Comparative Study of Chaga (Inonotus obliquus) Dietary Supplements Using Complementary Analytical Techniques
Abstract
:1. Introduction
2. Results
2.1. Physical Appearance of Whole Chaga and Commercial Products
2.2. Polysaccharides in Authentic Chaga Canker and Commercial Products
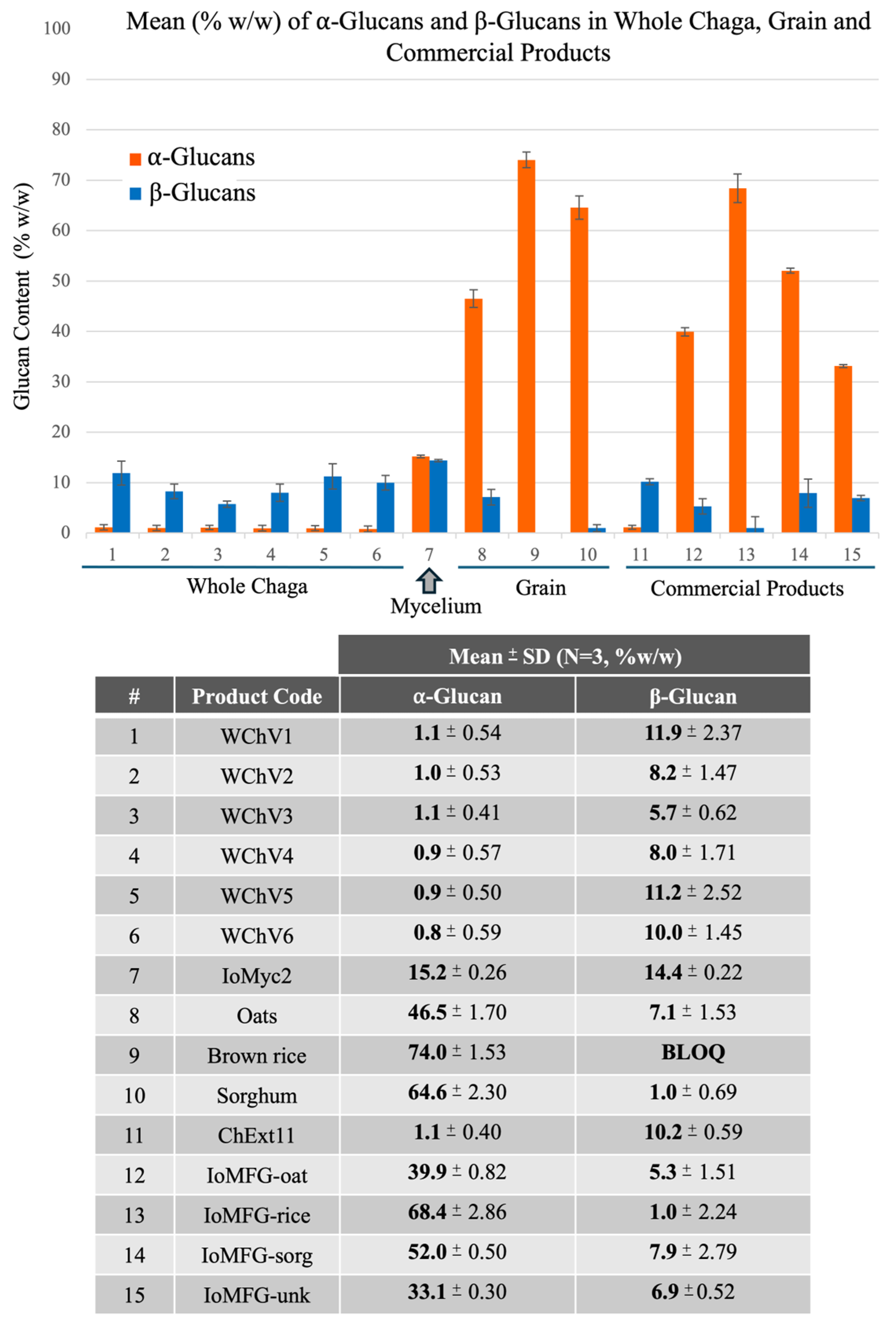
2.2.1. β-Glucan Quantification
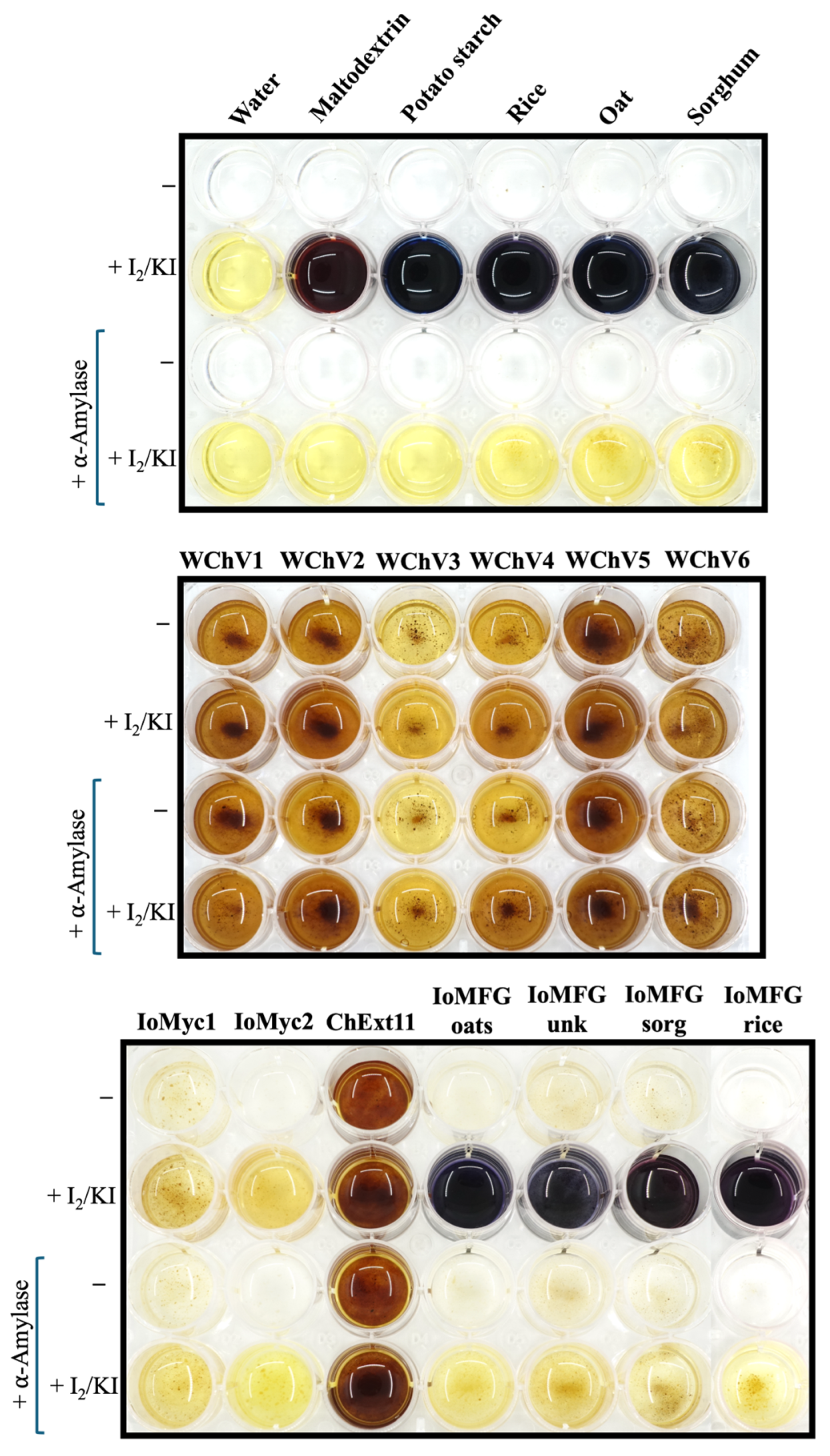
2.2.2. Starch Detection Using Lugol’s Reagent (I2/KI)
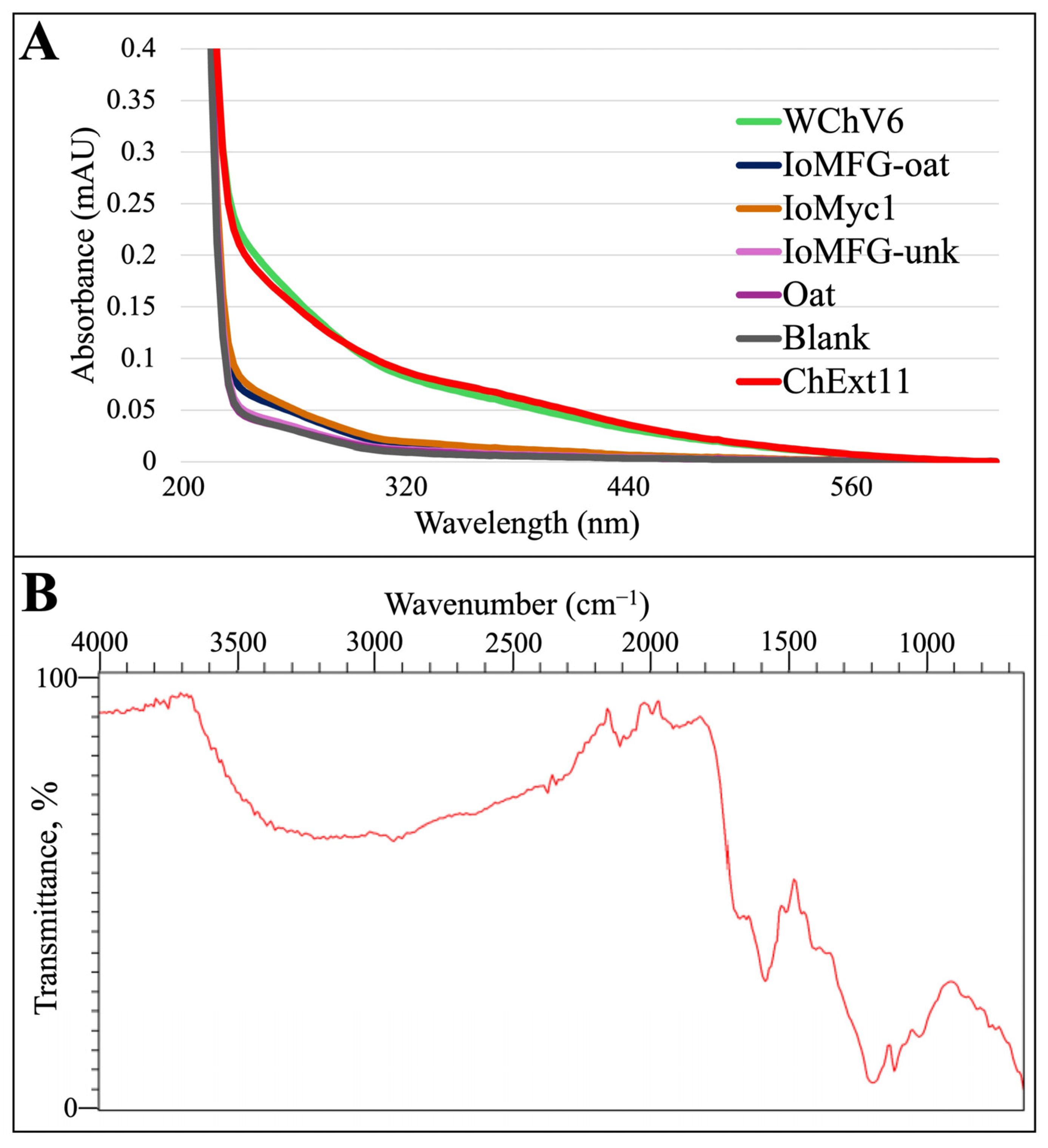
2.3. Melanin Evaluation in Chaga Dietary Supplements
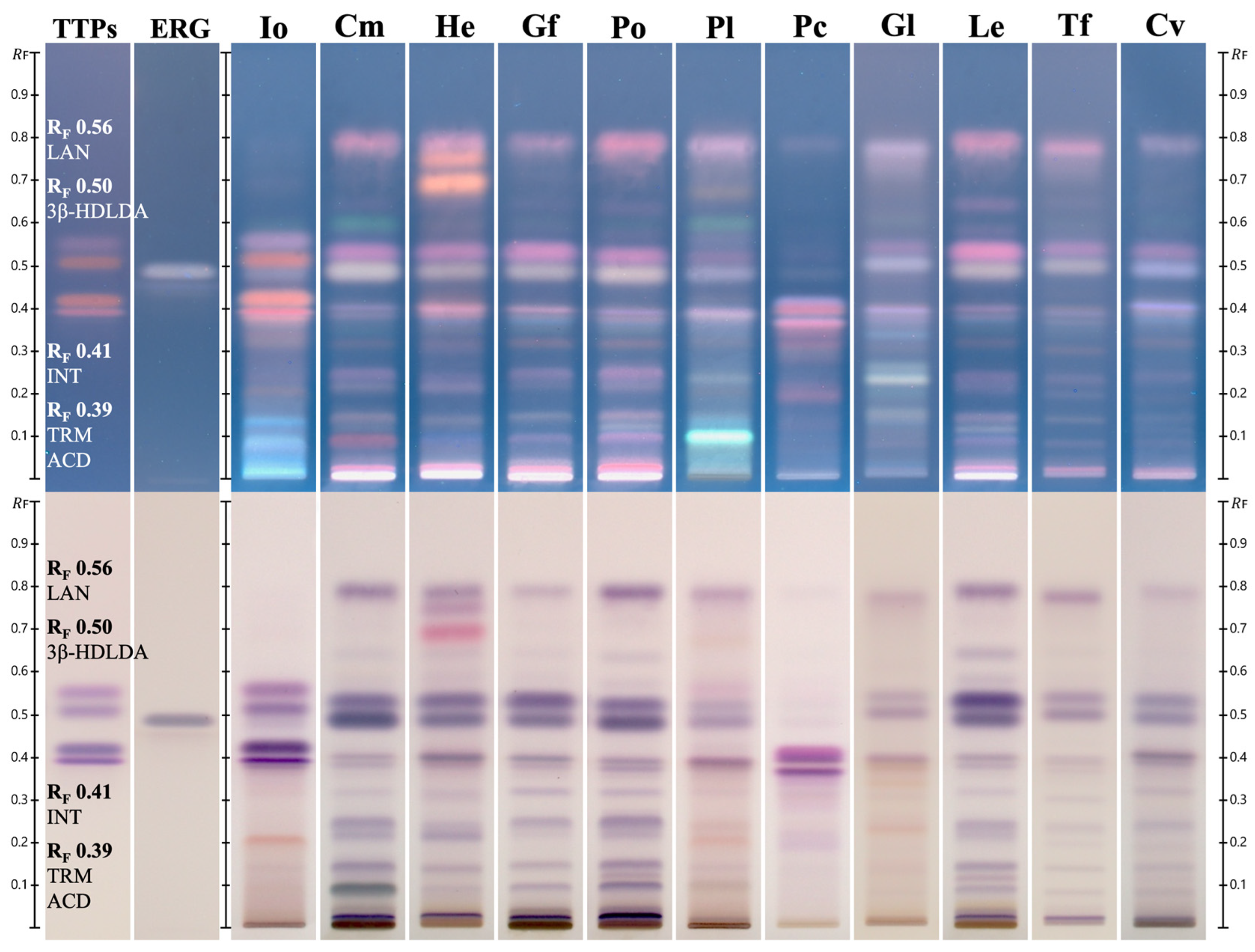
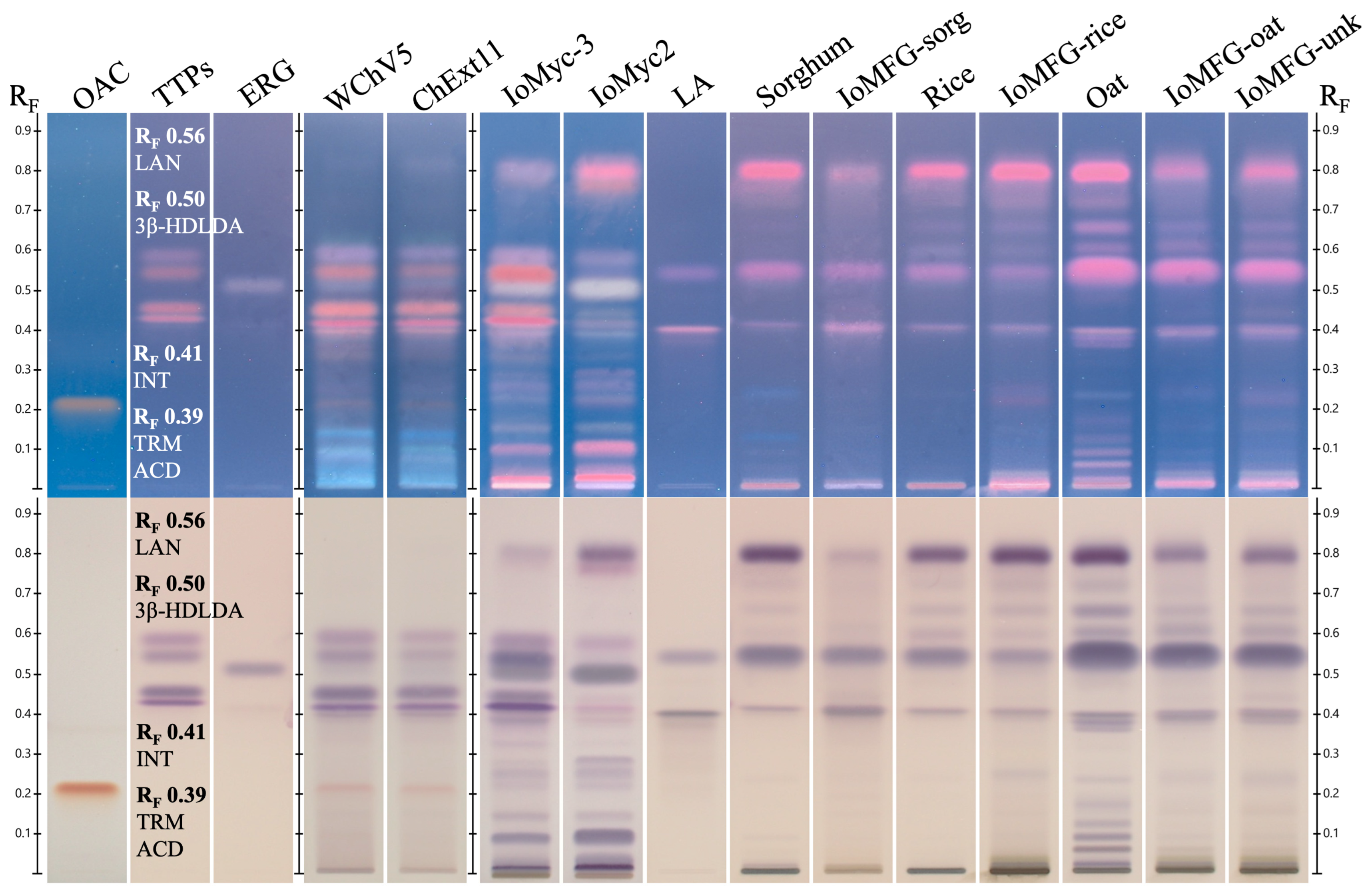
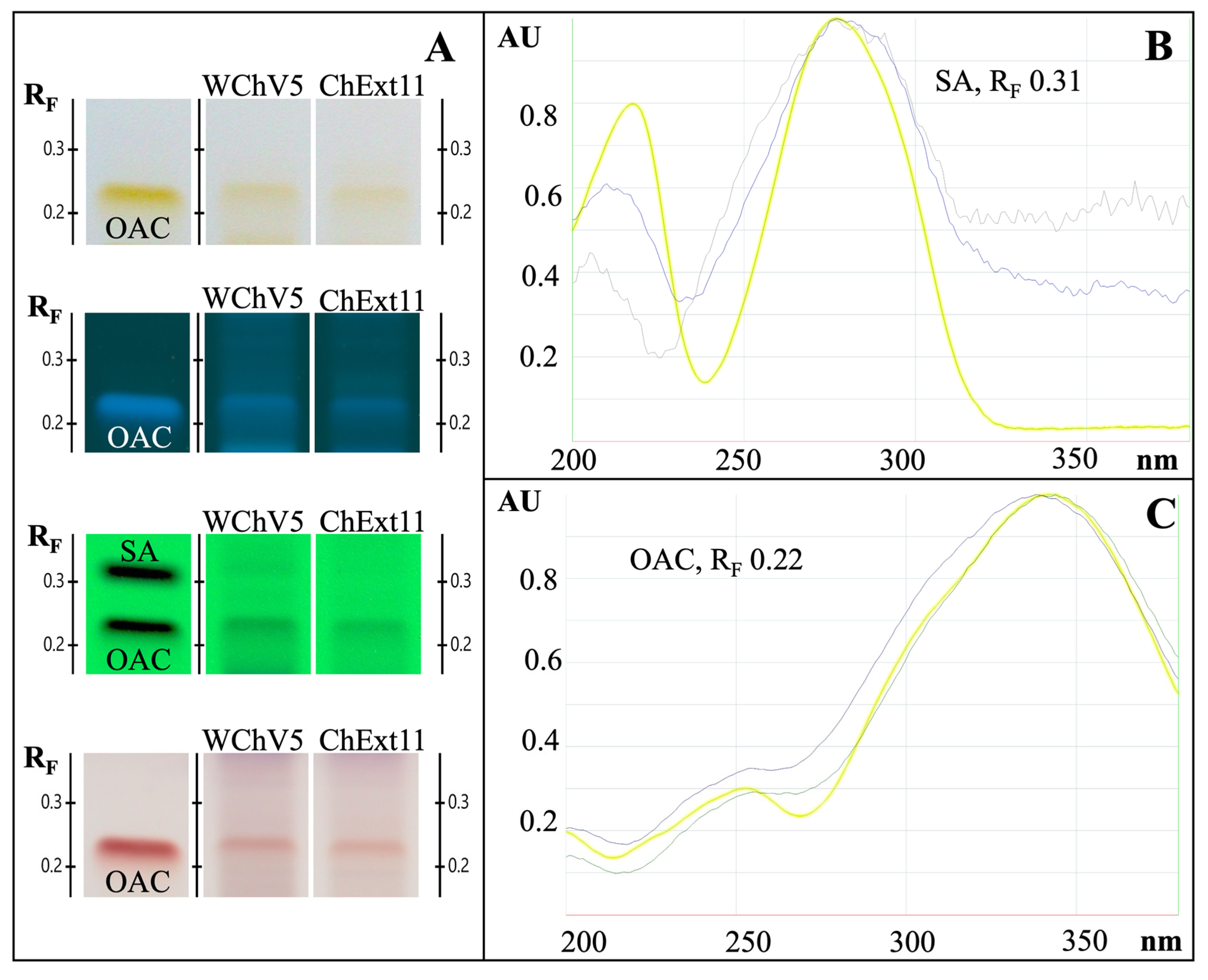
2.4. HPTLC Identification of Chaga
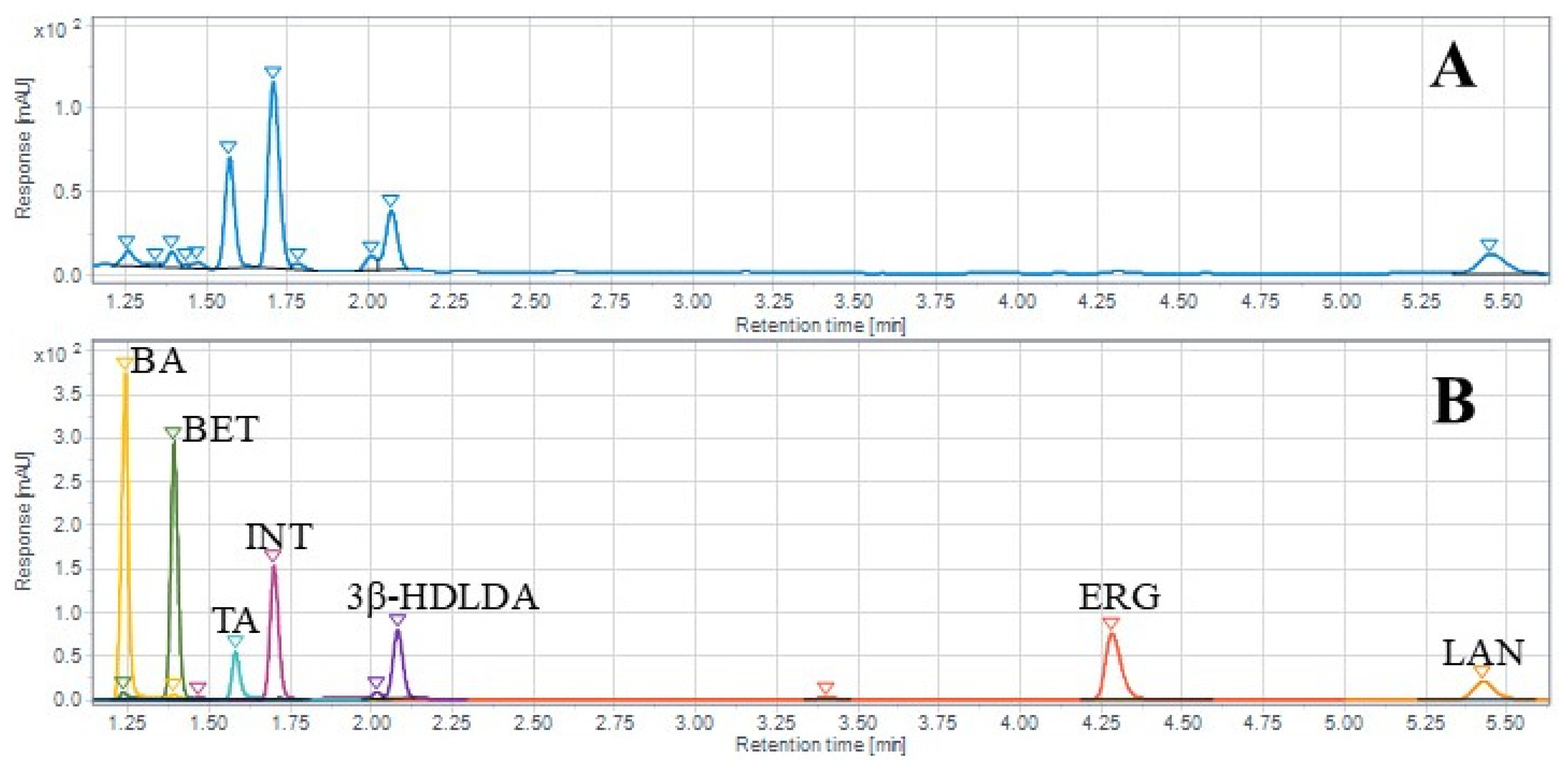
2.5. LC-ELSD/DAD/PDA Detection of Chaga Chemical Constituents
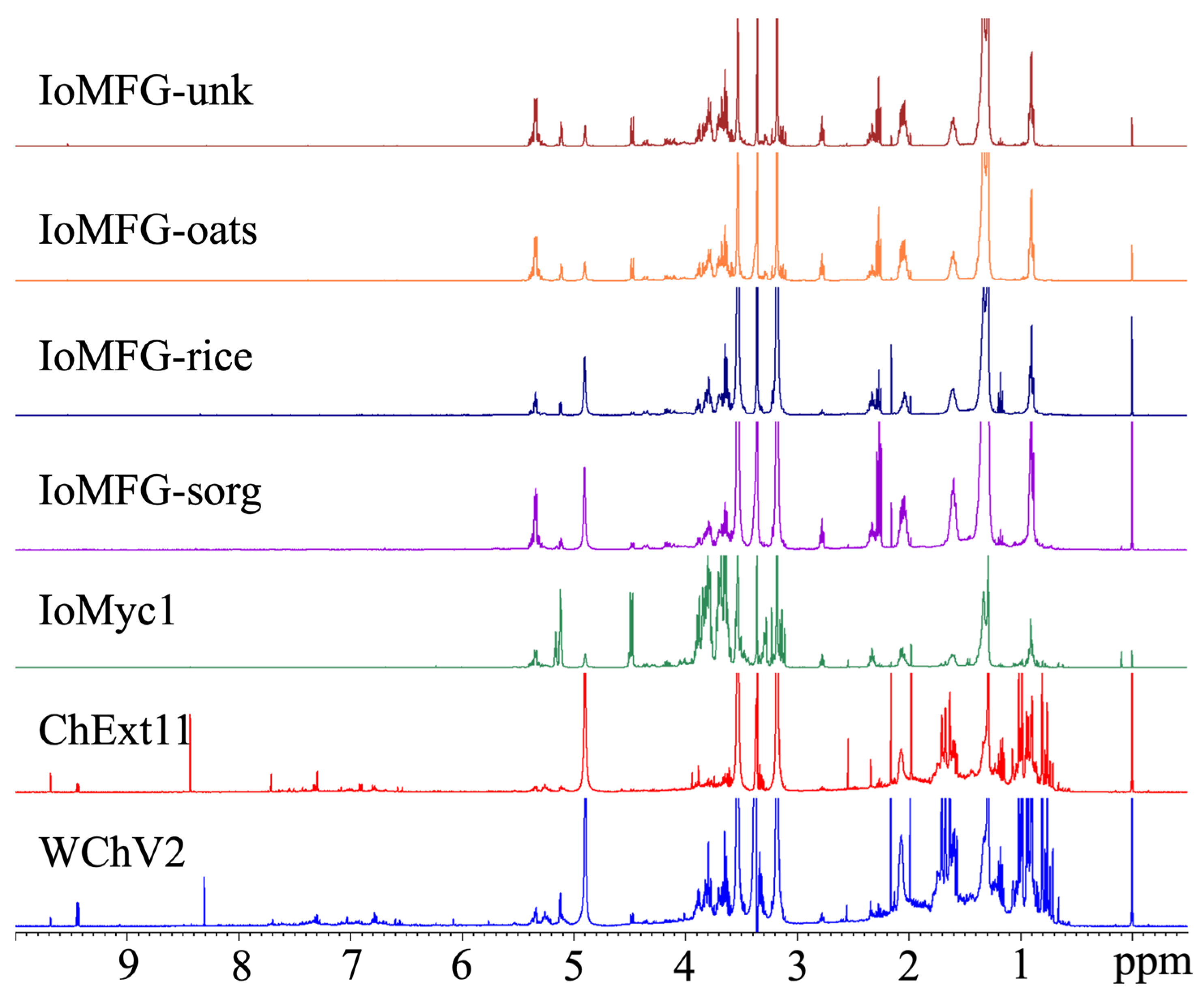
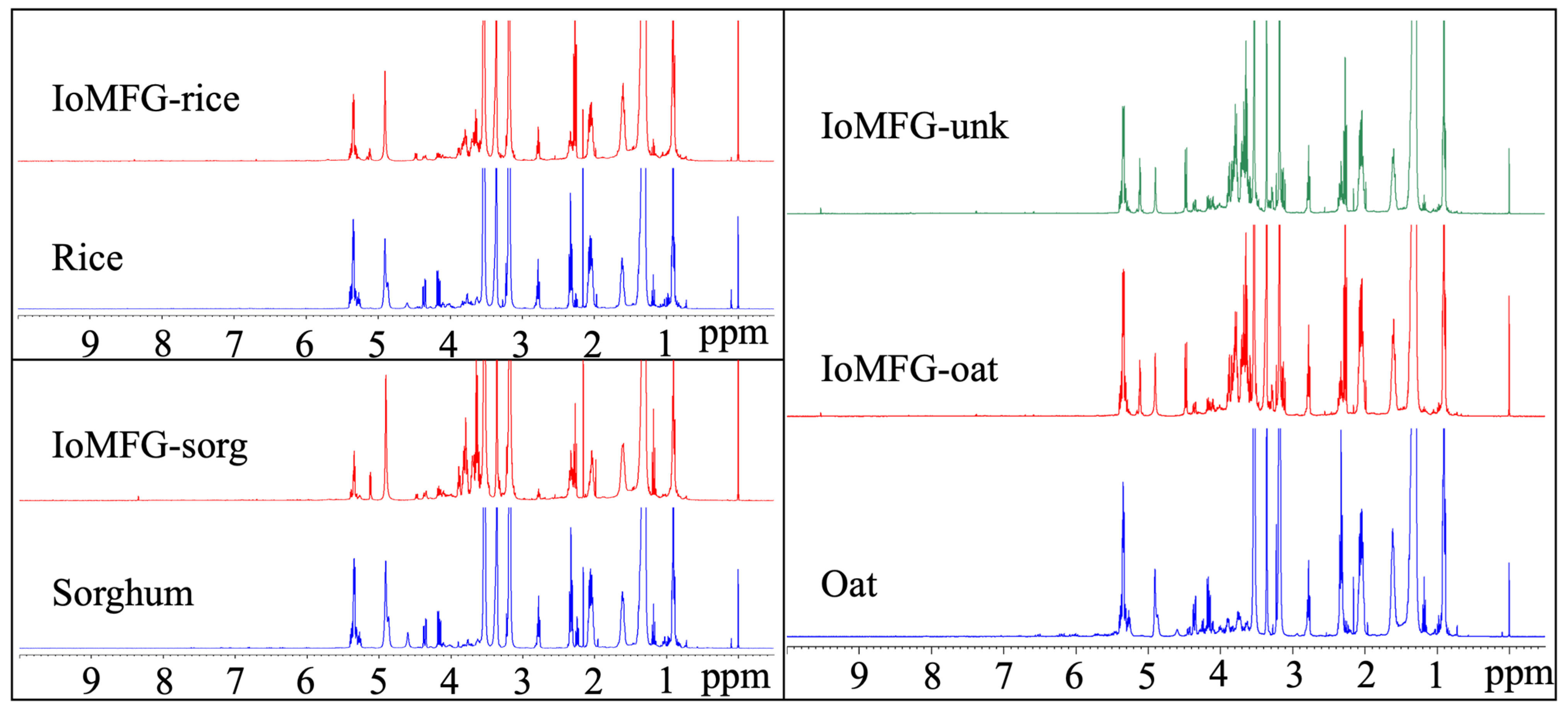
2.6. 1H NMR Spectral Fingerprinting of Chaga and Commercial Products
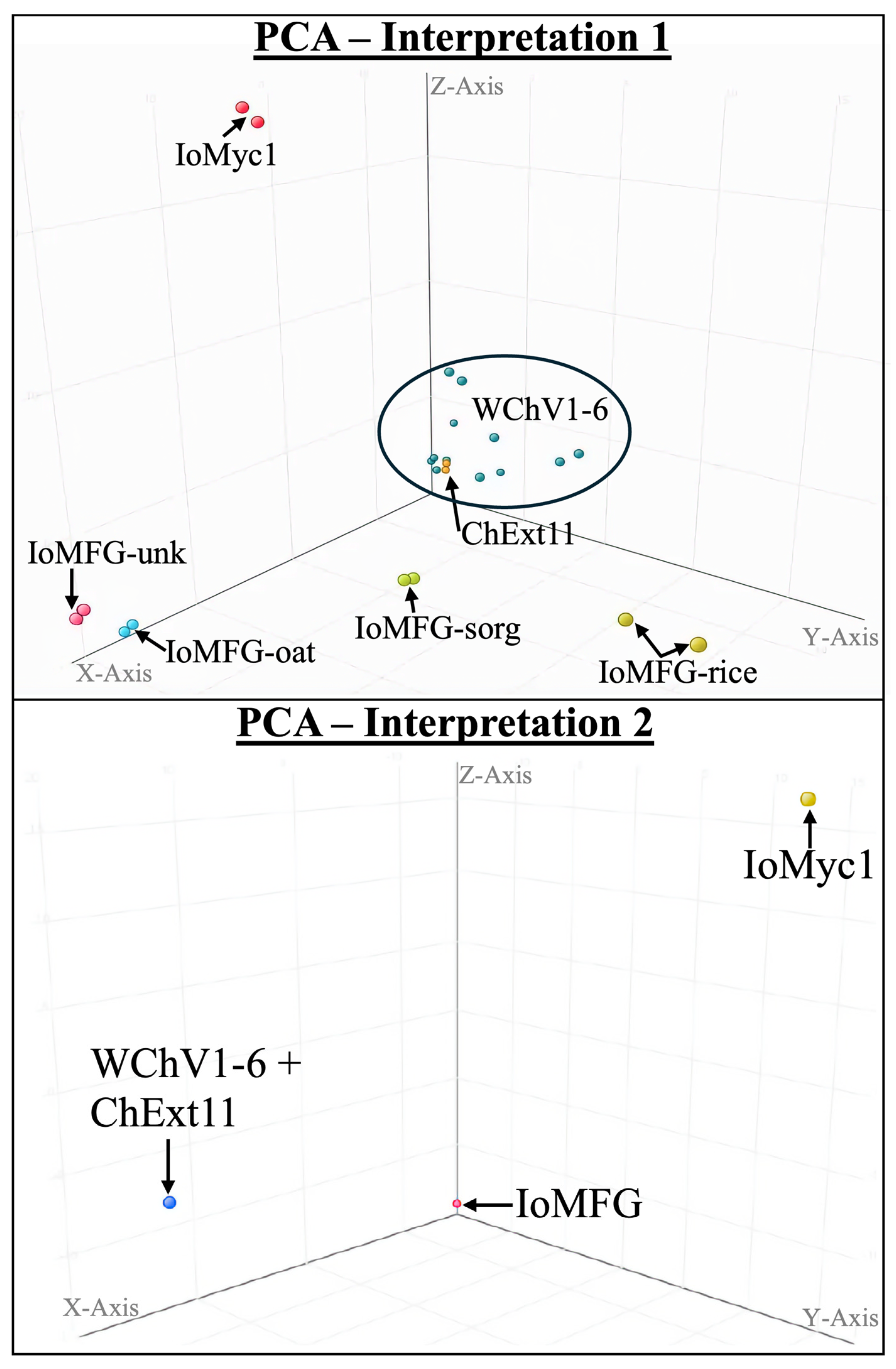
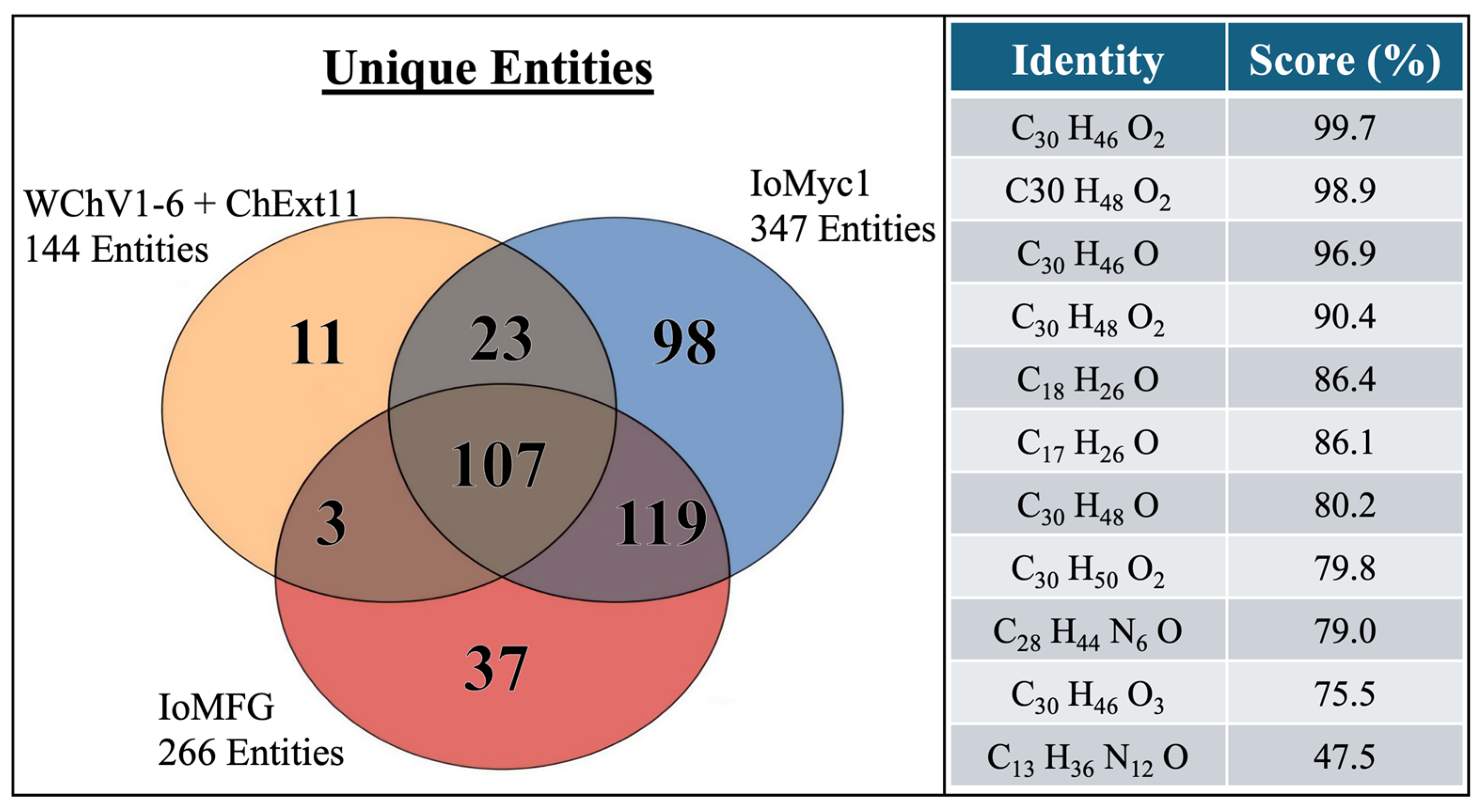
2.7. LC-QToF-MS Metabolomic Analysis of Chaga Dietary Supplements
3. Discussion
3.1. Analytical Methods and Their Limitations
3.2. Physical Appearance Differences Between Whole Chaga Canker and Fermented Grain Products
3.3. Polysaccharide Composition as a Key Differentiator
3.4. Melanin as a Marker for Chaga Identification
3.5. Triterpenoid and Sterol Profiles Distinguish Chaga Canker from Mycelia Fermented Grain
3.6. Phenolic and Fatty Acid Profiles Highlight Additional Compositional Differences
3.7. Metabolomic Analysis Confirms Authenticity Gaps
4. Materials and Methods
4.1. Origin of Whole Dried Chaga, Chaga Canker 1:1 Extract, Mycelia Fermented Grain, and Grain Substrate Reference Materials
4.2. Starch Detection with Lugol’s Reagent (I2/KI)
4.3. Polysaccharide Analysis: α- and β-Glucan Quantification
4.4. Melanin Extraction and Spectral Characterization
4.4.1. Melanin Extraction and Purification
4.4.2. Melanin Spectral Analysis
4.5. HPTLC Identification and Fingerprinting
4.5.1. Chemicals and Reagents
4.5.2. HPTLC Extraction Protocol
4.5.3. Chromatography System and HPTLC Fingerprint Analysis
4.6. UHPLC-ELSD/PDA Analysis of Triterpenoids, Sterols, and Fatty Acids
4.7. LC-DAD Triterpenoid Quantification
4.7.1. Preparation of Test Solutions and Standards for LC-DAD Quantification
4.7.2. HPLC-DAD Instrumentation and Chromatographic Conditions
4.7.3. Spectral Analysis and Compound Identification
4.7.4. Calibration Curves, Quantification, and Method Validation
4.8. 1H NMR Spectral Fingerprinting
4.8.1. Sample Preparation for 1H NMR
4.8.2. Data Acquisition and Processing
