Anion Exchange Membrane Based on BPPO/PECH with Net Structure for Acid Recovery via Diffusion Dialysis
Abstract
1. Introduction
2. Results and Discussion
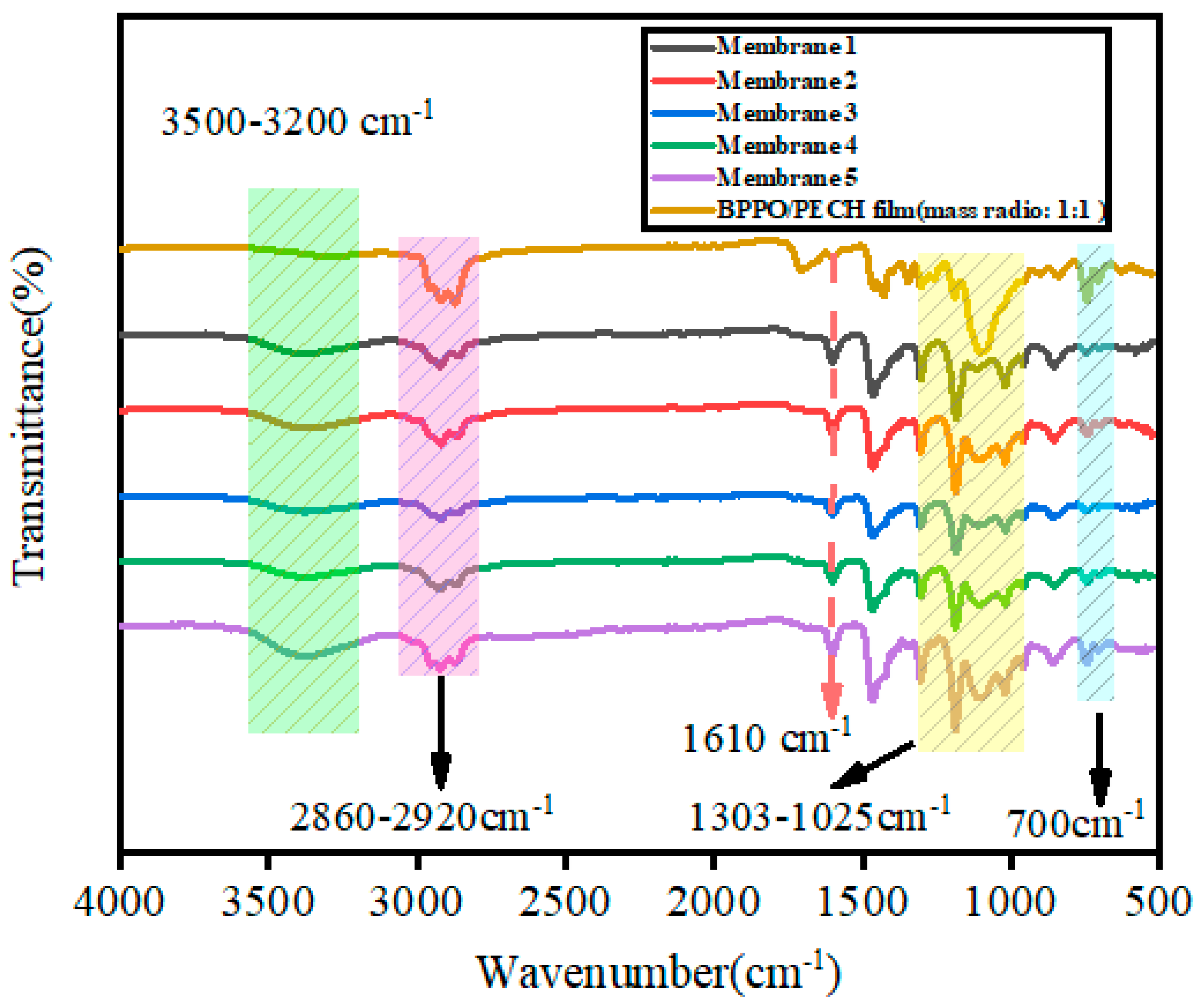
2.1. FTIR Spectra of the Synthesized Membranes
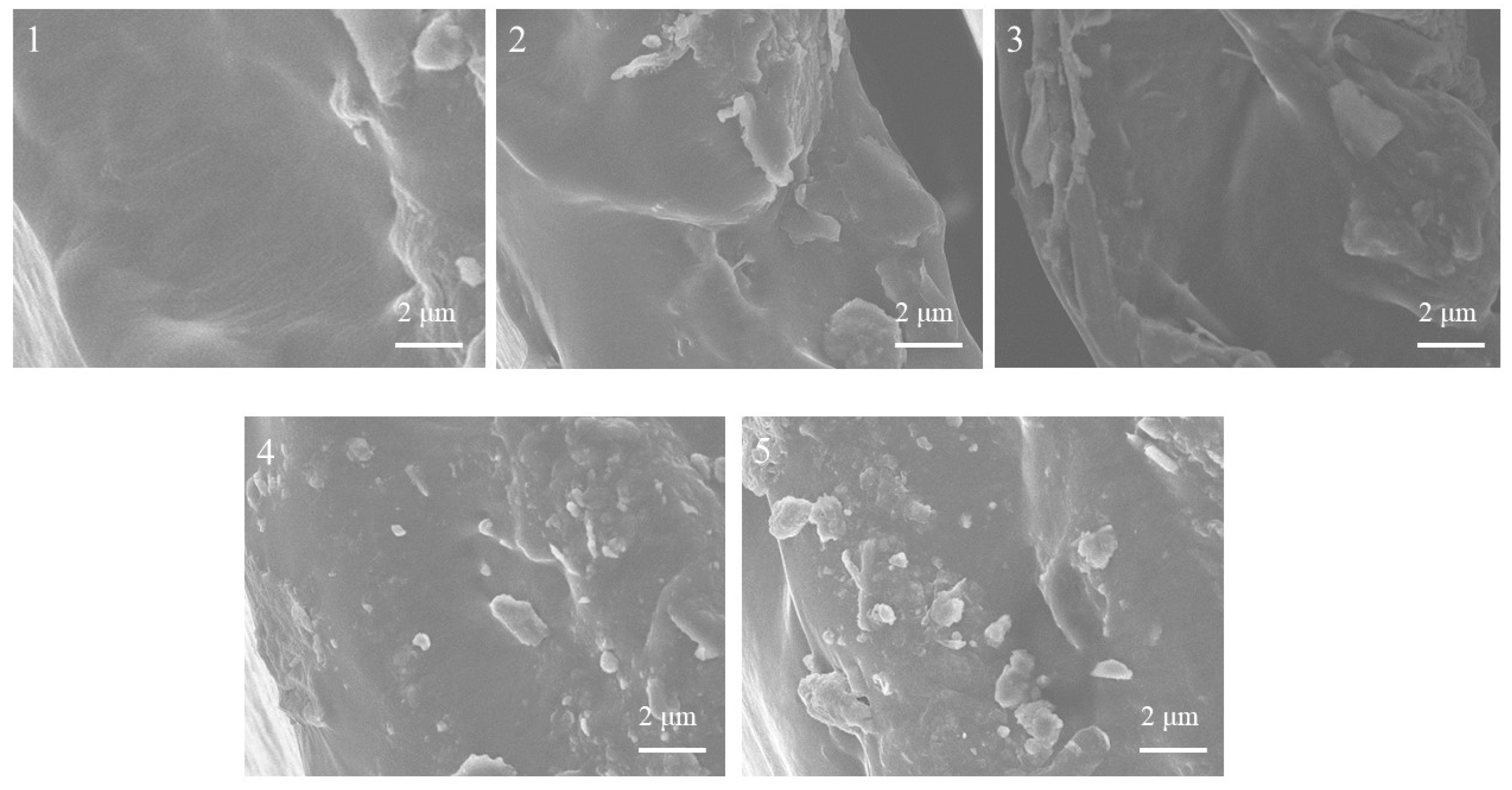
2.2. SEM Analysis of Membrane Morphology
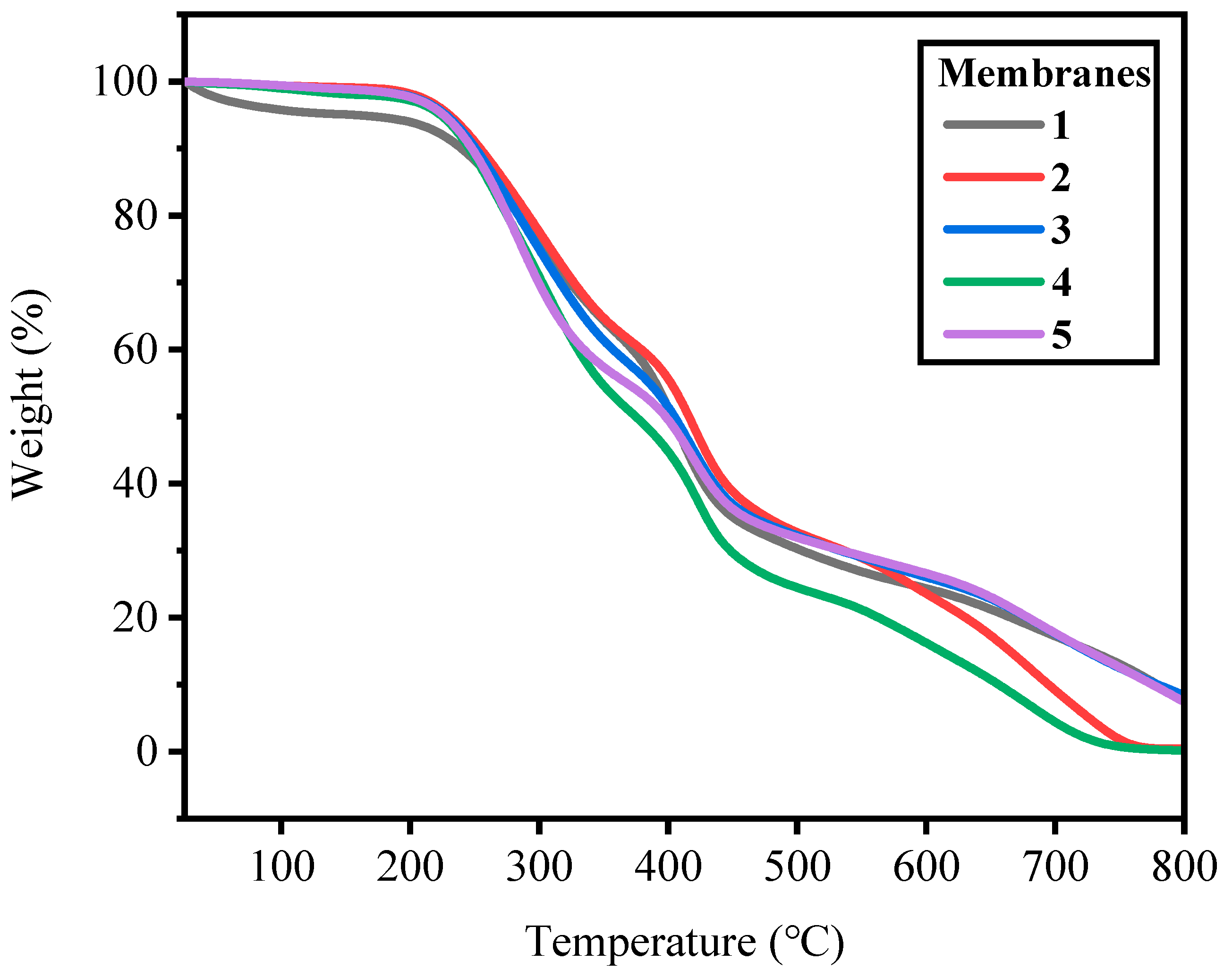
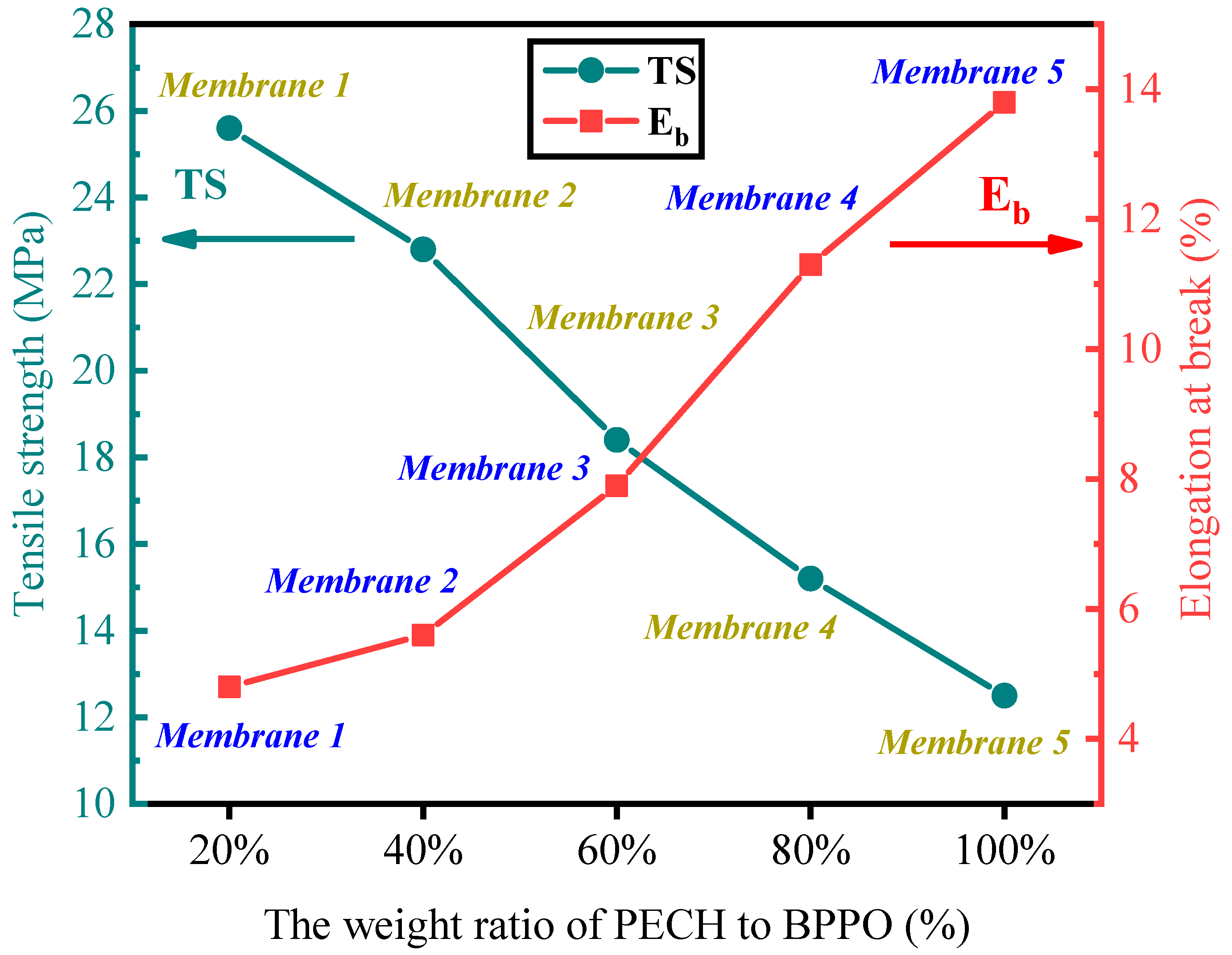
2.3. Thermal and Mechanical Properties of Membranes
2.4. Acid Resistance
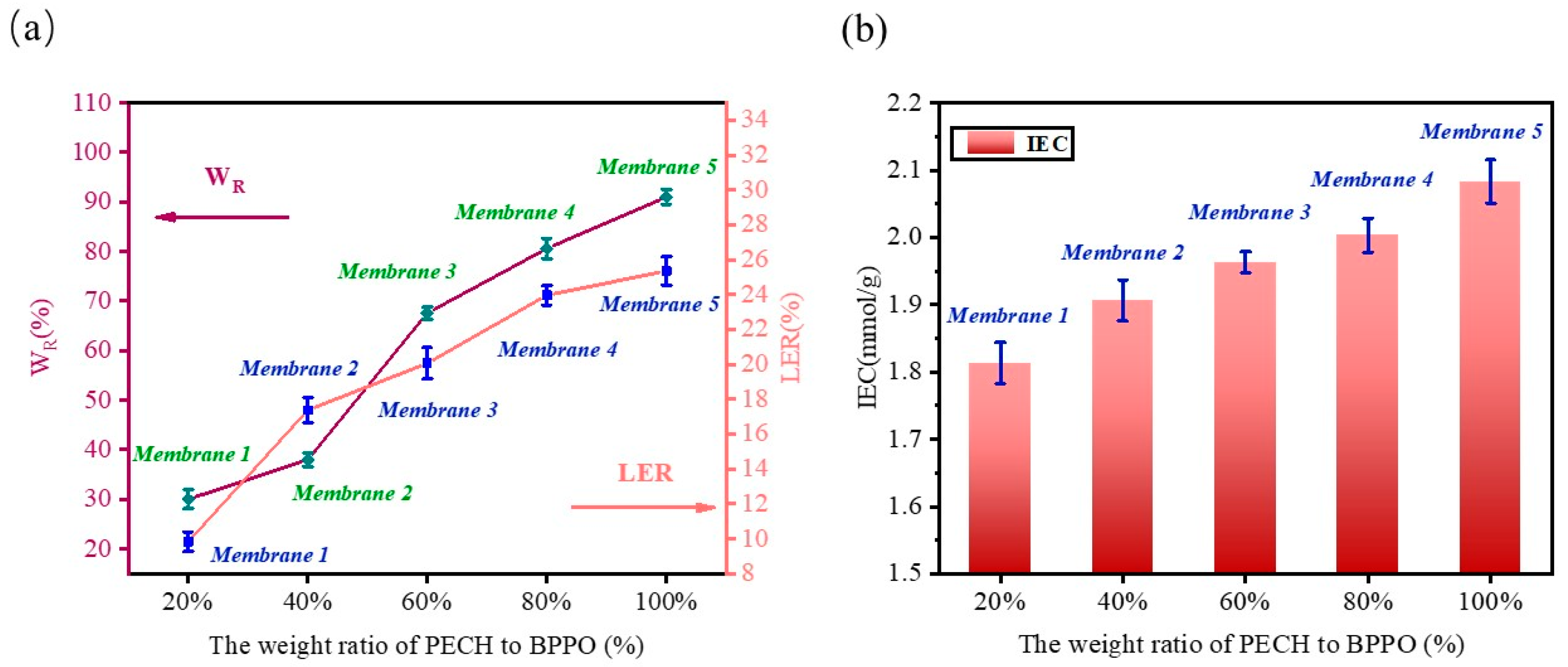
2.5. Water Uptake (WR) Linear Expansion Rate (LER) and Ion Exchange Capacity (IEC)
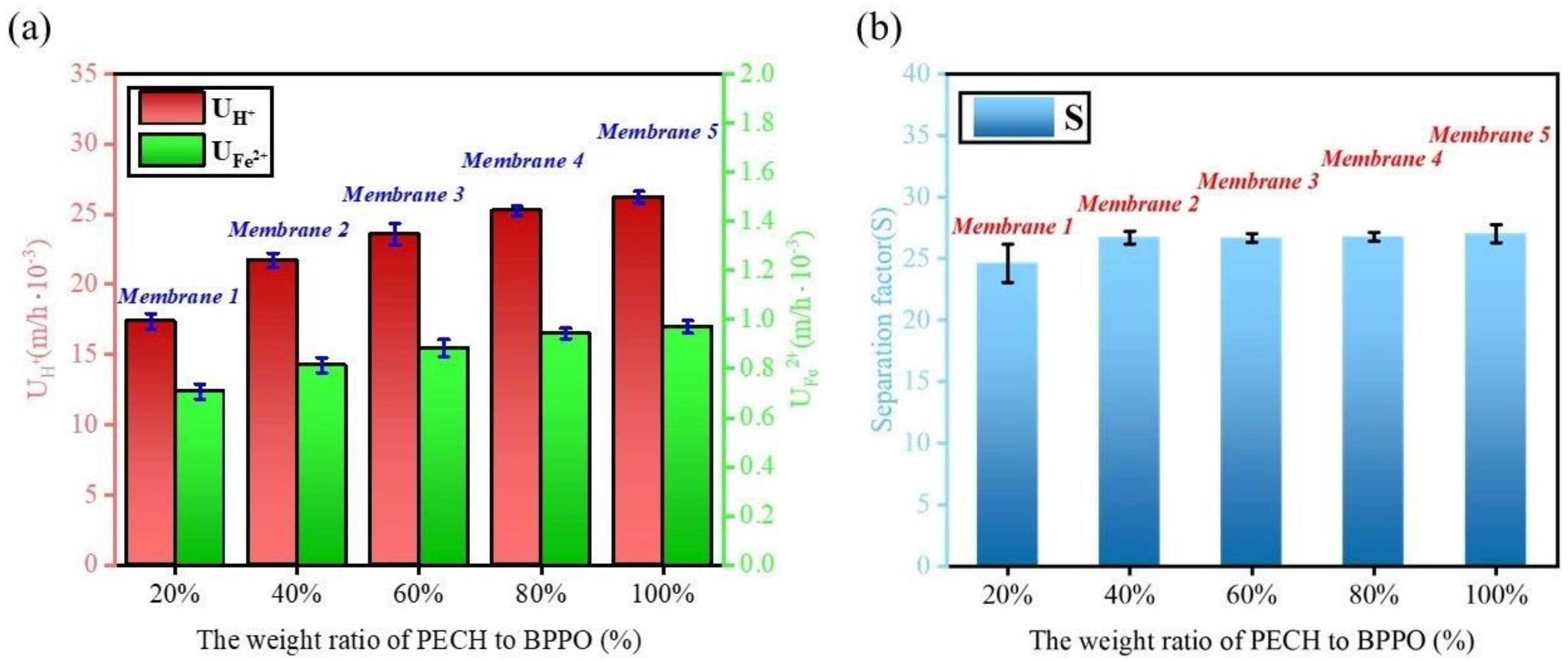
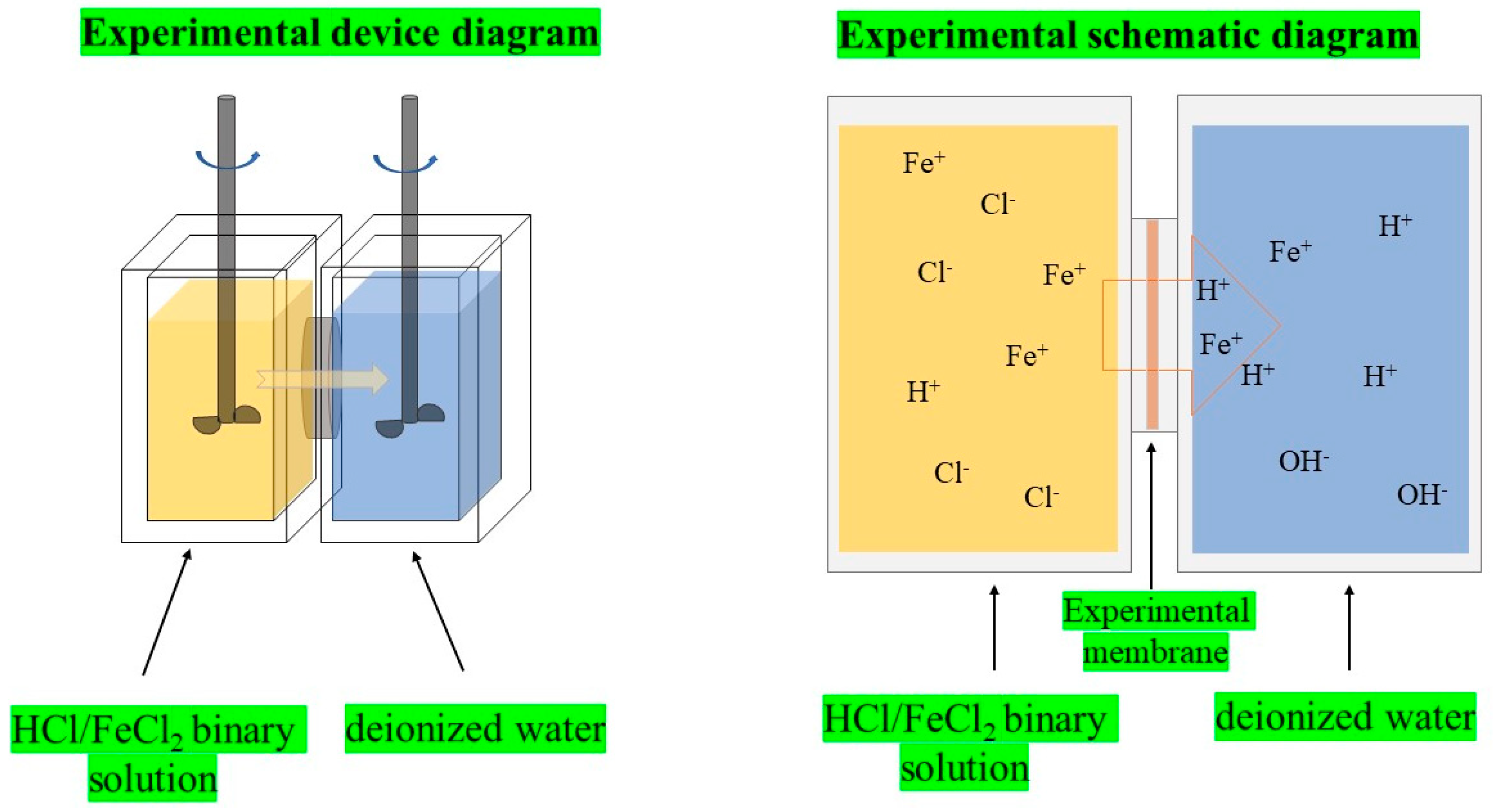
2.6. Diffusion Dialysis Performance
3. Materials and Methods
3.1. Materials
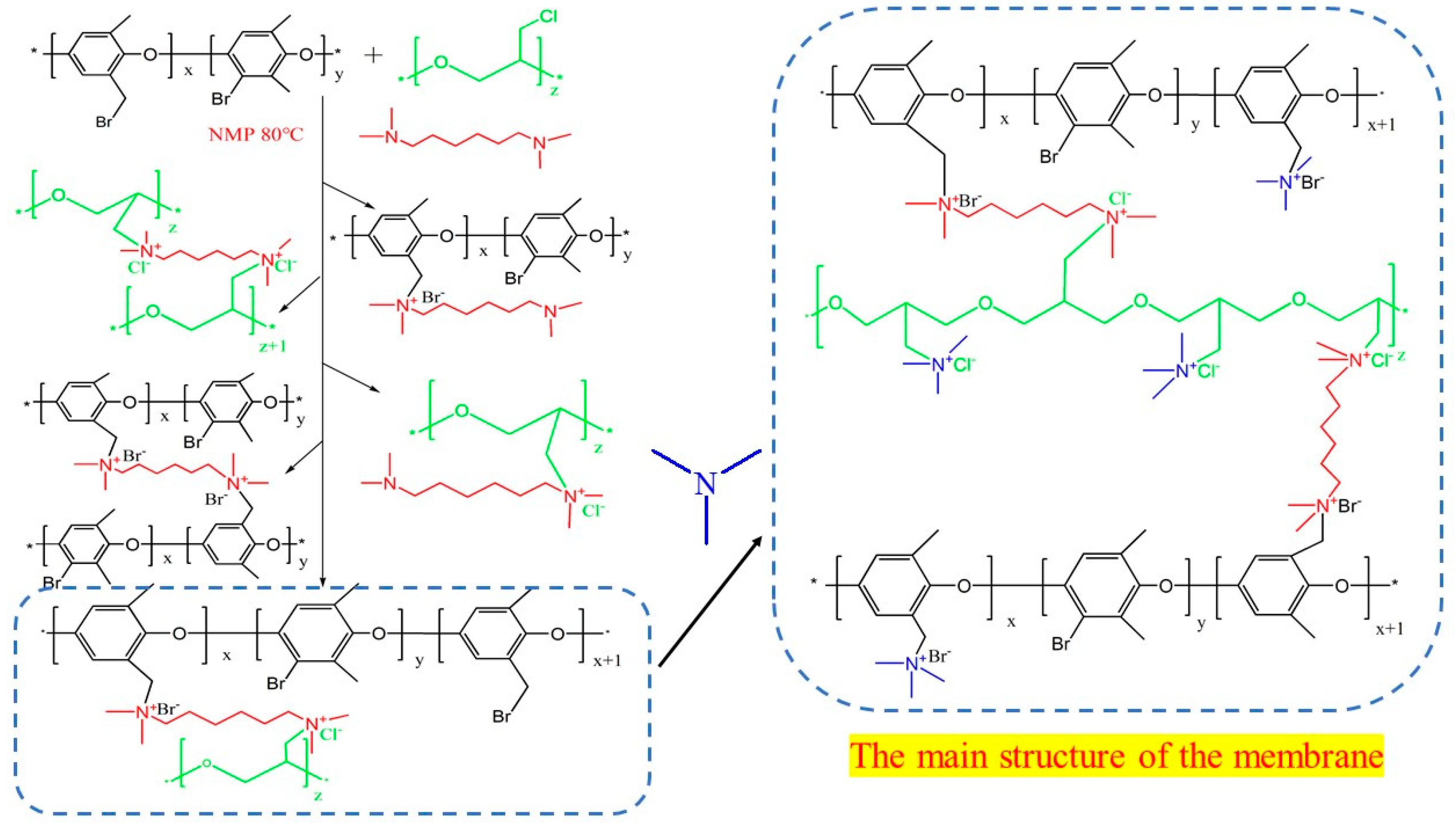
3.2. Reaction Equation Diagram for the Preparation of Ion Exchange Membranes
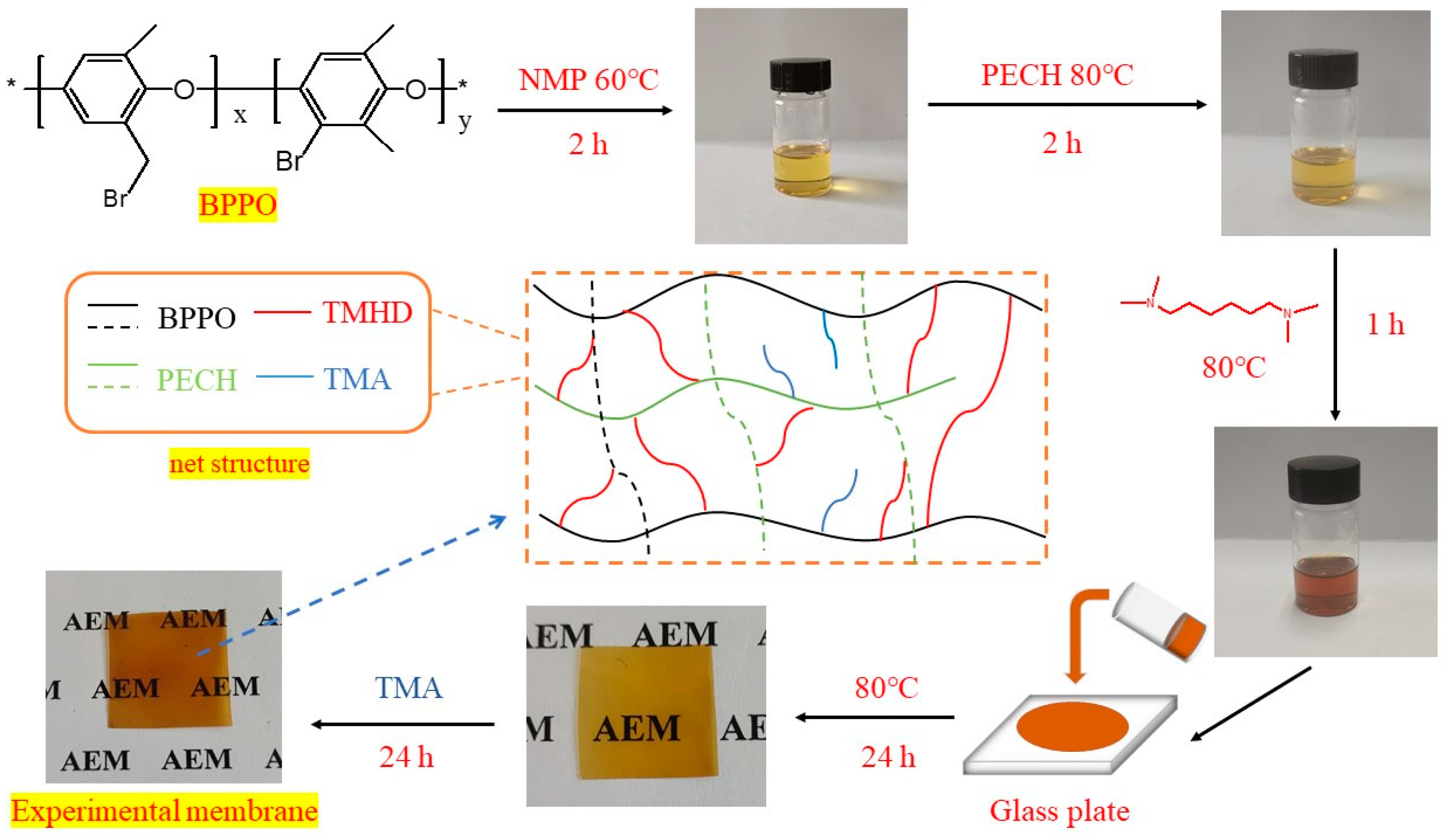
3.3. Preparation Process of Anion Exchange Membranes
3.4. Membrane Test
3.4.1. Membrane Morphology
3.4.2. FTIR Analysis
3.4.3. Thermal and Mechanical Properties of Membranes
3.4.4. Acid Resistance
3.4.5. Ion Exchange Capacity (IEC)
3.4.6. Water Uptake (WR) and Linear Expansion Rate (LER)
3.4.7. Diffusion Dialysis Performance (DD)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ramzan, M.; Raza, S.A.; Usman, M.; Sharma, G.D.; Iqbal, H.A. Environmental cost of non-renewable energy and economic progress: Do ICT and financial development mitigate some burden? J. Clean. Prod. 2022, 333, 130066. [Google Scholar] [CrossRef]
- Hussain, A.; Yan, H.; Ul Afsar, N.; Wang, H.; Yan, J.; Jiang, C.; Wang, Y.; Xu, T. Acid recovery from molybdenum metallurgical wastewater via selective electrodialysis and nanofiltration. Sep. Purif. Technol. 2022, 295, 121318. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Xia, Z.; Zhao, X.; Zhou, Z.; Ye, L. Green and circular method for chloride separation from acid wastewater: Application in zinc smelter. Sep. Purif. Technol. 2022, 283, 120221. [Google Scholar] [CrossRef]
