Mechanisms of and Potential Medications for Oxidative Stress in Ovarian Granulosa Cells: A Review
Abstract
1. Introduction
2. Mechanisms of Oxidative Stress in Granulosa Cells
2.1. PI3K/AKT
2.2. MAPK
2.3. FOXO Axis
2.4. Nrf2
2.5. Mitophagy
2.6. Inflammation
2.7. Other
3. Potential Medication for Granulosa Cells under Oxidative Stress
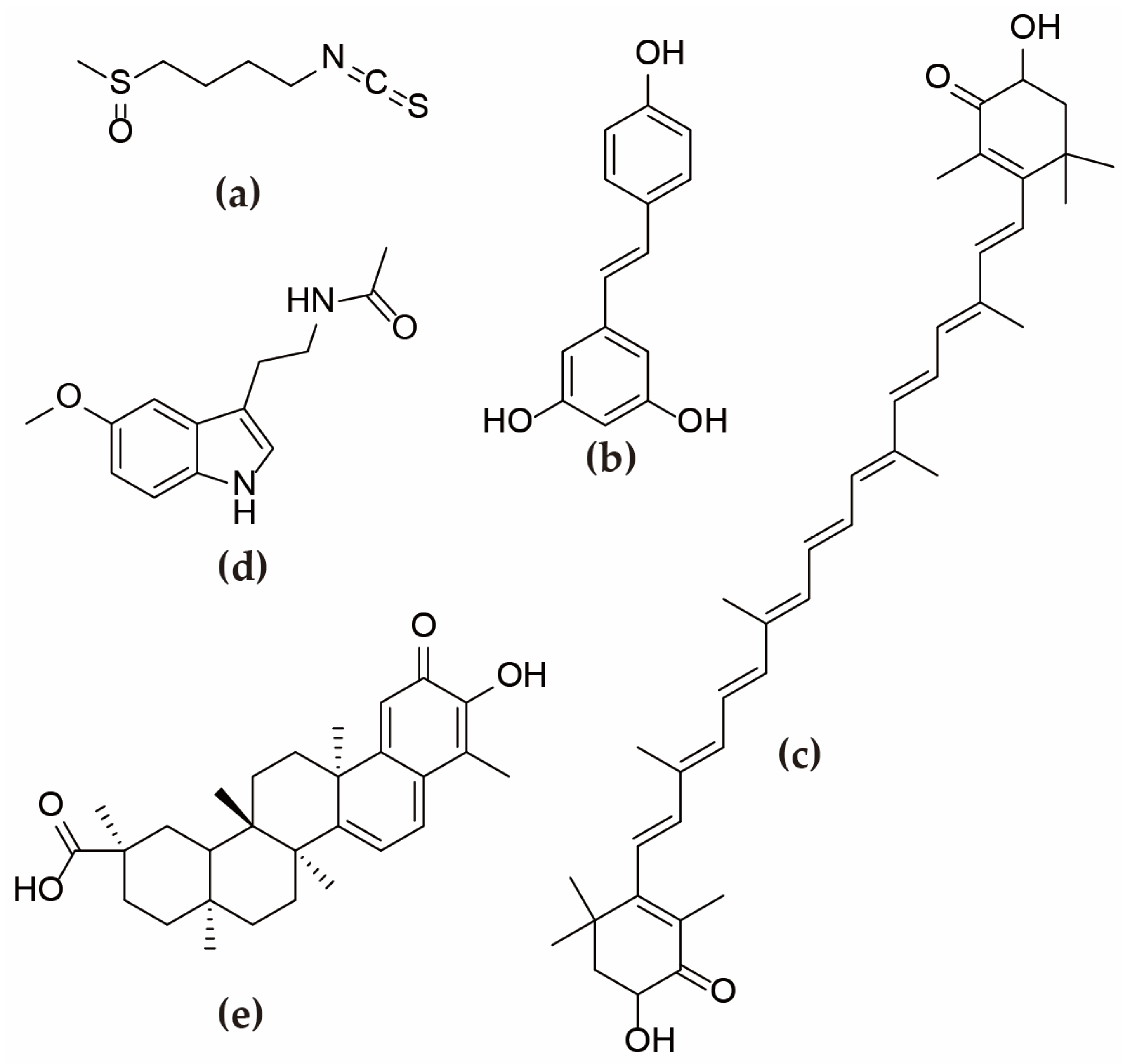
3.1. Sulforaphane
3.2. Periplaneta Americana Peptide
3.3. Resveratrol
3.4. Astaxanthin
3.5. Melatonin and Celastrol
3.6. Growth Hormone
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Adashi, E.Y.; Resnick, C.E.; D’Ercole, A.J.; Svoboda, M.E.; Van Wyk, J.J. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr. Rev. 1985, 6, 400–420. [Google Scholar] [CrossRef] [PubMed]
- McNatty, K.P.; Makris, A.; DeGrazia, C.; Osathanondh, R.; Ryan, K.J. The production of progesterone, androgens, and estrogens by granulosa cells, thecal tissue, and stromal tissue from human ovaries in vitro. J. Clin. Endocrinol. Metab. 1979, 49, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J. Intercommunication between mammalian oocytes and companion somatic cells. Bioessays 1991, 13, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Hernández, D.G.; Arreola-Mendoza, L.; Santacruz-Márquez, R.; García-Zepeda, S.P.; Parra-Forero, L.Y.; Olivares-Reyes, J.A.; Hernández-Ochoa, I. Bisphenol A alters oocyte maturation by prematurely closing gap junctions in the cumulus cell-oocyte complex. Toxicol. Appl. Pharmacol. 2018, 344, 13–22. [Google Scholar] [CrossRef]
- Grøndahl, M.L.; Nielsen, M.E.; Dal Canto, M.B.; Fadini, R.; Rasmussen, I.A.; Westergaard, L.G.; Kristensen, S.G.; Yding Andersen, C. Anti-Müllerian hormone remains highly expressed in human cumulus cells during the final stages of folliculogenesis. Reprod. Biomed. Online 2011, 22, 389–398. [Google Scholar] [CrossRef]
- Weenen, C.; Laven, J.S.; Von Bergh, A.R.; Cranfield, M.; Groome, N.P.; Visser, J.A.; Kramer, P.; Fauser, B.C.; Themmen, A.P. Anti-Müllerian hormone expression pattern in the human ovary: Potential implications for initial and cyclic follicle recruitment. Mol. Hum. Reprod. 2004, 10, 77–83. [Google Scholar] [CrossRef]
- Hirshfield, A.N. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 1991, 124, 43–101. [Google Scholar] [CrossRef]
- Manabe, N.; Matsuda-Minehata, F.; Goto, Y.; Maeda, A.; Cheng, Y.; Nakagawa, S.; Inoue, N.; Wongpanit, K.; Jin, H.; Gonda, H.; et al. Role of cell death ligand and receptor system on regulation of follicular atresia in pig ovaries. Reprod. Domest. Anim. 2008, 43 (Suppl. S2), 268–272. [Google Scholar] [CrossRef]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Y.; Yang, D.; Ren, D. Oxidative stress-induced apoptosis in granulosa cells involves JNK, p53 and Puma. Oncotarget 2017, 8, 25310–25322. [Google Scholar] [CrossRef]
- Alkadi, H. A Review on Free Radicals and Antioxidants. Infect. Disord. Drug. Targets 2020, 20, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Sarniak, A.; Lipińska, J.; Tytman, K.; Lipińska, S. Endogenous mechanisms of reactive oxygen species (ROS) generation. Postepy Hig. Med. Dosw. 2016, 70, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Devine, P.J.; Perreault, S.D.; Luderer, U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod. 2012, 86, 27. [Google Scholar] [CrossRef]
- Luderer, U. Ovarian toxicity from reactive oxygen species. Vitam. Horm. 2014, 94, 99–127. [Google Scholar] [CrossRef] [PubMed]
- Safaei, Z.; Bakhshalizadeh, S.; Nasr-Esfahani, M.H.; Akbari Sene, A.; Najafzadeh, V.; Soleimani, M.; Shirazi, R. Vitamin D3 affects mitochondrial biogenesis through mitogen-activated protein kinase in polycystic ovary syndrome mouse model. J. Cell. Physiol. 2020, 235, 6113–6126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Zhang, J.; Li, Y.; Chen, Y.X.; Wu, X.M.; Li, X.; Zhang, X.F.; Ma, L.Z.; Yang, Y.Z.; Zheng, K.M.; et al. Advanced Oxidation Protein Products Induce G1/G0-Phase Arrest in Ovarian Granulosa Cells via the ROS-JNK/p38 MAPK-p21 Pathway in Premature Ovarian Insufficiency. Oxid. Med. Cell. Longev. 2021, 2021, 6634718. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Kalantaridou, S.N.; Davis, S.R.; Nelson, L.M. Premature ovarian failure. Endocrinol. Metab. Clin. North Am. 1998, 27, 989–1006. [Google Scholar] [CrossRef]
- Ghahremani-Nasab, M.; Ghanbari, E.; Jahanbani, Y.; Mehdizadeh, A.; Yousefi, M. Premature ovarian failure and tissue engineering. J. Cell. Physiol. 2020, 235, 4217–4226. [Google Scholar] [CrossRef]
- Shareghi-Oskoue, O.; Aghebati-Maleki, L.; Yousefi, M. Transplantation of human umbilical cord mesenchymal stem cells to treat premature ovarian failure. Stem. Cell. Res. Ther. 2021, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Warenik-Szymankiewicz, A.; Słopień, R. Genetic aspects of premature ovarian failure. Endokrynol. Pol. 2005, 56, 359–361. [Google Scholar] [PubMed]
- Akdemir, Y.; Akpolat, M.; Elmas, O.; Kececi, M.; Buyukuysal, C.; Cetinkaya, B.; Guleryuz, N. Capsaicin prevents radiotherapy-induced premature ovarian failure in rats. Reprod. Fertil. Dev. 2022, 34, 350–361. [Google Scholar] [CrossRef]
- Méduri, G.; Massin, N.; Guibourdenche, J.; Bachelot, A.; Fiori, O.; Kuttenn, F.; Misrahi, M.; Touraine, P. Serum anti-Müllerian hormone expression in women with premature ovarian failure. Hum. Reprod. 2007, 22, 117–123. [Google Scholar] [CrossRef]
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [CrossRef] [PubMed]
- Osibogun, O.; Ogunmoroti, O.; Michos, E.D. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc. Med. 2020, 30, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Ullah, A.; Basit, S. Genetic Basis of Polycystic Ovary Syndrome (PCOS): Current Perspectives. Appl. Clin. Genet. 2019, 12, 249–260. [Google Scholar] [CrossRef]