- I.
- Spectral width: 8223 Hz (20 ppm)
- II.
- Scans: 64
- III.
- Recycle delay: 15 s
- IV.
- 90° pulse: Optimized per sample
4.9. LC QToF-MS Metabolomic Profiling
4.9.1. Sample Extraction for LC QToF-MS
4.9.2. Instrumentation and Analysis
4.9.3. Data Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kukulyanskaya, T.A.; Kurchenko, N.V.; Kurchenko, V.P.; Babitskaya, V.G. Physicochemical properties of melanins produced by the sterile form of Inonotus obliquus (“Chagi”) in natural and cultivated fungus. Appl. Biochem. Microbiol. 2002, 38, 58–61. [Google Scholar]
- Allen, E.A.; Morrison, D.J.; Wallis, G.W. Common Tree Diseases of British Columbia; Canadian Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 1996; p. 178. [Google Scholar]
- Kahlos, K. Studies on Triterpenes in Inonotus obliquus; Division of Pharmacology, School of Pharmacy, University of Helsinki: Helsinki, Finland, 1987. [Google Scholar]
- Reid, D.A. Inonotus obliquus (Pers. ex Fr.) Pilát in Britain. Trans. Br. Mycol. Soc. 1976, 67, 329–332. [Google Scholar]
- Glamočlija, J.; Ćirić, A.; Nikolić, M.; Fernandes, Â.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.; Soković, M.; van Griensven, L.J. Chemical characterization and biological activity of Chaga (Inonotus obliquus), a medicinal “mushroom”. J. Ethnopharmacol. 2015, 162, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Snell, W.H.; Dick, E.A. A Glossary of Mycology, 2nd ed.; Harvard University Press: Cambridge, MA, USA, 1971; p. 181. [Google Scholar]
- Gottesfeld, L.M.J. Use of cinder conk (Inonotus oblilquus) by the Gitskan of the northwestern British Columbia, Canada. J. Ethnobiol. 1992, 12, 153–156. [Google Scholar]
- Shashkina, M.Y.; Shashkin, P.N.; Sergeev, A.V. Chemical and medicobiological properties of chaga (review). Pharm. Chem. J. 2006, 40, 560–568. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skóra, B.; Pomianek, T.; Gmiński, J. Inonotus obliquus—From folk medicine to clinical use. J. Tradit. Complement. Med. 2020, 11, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Gu, Y.; Taka, A. Fungal medicine, Fuscoporia obliqua, as a traditional herbal medicine: Its bioactivities, in vivo testing and medicinal effects. Asian Biomed. 2008, 2, 459–469. [Google Scholar]
- Kim, Y.R. Immunomodulatory activity of the water extract from medicinal mushroom Inonotus obliquus. Mycobiology 2005, 33, 158–162. [Google Scholar]
- Shen, D.; Feng, Y.; Zhang, X.; Liu, J.; Gong, L.; Liao, H.; Li, R. In Vitro Immunomodulatory Effects of Inonotus obliquus Extracts on Resting M0 Macrophages and LPS-Induced M1 Macrophages. Evid. Based Complement. Alternat. Med. 2022, 2022, 8251344. [Google Scholar] [CrossRef]
