Insight into the Regulation of NDRG1 Expression
Abstract
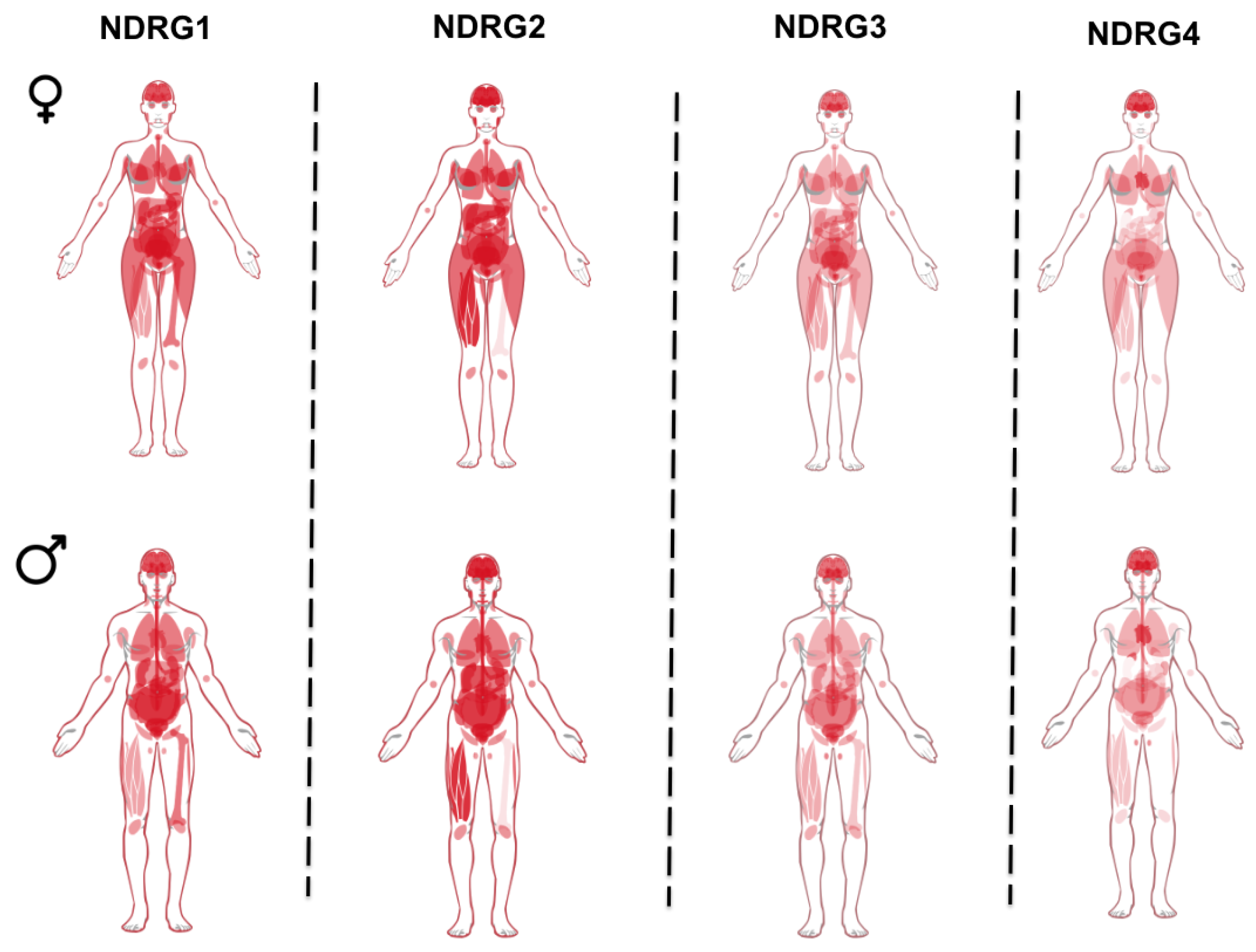
1. Introduction
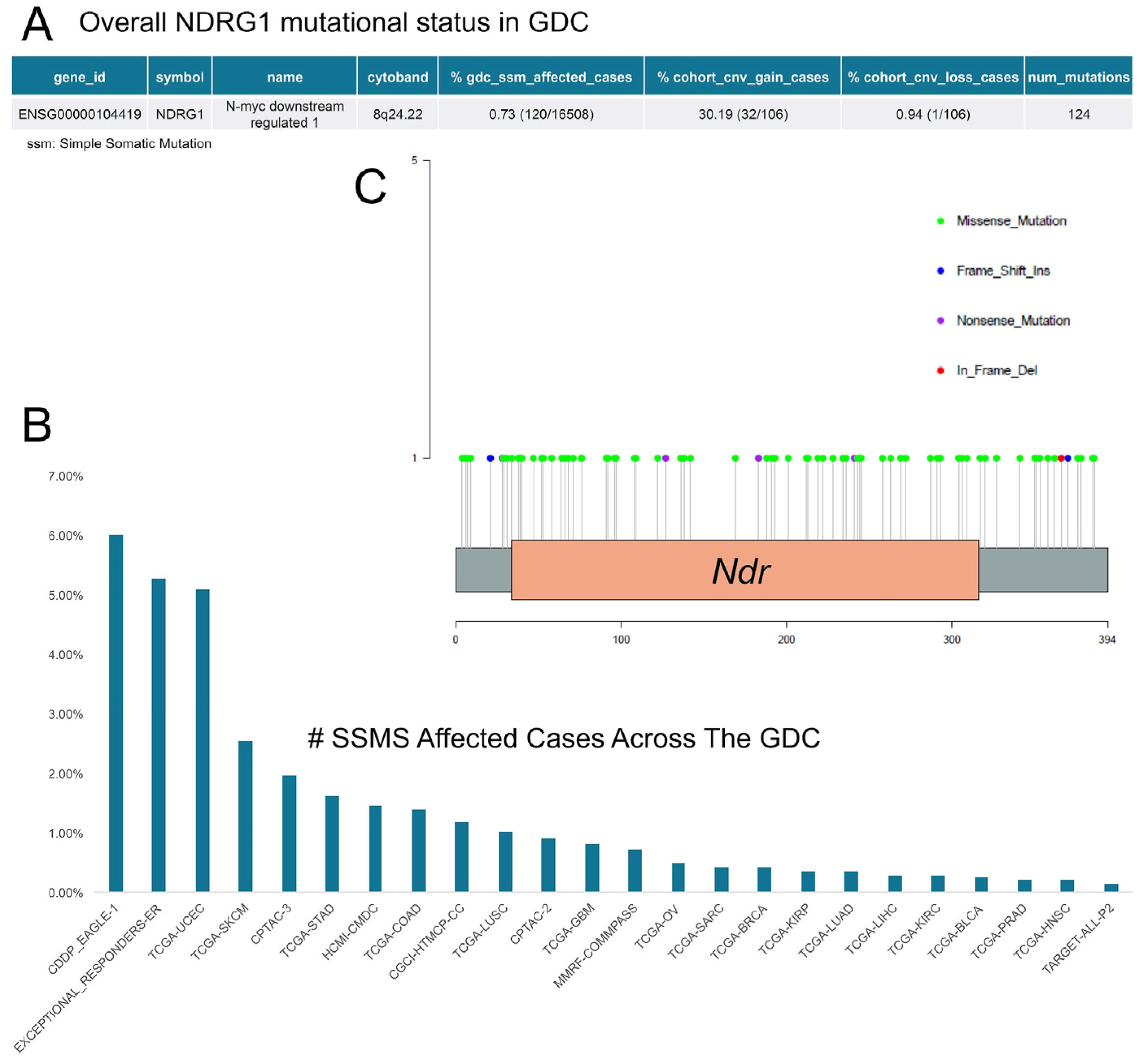
2. Regulation of NDRG1 Expression
3. Functional Roles of NDRG1
3.1. NDRG1 Regulation of Cell Differentiation
3.2. NDRG1 Regulation of Vesicular Trafficking
3.3. NDRG1, ER Stress, and Autophagy
3.4. NDRG1 Regulation of Senescence/Ageing
3.5. NDRG1 Regulation of Endothelial Function
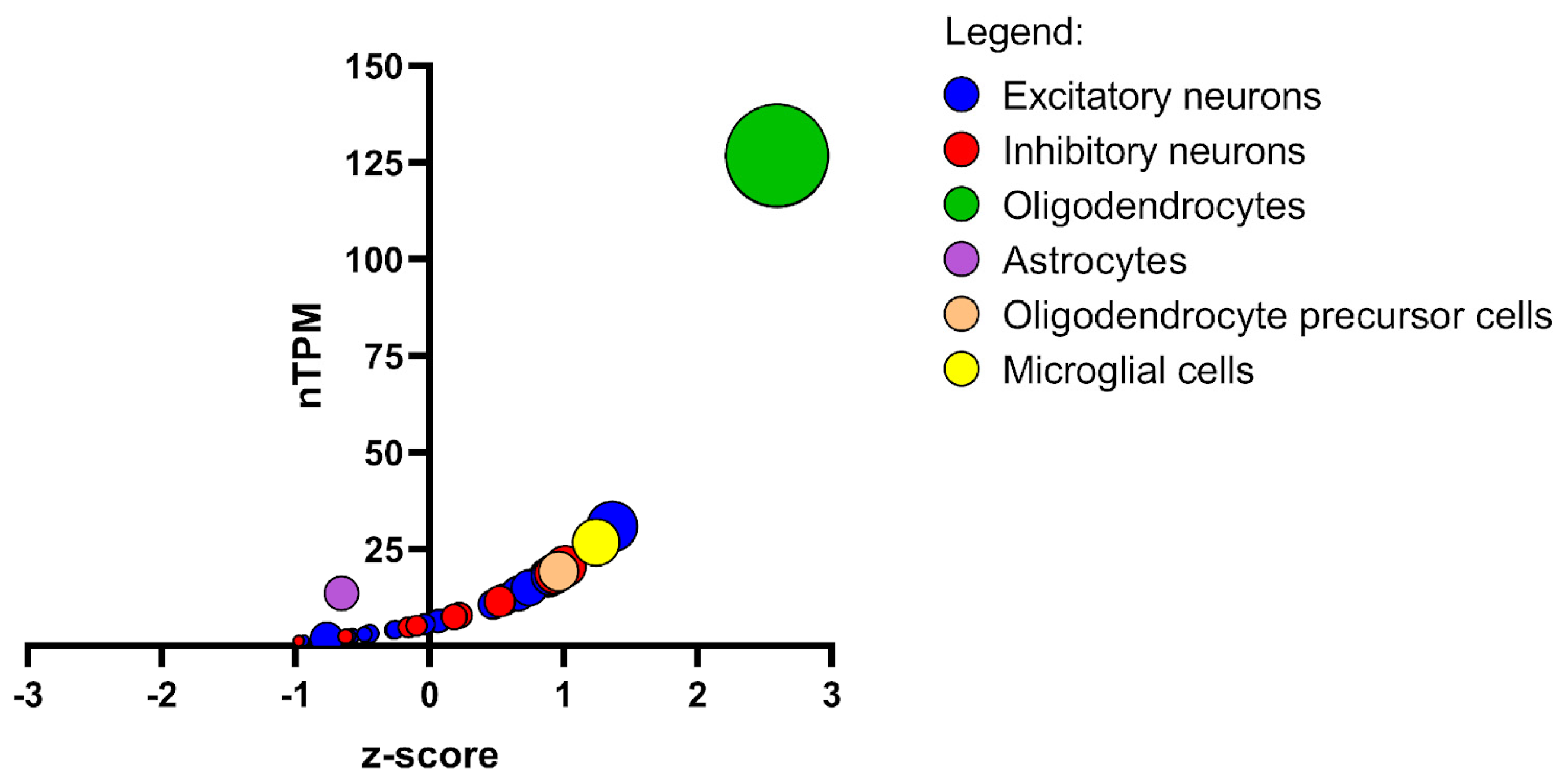
3.6. NDRG1 Regulation of Myelination
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qu, X.; Zhai, Y.; Wei, H.; Zhang, C.; Xing, G.; Yu, Y.; He, F. Characterization and Expression of Three Novel Differentiation-Related Genes Belong to the Human NDRG Gene Family. Mol. Cell. Biochem. 2002, 229, 35–44. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Kowalik-Jankowska, T.; Medici, S.; Peana, M.; Kozlowski, H. Copper(II) Binding to Cap43 Protein Fragments. Dalt. Trans. 2008, 44, 6127–6134. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Peana, M.; Medici, S.; Anedda, R. An NMR Study on Nickel Binding Sites in Cap43 Protein Fragments. Dalt. Trans. 2009, 5523–5534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Salnikow, K.; Costa, M. Cap43, a Novel Gene Specifically Induced by Ni2+ Compounds. Cancer Res. 1998, 58, 2182–2189. [Google Scholar]
- Salnikow, K.; Su, W.; Blagosklonny, M.V.; Costa, M. Carcinogenic Metals Induce Hypoxia-Inducible Factor-Stimulated Transcription by Reactive Oxygen Species-Independent Mechanism. Cancer Res. 2000, 60, 3375–3378. [Google Scholar]
- Park, K.C.; Menezes, S.V.; Kalinowski, D.S.; Sahni, S.; Jansson, P.J.; Kovacevic, Z.; Richardson, D.R. Identification of Differential Phosphorylation and Sub-Cellular Localization of the Metastasis Suppressor, NDRG1. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2644–2663. [Google Scholar] [CrossRef]
- Mustonen, V.; Muruganandam, G.; Loris, R.; Kursula, P.; Ruskamo, S. Crystal and Solution Structure of NDRG1, a Membrane-binding Protein Linked to Myelination and Tumour Suppression. FEBS J. 2021, 288, 3507–3529. [Google Scholar] [CrossRef]
- Zhou, R.-H.; Kokame, K.; Tsukamoto, Y.; Yutani, C.; Kato, H.; Miyata, T. Characterization of the Human NDRG Gene Family: A Newly Identified Member, NDRG4, Is Specifically Expressed in Brain and Heart. Genomics 2001, 73, 86–97. [Google Scholar] [CrossRef]
- Vaes, N.; Lentjes, M.H.F.M.; Gijbels, M.J.; Rademakers, G.; Daenen, K.L.; Boesmans, W.; Wouters, K.A.D.; Geuzens, A.; Qu, X.; Steinbusch, H.P.J.; et al. NDRG4, an Early Detection Marker for Colorectal Cancer, Is Specifically Expressed in Enteric Neurons. Neurogastroenterol. Motil. 2017, 29, e13095. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kokame, K.; Okuda, T.; Nakajo, Y.; Yanamoto, H.; Miyata, T. NDRG4 Protein-Deficient Mice Exhibit Spatial Learning Deficits and Vulnerabilities to Cerebral Ischemia. J. Biol. Chem. 2011, 286, 26158–26165. [Google Scholar] [CrossRef]
- Yang, M.; Feng, Y.; Liu, J.; Wang, H.; Wu, S.; Zhao, W.; Kim, P.; Zhou, X. SexAnnoDB, a knowledgebase of sex-specific regulations from multi-omics data of human cancers. Biol. Sex Differ. 2024, 15, 64. [Google Scholar] [CrossRef]
- Marques, R.B.; Dits, N.F.; Erkens-Schulze, S.; van Ijcken, W.F.; van Weerden, W.M.; Jenster, G. Modulation of androgen receptor signaling in hormonal therapy-resistant prostate cancer cell lines. PLoS ONE 2011, 6, e23144. [Google Scholar] [CrossRef]