- Siddiqui, S.; Mateen, S.; Ahmad, R.; Moin, S. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J. Assist. Reprod. Genet. 2022, 39, 2439–2473. [Google Scholar] [CrossRef]
- Rodriguez Paris, V.; Bertoldo, M.J. The Mechanism of Androgen Actions in PCOS Etiology. Med. Sci. 2019, 7, 89. [Google Scholar] [CrossRef]
- McFee, R.M.; Romereim, S.M.; Snider, A.P.; Summers, A.F.; Pohlmeier, W.E.; Kurz, S.G.; Cushman, R.A.; Davis, J.S.; Wood, J.R.; Cupp, A.S. A high-androgen microenvironment inhibits granulosa cell proliferation and alters cell identity. Mol. Cell. Endocrinol. 2021, 531, 111288. [Google Scholar] [CrossRef]
- Di Clemente, N.; Racine, C.; Pierre, A.; Taieb, J. Anti-Müllerian Hormone in Female Reproduction. Endocr. Rev. 2021, 42, 753–782. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, Z.; Mohammadi-Yeganeh, S.; Sameni, M.; Mirmotalebisohi, S.A.; Zali, H.; Salehi, M. Repurposing new drug candidates and identifying crucial molecules underlying PCOS Pathogenesis Based On Bioinformatics Analysis. Daru 2021, 29, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Kumariya, S.; Ubba, V.; Jha, R.K.; Gayen, J.R. Autophagy in ovary and polycystic ovary syndrome: Role, dispute and future perspective. Autophagy 2021, 17, 2706–2733. [Google Scholar] [CrossRef] [PubMed]
- Furat Rencber, S.; Kurnaz Ozbek, S.; Eraldemır, C.; Sezer, Z.; Kum, T.; Ceylan, S.; Guzel, E. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: An experimental study. J. Ovarian. Res. 2018, 11, 55. [Google Scholar] [CrossRef]
- Aglan, H.S.; Gebremedhn, S.; Salilew-Wondim, D.; Neuhof, C.; Tholen, E.; Holker, M.; Schellander, K.; Tesfaye, D. Regulation of Nrf2 and NF-κB during lead toxicity in bovine granulosa cells. Cell. Tissue. Res. 2020, 380, 643–655. [Google Scholar] [CrossRef]
- Yadav, A.K.; Yadav, P.K.; Chaudhary, G.R.; Tiwari, M.; Gupta, A.; Sharma, A.; Pandey, A.N.; Pandey, A.K.; Chaube, S.K. Autophagy in hypoxic ovary. Cell. Mol. Life Sci. 2019, 76, 3311–3322. [Google Scholar] [CrossRef]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Du, X.; Li, Q.; Cao, Q.; Wang, S.; Liu, H.; Li, Q. Integrated Analysis of miRNA-mRNA Interaction Network in Porcine Granulosa Cells Undergoing Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, 1041583. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Gao, Y.; Li, Q.; Cao, Y.; Shen, Y.; Chen, P.; Yan, J.; Li, J. Vitamin E regulates bovine granulosa cell apoptosis via NRF2-mediated defence mechanism by activating PI3K/AKT and ERK1/2 signalling pathways. Reprod. Domest. Anim. 2021, 56, 1066–1084. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Vander Heiden, M.G.; McCormick, F. The Metabolic Landscape of RAS-Driven Cancers from biology to therapy. Nat. Cancer 2021, 2, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Therachiyil, L.; Anand, A.; Azmi, A.; Bhat, A.; Korashy, H.M.; Uddin, S. Role of RAS signaling in ovarian cancer. F1000Res 2022, 11, 1253. [Google Scholar] [CrossRef] [PubMed]
- Sammad, A.; Luo, H.; Hu, L.; Zhao, S.; Gong, J.; Umer, S.; Khan, A.; Zhu, H.; Wang, Y. Joint Transcriptome and Metabolome Analysis Prevails the Biological Mechanisms Underlying the Pro-Survival Fight in In Vitro Heat-Stressed Granulosa Cells. Biology 2022, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Yan, J.; Wu, Y.; Wu, K.; Li, W. Morroniside suppresses hydrogen peroxide-stimulated autophagy and apoptosis in rat ovarian granulosa cells through the PI3K/AKT/mTOR pathway. Hum. Exp. Toxicol. 2021, 40, 577–586. [Google Scholar] [CrossRef]
- Feng, J.; Ma, W.W.; Li, H.X.; Pei, X.Y.; Deng, S.L.; Jia, H.; Ma, W.Z. Melatonin prevents cyclophosphamide-induced primordial follicle loss by inhibiting ovarian granulosa cell apoptosis and maintaining AMH expression. Front. Endocrinol. 2022, 13, 895095. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xi, Y.; Li, M.; Wu, Y.; Yan, W.; Dai, J.; Wu, M.; Ding, W.; Zhang, J.; Zhang, F.; et al. Maternal exposure to PM2.5 decreases ovarian reserve in neonatal offspring mice through activating PI3K/AKT/FoxO3a pathway and ROS-dependent NF-κB pathway. Toxicology 2022, 481, 153352. [Google Scholar] [CrossRef]
- Zhu, M.; Miao, S.; Zhou, W.; Elnesr, S.S.; Dong, X.; Zou, X. MAPK, AKT/FoxO3a and mTOR pathways are involved in cadmium regulating the cell cycle, proliferation and apoptosis of chicken follicular granulosa cells. Ecotoxicol. Environ. Saf. 2021, 214, 112091. [Google Scholar] [CrossRef]
- Xu, H.; Mu, X.; Ding, Y.; Tan, Q.; Liu, X.; He, J.; Gao, R.; Li, N.; Geng, Y.; Wang, Y.; et al. Melatonin alleviates benzo(a)pyrene-induced ovarian corpus luteum dysfunction by suppressing excessive oxidative stress and apoptosis. Ecotoxicol. Environ. Saf. 2021, 207, 111561. [Google Scholar] [CrossRef]
- Ding, H.; Li, Z.; Li, X.; Yang, X.; Zhao, J.; Guo, J.; Lu, W.; Liu, H.; Wang, J. FTO Alleviates CdCl(2)-Induced Apoptosis and Oxidative Stress via the AKT/Nrf2 Pathway in Bovine Granulosa Cells. Int. J. Mol. Sci. 2022, 23, 4948. [Google Scholar] [CrossRef]
- Gong, Y.; Luo, S.; Fan, P.; Zhu, H.; Li, Y.; Huang, W. Growth hormone activates PI3K/Akt signaling and inhibits ROS accumulation and apoptosis in granulosa cells of patients with polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2020, 18, 121. [Google Scholar] [CrossRef]
