Van Labeke, M.-C.; Leus, L.; Van Huylenbroeck, J. Drought tolerance screening of a Rosa population. In Proceedings of the II International Symposium on Woody Ornamentals of the Temperate Zone, Ghent, Belgium, 1–4 July 2012; Volume 990, pp. 121–127. [Google Scholar]
- Fascella, G.; Agnello, S.; Maggiore, P.; Zizzo, G.; Guarino, L. Effect of Controlled Irrigation Methods Using Climatic Parameters on Yield and Quality of Hydroponic Cut Roses. Acta Hortic. 2010, 870, 65–72. [Google Scholar] [CrossRef]
- Shangguan, Z.; Shao, M.; Dyckmans, J. Effects of nitrogen nutrition and water deficit on net photosynthetic rate and chlorophyll fluorescence in winter wheat. J. Plant Physiol. 2000, 156, 46–51. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Hu, Y.; Burucs, Z.; von Tucher, S.; Schmidhalter, U. Short-term effects of drought and salinity on mineral nutrient distribution along growing leaves of maize seedlings. Environ. Exp. Bot. 2007, 60, 268–275. [Google Scholar] [CrossRef]
- Tommasini, L.; Svensson, J.T.; Rodriguez, E.M.; Wahid, A.; Malatrasi, M.; Kato, K.; Wanamaker, S.; Resnik, J.; Close, T.J. Dehydrin gene expression provides an indicator of low temperature and drought stress: Transcriptome-based analysis of barley (Hordeum vulgare L.). Funct. Integr. Genom. 2008, 8, 387–405. [Google Scholar] [CrossRef]
- Kuchenbuch, R.; Claassen, N.; Jungk, A. Potassium availability in relation to soil moisture: I. Effect of soil moisture on potassium diffusion, root growth and potassium uptake of onion plants/Kaliumverfügbarkeit in Beziehung zur Bodenfeuchte: I. Wirkung des Wassergehaltes auf die K-Diffusion, das Wurzelwachstum. Plant Soil 1986, 95, 221–231. [Google Scholar]
- Martínez-Viveros, O.; Jorquera, M.A.; Crowley, D.; Gajardo, G.; Mora, M. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr. 2010, 10, 293–319. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Chattha, M.U.; Ullah, M.A.; Sulaman, S.; Nawaz, M.; Zhiqiang, W.; Yanqin, M.; Guoqin, H. The role of potassium in plants under drought stress: Mini review. J. Basic Appl. Sci. 2017, 13, 268–271. [Google Scholar] [CrossRef]
- Luo, D.; Li, J.; Luo, J.; Ma, Y.; Wang, Y.; Liu, W.; Rodriguez, L.G.; Yao, Y. Responses to solar UV-B exclusion and drought stress in two cultivars of chestnut rose with different leaf thickness. Forests 2022, 14, 50. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Kharal, M.A. Role of phytohormones in stress tolerance of plants. In Plant, Soil and Microbes: Volume 2: Mechanisms and Molecular Interactions; Hakeem, K.R., Akhtar, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 385–421. [Google Scholar]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, K.; Hou, X.; Li, H.; Wang, G.; Xu, Z.; Xiong, A. Transcriptome profiling reveals the association of multiple genes and pathways contributing to hormonal control in celery leaves. Acta Biochim. Biophy. Sin. 2019, 51, 524–534. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Ismail; Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Lee, I.-J. Endophytic fungus Aspergillus japonicus mediates host plant growth under normal and heat stress conditions. BioMed Res. Int. 2018, 2018, 7696831. [Google Scholar] [CrossRef] [PubMed]
- Ciura, J.; Kruk, J. Phytohormones as targets for improving plant productivity and stress tolerance. J. Plant Physiol. 2018, 229, 32–40. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Gnanasekaran, P.; Kumari, P.; Lakra, N.; Lal, S.K.; Pawar, J.; Narayan, O.P. An overview of recent advancement in phytohormones-mediated stress management and drought tolerance in crop plants. Plant Gene 2021, 25, 100264. [Google Scholar]
- Lee, M.; Jung, J.-H.; Han, D.-Y.; Seo, P.J.; Park, W.J.; Park, C.-M. Activation of a flavin monooxygenase gene YUCCA7 enhances drought resistance in Arabidopsis. Planta 2012, 235, 923–938. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Zhang, W.; Yan, S.; Wang, R.; Zhao, J.; Li, Y.; Qi, Z.; Sun, Z.; Zhu, Z. The putative auxin efflux carrier OsPIN3t is involved in the drought stress response and drought tolerance. Plant J. 2012, 72, 805–816. [Google Scholar] [CrossRef]
- Nir, I.; Moshelion, M.; Weiss, D. The Arabidopsis GIBBERELLIN METHYL TRANSFERASE 1 suppresses gibberellin activity, reduces whole-plant transpiration and promotes drought tolerance in transgenic tomato. Plant Cell Environ. 2014, 37, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Shi, H.; Shang, X.; Zhao, Z.; Fang, Y.; Wu, H.; Luo, P.; Cui, Y.; Chen, W. RhMED15a-like, a subunit of the Mediator complex, is involved in the drought stress response in Rosa hybrida. BMC Plant Biol. 2024, 24, 351. [Google Scholar] [CrossRef]