- Ali, A.; Nymann, M.C.; Christensen, M.L.; Quist-Jensen, C.A. Industrial Wastewater Treatment by Nanofiltration-a Case Study on the Anodizing Industry. Membranes 2020, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, P.; He, Y.; Hu, X.; Emmanuel, K. Branched Polyvinyl Alcohol Hybrid Membrane for Acid Recovery via Diffusion Dialysis. Chem. Eng. Technol. 2019, 42, 1180–1187. [Google Scholar] [CrossRef]
- Shehzad, M.A.; Yasmin, A.; Ge, X.; Wu, L.; Xu, T. A Review of Nanostructured Ion-Exchange Membranes. Adv. Mater. Technol. 2021, 6, 2001171. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Wu, L.; Ge, L.; Xu, T. Ion Exchange Membrane “ABC”—A Key Material for Upgrading Process Industries. Chin. J. Chem. 2021, 39, 825–837. [Google Scholar] [CrossRef]
- Song, W.; He, Y.; Shehzad, M.A.; Ge, X.; Ge, L.; Liang, X.; Wei, C.; Ge, Z.; Zhang, K.; Li, G.; et al. Exploring H-bonding interaction to enhance proton permeability of an acid-selective membrane. J. Membr. Sci. 2021, 637, 119650. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, W.; Liang, X.; Zhang, K.; Wang, H.; Ge, X.; Wei, C.; Song, W.; Ge, Z.; Wu, L.; et al. Flexible Bis-piperidinium Side Chains Construct Highly Conductive and Robust Anion-Exchange Membranes. ACS Appl. Energy Mater. 2021, 4, 9701–9711. [Google Scholar] [CrossRef]
- Alam, M.M.; Hossain, M.; Mei, Y.; Jiang, C.; Wang, Y.; Tang, C.Y.; Xu, T. An alkaline stable anion exchange membrane for electro-desalination. Desalination 2021, 497, 114779. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, F.; Wang, M.; Li, W.; Song, Y.; Liu, G.; Yang, H.; Gao, B.; Wu, H.; Jiang, Z. Hybrid membranes for pervaporation separations. J. Membr. Sci. 2017, 541, 329–346. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Zhao, L.; Zhang, G.; Wu, C.; Xu, T. QPPO/PVA anion exchange hybrid membranes from double crosslinking agents for acid recovery. J. Membr. Sci. 2013, 428, 95–103. [Google Scholar] [CrossRef]