- Saha, P.; Kumar, S.; Datta, K.; Tyagi, R.K. Upsurge in autophagy, associated with mifepristone-treated polycystic ovarian condition, is reversed upon thymoquinone treatment. J. Steroid. Biochem. Mol. Biol. 2021, 208, 105823. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Dai, W.; Liu, L.; Han, H.; Zhang, J.; Du, X.; Pei, X.; Fu, X. Metformin ameliorates polycystic ovary syndrome in a rat model by decreasing excessive autophagy in ovarian granulosa cells via the PI3K/AKT/mTOR pathway. Endocr. J. 2022, 69, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Rostami, S.; Nekoonam, S.; Rashidi, Z.; Sobhani, A.; Amidi, F. The Effect of Astaxanthin and Metformin on Oxidative Stress in Granulosa Cells of BALB C Mouse Model of Polycystic Ovary Syndrome. Reprod. Sci. 2021, 28, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; He, J.; Bai, Y.; He, Q.; Zhang, T.; Zhang, J.; Yang, G.; Xu, Z.; Hu, J.; Yao, G. Baicalin improves the functions of granulosa cells and the ovary in aged mice through the mTOR signaling pathway. J. Ovarian. Res. 2022, 15, 34. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Zhang, Z.; Xu, D.; Duan, J.; Li, X.; Yang, L.; Hua, R.; Cheng, J.; Li, Q. Isorhamnetin Promotes Estrogen Biosynthesis and Proliferation in Porcine Granulosa Cells via the PI3K/Akt Signaling Pathway. J. Agric. Food Chem. 2021, 69, 6535–6542. [Google Scholar] [CrossRef]
- Hepworth, E.M.W.; Hinton, S.D. Pseudophosphatases as Regulators of MAPK Signaling. Int. J. Mol. Sci. 2021, 22, 12595. [Google Scholar] [CrossRef]
- Díaz-Troya, S.; Pérez-Pérez, M.E.; Florencio, F.J.; Crespo, J.L. The role of TOR in autophagy regulation from yeast to plants and mammals. Autophagy 2008, 4, 851–865. [Google Scholar] [CrossRef]
- Sheller-Miller, S.; Richardson, L.; Martin, L.; Jin, J.; Menon, R. Systematic review of p38 mitogen-activated kinase and its functional role in reproductive tissues. Am. J. Reprod. Immunol. 2018, 80, e13047. [Google Scholar] [CrossRef]
- Bragado, P.; Armesilla, A.; Silva, A.; Porras, A. Apoptosis by cisplatin requires p53 mediated p38alpha MAPK activation through ROS generation. Apoptosis 2007, 12, 1733–1742. [Google Scholar] [CrossRef]
- Exil, V.; Ping, L.; Yu, Y.; Chakraborty, S.; Caito, S.W.; Wells, K.S.; Karki, P.; Lee, E.; Aschner, M. Activation of MAPK and FoxO by manganese (Mn) in rat neonatal primary astrocyte cultures. PLoS ONE 2014, 9, e94753. [Google Scholar] [CrossRef]
- Sammad, A.; Luo, H.; Hu, L.; Zhu, H.; Wang, Y. Transcriptome Reveals Granulosa Cells Coping through Redox, Inflammatory and Metabolic Mechanisms under Acute Heat Stress. Cells 2022, 11, 1443. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.T.; Dhanasekaran, N. Apoptosis of granulosa cells: A review on the role of MAPK-signalling modules. Reprod. Domest. Anim. 2003, 38, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lin, J.; Chen, C.; Nie, X.; Dou, F.; Chen, J.; Wang, Z.; Gong, Z. MicroRNA-146b-5p overexpression attenuates premature ovarian failure in mice by inhibiting the Dab2ip/Ask1/p38-Mapk pathway and γH2A.X phosphorylation. Cell. Prolif. 2021, 54, e12954. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hao, G.; Lin, X.; Zhao, Z.; Yang, A.; Cao, Y.; Zhang, S.; Fan, L.; Geng, J.; Zhang, Y.; et al. Morroniside Protects Human Granulosa Cells against H(2)O(2)-Induced Oxidative Damage by Regulating the Nrf2 and MAPK Signaling Pathways. Evid. Based. Complement Alternat. Med. 2022, 2022, 8099724. [Google Scholar] [CrossRef] [PubMed]
- Link, W. Introduction to FOXO Biology. Methods Mol. Biol. 2019, 1890, 1–9. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef]
- Ponugoti, B.; Dong, G.; Graves, D.T. Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp. Diabetes Res. 2012, 2012, 939751. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 2012, 11, 230–241. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, A.; Wu, Y.; Mi, Y.; Zhang, C. Protective Effect of Grape Seed Proanthocyanidins on Oxidative Damage of Chicken Follicular Granulosa Cells by Inhibiting FoxO1-Mediated Autophagy. Front. Cell. Dev. Biol. 2022, 10, 762228. [Google Scholar] [CrossRef]
- Barbe, A.; Ramé, C.; Mellouk, N.; Estienne, A.; Bongrani, A.; Brossaud, A.; Riva, A.; Guérif, F.; Froment, P.; Dupont, J. Effects of Grape Seed Extract and Proanthocyanidin B2 on In Vitro Proliferation, Viability, Steroidogenesis, Oxidative Stress, and Cell Signaling in Human Granulosa Cells. Int. J. Mol. Sci. 2019, 20, 4215. [Google Scholar] [CrossRef]
- Yun, H.R.; Jo, Y.H.; Kim, J.; Shin, Y.; Kim, S.S.; Choi, T.G. Roles of Autophagy in Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 3289. [Google Scholar] [CrossRef]
- Fu, Z.; Tindall, D.J. FOXOs, cancer and regulation of apoptosis. Oncogene 2008, 27, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Su, J.; Wang, Q.; Liu, K.; Fu, R.; Sui, S. Signaling pathways of Periplaneta americana peptide resist H(2)O(2)-induced apoptosis in pig-ovary granulosa cells through FoxO1. Theriogenology 2022, 183, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Ren, Q.L.; Chen, J.F.; Gao, B.W.; Wang, X.W.; Zhang, Z.J.; Wang, J.; Xu, Z.J.; Xing, B.S. Autophagy Contributes to Oxidative Stress-Induced Apoptosis in Porcine Granulosa Cells. Reprod. Sci. 2021, 28, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, J.; Ma, B.; Wang, J.; Chen, J. Role of Autophagy in Lysophosphatidylcholine-Induced Apoptosis of Mouse Ovarian Granulosa Cells. Int. J. Mol. Sci. 2022, 23, 1479. [Google Scholar] [CrossRef]
- Kaminskyy, V.O.; Zhivotovsky, B. Free radicals in cross talk between autophagy and apoptosis. Antioxid. Redox. Signal. 2014, 21, 86–102. [Google Scholar] [CrossRef]
- Yao, W.; Pan, Z.; Du, X.; Zhang, J.; Liu, H.; Li, Q. NORHA, a novel follicular atresia-related lncRNA, promotes porcine granulosa cell apoptosis via the miR-183-96-182 cluster and FoxO1 axis. J. Anim. Sci. Biotechnol. 2021, 12, 103. [Google Scholar] [CrossRef]
- Venugopal, R.; Jaiswal, A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA 1996, 93, 14960–14965. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef]