- Fotovati, A.; Fujii, T.; Yamaguchi, M.; Kage, M.; Shirouzu, K.; Oie, S.; Basaki, Y.; Ono, M.; Yamana, H.; Kuwano, M. 17β-Estradiol Induces Down-Regulation of Cap43/NDRG1/Drg-1, a Putative Differentiation-Related and Metastasis Suppressor Gene, in Human Breast Cancer Cells. Clin. Cancer Res. 2006, 12, 3010–3018. [Google Scholar] [CrossRef]
- Wang, D.; Tian, X.; Jiang, Y. NDRG1/Cap43 overexpression in tumor tissues and serum from lung cancer patients. J. Cancer Res. Clin. Oncol. 2012, 138, 1813–1820. [Google Scholar] [CrossRef]
- Deng, Y.T.; You, J.; He, Y.; Zhang, Y.; Li, H.Y.; Wu, X.R.; Cheng, J.Y.; Guo, Y.; Long, Z.W.; Chen, Y.L.; et al. Atlas of the plasma proteome in health and disease in 53,026 adults. Cell 2025, 188, 253–271.e7. [Google Scholar] [CrossRef]
- Shimono, A.; Okuda, T.; Kondoh, H. N-Myc-Dependent Repression of Ndr1, a Gene Identified by Direct Subtraction of Whole Mouse Embryo CDNAs between Wild Type and N-Myc Mutant. Mech. Dev. 1999, 83, 39–52. [Google Scholar] [CrossRef]
- Piquemal, D.; Joulia, D.; Balaguer, P.; Basset, A.; Marti, J.; Commes, T. Differential Expression of the RTP/Drg1/Ndr1 Gene Product in Proliferating and Growth Arrested Cells. Biochim. Biophys. Acta Mol. Cell Res. 1999, 1450, 364–373. [Google Scholar] [CrossRef]
- Kokame, K.; Kato, H.; Miyata, T. Homocysteine-Respondent Genes in Vascular Endothelial Cells Identified by Differential Display Analysis. J. Biol. Chem. 1996, 271, 29659–29665. [Google Scholar] [CrossRef]
- Park, H.; Adams, M.A.; Lachat, P.; Bosman, F.; Pang, S.C.; Graham, C.H. Hypoxia Induces the Expression of a 43-KDa Protein (PROXY-1) in Normal and Malignant Cells. Biochem. Biophys. Res. Commun. 2000, 276, 321–328. [Google Scholar] [CrossRef]
- Han, L.-L.; Hou, L.; Zhou, M.-J.; Ma, Z.; Lin, D.-L.; Wu, L.; Ge, Y. Aberrant NDRG1 Methylation Associated with Its Decreased Expression and Clinicopathological Significance in Breast Cancer. J. Biomed. Sci. 2013, 20, 52. [Google Scholar] [CrossRef]
- Mircetic, J.; Camgöz, A.; Abohawya, M.; Ding, L.; Dietzel, J.; Tobar, S.G.; Paszkowski-Rogacz, M.; Seidlitz, T.; Schmäche, T.; Mehnert, M.; et al. CRISPR/Cas9 Screen in Gastric Cancer Patient-Derived Organoids Reveals KDM1A-NDRG1 Axis as a Targetable Vulnerability. Small Methods 2023, 7, e2201605. [Google Scholar] [CrossRef]
- Kurdistani, S.K.; Arizti, P.; Reimer, C.L.; Sugrue, M.M.; Aaronson, S.A.; Lee, S.W. Inhibition of Tumor Cell Growth by RTP/Rit42 and Its Responsiveness to P53 and DNA Damage. Cancer Res. 1998, 58, 4439–4444. [Google Scholar]
- Cangul, H. Hypoxia Upregulates the Expression of the NDRG1 Gene Leading to Its Overexpression in Various Human Cancers. BMC Genet. 2004, 5, 27. [Google Scholar] [CrossRef]
- Lane, D.J.R.; Saletta, F.; Rahmanto, Y.S.; Kovacevic, Z.; Richardson, D.R. N-Myc Downstream Regulated 1 (NDRG1) Is Regulated by Eukaryotic Initiation Factor 3a (EIF3a) during Cellular Stress Caused by Iron Depletion. PLoS ONE 2013, 8, e57273. [Google Scholar] [CrossRef]
- Yuniati, L.; van der Meer, L.T.; Tijchon, E.; van Ingen Schenau, D.; van Emst, L.; Levers, M.; Palit, S.A.L.; Rodenbach, C.; Poelmans, G.; Hoogerbrugge, P.M.; et al. Tumor Suppressor BTG1 Promotes PRMT1-Mediated ATF4 Function in Response to Cellular Stress. Oncotarget 2016, 7, 3128–3143. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, Y.; Yang, X.; Ma, P.; Li, Y.; Wu, Y.; Chen, X.; Deng, X.; Yang, T.; Mao, X.; et al. Claudin-2 Promotes Colorectal Cancer Growth and Metastasis by Suppressing NDRG1 Transcription. Clin. Transl. Med. 2021, 11, e667. [Google Scholar] [CrossRef]