- Yan, J.; Wang, H.; Fu, R.; Fu, R.; Li, R.; Chen, B.; Jiang, C.; Ge, L.; Liu, Z.; Wang, Y.; et al. Ion exchange membranes for acid recovery: Diffusion Dialysis (DD) or Selective Electrodialysis (SED)? Desalination 2022, 531, 115690. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Xu, T.; Wu, Y. Diffusion dialysis-concept, principle and applications. J. Membr. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Swaby, S.; Urena, N.; Perez-Prior, M.T.; Varez, A.; Levenfeld, B. Synthesis and Characterization of Novel Anion Exchange Membranes Based on Semi-Interpenetrating Networks of Functionalized Polysulfone: Effect of Ionic Crosslinking. Polymers 2021, 13, 958. [Google Scholar] [CrossRef]
- Ji, W.; Afsar, N.U.; Wu, B.; Sheng, F.; Shehzad, M.A.; Ge, L.; Xu, T. In-situ crosslinked SPPO/PVA composite membranes for alkali recovery via diffusion dialysis. J. Membr. Sci. 2019, 590, 117267. [Google Scholar] [CrossRef]
- Hren, M.; Božič, M.; Fakin, D.; Kleinschek, K.S.; Gorgieva, S. Alkaline membrane fuel cells: Anion exchange membranes and fuels. Sustain. Energy Fuels 2021, 5, 604–637. [Google Scholar] [CrossRef]
- Abdi, Z.G.; Chen, J.C.; Chiu, T.H.; Yang, H.; Yu, H.H. Synthesis of ionic polybenzimidazoles with broad ion exchange capacity range for anion exchange membrane fuel cell application. J. Polym. Sci. 2021, 59, 2069–2081. [Google Scholar] [CrossRef]
- Alam, M.M.; Wang, Y.; Jiang, C.; Xu, T.; Liu, Y.; Xu, T. A Novel Anion Exchange Membrane for Bisulfite Anion Separation by Grafting a Quaternized Moiety through BPPO via Thermal-Induced Phase Separation. Int. J. Mol. Sci. 2020, 21, 5782. [Google Scholar] [CrossRef]
- Karakoc, E.; Guler, E. Comparison of Physicochemical Properties of Two Types of Polyepichlorohydrin-Based Anion Exchange Membranes for Reverse Electrodialysis. Membranes 2022, 12, 257. [Google Scholar] [CrossRef]
- Tiago, G.; Cristovao, M.B.; Marques, A.P.; Huertas, R.; Merino-Garcia, I.; Pereira, V.J.; Crespo, J.G.; Velizarov, S. A Study on Biofouling and Cleaning of Anion Exchange Membranes for Reverse Electrodialysis. Membranes 2022, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Han, M.; Dong, T.; Yao, J.; Han, L. Fouling dynamics of anion polyacrylamide on anion exchange membrane in electrodialysis. Desalination 2021, 507, 115036. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, S.; Peng, G.; Sotto, A.; Ruan, H.; Shen, J.; Gao, C. Novel crosslinked brominated polyphenylene oxide composite nanofiltration membranes with organic solvent permeability and swelling property. J. Membr. Sci. 2021, 620, 118784. [Google Scholar] [CrossRef]