- Esfandyari, S.; Aleyasin, A.; Noroozi, Z.; Taheri, M.; Khodarahmian, M.; Eslami, M.; Rashidi, Z.; Amidi, F. The Protective Effect of Sulforaphane against Oxidative Stress through Activation of NRF2/ARE Pathway in Human Granulosa Cells. Cell J. 2021, 23, 692–700. [Google Scholar] [CrossRef]
- Cyran, A.M.; Zhitkovich, A. HIF1, HSF1, and NRF2: Oxidant-Responsive Trio Raising Cellular Defenses and Engaging Immune System. Chem. Res. Toxicol. 2022, 35, 1690–1700. [Google Scholar] [CrossRef] [PubMed]
- Uruno, A.; Yamamoto, M. The KEAP1-NRF2 System and Neurodegenerative Diseases. Antioxid. Redox. Signal 2023, 38, 974–988. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Yamamoto, M. The KEAP1-NRF2 System in Cancer. Front. Oncol. 2017, 7, 85. [Google Scholar] [CrossRef]
- Liby, K.T.; Sporn, M.B. Synthetic oleanane triterpenoids: Multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol. Rev. 2012, 64, 972–1003. [Google Scholar] [CrossRef] [PubMed]
- Galan-Cobo, A.; Sitthideatphaiboon, P.; Qu, X.; Poteete, A.; Pisegna, M.A.; Tong, P.; Chen, P.-H.; Boroughs, L.K.; Rodriguez, M.L.M.; Zhang, W.; et al. LKB1 and KEAP1/NRF2 Pathways Cooperatively Promote Metabolic Reprogramming with Enhanced Glutamine Dependence in KRAS-Mutant Lung Adenocarcinoma. Cancer Res. 2019, 79, 3251–3267. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Goswami, D.; Adiseshaiah, P.P.; Burgan, W.; Yi, M.; Guerin, T.M.; Kozlov, S.V.; Nissley, D.V.; McCormick, F. Undermining Glutaminolysis Bolsters Chemotherapy While NRF2 Promotes Chemoresistance in KRAS-Driven Pancreatic Cancers. Cancer Res. 2020, 80, 1630–1643. [Google Scholar] [CrossRef]
- Alberghina, L.; Gaglio, D. Redox control of glutamine utilization in cancer. Cell Death Dis. 2014, 5, e1561. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B. Relationships between Stress Granules, Oxidative Stress, and Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 1809592. [Google Scholar] [CrossRef]
- Lian, X.J.; Gallouzi, I.-E. Oxidative Stress Increases the Number of Stress Granules in Senescent Cells and Triggers a Rapid Decrease in p21waf1/cip1 Translation. J. Biol. Chem. 2009, 284, 8877–8887. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Li, L.; Bai, L.; Wang, Y.; Zhang, J.; Wang, H. Inhibition of Nicotinamide adenine dinucleotide phosphate oxidase 4 attenuates cell apoptosis and oxidative stress in a rat model of polycystic ovary syndrome through the activation of Nrf-2/HO-1 signaling pathway. Mol. Cell. Endocrinol. 2022, 550, 111645. [Google Scholar] [CrossRef]
- Shaoyong, W.; Liu, Y.; Xu, B.; Pan, B.; Xianmi, X.; Wang, Y.; Jin, M. Exposure to BDE-47 causes female infertility risk and induces oxidative stress and lipotoxicity-mediated ovarian hormone secretion disruption in mice. Sci. Total. Environ. 2022, 842, 156885. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Cheng, G.; Xu, C.; Liu, H.; Wang, Y.; Li, N.; Fan, X.; Zhu, C.; Xia, W. Copper Nanoparticles Induce Oxidative Stress via the Heme Oxygenase 1 Signaling Pathway in vitro Studies. Int. J. Nanomed. 2021, 16, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Sohel, M.M.H.; Salman, M.A.; Ayvaz, A. Cellular and Transcriptional Adaptation of Bovine Granulosa Cells Under Ethanol-Induced Stress In Vitro. Alcohol. Alcohol. 2021, 56, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zheng, L.W.; Wang, Y.S.; Huang, J.C.; Yang, Z.Q.; Yue, Z.P.; Guo, B. Genistein exhibits therapeutic potential for PCOS mice via the ER-Nrf2-Foxo1-ROS pathway. Food. Funct. 2021, 12, 8800–8811. [Google Scholar] [CrossRef]
- Ji, R.; Jia, F.Y.; Chen, X.; Wang, Z.H.; Jin, W.Y.; Yang, J. Salidroside alleviates oxidative stress and apoptosis via AMPK/Nrf2 pathway in DHT-induced human granulosa cell line KGN. Arch. Biochem. Biophys. 2022, 715, 109094. [Google Scholar] [CrossRef]
- Taheri, M.; Roudbari, N.H.; Amidi, F.; Parivar, K. Investigating the effect of Sulforaphane on AMPK/AKT/NRF2 pathway in human granulosa-lutein cells under H(2)O(2)-induced oxidative stress. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 276, 125–133. [Google Scholar] [CrossRef]
- Taheri, M.; Hayati Roudbari, N.; Amidi, F.; Parivar, K. The protective effect of sulforaphane against oxidative stress in granulosa cells of patients with polycystic ovary syndrome (PCOS) through activation of AMPK/AKT/NRF2 signaling pathway. Reprod. Biol. 2021, 21, 100563. [Google Scholar] [CrossRef]
- Taqi, M.O.; Saeed-Zidane, M.; Gebremedhn, S.; Salilew-Wondim, D.; Tholen, E.; Neuhoff, C.; Hoelker, M.; Schellander, K.; Tesfaye, D. NRF2-mediated signaling is a master regulator of transcription factors in bovine granulosa cells under oxidative stress condition. Cell Tissue. Res. 2021, 385, 769–783. [Google Scholar] [CrossRef]
- Fan, L.; Guan, F.; Ma, Y.; Zhang, Y.; Li, L.; Sun, Y.; Cao, C.; Du, H.; He, M. N-Acetylcysteine improves oocyte quality through modulating the Nrf2 signaling pathway to ameliorate oxidative stress caused by repeated controlled ovarian hyperstimulation. Reprod. Fertil. Dev. 2022, 34, 736–750. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Zeng, Z.; Tang, L.; Zhao, S.; Zhou, F.; Zhou, L.; Xia, W.; Zhu, C.; Rao, M. Humanin regulates oxidative stress in the ovaries of polycystic ovary syndrome patients via the Keap1/Nrf2 pathway. Mol. Hum. Reprod. 2021, 27, gaaa081. [Google Scholar] [CrossRef]