- Mao, M.; Jia, Y.; Chen, Y.; Yang, J.; Xu, L.; Zhang, X.; Zhou, J.; Li, Z.; Chen, C.; Ju, S.; et al. HJURP Regulates Cell Proliferation and Chemo-Resistance via YAP1/NDRG1 Transcriptional Axis in Triple-Negative Breast Cancer. Cell Death Dis. 2022, 13, 396. [Google Scholar] [CrossRef]
- Holmes, B.; Benavides-Serrato, A.; Saunders, J.T.; Kumar, S.; Nishimura, R.N.; Gera, J. MTORC2-Mediated Direct Phosphorylation Regulates YAP Activity Promoting Glioblastoma Growth and Invasive Characteristics. Neoplasia 2021, 23, 951–965. [Google Scholar] [CrossRef]
- Vaidyanathan, S.; Salmi, T.M.; Sathiqu, R.M.; McConville, M.J.; Cox, A.G.; Brown, K.K. YAP Regulates an SGK1/MTORC1/SREBP-Dependent Lipogenic Program to Support Proliferation and Tissue Growth. Dev. Cell 2022, 57, 719–731.e8. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Alessi, D.R. MTOR Complex 2 (MTORC2) Controls Hydrophobic Motif Phosphorylation and Activation of Serum- and Glucocorticoid-Induced Protein Kinase 1 (SGK1). Biochem. J. 2008, 416, 375–385. [Google Scholar] [CrossRef]
- Brickley, D.R.; Agyeman, A.S.; Kopp, R.F.; Hall, B.A.; Harbeck, M.C.; Belova, L.; Volden, P.A.; Wu, W.; Roe, M.W.; Conzen, S.D. Serum-and Glucocorticoid-Induced Protein Kinase 1 (SGK1) Is Regulated by Store-Operated Ca2+ Entry and Mediates Cytoprotection against Necrotic Cell Death. J. Biol. Chem. 2013, 288, 32708–32719. [Google Scholar] [CrossRef]
- Sevinsky, C.J.; Khan, F.; Kokabee, L.; Darehshouri, A.; Maddipati, K.R.; Conklin, D.S. NDRG1 Regulates Neutral Lipid Metabolism in Breast Cancer Cells. Breast Cancer Res. 2018, 20, 55. [Google Scholar] [CrossRef]
- Li, C.; Qiu, S.; Liu, X.; Guo, F.; Zhai, J.; Li, Z.; Deng, L.; Ge, L.; Qian, H.; Yang, L.; et al. Extracellular Matrix-Derived Mechanical Force Governs Breast Cancer Cell Stemness and Quiescence Transition through Integrin-DDR Signaling. Signal Transduct. Target. Ther. 2023, 8, 247. [Google Scholar] [CrossRef]
- Stein, S.; Thomas, E.K.; Herzog, B.; Westfall, M.D.; Rocheleau, J.V.; Jackson, R.S.; Wang, M.; Liang, P. NDRG1 Is Necessary for P53-Dependent Apoptosis. J. Biol. Chem. 2004, 279, 48930–48940. [Google Scholar] [CrossRef]
- Croessmann, S.; Wong, H.Y.; Zabransky, D.J.; Chu, D.; Mendonca, J.; Sharma, A.; Mohseni, M.; Rosen, D.M.; Scharpf, R.B.; Cidado, J.; et al. NDRG1 Links P53 with Proliferation-Mediated Centrosome Homeostasis and Genome Stability. Proc. Natl. Acad. Sci. USA 2015, 112, 11583–11588. [Google Scholar] [CrossRef]
- Kim, K.; Ongusaha, P.P.; Hong, Y.-K.; Kurdistani, S.K.; Nakamura, M.; Lu, K.P.; Lee, S.W. Function of Drg1/Rit42 in P53-Dependent Mitotic Spindle Checkpoint. J. Biol. Chem. 2004, 279, 38597–38602. [Google Scholar] [CrossRef]
- Sun, X.; Luo, H.; Han, C.; Zhang, Y.; Yan, C. Identification of a Hypoxia-Related Molecular Classification and Hypoxic Tumor Microenvironment Signature for Predicting the Prognosis of Patients with Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 700062. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.-H.; Gao, G.-D.; Wang, G.; Qu, L.; Li, J.-G.; Wang, C.-M. HIF-1α up-Regulates NDRG1 Expression through Binding to NDRG1 Promoter, Leading to Proliferation of Lung Cancer A549 Cells. Mol. Biol. Rep. 2013, 40, 3723–3729. [Google Scholar] [CrossRef]
- Wouters, B.G.; Koritzinsky, M. Hypoxia Signalling through MTOR and the Unfolded Protein Response in Cancer. Nat. Rev. Cancer 2008, 8, 851–864. [Google Scholar] [CrossRef]
- Zhang, P.; Tchou-Wong, K.-M.; Costa, M. Egr-1 Mediates Hypoxia-Inducible Transcription of the NDRG1 Gene through an Overlapping Egr-1/Sp1 Binding Site in the Promoter. Cancer Res. 2007, 67, 9125–9133. [Google Scholar] [CrossRef]