- Song, K.-C.; Choi, B.-L.; Shin, J.-W.; Son, J.-Y.; Yoo, H.-S.; Cho, J.-H.; Lee, Y.-W.; Son, C.-G.; Cho, C.-K. Effects of Inonotus obliquus extracts on immunomodulating activity. Kor. J. Ori. Med. 2007, 28, 27–41. [Google Scholar]
- Park, Y.M.; Won, J.H.; Kim, Y.H.; Choi, J.W.; Park, H.J.; Lee, K.T. In vivo and in vitro anti-inflammatory and anti-nociceptive effects of the methanol extract of Inonotus obliquus. J. Ethnopharmacol. 2005, 101, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, H.; Dong, P.; Lu, X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013, 139, 503–508. [Google Scholar] [CrossRef]
- Alhallaf, W.; Perkins, L.B. The anti-Inflammatory properties of chaga extracts obtained by different extraction methods against LPS-Induced RAW 264.7. Molecules 2022, 27, 4207. [Google Scholar] [CrossRef] [PubMed]
- Ern, P.T.Y.; Quan, T.Y.; Yee, F.S.; Yin, A.C.Y. Therapeutic properties of Inonotus obliquus (Chaga mushroom): A review. Mycology 2023, 15, 144–161. [Google Scholar] [CrossRef]
- Drenkhan, R.; Kaldmäe, H.; Silm, M.; Adamson, K.; Bleive, U.; Aluvee, A.; Erik, M.; Raal, A. Comparative analyses of bioactive compounds in Inonotus obliquus conks growing on Alnus and Betula. Biomolecules 2022, 12, 1178. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Z.; Lei, Z.; Yang, Y.; Sugiura, N. Comparative study of antioxidant activity and antiproliferative effect of hot water and ethanol extracts from the mushroom Inonotus obliquus. J. Biosci. Bioeng. 2009, 107, 42–48. [Google Scholar] [CrossRef]
- Nakajima, Y.; Sato, Y.; Konishi, T. Antioxidant small phenolic ingredients in Inonotus obliquus (persoon) Pilat (Chaga). Chem. Pharm. Bull. 2007, 55, 1222–1226. [Google Scholar] [CrossRef]
- Zheng, W.; Miao, K.; Liu, Y.; Zhao, Y.; Zhang, M.; Pan, S.; Dai, Y. Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production. Appl. Microbiol. Biotechnol. 2010, 87, 1237–1254. [Google Scholar] [CrossRef] [PubMed]
- Maenaka, T.; Oshima, M.; Itokawa, Y.; Masubuchi, T.; Takagi, Y.; Choi, J.S.; Ishida, T.; Gu, Y. Effects of Fuscoporia obliqua on postprandial glucose excursion and endothelial dysfunction in type 2 diabetic patients. J. Tradit. Chin. Med. 2008, 28, 49–57. [Google Scholar] [CrossRef]
- Lu, X.; Chen, H.; Dong, P.; Fu, L.; Zhang, X. Phytochemical characteristics and hypoglycaemic activity of fraction from mushroom Inonotus obliquus. J. Sci. Food Agric. 2010, 90, 276–280. [Google Scholar] [CrossRef]