- Nie, Z.; Zhang, N.; Guo, L.; Lv, C.; Zhang, Y.; Wang, C.; Wu, H. Growth hormone improved oxidative stress in follicle fluid by influencing Nrf2/Keap1 expression in women of advanced age undergoing IVF. Gynecol. Endocrinol. 2022, 38, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. Faseb. J. 2015, 29, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Onishi, M.; Yamano, K.; Sato, M.; Matsuda, N.; Okamoto, K. Molecular mechanisms and physiological functions of mitophagy. Embo. J. 2021, 40, e104705. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Narendra, D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell. Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Shida, M.; Kitajima, Y.; Nakamura, J.; Yanagihara, K.; Baba, K.; Wakiyama, K.; Noshiro, H. Impaired mitophagy activates mtROS/HIF-1α interplay and increases cancer aggressiveness in gastric cancer cells under hypoxia. Int. J. Oncol. 2016, 48, 1379–1390. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Li, H.; Chen, B.; Liu, Z.; Wu, G.; Li, C.; Li, R.; Cao, Y.; Zhou, J.; et al. Sulforaphane Acts Through NFE2L2 to Prevent Hypoxia-Induced Apoptosis in Porcine Granulosa Cells via Activating Antioxidant Defenses and Mitophagy. J. Agric. Food Chem. 2022, 70, 8097–8110. [Google Scholar] [CrossRef]
- Fu, R.; Kong, C.; Wang, Q.; Liu, K.; Si, H.; Sun, R.; Tang, Y.; Sui, S. Small Peptides from Periplaneta americana Inhibits Oxidative Stress-Induced KGN Cell Apoptosis by Regulating Mitochondrial Function Through Bcl2L13. Reprod. Sci. 2022, 30, 473–486. [Google Scholar] [CrossRef]
- Jiang, Y.; Shen, M.; Chen, Y.; Wei, Y.; Tao, J.; Liu, H. Melatonin Represses Mitophagy to Protect Mouse Granulosa Cells from Oxidative Damage. Biomolecules 2021, 11, 968. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell. Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef]
- Katagiri, T.; Kameda, H.; Nakano, H.; Yamazaki, S. Regulation of T cell differentiation by the AP-1 transcription factor JunB. Immunol. Med. 2021, 44, 197–203. [Google Scholar] [CrossRef]
- Sato, R.; Shimizu, F.; Kuwahara, M.; Mizukami, Y.; Watanabe, K.; Maeda, T.; Sano, Y.; Takeshita, Y.; Koga, M.; Kusunoki, S.; et al. Autocrine TNF-α Increases Penetration of Myelin-Associated Glycoprotein Antibodies Across the Blood-Nerve Barrier in Anti-MAG Neuropathy. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200086. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Luo, S.; Feng, L.; Wang, J.; Mao, J.; Luo, B. Resveratrol regulates the inflammation and oxidative stress of granulosa cells in PCOS via targeting TLR2. J. Bioenerg. Biomembr. 2022, 54, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Ali, I.; Li, L.; Wang, G. N-acetylcysteine modulates non-esterified fatty acid-induced pyroptosis and inflammation in granulosa cells. Mol. Immunol. 2020, 127, 157–163. [Google Scholar] [CrossRef]
- Fan, H.; Wang, S.; Wang, H.; Sun, M.; Wu, S.; Bao, W. Melatonin Ameliorates the Toxicity Induced by Deoxynivalenol in Murine Ovary Granulosa Cells by Antioxidative and Anti-Inflammatory Effects. Antioxidants 2021, 10, 1045. [Google Scholar] [CrossRef] [PubMed]
- Al-Shahat, A.; Hulail, M.A.E.; Soliman, N.M.M.; Khamis, T.; Fericean, L.M.; Arisha, A.H.; Moawad, R.S. Melatonin Mitigates Cisplatin-Induced Ovarian Dysfunction via Altering Steroidogenesis, Inflammation, Apoptosis, Oxidative Stress, and PTEN/PI3K/Akt/mTOR/AMPK Signaling Pathway in Female Rats. Pharmaceutics 2022, 14, 2769. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, Y.; Hu, C.; Wang, Y.; Yan, Z.; Li, Z.; Wu, R. Mitochondria and oxidative stress in ovarian endometriosis. Free Radic. Biol. Med. 2019, 136, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Sun, H.; Mao, Z.; Zhang, L.; Chen, X.; Shi, Y.; Shang, Y. Vitamin D deficiency inhibits microRNA-196b-5p which regulates ovarian granulosa cell hormone synthesis, proliferation, and apoptosis by targeting RDX and LRRC17. Ann. Transl. Med. 2021, 9, 1775. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, D.; Tian, Y.; Wei, Q.; Amevor, F.K.; Sun, C.; Yu, C.; Yang, C.; Du, H.; Jiang, X.; et al. miRNA sequencing analysis of healthy and atretic follicles of chickens revealed that miR-30a-5p inhibits granulosa cell death via targeting Beclin1. J. Anim. Sci. Biotechnol. 2022, 13, 55. [Google Scholar] [CrossRef]
- Yang, Z.; Hong, W.; Zheng, K.; Feng, J.; Hu, C.; Tan, J.; Zhong, Z.; Zheng, Y. Chitosan Oligosaccharides Alleviate H(2)O(2)-stimulated Granulosa Cell Damage via HIF-1α Signaling Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 4247042. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, R.; Chen, L. Ferroptosis inhibitor ferrostatin-1 alleviates homocysteine-induced ovarian granulosa cell injury by regulating TET activity and DNA methylation. Mol. Med. Rep. 2022, 25, 130. [Google Scholar] [CrossRef]
- Mu, H.; Cai, S.; Wang, X.; Li, H.; Zhang, L.; Li, H.; Xiang, W. RNA binding protein IGF2BP1 meditates oxidative stress-induced granulosa cell dysfunction by regulating MDM2 mRNA stability in an m(6)A-dependent manner. Redox. Biol. 2022, 57, 102492. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Bussolati, S.; Torcianti, V.; Grasselli, F. Perfluorooctanoic Acid (PFOA) Induces Redox Status Disruption in Swine Granulosa Cells. Vet. Sci. 2022, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Sha, C.; Chen, L.; Lin, L.; Li, T.; Wei, H.; Yang, M.; Gao, W.; Zhao, D.; Chen, Q.; Liu, Y.; et al. TRDMT1 participates in the DNA damage repair of granulosa cells in premature ovarian failure. Aging 2021, 13, 15193–15213. [Google Scholar] [CrossRef]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013, 2013, 415078. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Russo, G.L.; Skalicka-Woźniak, K.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Nrf2 targeting by sulforaphane: A potential therapy for cancer treatment. Crit. Rev. Food Sci. Nutr. 2018, 58, 1391–1405. [Google Scholar] [CrossRef]
- Sohel, M.M.H.; Konca, Y.; Akyuz, B.; Arslan, K.; Sariozkan, S.; Cinar, M.U. Concentration dependent antioxidative and apoptotic effects of sulforaphane on bovine granulosa cells in vitro. Theriogenology 2017, 97, 17–26. [Google Scholar] [CrossRef]