- Gadzinowska, J.; Ostrowska, A.; Hura, K.; Dziurka, M.; Pawłowska, B.; Hura, T. Physiological traits determining high adaptation potential of sweet briar (Rosa rubiginosa L.) at early stage of growth to dry lands. Sci. Rep. 2019, 9, 19390. [Google Scholar] [CrossRef] [PubMed]
- Shahbani, Z.; Kosh-Khui, M.; Salehi, H.; Kafi, M.; Kamgar Haghighi, A.A.; Eshghi, S.; Omidi, M. Hormonal and Physiological Changes in Miniature Roses (Rosa chinensis Jacq. var. minima Rehd.) Exposed to Water Deficit and Salinity Stress Conditions. Gesunde Pflanz. 2023, 75, 1781–1797. [Google Scholar]
- Zhang, F.; Wang, P.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Effects of mycorrhizal fungi on root-hair growth and hormone levels of taproot and lateral roots in trifoliate orange under drought stress. Arch. Agron. Soil Sci. 2019, 65, 1316–1330. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Strzelak, A.; Ratajczak, A.; Adamiec, A.; Feleszko, W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: A mechanistic review. Int. J. Environ. Res. Public Health 2018, 15, 1033. [Google Scholar] [CrossRef]
- De Gara, L.; Foyer, C.H. Ying and Yang interplay between reactive oxygen and reactive nitrogen species controls cell functions. Plant Cell Environ. 2017, 40, 459–461. [Google Scholar] [CrossRef]
- Verma, G.; Srivastava, D.; Tiwari, P.; Chakrabarty, D. ROS modulation in crop plants under drought stress. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 311–336. [Google Scholar]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Fujita, M. Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (‘Triticum aestivum’ L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust. J. Crop Sci. 2012, 6, 1314–1323. [Google Scholar]
- Xu, Y.; Burgess, P.; Zhang, X.; Huang, B. Enhancing cytokinin synthesis by overexpressing ipt alleviated drought inhibition of root growth through activating ROS-scavenging systems in Agrostis stolonifera. J. Exp. Bot. 2016, 67, 1979–1992. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Mellacheruvu, S.; Tamirisa, S.; Vudem, D.R.; Khareedu, V.R. Pigeonpea hybrid-proline-rich protein (CcHyPRP) confers biotic and abiotic stress tolerance in transgenic rice. Front. Plant Sci. 2016, 6, 1167. [Google Scholar]
- Tang, Y.; Bao, X.; Zhi, Y.; Wu, Q.; Guo, Y.; Yin, X.; Zeng, L.; Li, J.; Zhang, J.; He, W.; et al. Overexpression of a MYB family gene, OsMYB6, increases drought and salinity stress tolerance in transgenic rice. Front. Plant Sci. 2019, 10, 168. [Google Scholar]
- Niu, Y.; Wang, Y.; Li, P.; Zhang, F.; Liu, H.; Zheng, G. Drought stress induces oxidative stress and the antioxidant defense system in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. Acta Physiol. Plant. 2013, 35, 1189–1200. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Y.; Yu, S.; Huang, Y.; Liu, H.; Chen, W.; He, X. Effects of drought stress on growth, physiology and secondary metabolites of Two Adonis species in Northeast China. Sci. Hortic. 2020, 259, 108795. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Lee, D.-J.; Cheema, S.A.; Aziz, T. DROUGHT STRESS: Comparative Time Course Action of the Foliar Applied Glycinebetaine, Salicylic Acid, Nitrous Oxide, Brassinosteroids and Spermine in Improving Drought Resistance of Rice. J. Agron. Crop Sci. 2010, 196, 336–345. [Google Scholar]
- Adamipour, N.; Khosh-Khui, M.; Salehi, H.; Razi, H.; Karami, A.; Moghadam, A. Role of genes and metabolites involved in polyamines synthesis pathways and nitric oxide synthase in stomatal closure on Rosa damascena Mill. under drought stress. Plant Physiol. Biochem. 2020, 148, 53–61. [Google Scholar] [CrossRef]
- Jin, J.; Shan, N.; Ma, N.; Bai, J.; Gao, J. Regulation of ascorbate peroxidase at the transcript level is involved in tolerance to postharvest water deficit stress in the cut rose (Rosa hybrida L.) cv. Samantha. Postharvest Biol. Technol. 2006, 40, 236–243. [Google Scholar] [CrossRef]
- Jiang, Y.; Khan, M.A.; Wang, Z.; Liu, J.; Xue, J.; Gao, J.; Zhang, C. Cu/ZnSOD involved in tolerance to dehydration in cut rose (Rosa hybrida). Postharvest Biol. Technol. 2015, 100, 187–195. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global plant stress signaling: Reactive oxygen species at the cross-road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Ahmad, A.; Siddiqi, T.; Ahmad, P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol. Plant. 2013, 35, 1039–1050. [Google Scholar] [CrossRef]