- Goel, P.; Mandal, P.; Bhuvanesh, E.; Shahi, V.K.; Chattopadhyay, S. Temperature resistant cross-linked brominated poly phenylene oxide-functionalized graphene oxide nanocomposite anion exchange membrane for desalination. Sep. Purif. Technol. 2021, 255, 117730. [Google Scholar] [CrossRef]
- Afsar, N.U.; Erigene, B.; Irfan, M.; Wu, B.; Xu, T.; Ji, W.; Emmanuel, K.; Ge, L.; Xu, T. High performance anion exchange membrane with proton transport pathways for diffusion dialysis. Sep. Purif. Technol. 2018, 193, 11–20. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Wu, C.; Han, X.; Xu, C. Anion exchange membranes with excellent monovalent anion perm-selectivity for electrodialysis applications. Chem. Eng. Res. Des. 2020, 158, 24–32. [Google Scholar] [CrossRef]
- Mondal, A.N.; Cheng, C.; Yao, Z.; Pan, J.; Hossain, M.M.; Khan, M.I.; Yang, Z.; Wu, L.; Xu, T. Novel quaternized aromatic amine based hybrid PVA membranes for acid recovery. J. Membr. Sci. 2015, 490, 29–37. [Google Scholar] [CrossRef]
- Gu, Y.; Zhao, J.; Zhou, H.; Jiang, H.; Li, J.; Zhang, B. Engineering robust RGO/PVA composite membrane for acid recovery via electron beam irradiation. Carbon 2022, 191, 243–254. [Google Scholar] [CrossRef]
- Yue, X.; Wu, W.; Chen, G.; Yang, C.; Liao, S.; Li, X. Influence of 2,2′,6,6′-tetramethyl biphenol-based anion-exchange membranes on the diffusion dialysis of hydrochloride acid. J. Appl. Polym. Sci. 2017, 134, 45333. [Google Scholar] [CrossRef]
- Shukla, G.; Ferrier, R.C. The versatile, functional polyether, polyepichlorohydrin: History, synthesis, and applications. J. Polym. Sci. 2021, 59, 2704–2718. [Google Scholar] [CrossRef]
- Ge, Q.; Ning, Y.; Wu, L.; Ge, L.; Liu, X.; Yang, Z.; Xu, T. Enhancing acid recovery efficiency by implementing oligomer ionic bridge in the membrane matrix. J. Membr. Sci. 2016, 518, 263–272. [Google Scholar] [CrossRef]