- Sohel, M.M.H.; Amin, A.; Prastowo, S.; Linares-Otoya, L.; Hoelker, M.; Schellander, K.; Tesfaye, D. Sulforaphane protects granulosa cells against oxidative stress via activation of NRF2-ARE pathway. Cell. Tissue. Res. 2018, 374, 629–641. [Google Scholar] [CrossRef]
- Pomés, A.; Mueller, G.A.; Randall, T.A.; Chapman, M.D.; Arruda, L.K. New Insights into Cockroach Allergens. Curr. Allergy Asthma. Rep. 2017, 17, 25. [Google Scholar] [CrossRef]
- Mosaheb, M.; Khan, N.A.; Siddiqui, R. Cockroaches, locusts, and envenomating arthropods: A promising source of antimicrobials. Iran. J. Basic Med. Sci. 2018, 21, 873–877. [Google Scholar] [CrossRef]
- Kim, I.W.; Lee, J.H.; Seo, M.; Lee, H.J.; Baek, M.; Kim, M.A.; Shin, Y.P.; Kim, S.H.; Kim, I.; Hwang, J.S. Anti-Inflammatory Activity of Antimicrobial Peptide Periplanetasin-5 Derived from the Cockroach Periplaneta americana. J. Microbiol. Biotechnol. 2020, 30, 1282–1289. [Google Scholar] [CrossRef]
- Wang, Q.; Fu, R.; Kong, C.; Liu, K.; Si, H.; Sui, S. The protective effect of small peptides from Periplaneta americana on hydrogen peroxide-induced apoptosis of granular cells. Vitr. Cell Dev. Biol. Anim. 2021, 57, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Liu, K.; Wang, Q.; Fu, R.; Si, H.; Sui, S. Periplaneta americana peptide decreases apoptosis of pig-ovary granulosa cells induced by H(2) O(2) through FoxO1. Reprod. Domest. Anim. 2021, 56, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Minno, G.D.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef]
- Chupradit, S.; Bokov, D.; Zamanian, M.Y.; Heidari, M.; Hakimizadeh, E. Hepatoprotective and therapeutic effects of resveratrol: A focus on anti-inflammatory and antioxidative activities. Fundam. Clin. Pharmacol. 2022, 36, 468–485. [Google Scholar] [CrossRef]
- Sawda, C.; Moussa, C.; Turner, R.S. Resveratrol for Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2017, 1403, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and Its Effects on the Vascular System. Int. J. Mol. Sci. 2019, 20, 1523. [Google Scholar] [CrossRef]
- Moreira-Pinto, B.; Costa, L.; Felgueira, E.; Fonseca, B.M.; Rebelo, I. Low Doses of Resveratrol Protect Human Granulosa Cells from Induced-Oxidative Stress. Antioxidants 2021, 10, 561. [Google Scholar] [CrossRef]
- Li, N.; Liu, L. Mechanism of resveratrol in improving ovarian function in a rat model of premature ovarian insufficiency. J. Obstet. Gynaecol. Res. 2018, 44, 1431–1438. [Google Scholar] [CrossRef]
- Nie, Z.; Hua, R.; Zhang, Y.; Zhang, N.; Zhang, Y.; Li, Q.; Wu, H. Resveratrol protects human luteinised granulosa cells against hydrogen peroxide-induced oxidative injury through the Sirt1. Reprod. Fertil. Dev. 2021, 33, 831–840. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, R.; Kumari, A.; Panwar, A. Astaxanthin: A super antioxidant from microalgae and its therapeutic potential. J. Basic Microbiol. 2022, 62, 1064–1082. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S.; Name, J.J. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases (Review). Int. J. Mol. Med. 2021, 47, 37–48. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Yaribeygi, H.; Sathyapalan, T.; Sahebkar, A. Astaxanthin and Nrf2 Signaling Pathway: A Novel Target for New Therapeutic Approaches. Mini. Rev. Med. Chem. 2022, 22, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.T.; Kotani, K. Astaxanthin as a Potential Protector of Liver Function: A Review. J. Clin. Med. Res. 2016, 8, 701–704. [Google Scholar] [CrossRef]
- Abdel-Ghani, M.A.; Yanagawa, Y.; Balboula, A.Z.; Sakaguchi, K.; Kanno, C.; Katagiri, S.; Takahashi, M.; Nagano, M. Astaxanthin improves the developmental competence of in vitro-grown oocytes and modifies the steroidogenesis of granulosa cells derived from bovine early antral follicles. Reprod. Fertil. Dev. 2019, 31, 272–281. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Z.; Liu, S.; Gao, F.; Zhang, J.; Peng, Z.; Wang, L.; Pan, X. Astaxanthin improves the development of the follicles and oocytes through alleviating oxidative stress induced by BPA in cultured follicles. Sci. Rep. 2022, 12, 7853. [Google Scholar] [CrossRef]
- Gharaei, R.; Alyasin, A.; Mahdavinezhad, F.; Samadian, E.; Ashrafnezhad, Z.; Amidi, F. Randomized controlled trial of astaxanthin impacts on antioxidant status and assisted reproductive technology outcomes in women with polycystic ovarian syndrome. J. Assist. Reprod. Genet. 2022, 39, 995–1008. [Google Scholar] [CrossRef]
- Eslami, M.; Esfandyari, S.; Aghahosseini, M.; Rashidi, Z.; Hosseinishental, S.H.; Brenjian, S.; Sobhani, A.; Amidi, F. Astaxanthin Protects Human Granulosa Cells against Oxidative Stress through Activation of NRF2/ARE Pathway and Its Downstream Phase II Enzymes. Cell J. 2021, 23, 319–328. [Google Scholar] [CrossRef]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Patel, K.K.; Dehari, D.; Agrawal, A.K.; Singh, S. Melatonin and its ubiquitous anticancer effects. Mol. Cell. Biochem. 2019, 462, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Bekała, A.; Płotek, W.; Siwicka-Gieroba, D.; Sołek-Pastuszka, J.; Bohatyrewicz, R.; Biernawska, J.; Kotfis, K.; Bielacz, M.; Jaroszyński, A.; Dabrowski, W. Melatonin and the Brain-Heart Crosstalk in Neurocritically Ill Patients-From Molecular Action to Clinical Practice. Int. J. Mol. Sci. 2022, 23, 7094. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Asada, H.; Yamagata, Y.; et al. Melatonin as a free radical scavenger in the ovarian follicle. Endocr. J. 2013, 60, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Li, S.; Zou, H.; Ji, D.; Lv, M.; Zhou, P.; Wei, Z.; Zhang, Z.; Cao, Y. Melatonin alleviates deoxynivalenol-induced apoptosis of human granulosa cells by reducing mutually accentuated FOXO1 and ER stress‡. Biol. Reprod. 2021, 105, 554–566. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Mukherjee, T.; Bishayee, A. Molecular targets of celastrol in cancer: Recent trends and advancements. Crit. Rev. Oncol. Hematol. 2018, 128, 70–81. [Google Scholar] [CrossRef]