- Mishra, S.S.; Panda, D. Leaf traits and antioxidant defense for drought tolerance during early growth stage in some popular traditional rice landraces from Koraput, India. Rice Sci. 2017, 24, 207–217. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Zlatev, Z.; Lidon, F.C. An overview on drought induced changes in plant growth, water relations and photosynthesis. Emir. J. Food Agric. (EJFA) 2012, 24. [Google Scholar]
- Kumar, S.; Sachdeva, S.; Bhat, K.; Vats, S. Plant responses to drought stress: Physiological, biochemical and molecular basis. In Biotic and Abiotic Stress Tolerance in Plants; Springer: Singapore, 2018; pp. 1–25. [Google Scholar]
- Xiang, Z.; Xu, G.; Jiang, W. Drought stress on physiological and biochemical processes in four spiny plant species. J. Zhejiang AF Univ. 2007, 24, 7. [Google Scholar]
- Grebennikova, O.; Pilkevich, R.; Gubanova, T.; Plugatar, S. Physiological and biochemical parameters of drought tolerance of some genotypes of garden roses. In BIO Web of Conferences, Proceedings of the International Scientific and Practical Conference "VAVILOV READINGS-2023" (VVRD 2023), Online, 25–26 May 2023; EDP Sciences: Les Ulis, France, 2023; p. 02014. [Google Scholar]
- Meena, Y.K.; Kaur, N. Towards an understanding of physiological and biochemical mechanisms of drought tolerance in plant. Annu. Res. Rev. Biol. 2019, 31, 1–13. [Google Scholar] [CrossRef]
- Morgan, P. Effects of abiotic stresses on plant hormone systems. In Stress Responses in Plants: Adaptation Mechanisms; Alscher, R.G., Cumming, J., Eds.; Wiley-Liss, Inc.: New York, NY, USA, 1990; pp. 313–314. [Google Scholar]
- Turner, N.C. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Marín-de la Rosa, N.; Lin, C.W.; Kang, Y.J.; Dhondt, S.; Gonzalez, N.; Inzé, D.; Falter-Braun, P. Drought resistance is mediated by divergent strategies in closely related Brassicaceae. New Phytol. 2019, 223, 783–797. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nam, N.H.; Chauhan, Y.S.; Johansen, C. Osmotic adjustment, water relations and carbohydrate remobilization in pigeonpea under water deficits. J. Plant Physiol. 2000, 157, 651–659. [Google Scholar] [CrossRef]
- Martìnez, J.-P.; Lutts, S.; Schanck, A.; Bajji, M.; Kinet, J.-M. Is osmotic adjustment required for water stress resistance in the Mediterranean shrub Atriplex halimus L? J. Plant Physiol. 2004, 161, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Ferchichi, S.; Hessini, K.; Dell’Aversana, E.; D’Amelia, L.; Woodrow, P.; Ciarmiello, L.F.; Fuggi, A.; Carillo, P. Hordeum vulgare and Hordeum maritimum respond to extended salinity stress displaying different temporal accumulation pattern of metabolites. Funct. Plant Biol. 2018, 45, 1096–1109. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Andrew Riseman, A.; Charlotte Jensen, C.; Michelle Williams, M. Stomatal conductivity and osmotic adjustment during acclimation to multiple cycles of drought stress in potted miniature rose (Rosa × hybrida). J. Hortic. Sci. Biotechnol. 2001, 76, 138–144. [Google Scholar] [CrossRef]
- Yang, S.; Chen, K.; Wang, S.; Gong, M. Osmoregulation as a key factor in drought hardening-induced drought tolerance in Jatropha curcas. Biol. Plant. 2015, 59, 529–536. [Google Scholar] [CrossRef]
- Surówka, E.; Hura, T. Osmoprotectants and Nonenzymatic Antioxidants in Halophytes. In Handbook of Halophytes: From Molecules to Ecosystems Towards Biosaline Agriculture; Grigore, M.-N., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1901–1930. [Google Scholar]
- Pal, P.K. Evaluation, genetic diversity, recent development of distillation method, challenges and opportunities of Rosa damascena: A review. J. Essent. Oil Bear. Plants 2013, 16, 1–10. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, A.K.; Yadav, A.K. Volatile constituents of essential oil and rose water of damask rose (Rosa damascena Mill.) cultivars from North Indian hills. Nat. Prod. Res. 2011, 25, 1577–1584. [Google Scholar] [CrossRef]
- Singh, S. Rose Oil Market Research Report Information By Application, By Product Type, By Distribution Channel, By Extraction Method, And By Region—Market Forecast Till 2032. Available online: https://www.marketresearchfuture.com/reports/rose-oil-market-7243 (accessed on 18 April 2025).