- Bertolotti, B.; Messaoudi, H.; Chikh, L.; Vancaeyzeele, C.; Alfonsi, S.; Fichet, O. Stability in alkaline aqueous electrolyte of air electrode protected with fluorinated interpenetrating polymer network membrane. J. Power Sources 2015, 274, 488–495. [Google Scholar] [CrossRef]
- Hu, M.; Ding, L.; Shehzad, M.A.; Ge, Q.; Liu, Y.; Yang, Z.; Wu, L.; Xu, T. Comb-shaped anion exchange membrane with densely grafted short chains or loosely grafted long chains? J. Membr. Sci. 2019, 585, 150–156. [Google Scholar] [CrossRef]
- Khan, M.I.; Zheng, C.; Mondal, A.N.; Hossain, M.M.; Wu, B.; Emmanuel, K.; Wu, L.; Xu, T. Preparation of anion exchange membranes from BPPO and dimethylethanolamine for electrodialysis. Desalination 2017, 402, 10–18. [Google Scholar] [CrossRef]
- Khan, M.I.; Mondal, A.N.; Cheng, C.; Pan, J.; Emmanuel, K.; Wu, L.; Xu, T. Porous BPPO-based membranes modified by aromatic amine for acid recovery. Sep. Purif. Technol. 2016, 157, 27–34. [Google Scholar] [CrossRef]
- Chen, W.; Shen, H.; Gong, Y.; Li, P.; Cheng, C. Anion exchange membranes with efficient acid recovery obtained by quaternized poly epichlorohydrin and polyvinyl alcohol during diffusion dialysis. J. Membr. Sci. 2023, 674, 121514. [Google Scholar] [CrossRef]
- Peng, L.; Huang, X.; Liu, D.; Miao, J.; Wu, B.; Cao, M.; Ge, Q.; Yang, B.; Su, L.; Xia, R.; et al. Preparation of Polyvinyl Alcohol (PVA)-Based Composite Membranes Using Carboxyl-Type Boronic Acid Copolymers for Alkaline Diffusion Dialysis. Polymers 2020, 12, 2360. [Google Scholar] [CrossRef]
- Khan, M.I.; Khraisheh, M.; AlMomani, F. Innovative BPPO Anion Exchange Membranes Formulation Using Diffusion Dialysis-Enhanced Acid Regeneration System. Membranes 2021, 11, 311. [Google Scholar] [CrossRef]
- Khan, M.I.; Khraisheh, M.; Almomani, F. Fabrication and characterization of pyridinium functionalized anion exchange membranes for acid recovery. Sci. Total Environ. 2019, 686, 90–96. [Google Scholar] [CrossRef]
- Lan, Y.; Zhou, D.; Lai, L.; Qi, H.; Xia, L.; Depuydt, S.; Van der Bruggen, B.; Zhao, Y. A monovalent selective anion exchange membrane made by poly(2,6-dimethyl-1,4-phenyl oxide) for bromide recovery. Sep. Purif. Technol. 2023, 305, 122377. [Google Scholar] [CrossRef]