- Lee, J.H.; Hyun, C.K. Insulin-sensitizing and beneficial lipid-metabolic effects of the water-soluble melanin complex extracted from Inonotus obliquus. Phytother. Res. 2014, 28, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Z.; Pan, Y.; Gao, X.; Chen, H. Anti-diabetic effects of Inonotus obliquus polysaccharides-chromium (III) complex in type 2 diabetic mice and its sub-acute toxicity evaluation in normal mice. Food Chem. Toxicol. 2017, 108, 498–509. [Google Scholar] [CrossRef]
- Chung, M.J.; Chung, C.K.; Jeong, Y.; Ham, S.S. Anticancer activity of subfractions containing pure compounds of Chaga mushroom (Inonotus obliquus) extract in human cancer cells and in Balbc/c mice bearing Sarcoma-180 cells. Nutr. Res. Pract. 2010, 4, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Géry, A.; Dubreule, C.; André, V.; Rioult, J.P.; Bouchart, V.; Heutte, N.; Eldin de Pécoulas, P.; Krivomaz, T.; Garon, D. Chaga (Inonotus obliquus), a Future Potential Medicinal Fungus in Oncology? A chemical study and a comparison of the cytotoxicity against human lung adenocarcinoma cells (A549) and human bronchial epithelial cells (BEAS-2B). Integr. Cancer Ther. 2018, 17, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Jang, J.E.; Mishra, S.K.; Lee, H.J.; Nho, C.W.; Shin, D.; Jin, M.; Kim, M.K.; Choi, C.; Oh, S.H. Ergosterol peroxide from Chaga mushroom (Inonotus obliquus) exhibits anti-cancer activity by down-regulation of the β-catenin pathway in colorectal cancer. J. Ethnopharmacol. 2015, 173, 303–312. [Google Scholar] [CrossRef]
- Kahlos, K.; Kangas, L.; Hiltunen, R. Antitumour tests of inotodiol from the fungus Inonotus obliquus. Acta. Pharm. Finica 1986, 95, 173–177. [Google Scholar]
- Nikitina, S.A.; Khabibrakhmanova, V.R.; Sysoeva, M.A. Khimicheskiĭ sostav i biologicheskaia aktivnost’ triterpenovykh i steroidnykh soedineniĭ chagi [Composition and biological activity of triterpenes and steroids from Inonotus obliquus (chaga)]. Biomed Khim. 2016, 62, 369–375. (In Russian) [Google Scholar] [CrossRef]
- Nomura, M.; Takahashi, T.; Uesugi, A.; Tanaka, R.; Kobayashi, S. Inotodiol, a lanostane triterpenoid, from Inonotus obliquus inhibits cell proliferation through caspase-3-dependent apoptosis. Anticancer Res. 2008, 28, 2691–2696. [Google Scholar]
- Plehn, S.; Wagle, S.; Rupasinghe, H.P.V. Chaga mushroom triterpenoids as adjuncts to minimally invasive cancer therapies: A review. Curr. Res. Toxicol. 2023, 5, 100137. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal plants of the Russian Pharmacopoeia; their history and applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, T.; Chen, X.H.; Zhang, G.; Curtis, R.B.; Lu, Z.H.; Jiang, J.H. In vitro antitumor activity and structure characterization of ethanol extracts from wild and cultivated Chaga medicinal mushroom, Inonotus obliquus (Pers.:Fr.) Pilát (Aphyllophoromycetideae). Int. J. Med. Mushrooms 2011, 13, 21–30. [Google Scholar] [CrossRef]