- Moein, M.; Ghasemia, Y.; Karami, F.; Tavallali, H. Composition of the Essential Oil of Rosa damascena Mill. from South of Iran: Composition of the essential oil of Rosa damascenea. Iran. J. Pharm. Sci. 2010, 6, 59–62. [Google Scholar]
- Farooqi, A.H.A.; Sharma, S. Effect of growth retardants on flowering of Rosa damascena Mill. In Proceedings of the International Congress of Plant Physiology, New Dehli, India, 15–20 February 1988; Volume 2, pp. 1369–1372. [Google Scholar]
- Kumar, R.; Sharma, S.K.; Sood, S.; Agnihotri, V.K.; Singh, B. Effect of diurnal variability and storage conditions on essential oil content and quality of damask rose (Rosa damascena Mill.) flowers in north western Himalayas. Sci. Hortic. 2013, 154, 102–108. [Google Scholar] [CrossRef]
- Ucar, Y.; Kazaz, S.; Eraslan, F.; Baydar, H. Effects of different irrigation water and nitrogen levels on the water use, rose flower yield and oil yield of Rosa damascena. Agric. Water Manag. 2017, 182, 94–102. [Google Scholar] [CrossRef]
- Ames, G.R.; Matthews, W.S.A. The distillation of essential oils. Trop. Sci. 1968, 10, 136–148. [Google Scholar]
- Yousefi, B. Screening of Rosa damascena Mill. landraces for flower yield and essential oil content in cold climates. Folia Hortic. 2016, 28, 31–40. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Rezaei Nejad, A. The influence of nitrogen-fertilizer and harvest time on the productivity of Thymus vulgaris L. Int. J. Hortic. Sci. 2000, 6, 43–46. [Google Scholar] [CrossRef]
- Kiymaz, S.; Altun, B.; Ertek, A. Effect of different water regimes and nitrogen applications on the growth, yield, essential oil content, and quality parameters of the oil rose (Rosa damascena Mill.). J. Plant Nutr. 2022, 45, 2108–2122. [Google Scholar] [CrossRef]
- Uçar, Y.; Kazaz, S.; Eraslan İnal, F.; Baydar, H.; Erbaş, S. Response of Rose (Rosa damascena Mill.) Oil Components to Different Irrigation Water and Nitrogen Applications. Ziraat Fakültesi Derg. 2024, 19, 128–140. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Y.; Wu, H.; Zhang, S.; Yang, R.; Liu, X. RhRab5ip, a new interactor of RhPIP1;1, was involved in flower opening of cut rose during water deficit. Plant Physiol. Biochem. 2022, 181, 61–70. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Miller, W.B. Nonrefrigerated dry storage can have negative effects on postharvest quality of cut Lilium. HortScience 2022, 57, 1475–1479. [Google Scholar] [CrossRef]
- Liu, D.; Liu, X.; Meng, Y.; Sun, C.; Tang, H.; Jiang, Y.; Khan, M.A.; Xue, J.; Ma, N.; Gao, J. An organ-specific role for ethylene in rose petal expansion during dehydration and rehydration. J. Exp. Bot. 2013, 64, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Woltering, E.J.; Paillart, M.J. Effect of cold storage on stomatal functionality, water relations and flower performance in cut roses. Postharvest Biol. Technol. 2018, 136, 66–73. [Google Scholar] [CrossRef]
- Ha, S.T.; Lim, J.-H.; In, B.-C. Extension of the vase life of cut roses by both improving water relations and repressing ethylene responses. Hortic. Sci. Technol. 2019, 37, 65–77. [Google Scholar] [CrossRef]
- Sukpitak, C.; Seraypheap, K.; Muñoz, P.; Munné-Bosch, S. Influence of water deficit on the longevity of ethylene-sensitive and ethylene-insensitive flowers. Environ. Exp. Bot. 2024, 219, 105647. [Google Scholar] [CrossRef]
- Dai, F.; Zhang, C.; Jiang, X.; Kang, M.; Yin, X.; Lü, P.; Zhang, X.; Zheng, Y.; Gao, J. RhNAC2 and RhEXPA4 are involved in the regulation of dehydration tolerance during the expansion of rose petals. Plant Physiol. 2012, 160, 2064–2082. [Google Scholar] [CrossRef]
- Chu, X.; Wang, C.; Chen, X.; Lu, W.; Li, H.; Wang, X.; Hao, L.; Guo, X. The cotton WRKY gene GhWRKY41 positively regulates salt and drought stress tolerance in transgenic Nicotiana benthamiana. PLoS ONE 2015, 10, e0143022. [Google Scholar] [CrossRef]
- Khazaei, H.; Street, K.; Bari, A.; Mackay, M.; Stoddard, F.L. The FIGS (Focused Identification of Germplasm Strategy) approach identifies traits related to drought adaptation in Vicia faba genetic resources. PLoS ONE 2013, 8, e63107. [Google Scholar] [CrossRef]
- Singh, B.; Bohra, A.; Mishra, S.; Joshi, R.; Pandey, S. Embracing new-generation ‘omics’ tools to improve drought tolerance in cereal and food-legume crops. Biol. Plant. 2015, 59, 413–428. [Google Scholar] [CrossRef]
- Hu, H.; Xiong, L. Genetic engineering and breeding of drought-resistant crops. Annu. Rev. Plant Biol. 2014, 65, 715–741. [Google Scholar] [CrossRef]
- Pérez-Clemente, R.M.; Vives, V.; Zandalinas, S.I.; López-Climent, M.F.; Muñoz, V.; Gómez-Cadenas, A. Biotechnological approaches to study plant responses to stress. BioMed Res. Int. 2013, 2013, 654120. [Google Scholar] [CrossRef]