- Salnikow, K.; Kluz, T.; Costa, M.; Piquemal, D.; Demidenko, Z.N.; Xie, K.; Blagosklonny, M.V. The Regulation of Hypoxic Genes by Calcium Involves C-Jun/AP-1, Which Cooperates with Hypoxia-Inducible Factor 1 in Response to Hypoxia. Mol. Cell. Biol. 2002, 22, 1734–1741. [Google Scholar] [CrossRef]
- Askautrud, H.A.; Gjernes, E.; Gunnes, G.; Sletten, M.; Ross, D.T.; Børresen-Dale, A.L.; Iversen, N.; Tranulis, M.A.; Frengen, E. Global Gene Expression Analysis Reveals a Link between NDRG1 and Vesicle Transport. PLoS ONE 2014, 9, e87268. [Google Scholar] [CrossRef]
- Weiler, M.; Blaes, J.; Pusch, S.; Sahm, F.; Czabanka, M.; Luger, S.; Bunse, L.; Solecki, G.; Eichwald, V.; Jugold, M.; et al. MTOR Target NDRG1 Confers MGMT-Dependent Resistance to Alkylating Chemotherapy. Proc. Natl. Acad. Sci. USA 2014, 111, 409–414. [Google Scholar] [CrossRef]
- Park, J.S.; Gabel, A.M.; Kassir, P.; Kang, L.; Chowdhary, P.K.; Osei-Ntansah, A.; Tran, N.D.; Viswanathan, S.; Canales, B.; Ding, P.; et al. N-Myc Downstream Regulated Gene 1 (Ndrg1) Functions as a Molecular Switch for Cellular Adaptation to Hypoxia. Elife 2022, 11, e74031. [Google Scholar] [CrossRef]
- Pearce, L.R.; Sommer, E.M.; Sakamoto, K.; Wullschleger, S.; Alessi, D.R. Protor-1 Is Required for Efficient MTORC2-Mediated Activation of SGK1 in the Kidney. Biochem. J. 2011, 436, 169–179. [Google Scholar] [CrossRef]
- Murray, J.T.; Campbell, D.G.; Morrice, N.; Auld, G.C.; Shpiro, N.; Marquez, R.; Peggie, M.; Bain, J.; Bloomberg, G.B.; Grahammer, F.; et al. Exploitation of KESTREL to Identify NDRG Family Members as Physiological Substrates for SGK1 and GSK3. Biochem. J. 2004, 384, 477–488. [Google Scholar] [CrossRef]
- You, G.-R.; Chang, J.T.; Li, H.-F.; Cheng, A.-J. Multifaceted and Intricate Oncogenic Mechanisms of NDRG1 in Head and Neck Cancer Depend on Its C-Terminal 3R-Motif. Cells 2022, 11, 1581. [Google Scholar] [CrossRef]
- Humphrey, S.J.; Yang, G.; Yang, P.; Fazakerley, D.J.; Stöckli, J.; Yang, J.Y.; James, D.E. Dynamic Adipocyte Phosphoproteome Reveals That Akt Directly Regulates MTORC2. Cell Metab. 2013, 17, 1009–1020. [Google Scholar] [CrossRef]
- Kazyken, D.; Magnuson, B.; Bodur, C.; Acosta-Jaquez, H.A.; Zhang, D.; Tong, X.; Barnes, T.M.; Steinl, G.K.; Patterson, N.E.; Altheim, C.H.; et al. AMPK Directly Activates MTORC2 to Promote Cell Survival during Acute Energetic Stress. Sci. Signal. 2019, 12, eaav3249. [Google Scholar] [CrossRef]
- Cockfield, J.A.; Schafer, Z.T. SGK1-Regulated Metabolism: Key for the Survival of Cells Detached from the Extracellular Matrix. Mol. Cell. Oncol. 2021, 8, 1976583. [Google Scholar] [CrossRef]
- Merhi, A.; Delrée, P.; Marini, A.M. The Metabolic Waste Ammonium Regulates MTORC2 and MTORC1 Signaling. Sci. Rep. 2017, 7, 44602. [Google Scholar] [CrossRef]
- López-Tejada, A.; Griñán-Lisón, C.; González-González, A.; Cara, F.E.; Luque, R.J.; Rosa-Garrido, C.; Blaya-Cánovas, J.L.; Navarro-Ocón, A.; Valenzuela-Torres, M.; Parra-López, M.; et al. TGFβ Governs the Pleiotropic Activity of NDRG1 in Triple-Negative Breast Cancer Progression. Int. J. Biol. Sci. 2023, 19, 204–224. [Google Scholar] [CrossRef]
- Sommer, E.M.; Dry, H.; Cross, D.; Guichard, S.; Davies, B.R.; Alessi, D.R. Elevated SGK1 Predicts Resistance of Breast Cancer Cells to Akt Inhibitors. Biochem. J. 2013, 452, 499–508. [Google Scholar] [CrossRef]