- Zhao, F.; Mai, Q.; Ma, J.; Xu, M.; Wang, X.; Cui, T.; Qiu, F.; Han, G. Triterpenoids from Inonotus obliquus and their antitumor activities. Fitoterapia 2015, 101, 34–40. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, W. Deciphering the antitumoral potential of the bioactive metabolites from medicinal mushroom Inonotus obliquus. J. Ethnopharmacol. 2021, 265, 113321. [Google Scholar] [CrossRef]
- Kim, J.; Yang, S.C.; Hwang, A.Y.; Cho, H.; Hwang, K.T. Composition of triterpenoids in Inonotus obliquus and their anti-proliferative activity on cancer cell lines. Molecules 2020, 25, 4066. [Google Scholar] [CrossRef] [PubMed]
- Duru, K.C.; Kovaleva, E.G.; Danilova, I.G.; van der Bijl, P. The pharmacological potential and possible molecular mechanisms of action of Inonotus obliquus from preclinical studies. Phytother. Res. 2019, 33, 1966–1980. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, Y.; Xue, Z.; Li, N.; Liu, J.; Chen, H. Recent Developments in Inonotus obliquus (Chaga mushroom) polysaccharides: Isolation, structural characteristics, biological activities and application. Polymers 2021, 13, 1441. [Google Scholar] [CrossRef]
- Wold, C.W.; Kjeldsen, C.; Corthay, A.; Rise, F.; Christensen, B.E.; Duus, J.Ø.; Inngjerdingen, K.T. Structural characterization of bioactive heteropolysaccharides from the medicinal fungus Inonotus obliquus (Chaga). Carbohydr. Polym. 2018, 185, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Babitskaya, V.G.; Scherba, V.V.; Ikonnikova, N.V.; Bisko, N.A.; Mitropolskaya, N.Y. Melanin complex from medicinal mushroom Inonotus obliquus 9Pers.: Fr.) Pilát (Chaga) (Aphyllophoromycetideae). Int. J. Med. Mushrooms 2002, 4, 139–145. [Google Scholar]
- Peng, H.; Shahidi, F. Bioactive compounds and bioactive properties of Chaga (Inonotus obliquus) mushroom: A review. JFB 2020, 12, 9–75. [Google Scholar] [CrossRef]
- Wu, D.T.; Deng, Y.; Chen, L.X.; Zhao, J.; Bzhelyansky, A.; Li, S.P. Evaluation on quality consistency of Ganoderma lucidum dietary supplements collected in the United States. Sci. Rep. 2017, 7, 7792. [Google Scholar] [CrossRef]
- Beltrame, G.; Trygg, J.; Hemming, J.; Han, Z.; Yang, B. Comparison of Polysaccharides Extracted from Cultivated Mycelium of Inonotus obliquus with Polysaccharide Fractions Obtained from Sterile Conk (Chaga) and Birch Heart Rot. J. Fungi 2021, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Lante, A.; Canazza, E.; Tessari, P. Beta-Glucans of Cereals: Functional and Technological Properties. Nutrients 2023, 15, 2124. [Google Scholar] [CrossRef] [PubMed]