- Liao, J.; Xu, J.; Ruan, H.; Mu, J.; Jie, X.; Li, W.; Xu, Y.; Shen, J. Exploring the organic solvent resistance of anion exchange membranes based on poly(2,6-dimethyl-1,4-phenyl oxide) for electrodialysis desalination. Desalination 2023, 546, 116202. [Google Scholar] [CrossRef]
- Khan, M.I.; Shanableh, A.; Osman, S.M.; Lashari, M.H.; Manzoor, S.; Rehman, A.U.; Luque, R. Fabrication of trimethylphosphine-functionalized anion exchange membranes for desalination application via electrodialysis process. Chemosphere 2022, 308, 136330. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-B.; Kim, D.-H.; Kang, M.-S. Thin Reinforced Poly(2,6-dimethyl-1,4-phenylene oxide)-based Anion-exchange Membranes with High Mechanical and Chemical Stabilities. Chem. Lett. 2019, 48, 1500–1503. [Google Scholar] [CrossRef]
- Mondal, A.N.; Hou, J.; He, Y.; Wu, L.; Ge, L.; Xu, T. Preparation of click-driven cross-linked anion exchange membranes with low water uptake. Particuology 2020, 48, 65–73. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Yang, S.; Pan, J.; Yan, R.; Bruggen, B.V.D.; Sotto, A.; Gao, C.; Shen, J. Internal cross-linked anion exchange membranes with improved dimensional stability for electrodialysis. J. Membr. Sci. 2017, 542, 280–288. [Google Scholar] [CrossRef]
- Cheng, C.L.; Yang, Z.J.; Pan, J.F.; Tong, B.; Xu, T.W. Facile and cost effective PVA based hybrid membrane fabrication for acid recovery. Sep. Purif. Technol. 2014, 136, 250–257. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Islam, A.; Butt, M.A.; Mannan, H.A.; Khan, R.U.; Kamran, K.; Bashir, S.; Iqbal, J.; Al-Ghamdi, A.A.; Al-Sehemi, A.G. Quaternized Diaminobutane/Poly(vinyl alcohol) Cross-Linked Membranes for Acid Recovery via Diffusion Dialysis. Membranes 2021, 11, 786. [Google Scholar] [CrossRef]
- Hao, J.W.; Wu, Y.H.; Ran, J.; Wu, B.; Xu, T.W. A simple and green preparation of PVA-based cation exchange hybrid membranes for alkali recovery. J. Membr. Sci. 2013, 433, 10–16. [Google Scholar] [CrossRef]