- McCaig, C.; Potter, L.; Abramczyk, O.; Murray, J.T. Phosphorylation of NDRG1 Is Temporally and Spatially Controlled during the Cell Cycle. Biochem. Biophys. Res. Commun. 2011, 411, 227–234. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Mattar, P.; Toledo, M.; Bains, H.; Kalyani, M.; Aoun, M.L.; Sharma, M.; McIntire, L.B.J.; Gunther-Cummins, L.; Macaluso, F.P.; et al. MTORC2–NDRG1–CDC42 Axis Couples Fasting to Mitochondrial Fission. Nat. Cell Biol. 2023, 25, 989–1003. [Google Scholar] [CrossRef]
- Ho, J.J.D.; Wang, M.; Audas, T.E.; Kwon, D.; Carlsson, S.K.; Timpano, S.; Evagelou, S.L.; Brothers, S.; Gonzalgo, M.L.; Krieger, J.R.; et al. Systemic Reprogramming of Translation Efficiencies on Oxygen Stimulus. Cell Rep. 2016, 14, 1293–1300. [Google Scholar] [CrossRef]
- Luo, E.-C.; Chang, Y.-C.; Sher, Y.-P.; Huang, W.-Y.; Chuang, L.-L.; Chiu, Y.-C.; Tsai, M.-H.; Chuang, E.Y.; Lai, L.-C. MicroRNA-769-3p Down-Regulates NDRG1 and Enhances Apoptosis in MCF-7 Cells During Reoxygenation. Sci. Rep. 2014, 4, 5908. [Google Scholar] [CrossRef]
- Candiello, E.; Reato, G.; Verginelli, F.; Gambardella, G.; D Ambrosio, A.; Calandra, N.; Orzan, F.; Iuliano, A.; Albano, R.; Sassi, F.; et al. MicroRNA 483-3p Overexpression Unleashes Invasive Growth of Metastatic Colorectal Cancer via NDRG1 Downregulation and Ensuing Activation of the ERBB3/AKT Axis. Mol. Oncol. 2023, 17, 1280–1301. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Teng, Z.; Zhang, Z.; Xu, Y. Overexpressed MicroRNA-182 Promotes Proliferation and Invasion in Prostate Cancer PC-3 Cells by Down-Regulating N-Myc Downstream Regulated Gene 1 (NDRG1). PLoS ONE 2013, 8, e68982. [Google Scholar] [CrossRef]
- Lin, H.-C.; Yeh, C.-C.; Chao, L.-Y.; Tsai, M.-H.; Chen, H.-H.; Chuang, E.Y.; Lai, L.-C. The Hypoxia-Responsive LncRNA NDRG-OT1 Promotes NDRG1 Degradation via Ubiquitin-Mediated Proteolysis in Breast Cancer Cells. Oncotarget 2018, 9, 10470–10482. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, J.H. SUMO Modification Regulates the Protein Stability of NDRG1. Biochem. Biophys. Res. Commun. 2015, 459, 161–165. [Google Scholar] [CrossRef]
- Joshi, V.; Stacey, A.; Feng, Y.; Kalita-de Croft, P.; Duijf, P.H.; Simpson, P.T.; Lakhani, S.R.; McCart Reed, A.E. NDRG1 Is a Prognostic Biomarker in Breast Cancer and Breast Cancer Brain Metastasis. J. Pathol. Clin. Res. 2024, 10, e12364. [Google Scholar] [CrossRef]
- Guan, R.J.; Ford, H.L.; Fu, Y.; Li, Y.; Shaw, L.M.; Pardee, A.B. Drg-1 as a Differentiation-Related, Putative Metastatic Suppressor Gene in Human Colon Cancer. Cancer Res. 2000, 60, 749–755. [Google Scholar]
- van Belzen, N.; Dinjens, W.N.; Diesveld, M.P.; Groen, N.A.; van der Made, A.C.; Nozawa, Y.; Vlietstra, R.; Trapman, J.; Bosman, F.T. A Novel Gene Which Is Up-Regulated during Colon Epithelial Cell Differentiation and down-Regulated in Colorectal Neoplasms. Lab. Investig. 1997, 77, 85–92. [Google Scholar]
- Bandyopadhyay, S.; Pai, S.K.; Gross, S.C.; Hirota, S.; Hosobe, S.; Miura, K.; Saito, K.; Commes, T.; Hayashi, S.; Watabe, M.; et al. The Drg-1 Gene Suppresses Tumor Metastasis in Prostate Cancer. Cancer Res. 2003, 63, 1731–1736. [Google Scholar]
- Cai, K.; El-Merahbi, R.; Loeffler, M.; Mayer, A.E.; Sumara, G. Ndrg1 Promotes Adipocyte Differentiation and Sustains Their Function. Sci. Rep. 2017, 7, 7191. [Google Scholar] [CrossRef]
- Li, A.; Zhu, X.; Wang, C.; Yang, S.; Qiao, Y.; Qiao, R.; Zhang, J. Upregulation of NDRG1 Predicts Poor Outcome and Facilitates Disease Progression by Influencing the EMT Process in Bladder Cancer. Sci. Rep. 2019, 9, 5166. [Google Scholar] [CrossRef]
- Zheng, P.; Wu, Y.; Wang, Y.; Hu, F. Disulfiram Suppresses Epithelial-Mesenchymal Transition (EMT), Migration and Invasion in Cervical Cancer through the HSP90A/NDRG1 Pathway. Cell. Signal. 2023, 109, 110771. [Google Scholar] [CrossRef]