- Henrion, M.; Francey, C.; Lê, K.-A.; Lamothe, L. Cereal B-Glucans: The Impact of Processing and How It Affects Physiological Responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef]
- Phuwadolpaisarn, P. Comparison of β-Glucan Content in Milled Rice, Rice Husk and Rice Bran from Rice Cultivars Grown in Different Locations of Thailand and the Relationship between β-Glucan and Amylose Contents. Molecules 2021, 26, 6368. [Google Scholar] [CrossRef]
- Wold, C.W.; Gerwick, W.H.; Wangensteen, H.; Inngjerdingen, K.T. Bioactive triterpenoids and water-soluble melanin from Inonotus obliquus (Chaga) with immunomodulatory activity. JFF 2020, 71, 104025. [Google Scholar] [CrossRef]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal Mushrooms: Their Bioactive Components, Nutritional Value and Application in Functional Food Production—A Review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef] [PubMed]
- Sari, M.; Prange, A.; Lelley, J.I.; Hambitzer, R. Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chem. 2017, 216, 45–51. [Google Scholar] [CrossRef]
- McCleary, B.V.; Draga, A. Measurement of β-Glucan in Mushrooms and Mycelial Products. J. AOAC Int. 2016, 2, 364–373. [Google Scholar] [CrossRef]
- Rhee, S.J.; Cho, S.Y.; Kim, K.M.; Cha, D.-S.; Park, H.-J. A comparative study of analytical methods for alkali-soluble ß-glucan in medicinal mushroom Chaga (Inonotus obliquus). LWT 2008, 41, 545–549. [Google Scholar] [CrossRef]
- Morris, D.L. Colorimetric determination of glycogen; disadvantage of the iodine method. J. Biol. Chem. 1946, 166, 199–203. [Google Scholar]
- Quain, D.E. The determination of glycogen in yeast. J. Inst. Brew. 1981, 87, 289–291. [Google Scholar]
- Brust, H.; Orzechowski, S.; Fettke, J. Starch and Glycogen Analyses: Methods and Techniques. Biomolecules 2020, 10, 1020. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, W.; Zhang, F.; Hu, X.; Yuan, Y.; Wu, X.; Fu, J. Differences between water-soluble and water-insoluble melanin derived from Inonotus hispidus mushroom. Food Chem. X 2022, 16, 100498. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.S.; Kim, S.H.; Moon, S.Y.; Chung, M.J.; Cui, C.B.; Han, E.K.; Chung, C.K.; Choe, M. Antimutagenic effects of subfractions of Chaga mushroom (Inonotus obliquus) extract. Mutat Res. 2009, 672, 55–59. [Google Scholar] [CrossRef]