- Eti, M.; Cihanoglu, A.; Guler, E.; Gomez-Coma, L.; Altiok, E.; Arda, M.; Ortiz, I.; Kabay, N. Further Development of Polyepichlorohydrin Based Anion Exchange Membranes for Reverse Electrodialysis by Tuning Cast Solution Properties. Membranes 2022, 12, 1192. [Google Scholar] [CrossRef]
- Qian, J.; Cai, S.; Hu, J.; Wang, C.; Li, G. Preparation and Properties of Quaternary Ammonium Anion Exchange Membranes with Flexible Side Chains for the Vanadium Redox Flow Battery. Ind. Eng. Chem. Res. 2023, 62, 2719–2728. [Google Scholar] [CrossRef]
- Bendova, H.; Dusek, L. Treatment of Spent Pickling Solutions by Diffusion Dialysis Using Anion-Exchange Membrane Neosepta-AFN. Membranes 2022, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Patnaik, P.; Mondal, R.; Sarkar, S.; Chatterjee, U. GO-anchored imidazolium based cross-linked composite anion exchange membranes for the enhancement in acid recovery via diffusion dialysis. Chem. Eng. J. 2023, 451, 138901. [Google Scholar] [CrossRef]
- Khan, M.I.; Shanableh, A.; Khraisheh, M.; AlMomani, F. Synthesis of Porous BPPO-Based Anion Exchange Membranes for Acid Recovery via Diffusion Dialysis. Membranes 2022, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Shen, H.Y.; Gong, Y.F.; Chen, W.; Li, P. Auxiliary functional group diffusion dialysis membranes for acid recovery. J. Polym. Sci. 2022, 60, 3043–3053. [Google Scholar] [CrossRef]
- Ge, L.; Mondal, A.N.; Liu, X.; Wu, B.; Yu, D.; Li, Q.; Miao, J.; Ge, Q.; Xu, T. Advanced charged porous membranes with ultrahigh selectivity and permeability for acid recovery. J. Membr. Sci. 2017, 536, 11–18. [Google Scholar] [CrossRef]
- Lin, X.; Shamsaei, E.; Kong, B.; Liu, J.Z.; Hu, Y.; Xu, T.; Wang, H. Porous diffusion dialysis membranes for rapid acid recovery. J. Membr. Sci. 2016, 502, 76–83. [Google Scholar] [CrossRef]
- Cheng, C.L.; Chen, W.; Tong, B.; Hu, X.H.; Li, P. Simple and cost-effective to fabricate P(VC/VAC) based anion exchange membranes for acid recovery via diffusion dialysis. Desalin. Water Treat. 2021, 213, 139–147. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, W.; Shen, H.Y.; Cheng, C. Semi-interpenetrating Polymer-Network Anion Exchange Membrane Based on Quaternized Polyepichlorohydrin and Polyvinyl Alcohol for Acid Recovery by Diffusion Dialysis. Ind. Eng. Chem. Res. 2023, 62, 5624–5634. [Google Scholar] [CrossRef]