- Liu, J.-H.; Peng, T.; Dai, W. Critical cis-acting elements and interacting transcription factors: Key players associated with abiotic stress responses in plants. Plant Mol. Biol. Rep. 2014, 32, 303–317. [Google Scholar] [CrossRef]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Huang, G.-T.; Ma, S.-L.; Bai, L.-P.; Zhang, L.; Ma, H.; Jia, P.; Liu, J.; Zhong, M.; Guo, Z.-F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012, 39, 969–987. [Google Scholar] [CrossRef]
- Danquah, A.; De Zélicourt, A.; Colcombet, J.; Hirt, H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef]
- Hirayama, T.; Shinozaki, K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef]
- Mizoi, J.; Ohori, T.; Moriwaki, T.; Kidokoro, S.; Todaka, D.; Maruyama, K.; Kusakabe, K.; Osakabe, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. GmDREB2A;2, a canonical DEHYDRATION-RESPONSIVE ELEMENT-BINDING PROTEIN2-type transcription factor in soybean, is posttranslationally regulated and mediates dehydration-responsive element-dependent gene expression. Plant Physiol. 2013, 161, 346–361. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Hou, X. Genome-wide analysis of the AP2/ERF transcription factor superfamily in Chinese cabbage (Brassica rapa ssp. pekinensis). BMC Genom. 2013, 14, 573. [Google Scholar] [CrossRef]
- Anbazhagan, K.; Bhatnagar-Mathur, P.; Vadez, V.; Dumbala, S.R.; Kishor, P.K.; Sharma, K.K. DREB1A overexpression in transgenic chickpea alters key traits influencing plant water budget across water regimes. Plant Cell Rep. 2015, 34, 199–210. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Khan, S.A.; Li, M.Z.; Wang, S.M.; Yin, H.J. Revisiting the Role of Plant Transcription Factors in the Battle against Abiotic Stress. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Gong, P.; Zhang, J.; Li, H.; Yang, C.; Zhang, C.; Zhang, X.; Khurram, Z.; Zhang, Y.; Wang, T.; Fei, Z.; et al. Transcriptional profiles of drought-responsive genes in modulating transcription signal transduction, and biochemical pathways in tomato. J. Exp. Bot. 2010, 61, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Lindemose, S.; O’Shea, C.; Jensen, M.K.; Skriver, K. Structure, function and networks of transcription factors involved in abiotic stress responses. Int. J. Mol. Sci. 2013, 14, 5842–5878. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.K.; Kjaersgaard, T.; Nielsen, M.M.; Galberg, P.; Petersen, K.; O’shea, C.; Skriver, K. The Arabidopsis thaliana NAC transcription factor family: Structure–function relationships and determinants of ANAC019 stress signalling. Biochem. J. 2010, 426, 183–196. [Google Scholar] [CrossRef]
- Olsen, A.N.; Ernst, H.A.; Leggio, L.L.; Skriver, K. NAC transcription factors: Structurally distinct, functionally diverse. Trends Plant Sci. 2005, 10, 79–87. [Google Scholar] [CrossRef]
- Nakashima, K.; Takasaki, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. NAC transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2012, 1819, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Puranik, S.; Sahu, P.P.; Srivastava, P.S.; Prasad, M. NAC proteins: Regulation and role in stress tolerance. Trends Plant Sci. 2012, 17, 369–381. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Li, M.; Yan, Y.; Liu, X.; Li, L. Dual function of NAC072 in ABF3-mediated ABA-responsive gene regulation in Arabidopsis. Front. Plant Sci. 2016, 7, 1075. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, C.; Lü, P.; Jiang, G.; Liu, X.; Dai, F.; Gao, J. Rh NAC 3, a stress-associated NAC transcription factor, has a role in dehydration tolerance through regulating osmotic stress-related genes in rose petals. Plant Biotechnol. J. 2014, 12, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Li, S.; Li, W.; Hao, Q.; Wan, X.; Wang, K.; Liu, Q.; Liu, Q.; Jiang, X. RhNAC31, a novel rose NAC transcription factor, enhances tolerance to multiple abiotic stresses in Arabidopsis. Acta Physiol. Plant. 2019, 41, 1–16. [Google Scholar] [CrossRef]
- Geng, L.; Yu, S.; Zhang, Y.; Su, L.; Lu, W.; Zhu, H.; Jiang, X. Transcription factor RcNAC091 enhances rose drought tolerance through the abscisic acid–dependent pathway. Plant Physiol. 2023, 193, 1695–1712. [Google Scholar] [CrossRef]
- Fujita, M.; Fujita, Y.; Maruyama, K.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Tran, L.S.P.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J. 2004, 39, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, Z.; Wang, H.; Zhao, X.; Su, L.; Geng, L.; Lu, Y.; Tong, B.; Liu, Q.; Jiang, X. Genome-wide analysis of BURP genes and identification of a BURP-V gene RcBURP4 in Rosa chinensis. Plant Cell Rep. 2022, 41, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Agarwal, P.K.; Joshi, A.J.; Sopory, S.K.; Reddy, M.K. Overexpression of PgDREB2A transcription factor enhances abiotic stress tolerance and activates downstream stress-responsive genes. Mol. Biol. Rep. 2010, 37, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2012, 1819, 86–96. [Google Scholar] [CrossRef]