- Hu, Z.-Y.; Xie, W.-B.; Yang, F.; Xiao, L.-W.; Wang, X.-Y.; Chen, S.-Y.; Li, Z.-G. NDRG1 Attenuates Epithelial–Mesenchymal Transition of Nasopharyngeal Cancer Cells via Blocking Smad2 Signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1876–1886. [Google Scholar] [CrossRef]
- Mi, L.; Zhu, F.; Yang, X.; Lu, J.; Zheng, Y.; Zhao, Q.; Wen, X.; Lu, A.; Wang, M.; Zheng, M.; et al. The Metastatic Suppressor NDRG1 Inhibits EMT, Migration and Invasion through Interaction and Promotion of Caveolin-1 Ubiquitylation in Human Colorectal Cancer Cells. Oncogene 2017, 36, 4323–4335. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, D.; Yue, F.; Zheng, M.; Kovacevic, Z.; Richardson, D.R. The Iron Chelators Dp44mT and DFO Inhibit TGF-β-Induced Epithelial-Mesenchymal Transition via Up-Regulation of N-Myc Downstream-Regulated Gene 1 (NDRG1). J. Biol. Chem. 2012, 287, 17016–17028. [Google Scholar] [CrossRef]
- Xi, R.; Pun, I.H.Y.; Menezes, S.V.; Fouani, L.; Kalinowski, D.S.; Huang, M.L.H.; Zhang, X.; Richardson, D.R.; Kovacevic, Z. Novel Thiosemicarbazones Inhibit Lysine-Rich Carcinoembryonic Antigen–Related Cell Adhesion Molecule 1 (CEACAM1) Coisolated (LYRIC) and the LYRIC-Induced Epithelial-Mesenchymal Transition via Upregulation of N-Myc Downstream-Regulated Gene 1 (NDRG1). Mol. Pharmacol. 2017, 91, 499–517. [Google Scholar] [CrossRef]
- Kachhap, S.K.; Faith, D.; Qian, D.Z.; Shabbeer, S.; Galloway, N.L.; Pili, R.; Denmeade, S.R.; DeMarzo, A.M.; Carducci, M.A. The N-Myc Down Regulated Gene1 (NDRG1) Is a Rab4a Effector Involved in Vesicular Recycling of E-Cadherin. PLoS ONE 2007, 2, e844. [Google Scholar] [CrossRef]
- Sahni, S.; Bae, D.-H.; Lane, D.J.R.; Kovacevic, Z.; Kalinowski, D.S.; Jansson, P.J.; Richardson, D.R. The Metastasis Suppressor, N-Myc Downstream-Regulated Gene 1 (NDRG1), Inhibits Stress-Induced Autophagy in Cancer Cells. J. Biol. Chem. 2014, 289, 9692–9709. [Google Scholar] [CrossRef]
- Merlot, A.M.; Porter, G.M.; Sahni, S.; Lim, E.G.; Peres, P.; Richardson, D.R. The Metastasis Suppressor, NDRG1, Differentially Modulates the Endoplasmic Reticulum Stress Response. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2094–2110. [Google Scholar] [CrossRef]
- Fares, J.; Petrosyan, E.; Kanojia, D.; Dmello, C.; Cordero, A.; Duffy, J.T.; Yeeravalli, R.; Sahani, M.H.; Zhang, P.; Rashidi, A.; et al. Metixene Is an Incomplete Autophagy Inducer in Preclinical Models of Metastatic Cancer and Brain Metastases. J. Clin. Investig. 2023, 133, e161142. [Google Scholar] [CrossRef]
- Chen, X.; Iliopoulos, D.; Zhang, Q.; Tang, Q.; Greenblatt, M.B.; Hatziapostolou, M.; Lim, E.; Tam, W.L.; Ni, M.; Chen, Y.; et al. XBP1 Promotes Triple-Negative Breast Cancer by Controlling the HIF1α Pathway. Nature 2014, 508, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.-J.; Chua, M.-S.; So, S.K. Suppressing N-Myc Downstream Regulated Gene 1 Reactivates Senescence Signaling and Inhibits Tumor Growth in Hepatocellular Carcinoma. Carcinogenesis 2014, 35, 915–922. [Google Scholar] [CrossRef]
- Sahin, P.; McCaig, C.; Jeevahan, J.; Murray, J.T.; Hainsworth, A.H. The Cell Survival Kinase SGK1 and Its Targets FOXO3a and NDRG1 in Aged Human Brain. Neuropathol. Appl. Neurobiol. 2013, 39, 623–633. [Google Scholar] [CrossRef]
- Lee, H.-K.; Kwon, B.; Lemere, C.A.; de la Monte, S.; Itamura, K.; Ha, A.Y.; Querfurth, H.W. MTORC2 (Rictor) in Alzheimer’s Disease and Reversal of Amyloid-β Expression-Induced Insulin Resistance and Toxicity in Rat Primary Cortical Neurons. J. Alzheimer’s Dis. 2017, 56, 1015–1036. [Google Scholar] [CrossRef]