- Upska, K.; Klavins, L.; Radenkovs, V.; Nikolajeva, V.; Faven, L.; Isosaari, E.; Lauberts, M.; Viksna, A.; Klavins, M. Extraction possibilities of lipid fraction and authentication assessment of chaga (Inonotus obliquus). Biomass Conv. Bioref. 2023, 13, 14005–14021. [Google Scholar] [CrossRef]
- Duan, Y.; Han, H.; Qi, J.; Gao, J.-M.; Xu, Z.; Wang, P.; Zang, J.; Liu, C. Genome sequencing of Inonotus obliquus reveals inshights into candidate genes involved in secondary metabolite biosynthesis. BMC Genom. 2022, 23, 314. [Google Scholar] [CrossRef]
- Papoutsis, K.; Grasso, S.; Menon, A.; Brunton, N.; Lyng, J.; Jacquier, J.-C.; Bhuyan, D.J. Recovery of ergosterol and vitamin D2 from mushroom waste—Potential valorization by food and pharmaceutical industries. Trends Food Sci. Technol. 2020, 99, 351–366. [Google Scholar] [CrossRef]
- Trinh, T.A.; Seo, Y.H.; Choi, S.; Lee, J.; Kang, K.S. Protective Effect of Osmundacetone against Neurological Cell Death Caused by Oxidative Glutamate Toxicity. Biomolecules 2021, 11, 328. [Google Scholar] [CrossRef]
- Fordjour, E.; Manful, C.F.; Javed, R.; Galagedara, L.W.; Cuss, C.W.; Cheema, M.; Thomas, R. Chaga mushroom: A super-fungus with countless facets and untapped potential. Front. Pharmacol. 2023, 14, 1273786. [Google Scholar] [CrossRef]
- Hwang, A.Y.; Yang, S.C.; Kim, J.; Lim, T.; Cho, H.; Hwang, K.T. Effects of non-traditional extraction methods on extracting bioactive compounds from chaga mushroom (Inonotus obliquus) compared to hot water extraction. LWT 2019, 110, 80–84. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Critch, A.L.; Manful, C.F.; Rajakaruna, A.; Vidal, N.P.; Pham, T.H.; Cheema, M.; Thomas, R. Effects of pH and Temperature on Water under Pressurized Conditions in the Extraction of Nutraceuticals from Chaga (Inonotus obliquus) Mushroom. Antioxidants 2021, 10, 1322. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wang, X.; Shi, Y.; Li, L.; Chu, J.; Li, J.; Lin, W.; Yu, T.; Hou, D. Integrated omic profiling of the medicinal mushroom Inonotus obliquus under submerged conditions. BMC Genom. 2023, 24, 554. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, M.; Zhao, Y.; Miao, K.; Pan, S.; Cao, F.; Dai, Y. Analysis of antioxidant metabolites by solvent extraction from sclerotia of Inonotus obliquus (Chaga). Phytochem. Anal. 2011, 2, 95–102. [Google Scholar] [CrossRef]
- Human Metabolome Dadatabse. Available online: https://hmdb.ca/ (accessed on 20 February 2025).
- Uffelman, C.N.; Doenges, K.A.; Armstrong, M.L.; Quinn, K.; Reisdorph, R.M.; Tang, M.; Krebs, N.F.; Reisdorph, N.A.; Campbell, W.W. Metabolomics Profiling of White Button, Crimini, Portabella, Lion’s Mane, Maitake, Oyster, and Shiitake Mushrooms Using Untargeted Metabolomics and Targeted Amino Acid Analysis. Foods 2023, 12, 2985. [Google Scholar] [CrossRef]