- Du, C.; Hu, K.; Xian, S.; Liu, C.; Fan, J.; Tu, J.; Fu, T. Dynamic transcriptome analysis reveals AP2/ERF transcription factors responsible for cold stress in rapeseed (Brassica napus L.). Mol. Genet. Genom. 2016, 291, 1053–1067. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Y.; Zou, J.; Fang, Y.; Wang, L.; Wang, M.; Jiang, X.; Liu, Y.; Gao, J.; Zhang, C. A RhABF2/Ferritin module affects rose (Rosa hybrida) petal dehydration tolerance and senescence by modulating iron levels. Plant J. 2017, 92, 1157–1169. [Google Scholar] [CrossRef]
- Ma, J.; Li, M.-Y.; Wang, F.; Tang, J.; Xiong, A.-S. Genome-wide analysis of Dof family transcription factors and their responses to abiotic stresses in Chinese cabbage. BMC Genom. 2015, 16, 33. [Google Scholar] [CrossRef]
- Chen, P.; Yan, M.; Li, L.; He, J.; Zhou, S.; Li, Z.; Niu, C.; Bao, C.; Zhi, F.; Ma, F.; et al. The apple DNA-binding one zinc-finger protein MdDof54 promotes drought resistance. Hortic. Res. 2020, 7, 195. [Google Scholar] [CrossRef]
- Cao, L.; Ye, F.; Fahim, A.M.; Ma, C.; Pang, Y.; Zhang, X.; Zhang, Q.; Lu, X. Transcription factor ZmDof22 enhances drought tolerance by regulating stomatal movement and antioxidant enzymes activities in maize (Zea mays L.). Theor. Appl. Genet. 2024, 137, 132. [Google Scholar] [CrossRef]
- Zhao, C.; Bai, H.; Li, C.; Pang, Z.; Xuan, L.; Lv, D.; Niu, S. Genome-Wide Identification of the DOF Gene Family in Kiwifruit (Actinidia chinensis) and Functional Validation of AcDOF22 in Response to Drought Stress. Int. J. Mol. Sci. 2024, 25, 9103. [Google Scholar] [CrossRef]
- Nan, H.; Ludlow, R.A.; Lu, M.; An, H. Genome-wide analysis of Dof genes and their response to abiotic stress in rose (Rosa chinensis). Front. Genet. 2021, 12, 538733. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Osbourn, A.; Ma, P. MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol. Plant 2015, 8, 689–708. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Xie, N.; Li, Y.; Zhao, Z.; Luo, P.; Cui, Y.; Rao, X.; Chen, W. Mediator Subunit RhMED15a Regulates Drought Tolerance in Rose. Horticulturae 2024, 10, 84. [Google Scholar] [CrossRef]
- Hou, Y.; Fan, C.; Sun, J.; Chang, Y.; Lu, J.; Sun, J.; Wang, C.; Liu, J. Genome-wide Identification, evolution, and expression analysis of the TCP gene family in rose (Rosa chinensis Jacq.). Horticulturae 2022, 8, 961. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cheon, K.-S.; Kim, S.Y.; Kim, J.H.; Kwon, O.H.; Lee, H.J.; Kim, W.H. Expression of SOD2 enhances tolerance to drought stress in roses. Hortic. Environ. Biotechnol. 2020, 61, 569–576. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.; Zou, J.; Zhang, X.; Jiang, L.; Liu, K.; Lü, P.; Gao, J.; Zhang, C. The RhHB1/RhLOX4 module affects the dehydration tolerance of rose flowers (Rosa hybrida) by fine-tuning jasmonic acid levels. Hortic. Res. 2020, 7, 74. [Google Scholar] [CrossRef]
- Allen, B.L.; Taatjes, D.J. The Mediator complex: A central integrator of transcription. Nat. Rev. Mol. Cell Biol. 2015, 16, 155–166. [Google Scholar] [CrossRef]
- Buendía-Monreal, M.; Gillmor, C.S. Mediator: A key regulator of plant development. Dev. Biol. 2016, 419, 7–18. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Qu, L.J. Plant Mediator complex and its critical functions in transcription regulation. J. Integr. Plant Biol. 2016, 58, 106–118. [Google Scholar] [CrossRef]
- Chen, J.; Yang, S.; Fan, B.; Zhu, C.; Chen, Z. The mediator complex: A central coordinator of plant adaptive responses to environmental stresses. Int. J. Mol. Sci. 2022, 23, 6170. [Google Scholar] [CrossRef]
- Li, S.; Xu, J.; Cao, Y.; Wu, J.; Liu, Q.; Zhang, D. Genome-Wide Analyses of CCHC Family Genes and Their Expression Profiles under Drought Stress in Rose (Rosa chinensis). Int. J. Mol. Sci. 2024, 25, 8983. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, S.; Ren, Y.; You, Y.; Wang, Z.; Zhang, Y.; Zhu, X.; Hu, P. Genome-wide identification of HSP90 gene family in Rosa chinensis and its response to salt and drought stresses. 3 Biotech 2024, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhao, J.; Huang, W.; Liu, C.; Hao, X.; Gao, C.; Deng, M.; Wen, J. Genome-Wide Identification of WRKY Transcription Factor Family in Chinese Rose and Response to Drought, Heat, and Salt Stress. Genes 2024, 15, 800. [Google Scholar] [CrossRef] [PubMed]
- Dar, R.A.; Nisar, S.; Tahir, I. Ethylene: A key player in ethylene sensitive flower senescence: A review. Sci. Hortic. 2021, 290, 110491. [Google Scholar] [CrossRef]
- Ichimura, K.; Mizoguchi, T.; Yoshida, R.; Yuasa, T.; Shinozaki, K. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 2000, 24, 655–665. [Google Scholar] [CrossRef]
- Tsugama, D.; Liu, S.; Takano, T. Drought-induced activation and rehydration-induced inactivation of MPK6 in Arabidopsis. Biochem. Biophys. Res. Commun. 2012, 426, 626–629. [Google Scholar] [CrossRef]