- Béguin, E.P.; Janssen, E.F.J.; Hoogenboezem, M.; Meijer, A.B.; Hoogendijk, A.J.; van den Biggelaar, M. Flow-induced Reorganization of Laminin-integrin Networks Within the Endothelial Basement Membrane Uncovered by Proteomics. Mol. Cell. Proteom. 2020, 19, 1179–1192. [Google Scholar] [CrossRef]
- Zhang, G.; Qin, Q.; Zhang, C.; Sun, X.; Kazama, K.; Yi, B.; Cheng, F.; Guo, Z.-F.; Sun, J. NDRG1 Signaling Is Essential for Endothelial Inflammation and Vascular Remodeling. Circ. Res. 2023, 132, 306–319. [Google Scholar] [CrossRef]
- Watari, K.; Shibata, T.; Fujita, H.; Shinoda, A.; Murakami, Y.; Abe, H.; Kawahara, A.; Ito, H.; Akiba, J.; Yoshida, S.; et al. NDRG1 Activates VEGF-A-Induced Angiogenesis through PLCγ1/ERK Signaling in Mouse Vascular Endothelial Cells. Commun. Biol. 2020, 3, 107. [Google Scholar] [CrossRef]
- Yang, H.; Qin, X.; Zhang, R.; Miao, J.; Cui, J.; Li, Y.; Miao, X. The Role of NDRG1 Expression in Vasculogenic Mimicry of High-Grade Gliomas. J. Cancer 2024, 15, 6631–6643. [Google Scholar] [CrossRef]
- Okuda, T.; Higashi, Y.; Kokame, K.; Tanaka, C.; Kondoh, H.; Miyata, T. Ndrg1 -Deficient Mice Exhibit a Progressive Demyelinating Disorder of Peripheral Nerves. Mol. Cell. Biol. 2004, 24, 3949–3956. [Google Scholar] [CrossRef]
- Kalaydjieva, L.; Gresham, D.; Gooding, R.; Heather, L.; Baas, F.; de Jonge, R.; Blechschmidt, K.; Angelicheva, D.; Chandler, D.; Worsley, P.; et al. N-Myc Downstream-Regulated Gene 1 Is Mutated in Hereditary Motor and Sensory Neuropathy–Lom. Am. J. Hum. Genet. 2000, 67, 47–58. [Google Scholar] [CrossRef]
- Echaniz-Laguna, A.; Degos, B.; Bonnet, C.; Latour, P.; Hamadouche, T.; Lévy, N.; Leheup, B. NDRG1-Linked Charcot-Marie-Tooth Disease (CMT4D) with Central Nervous System Involvement. Neuromuscul. Disord. 2007, 17, 163–168. [Google Scholar] [CrossRef]
- Hunter, M.; Angelicheva, D.; Tournev, I.; Ingley, E.; Chan, D.C.; Watts, G.F.; Kremensky, I.; Kalaydjieva, L. NDRG1 Interacts with APO A-I and A-II and Is a Functional Candidate for the HDL-C QTL on 8q24. Biochem. Biophys. Res. Commun. 2005, 332, 982–992. [Google Scholar] [CrossRef]
- Pietiäinen, V.; Vassilev, B.; Blom, T.; Wang, W.; Nelson, J.; Bittman, R.; Bäck, N.; Zelcer, N.; Ikonen, E. NDRG1 Functions in LDL Receptor Trafficking by Regulating Endosomal Recycling and Degradation. J. Cell Sci. 2013, 126, 3961–3971. [Google Scholar] [CrossRef]
- Berger, P.; Sirkowski, E.E.; Scherer, S.S.; Suter, U. Expression Analysis of the N-Myc Downstream-Regulated Gene 1 Indicates That Myelinating Schwann Cells Are the Primary Disease Target in Hereditary Motor and Sensory Neuropathy-Lom. Neurobiol. Dis. 2004, 17, 290–299. [Google Scholar] [CrossRef]
- Hirata, K.; Masuda, K.; Morikawa, W.; He, J.; Kuraoka, A.; Kuwano, M.; Kawabuchi, M. N-myc Downstream-regulated Gene 1 Expression in Injured Sciatic Nerves. Glia 2004, 47, 325–334. [Google Scholar] [CrossRef]
- Vergara, D.; Romano, A.; Stanca, E.; La Pesa, V.; Aloisi, L.; De Domenico, S.; Franck, J.; Cicalini, I.; Giudetti, A.; Storelli, E.; et al. Proteomic Expression Profile of Injured Rat Peripheral Nerves Revealed Biological Networks and Processes Associated with Nerve Regeneration. J. Cell. Physiol. 2018, 233, 6207–6223. [Google Scholar] [CrossRef]
- Heller, B.A.; Ghidinelli, M.; Voelkl, J.; Einheber, S.; Smith, R.; Grund, E.; Morahan, G.; Chandler, D.; Kalaydjieva, L.; Giancotti, F.; et al. Functionally Distinct PI 3-Kinase Pathways Regulate Myelination in the Peripheral Nervous System. J. Cell Biol. 2014, 204, 1219–1236. [Google Scholar] [CrossRef]