- Saito, K.; Davis, K.C.; Morgan, D.A.; Toth, B.A.; Jiang, J.; Singh, U.; Berglund, E.D.; Grobe, J.L.; Rahmouni, K.; Cui, H. Celastrol Reduces Obesity in MC4R Deficiency and Stimulates Sympathetic Nerve Activity Affecting Metabolic and Cardiovascular Functions. Diabetes 2019, 68, 1210–1220. [Google Scholar] [CrossRef]
- Venkatesha, S.H.; Moudgil, K.D. Celastrol and Its Role in Controlling Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 267–289. [Google Scholar] [CrossRef]
- Martín-Ramírez, R.; González-Fernández, R.; Hernández, J.; Martín-Vasallo, P.; Palumbo, A.; Ávila, J. Celastrol and Melatonin Modify SIRT1, SIRT6 and SIRT7 Gene Expression and Improve the Response of Human Granulosa-Lutein Cells to Oxidative Stress. Antioxidants 2021, 10, 1871. [Google Scholar] [CrossRef]
- Tritos, N.A.; Klibanski, A. Effects of Growth Hormone on Bone. Prog. Mol. Biol. Transl. Sci. 2016, 138, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Strobl, J.S.; Thomas, M.J. Human growth hormone. Pharmacol. Rev. 1994, 46, 1–34. [Google Scholar] [PubMed]
- Chanson, P. The heart in growth hormone (GH) deficiency and the cardiovascular effects of GH. Ann. Endocrinol. 2021, 82, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.J.; Tsarfaty, G.; Longo, D.L. Growth hormone exerts hematopoietic growth-promoting effects in vivo and partially counteracts the myelosuppressive effects of azidothymidine. Blood 1992, 80, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Spiliotis, B.E. Growth hormone insufficiency and its impact on ovarian function. Ann. N. Y. Acad. Sci. 2003, 997, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, H.; Yu, Q.; Liu, H.; Huang, T.; Zhao, S.; Ma, J.; Zhao, H. Growth Hormone Promotes in vitro Maturation of Human Oocytes. Front. Endocrinol. 2019, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Yovich, J.L.; Stanger, J.D. Growth hormone supplementation improves implantation and pregnancy productivity rates for poor-prognosis patients undertaking IVF. Reprod. Biomed. Online 2010, 21, 37–49. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, K.; Xiong, D.; Wei, J.; Tan, H.; Qin, S. Growth hormone alleviates oxidative stress and improves the IVF outcomes of poor ovarian responders: A randomized controlled trial. Reprod. Biol. Endocrinol. 2020, 18, 91. [Google Scholar] [CrossRef]
- Feng, P.; Xie, Q.; Liu, Z.; Guo, Z.; Tang, R.; Yu, Q. Study on the Reparative Effect of PEGylated Growth Hormone on Ovarian Parameters and Mitochondrial Function of Oocytes From Rats With Premature Ovarian Insufficiency. Front. Cell. Dev. Biol. 2021, 9, 649005. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Zhang, Y.; Zhang, J.; Xu, W.; Wu, C.; Zhou, P. Growth hormone protects against ovarian granulosa cell apoptosis: Alleviation oxidative stress and enhancement mitochondrial function. Reprod. Biol. 2021, 21, 100504. [Google Scholar] [CrossRef]
- Jiao, J.H.; Gao, L.; Yong, W.L.; Kou, Z.Y.; Ren, Z.Q.; Cai, R.; Chu, G.Y.; Pang, W.J. Resveratrol improves estrus disorder induced by bisphenol A through attenuating oxidative stress, autophagy, and apoptosis. J. Biochem. Mol. Toxicol. 2022, 36, e23120. [Google Scholar] [CrossRef] [PubMed]
- Martín-Ramírez, R.; González-Fernández, R.; Rotoli, D.; Hernández, J.; Martín-Vasallo, P.; Palumbo, A.; Ávila, J. Celastrol Prevents Oxidative Stress Effects on FSHR, PAPP, and CYP19A1 Gene Expression in Cultured Human Granulosa-Lutein Cells. Int. J. Mol. Sci. 2021, 22, 3596. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Luo, S.; Fan, P.; Jin, S.; Zhu, H.; Deng, T.; Quan, Y.; Huang, W. Growth hormone alleviates oxidative stress and improves oocyte quality in Chinese women with polycystic ovary syndrome: A randomized controlled trial. Sci. Rep. 2020, 10, 18769. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.T.; Yin, H.; Hu, C.; Zeng, J.; Shi, X.; Chen, S.; Zhang, K.; Zheng, W.; Wu, W.; Liu, S. Tilapia skin peptides restore cyclophosphamide-induced premature ovarian failure via inhibiting oxidative stress and apoptosis in mice. Food Funct. 2022, 13, 1668–1679. [Google Scholar] [CrossRef]
- Marvaldi, C.; Martin, D.; Conte, J.G.; Gottardo, M.F.; Pidre, M.L.; Imsen, M.; Irizarri, M.; Manuel, S.L.; Duncan, F.E.; Romanowski, V.; et al. Mitochondrial humanin peptide acts as a cytoprotective factor in granulosa cell survival. Reproduction 2021, 161, 581–591. [Google Scholar] [CrossRef]
- Li, W.; Yin, X.; Yan, Y.; Liu, C.; Li, G. Kurarinone attenuates hydrogen peroxide-induced oxidative stress and apoptosis through activating the PI3K/Akt signaling by upregulating IGF1 expression in human ovarian granulosa cells. Environ. Toxicol. 2022, 38, 28–38. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Deng, L. Chrysin reduces inflammation and oxidative stress and improves ovarian function in D-gal-induced premature ovarian failure. Bioengineered 2022, 13, 8291–8301. [Google Scholar] [CrossRef]
- Kolesarova, A.; Michalcova, K.; Roychoudhury, S.; Baldovska, S.; Tvrda, E.; Vasicek, J.; Chrenek, P.; Sanislo, L.; Kren, V. Antioxidative effect of dietary flavonoid isoquercitrin on human ovarian granulosa cells HGL5 in vitro. Physiol. Res. 2021, 70, 745–754. [Google Scholar] [CrossRef]
- Zhou, P.; Deng, F.; Yang, Z.; Cao, C.; Zhao, H.; Liu, F.; Zhong, K.; Fu, L.; Peng, T.; Sun, D.; et al. Ginsenoside Rb1 inhibits oxidative stress-induced ovarian granulosa cell injury through Akt-FoxO1 interaction. Sci. China. Life. Sci. 2022, 65, 2301–2315. [Google Scholar] [CrossRef]

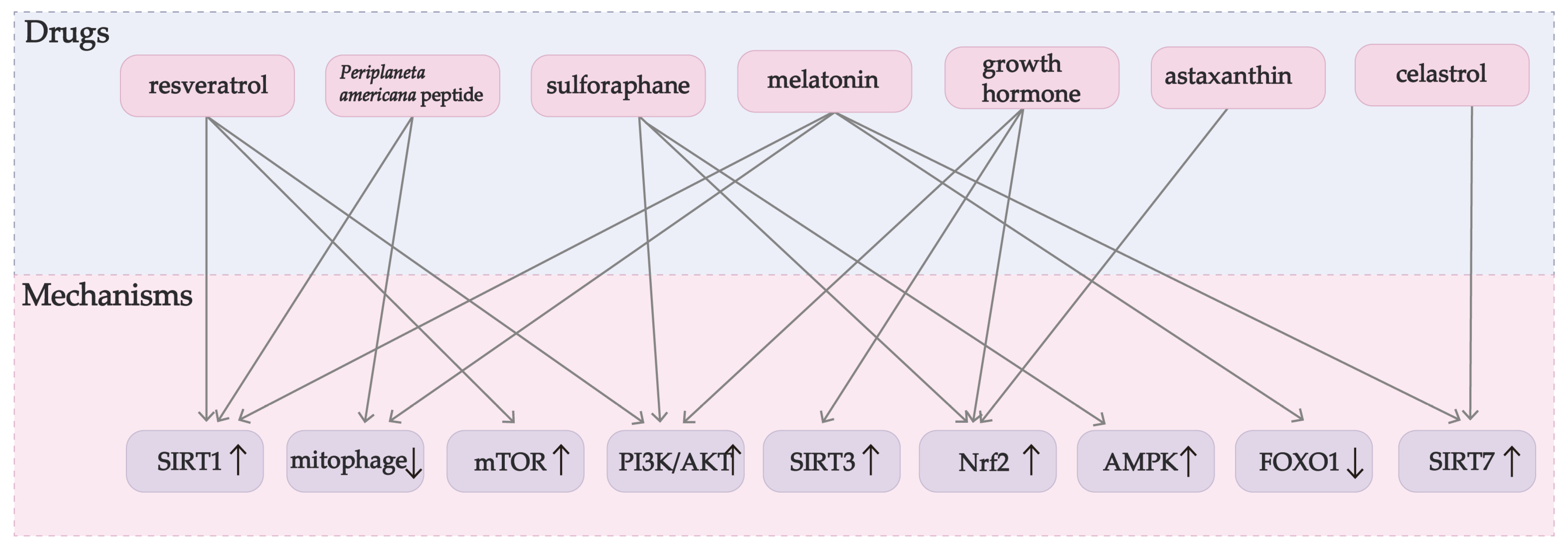
| Ingredient | Model | Mechanism | Effect |
|---|---|---|---|