- Xu, J.; Chua, N.H. Dehydration stress activates Arabidopsis MPK6 to signal DCP1 phosphorylation. EMBO J. 2012, 31, 1975–1984. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Q.; Wang, Q.; Feng, M.; Li, Y.; Meng, Y.; Zhang, Y.; Liu, G.; Ma, Z.; Wu, H. RhMKK9, a rose MAP KINASE KINASE gene, is involved in rehydration-triggered ethylene production in rose gynoecia. BMC Plant Biol. 2017, 17, 51. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef]
- Zhu, X.C.; Song, F.B.; Xu, H.W. Arbuscular mycorrhizae improves low temperature stress in maize via alterations in host water status and photosynthesis. Plant Soil 2010, 331, 129–137. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought tolerance: Role of organic osmolytes, growth regulators, and mineral nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment, 1st ed.; Parvaiz Ahmad, M.R.W., Ed.; Springer Science+Business Media: New York, NY, USA, 2014; Volume 1, pp. 25–55. [Google Scholar]
- Behrooz, A.; Vahdati, K.; Rejali, F.; Lotfi, M.; Sarikhani, S.; Leslie, C. Arbuscular mycorrhiza and plant growth-promoting bacteria alleviate drought stress in walnut. HortScience 2019, 54, 1087–1092. [Google Scholar] [CrossRef]
- Boutasknit, A.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Ben-Laouane, R.; Douira, A.; El Modafar, C.; Mitsui, T.; Wahbi, S.; Meddich, A. Arbuscular mycorrhizal fungi mediate drought tolerance and recovery in two contrasting carob (Ceratonia siliqua L.) ecotypes by regulating stomatal, water relations, and (in) organic adjustments. Plants 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Zobel, M.; Öpik, M. Plant and arbuscular mycorrhizal fungal (AMF) communities–which drives which? J. Veg. Sci. 2014, 25, 1133–1140. [Google Scholar] [CrossRef]
- Reynolds, W.; Drury, C.; Yang, X.; Tan, C.; Yang, J. Impacts of 48 years of consistent cropping, fertilization and land management on the physical quality of a clay loam soil. Can. J. Soil Sci. 2014, 94, 403–419. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Zou, Y.-N. Arbuscular mycorrhizal fungi and tolerance of drought stress in plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Springer: Singapore, 2017; pp. 25–41. [Google Scholar]
- Ren, A.-T.; Zhu, Y.; Chen, Y.-L.; Ren, H.-X.; Li, J.-Y.; Abbott, L.K.; Xiong, Y.-C. Arbuscular mycorrhizal fungus alters root-sourced signal (abscisic acid) for better drought acclimation in Zea mays L. seedlings. Environ. Exp. Bot. 2019, 167, 103824. [Google Scholar] [CrossRef]
- Zhang, M.C.; Duan, L.S.; Zhai, L.X.; Li, J.M.; Tian, X.L.; Wang, B.M.; He, Z.P.; Li, Z.H. Effect of plant growth regulators on water deficit induced yield loss in soybean. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004. [Google Scholar]
- Augé, R.M.; Foster, J.G.; Loescher, W.H.; Stodola, A.J. Symplastic molality of free amino acids and sugars in Rosa roots with regard to VA mycorrhizae and drought. Symbiosis 1992, 12, 1–17. [Google Scholar]
- Pinior, A.; Grunewaldt-Stöcker, G.; von Alten, H.; Strasser, R.J. Mycorrhizal impact on drought stress tolerance of rose plants probed by chlorophyll a fluorescence, proline content and visual scoring. Mycorrhiza 2005, 15, 596–605. [Google Scholar] [CrossRef]
- Augé, R.M.; Schekel, K.A.; Wample, R.L. Osmotic adjustment in leaves of VA mycorrhizal and nonmycorrhizal rose plants in response to drought stress. Plant Physiol. 1986, 82, 765–770. [Google Scholar] [CrossRef]
- Augé, R.M.; Schekel, K.A.; Wample, R.L. Greater leaf conductance of well-watered VA mycorrhizal rose plants is not related to phosphorus nutrition. New Phytol. 1986, 103, 107–116. [Google Scholar] [CrossRef]
- Green, C.D.; Stodola, A.; Augé, R.M. Transpiration of detached leaves from mycorrhizal and nonmycorrhizal cowpea and rose plants given varying abscisic acid, pH, calcium, and phosphorus. Mycorrhiza 1998, 8, 93–99. [Google Scholar] [CrossRef]
- Tripathy, L.; Dash, S.; Giri, T. Impact of different mulch materials on growth and flowering of rose cv. Mainu parle. J. Crop Weed 2019, 15, 28–31. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J.; Poree, F.; Schaeufele, R.; Helmke, H.; Frackenpohl, J.; Lehr, S.; von Koskull-Döring, P.; Christmann, A.; Schnyder, H.; et al. Abscisic acid receptors and coreceptors modulate plant water use efficiency and water productivity. Plant Physiol. 2019, 180, 1066–1080. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Nadira, U.A.; Bibi, N.; Zhang, G.; Wu, F. Tolerance to Combined Stress of Drought and Salinity in Barley. In Combined Stresses in Plants: Physiological, Molecular, and Biochemical Aspects; Mahalingam, R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 93–121. [Google Scholar]
- Monteiro, J.; Nell, T.A.; Barrett, J. Postproduction of potted miniature rose: Flower respiration and single flower longevity. J. Am. Soc. Hortic. Sci. 2001, 126, 134–139. [Google Scholar] [CrossRef]