- Yang, G.; Huang, L.; Jia, H.; Aikemu, B.; Zhang, S.; Shao, Y.; Hong, H.; Yesseyeva, G.; Wang, C.; Li, S.; et al. NDRG1 Enhances the Sensitivity of Cetuximab by Modulating EGFR Trafficking in Colorectal Cancer. Oncogene 2021, 40, 5993–6006. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Vondrackova, M.; Potomova, P.; Sandoval-Acuña, C.; Smigova, J.; Klanicova, K.; Rosel, D.; Brabek, J.; Stursa, J.; Werner, L.; et al. NDRG1 Acts as an Oncogene in Triple-Negative Breast Cancer and Its Loss Sensitizes Cells to Mitochondrial Iron Chelation. Front. Pharmacol. 2024, 15, 1422369. [Google Scholar] [CrossRef]
- Tu, L.C.; Yan, X.; Hood, L.; Lin, B. Proteomics Analysis of the Interactome of N-Myc Downstream Regulated Gene 1 and Its Interactions with the Androgen Response Program in Prostate Cancer Cells. Mol. Cell. Proteom. 2007, 6, 575–588. [Google Scholar] [CrossRef]
- Li, C.; Lv, J.; Wumaier, G.; Zhao, Y.; Dong, L.; Zeng, Y.; Zhu, N.; Zhang, X.; Wang, J.; Xia, J.; et al. NDRG1 Promotes Endothelial Dysfunction and Hypoxia-Induced Pulmonary Hypertension by Targeting TAF15. Precis. Clin. Med. 2023, 6, pbad024. [Google Scholar] [CrossRef]
- Saponaro, C.; Damato, M.; Stanca, E.; Aboulouard, S.; Zito, F.A.; De Summa, S.; Traversa, D.; Schirosi, L.; Bravaccini, S.; Pirini, F.; et al. Unraveling the Protein Kinase C/NDRG1 Signaling Network in Breast Cancer. Cell Biosci. 2024, 14, 156. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the Regulation of Protein Abundance from Proteomic and Transcriptomic Analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, S.-C.; Tan, J.-L.; Wang, J.; Bai, X.; Dong, Z.-Y.; Zhang, Q.-X. Inferring Homologous Recombination Deficiency of Ovarian Cancer From the Landscape of Copy Number Variation at Subchromosomal and Genetic Resolutions. Front. Oncol. 2021, 11, 772604. [Google Scholar] [CrossRef]
- Chiang, C.T.; Demetriou, A.N.; Ung, N.; Choudhury, N.; Ghaffarian, K.; Ruderman, D.L.; Mumenthaler, S.M. MTORC2 Contributes to the Metabolic Reprogramming in EGFR Tyrosine-Kinase Inhibitor Resistant Cells in Non-Small Cell Lung Cancer. Cancer Lett. 2018, 434, 152–159. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saponaro, C.; Gammaldi, N.; Cavallo, V.; Ramírez-Morales, M.A.; Zito, F.A.; Sonnessa, M.; Vari, F.; Serra, I.; De Summa, S.; Giudetti, A.M.; et al. Insight into the Regulation of NDRG1 Expression. Int. J. Mol. Sci. 2025, 26, 3582. https://doi.org/10.3390/ijms26083582
Saponaro C, Gammaldi N, Cavallo V, Ramírez-Morales MA, Zito FA, Sonnessa M, Vari F, Serra I, De Summa S, Giudetti AM, et al. Insight into the Regulation of NDRG1 Expression. International Journal of Molecular Sciences. 2025; 26(8):3582. https://doi.org/10.3390/ijms26083582
Chicago/Turabian StyleSaponaro, Concetta, Nicola Gammaldi, Viviana Cavallo, Maria Antonieta Ramírez-Morales, Francesco Alfredo Zito, Margherita Sonnessa, Francesco Vari, Ilaria Serra, Simona De Summa, Anna Maria Giudetti, and et al. 2025. "Insight into the Regulation of NDRG1 Expression" International Journal of Molecular Sciences 26, no. 8: 3582. https://doi.org/10.3390/ijms26083582
APA StyleSaponaro, C., Gammaldi, N., Cavallo, V., Ramírez-Morales, M. A., Zito, F. A., Sonnessa, M., Vari, F., Serra, I., De Summa, S., Giudetti, A. M., Trerotola, M., & Vergara, D. (2025). Insight into the Regulation of NDRG1 Expression. International Journal of Molecular Sciences, 26(8), 3582. https://doi.org/10.3390/ijms26083582