| Sulforaphane [80,96,97,106] | Porcine GCs | Increases antioxidant capacity and mitophagy through NFE2L2. | Inhibits granule cell apoptosis. |
| Human GCs | Upregulates the expression of antioxidants through the Nrf2/ARE pathway. | Reduces ROS production and apoptosis. | |
| Human granulosa-lutein cells | AMPK/AKT/Nrf2 pathway. | Reduces ROS and apoptosis levels. | |
| Periplaneta americana peptide [73,107,132] | KGN cells | Increases the expression of Bcl2L13 and its interaction with LC3B. | Promotes mitophagy and reduces apoptosis. |
| Pig ovary GCs | FOXO1 pathway. | Reduces apoptosis. | |
| Resveratrol [112,138,140,171] | PCOS cellular model | Inhibits the expression of TLR2 in GCs. | Alleviates inflammation and OS. |
| Female mice | Alleviates BPA-induced GC OS, autophagy, and apoptosis levels. | ||
| Human luteinized GCs | Increases antioxidant enzyme activity through the Sirt1. | Reduces apoptosis rate and ROS level. | |
| Human GCs | Reduces ROS/RNS formation after OS. | Protects GCs. | |
| Astaxanthin [148,149,150] | PCOS patients | Activates the Nrf2 axis. | Upregulates antioxidant levels and total antioxidant capacity. |
| Mouse follicles | Improves the antioxidant capacity and reduces OS in follicles and oocytes. | ||
| Human GCs | Activates Nrf2/ARE pathway. | Inhibits ROS production and cell death. | |
| Melatonin [45,108,114,156,160] | Mouse GCs | Inhibits mitophagy through the MEL-PINK1-Parkin pathway. | Reduces GC death. |
| Female mice | Reduces ovarian GC apoptosis and maintains AMH expression. | ||
| Murine ovary GCs | Antioxidant and anti-inflammatory effects. | Reduces oxidative damage. | |
| Human GCs | Reduces ER stress and inhibits the FOXO1 pathway. | Alleviates GC apoptosis. | |
| Mice and mouse ovarian granule cells | Inhibits excessive OS and apoptosis. | ||
| Human granulosa-lutein cells | SIRT1, SIRT6, and SIRT7 gene expression. | Improves cell viability. | |
| Celastrol [160,172] | Human granulosa-lutein cells | Induces SIRT7 gene expression. | Improves cell viability. |
| Human granulosa-lutein cells | Regulates human granulosa-lutein cells gene expression and regulates OS imbalance. | ||
| Growth hormone [50,101,168,169,170,173] | Rats with POI | Promotes the balance between OS and cellular oxidant detoxification. | Alleviates OS and apoptosis of GCs. |
| KGN cells | SIRT3-SOD2 pathway. | Reduces OS and enhances mitochondrial function, and inhibits apoptosis of GCs. | |
| Older women undergoing IVF | Nrf2/KEAP1 pathway. | Alleviates OS in follicle fluid. | |
| Poor ovarian responders | Reduces OS by improving antioxidant capacity and reducing ROS. | ||
| PCOS patients | Improves mitochondrial dysfunction and relieves OS. | ||
| PCOS patients | Activates the PI3K/AKT pathway. | Reduces ROS levels and apoptosis. | |
| Vitamin D [117] | Mouse ovaries | miR-196b-5p. | Regulates OS and hormone synthesis of GC. |
| Vitamin E [40] | Bovine GCs | Upregulates Nrf2-mediated defense system by activating the PI3K/AKT and ERK1/2 pathways. | Promotes GC proliferation and inhibits apoptosis. |
| Tilapia skin peptides [174] | Mouse with POF | Modulates the Bcl-2/BAX/CASP-3 apoptosis pathway and enhances the Nrf2/HO-1 pathway. | Attenuates OS and apoptosis. |
| Grape seed proanthocyanidins [69] | Chicken follicular GCs | Inhibits FOXO1 and activates the PI3K-AKT pathway. | Reduces GCs’ autophagy and oxidative damage. |
| Morroniside [44,64] | Rat ovarian GCs | PI3K/AKT/mTOR pathway. | Reduces apoptosis and autophagy of rat GCs. |
| Human GCs | Regulates the Nrf2 and MAPK pathways. | Inhibits GC apoptosis. | |
| Humanin [100,175] | KGN cells | Increases cell viability and reduces apoptosis. | |
| PCOS patients | Regulates the KEAP1/Nrf2 signaling pathway. | Alleviates OS. | |
| Metformin [52] | Rats with PCOS | PI3K/AKT/mTOR pathway. | Reduce autophagy and OS. |
| Kurarinone [176] | KGN cells | Activates the PI3K/AKT pathway. | Alleviate OS and apoptosis. |
| Isorhamnetin [55] | Porcine GCs | Activates the PI3K/AKT pathway. | Promotes cell proliferation, inhibits apoptosis, and regulates hormone synthesis. |
| Chrysin [177] | Mice ovarian GCs | Reduces inflammation and OS. | |
| Baicalin [54] | Human GCs/mice ovaries/older mice | mTOR pathway. | Enhances the viability and viability of granule cells. |
| Dietary flavonoid isoquercitrin [178] | Human ovarian GCs HGL5 | Inhibits ROS production. | Reduces OS. |
| Ginsenoside Rb1 [179] | Ovarian GCs from women and mice | Activates AKT phosphorylation and enhances AKT-FOXO1 interaction. | Inhibits OS. |
| Genistein [94] | Mice with PCOS | Increases the expression of Nrf2 and FOXO1 through the ER-Nrf2-FOXO1-ROS pathway. | Alleviates OS. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Jia, Y.; Meng, S.; Luo, Y.; Yang, Q.; Pan, Z. Mechanisms of and Potential Medications for Oxidative Stress in Ovarian Granulosa Cells: A Review. Int. J. Mol. Sci. 2023, 24, 9205. https://doi.org/10.3390/ijms24119205
Liu S, Jia Y, Meng S, Luo Y, Yang Q, Pan Z. Mechanisms of and Potential Medications for Oxidative Stress in Ovarian Granulosa Cells: A Review. International Journal of Molecular Sciences. 2023; 24(11):9205. https://doi.org/10.3390/ijms24119205
Chicago/Turabian StyleLiu, Siheng, Yunbing Jia, Shirui Meng, Yiran Luo, Qi Yang, and Zezheng Pan. 2023. "Mechanisms of and Potential Medications for Oxidative Stress in Ovarian Granulosa Cells: A Review" International Journal of Molecular Sciences 24, no. 11: 9205. https://doi.org/10.3390/ijms24119205
APA StyleLiu, S., Jia, Y., Meng, S., Luo, Y., Yang, Q., & Pan, Z. (2023). Mechanisms of and Potential Medications for Oxidative Stress in Ovarian Granulosa Cells: A Review. International Journal of Molecular Sciences, 24(11), 9205. https://doi.org/10.3390/ijms24119205