- Megazyme® by Neogen. Available online: https://www.megazyme.com/beta-glucan-assay-kit-yeast-mushroom (accessed on 18 February 2025).
- Singla, S.; Htut, K.Z.; Zhu, R.; Davis, A.; Ma, J.; Ni, Q.Z.; Burkart, M.D.; Maurer, C.; Miyoshi, T.; Dhinojwala, A. Isolation and Characterization of Allomelanin from Pathogenic Black Knot Fungus-a Sustainable Source of Melanin. ACS Omega 2021, 6, 35514–35522. [Google Scholar] [CrossRef]



| Composition | Sample Code | Absorbance | Dilution Factor |
|---|---|---|---|
| Whole Chaga Canker | WChV1 | 0.4768 | 2 |
| WChV2 | 0.6565 | 1 | |
| WChV3 | 0.4854 | 2 | |
| WChV4 | 0.5298 | 1 | |
| WChV5 | 0.7053 | 2 | |
| WChV6 | 0.7685 | 1 | |
| Chaga 1:1 Extract | ChExt11 | 0.6580 | 2 |
| I. obliquus Mycelium | IoMyc1 | 0.0313 | 1 |
| Fermented Grain | IoMFG-oat | 0.0417 | 1 |
| IoMFG-unk | 0.0492 | 1 | |
| IoMFG-sorg | 0.0457 | 1 | |
| IoMFG-rice | 0.0179 | 1 |
| Statistic | Inotodiol | Trametenolic Acid | 3β-hydroxylanosta- 8,24-dien-21-al | Lanosterol |
|---|---|---|---|---|
| RRF | NA | 1.28 | 1.39 | 1.43 |
| Mean% | 0.19 | 0.09 | 0.09 | 0.06 |
| SD | 0.080 | 0.022 | 0.030 | 0.017 |
| LOD (ng) | 19.9 | 16.7 | 8.4 | 3.0 |
| LOQ (ng) | 60.4 | 50.6 | 25.3 | 9.0 |
| R2 | 1.0000 | 0.9998 | 0.9999 | 0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Windsor, C.; Kreynes, A.E.; Chilton, J.S.; Chioffi, W.A.; Krishnamurthy, A.; Ishii, M. Comparative Study of Chaga (Inonotus obliquus) Dietary Supplements Using Complementary Analytical Techniques. Int. J. Mol. Sci. 2025, 26, 2970. https://doi.org/10.3390/ijms26072970
Windsor C, Kreynes AE, Chilton JS, Chioffi WA, Krishnamurthy A, Ishii M. Comparative Study of Chaga (Inonotus obliquus) Dietary Supplements Using Complementary Analytical Techniques. International Journal of Molecular Sciences. 2025; 26(7):2970. https://doi.org/10.3390/ijms26072970
Chicago/Turabian StyleWindsor, Coleton, Anna E. Kreynes, Jeff S. Chilton, William A. Chioffi, Arun Krishnamurthy, and Melissa Ishii. 2025. "Comparative Study of Chaga (Inonotus obliquus) Dietary Supplements Using Complementary Analytical Techniques" International Journal of Molecular Sciences 26, no. 7: 2970. https://doi.org/10.3390/ijms26072970
APA StyleWindsor, C., Kreynes, A. E., Chilton, J. S., Chioffi, W. A., Krishnamurthy, A., & Ishii, M. (2025). Comparative Study of Chaga (Inonotus obliquus) Dietary Supplements Using Complementary Analytical Techniques. International Journal of Molecular Sciences, 26(7), 2970. https://doi.org/10.3390/ijms26072970