- Ashkavand, P.; Zarafshar, M.; Tabari, M.; Mirzaie, J.; Nikpour, A.; Bordbar, S.K.; Struve, D.; Striker, G.G. Application of SiO2 nanoparticles as pretreatment alleviates the impact of drought on the physiological performance of Prunus mahaleb (Rosaceae). Boletín Soc. Argent. Botánica 2018, 53, 1–10. [Google Scholar] [CrossRef]
- Kandhol, N.; Jain, M.; Tripathi, D.K. Nanoparticles as potential hallmarks of drought stress tolerance in plants. Physiol. Plant 2022, 174, e13665. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Hasan, M.M.; Hammami, I.; Alghamdi, A.I.; Alshehri, D.; Alatawi, H.A. Green synthesized metal oxide nanoparticles mediate growth regulation and physiology of crop plants under drought stress. Plants 2021, 10, 1730. [Google Scholar] [CrossRef]
- Siddiqui, H.; Ahmed, K.B.M.; Sami, F.; Hayat, S. Silicon nanoparticles and plants: Current knowledge and future perspectives. In Sustainable Agriculture Reviews 41: Nanotechnology for Plant Growth and Development; Springer: Cham, Switzerland, 2020; pp. 129–142. [Google Scholar]
- Wang, H.-Z.; Zhang, L.-H.; Ma, J.; Li, X.-Y.; Li, Y.; Zhang, R.-P.; Wang, R.-Q. Effects of water stress on reactive oxygen species generation and protection system in rice during grain-filling stage. Agric. Sci. China 2010, 9, 633–641. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Kouna, T.; Antonopoulou, C.-I.; Therios, I. Exogenous proline induces soluble sugar accumulation and alleviates drought stress effects on photosystem II functioning of Arabidopsis thaliana leaves. Plant Growth Regul. 2011, 65, 315–325. [Google Scholar] [CrossRef]
- Ahmed, M.; Asif, M.; Hassan, F.-u. Augmenting drought tolerance in sorghum by silicon nutrition. Acta Physiol. Plant. 2014, 36, 473–483. [Google Scholar] [CrossRef]
- Keller, C.; Rizwan, M.; Davidian, J.-C.; Pokrovsky, O.; Bovet, N.; Chaurand, P.; Meunier, J.-D. Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 µM Cu. Planta 2015, 241, 847–860. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Qin, H.; Ding, H.; Li, Y.; Guo, T. Silicon application alleviates drought stress in wheat through transcriptional regulation of multiple antioxidant defense pathways. J. Plant Growth Regul. 2016, 35, 1–10. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Pessarakli, M. Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. J. Plant Nutr. 2016, 39, 1001–1015. [Google Scholar] [CrossRef]
- Hassan, F.; Ali, E.; Alamer, K. Exogenous application of polyamines alleviates water stress-induced oxidative stress of Rosa damascena Miller var. trigintipetala Dieck. South Afr. J. Bot. 2018, 116, 96–102. [Google Scholar] [CrossRef]
- Moftah, A.E.; Al-Humaid, A.I. Effects of Kaolin and Pinolene film-forming polymers on water relations and photosynthetic rate of tuberose (Polianthes tuberosa L.) plants under water deficit conditions. J. Appl. Hortic. 2004, 6, 16–22. [Google Scholar] [CrossRef]
- Giday, H.; Fanourakis, D.; Kjaer, K.H.; Fomsgaard, I.S.; Ottosen, C.-O. Foliar abscisic acid content underlies genotypic variation in stomatal responsiveness after growth at high relative air humidity. Ann. Bot. 2013, 112, 1857–1867. [Google Scholar] [CrossRef]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef]
- Kettlewell, P.S.; Heath, W.L.; Haigh, I.M. Yield enhancement of droughted wheat by film antitranspirant application: Rationale and evidence. Agric. Sci. 2010, 1, 143. [Google Scholar] [CrossRef][Green Version]
- Fanourakis, D.; Giday, H.; Li, T.; Kambourakis, E.; Ligoxigakis, E.K.; Papadimitriou, M.; Strataridaki, A.; Bouranis, D.; Fiorani, F.; Heuvelink, E.; et al. Antitranspirant compounds alleviate the mild-desiccation-induced reduction of vase life in cut roses. Postharvest Biol. Technol. 2016, 117, 110–117. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Perik, R.J.; Belde, P.J.M. Effects of surfactants on the longevity of dry-stored cut flowering stems of rose, Bouvardia, and Astilbe. Postharvest Biol. Technol. 1993, 3, 69–76. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Abadie, P.; Belde, P.J.M. Alkylethoxylate surfactants for rehydration of roses and Bouvardia flowers. Postharvest Biol. Technol. 2002, 24, 327–333. [Google Scholar] [CrossRef]
- Younis, A.; Bhatti, M.Z.M.; Riaz, A.; Tariq, U.; Arfan, M.; Nadeem, M.; Ahsan, M. Effect of different types of mulching on growth and flowering of Freesia alba cv. Aurora. Pak. J. Agric. Sci. 2012, 49, 429–433. [Google Scholar]
- Horo, P.; Kullu, N.; Hembrom, P.; Minz, A. Effect of mulching in rose (Rosa hybrida) cv. Maine parle. J. Pharmacogn. Phytochem. 2018, 7, 2415–2417. [Google Scholar]
- Sardar, H.; Akhtar, G.; Akram, A.; Naseem, K. Influence of mulching materials on soil conditions, weeds, plant growth and flower yield of Rosa centifolia. Int. J. Agric. Appl. Sci 2016, 8, 64–71. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).