| Weight Retention Rate/% | Membrane 1 | Membrane 2 | Membrane 3 | Membrane 4 | Membrane 5 |
|---|---|---|---|---|---|
| 7 d | 98.6 | 98.5 | 98.4 | 98.5 | 98.2 |
| 14 d | 98.8 | 98.6 | 98.4 | 98.5 | 98.1 |
| Membranes | WR/% | LER/% | IEC/mmol·g−1 | Refs. |
|---|---|---|---|---|
| Membranes 1–5 | 30.05–91.04 | 9.8–25.4 | 1.81–2.08 | This work |
| PVA/EPTAC | 196–267 | 35–44 | 0.58–1.15 | [46] |
| QDAB/PVA | 47.8–71.3 | 68.2–204.6 | 0.86–1.46 | [47] |
| PVA/THOPS | 38–86 | 15–25 | 0.70–1.56 | [48] |
| Membrane | UH+ (10−3 m/h) | S | Refs. |
|---|---|---|---|
| Membranes 1–5 | 17.3–26.2 | 24.6–27.0 | This work |
| BPPO/DMAP | 0.37–20.3 | 73–351 | [25] |
| BPPO/TPA | 5.6–10.4 | 21.9–38.8 | [35] |
| BPPO/TMA | 4.3–12 | 13.14–32.87 | [53] |
| BPPO/PEI | 63–70 | 11–20 | [56] |
| DF-120B | 4.00 | 24.30 | [27] |
| Number | Membrane 1 | Membrane 2 | Membrane 3 | Membrane 4 | Membrane 5 | BPPO/PECH Film |
|---|---|---|---|---|---|---|
| BPPO | 0.25 g | 0.25 g | 0.25 g | 0.25 g | 0.25 g | 0.25 g |
| PECH | 0.05 g | 0.10 g | 0.15 g | 0.20 g | 0.25 g | 0.25 g |
| TMHD | 0.12 g | 0.14 g | 0.16 g | 0.18 g | 0.20 g | |
| TMA | 0.02 g | 0.02 g | 0.02 g | 0.02 g | 0.02 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Gong, Y.; Chen, W.; Wei, X.; Li, P.; Cheng, C. Anion Exchange Membrane Based on BPPO/PECH with Net Structure for Acid Recovery via Diffusion Dialysis. Int. J. Mol. Sci. 2023, 24, 8596. https://doi.org/10.3390/ijms24108596
Shen H, Gong Y, Chen W, Wei X, Li P, Cheng C. Anion Exchange Membrane Based on BPPO/PECH with Net Structure for Acid Recovery via Diffusion Dialysis. International Journal of Molecular Sciences. 2023; 24(10):8596. https://doi.org/10.3390/ijms24108596
Chicago/Turabian StyleShen, Haiyang, Yifei Gong, Wei Chen, Xianbiao Wei, Ping Li, and Congliang Cheng. 2023. "Anion Exchange Membrane Based on BPPO/PECH with Net Structure for Acid Recovery via Diffusion Dialysis" International Journal of Molecular Sciences 24, no. 10: 8596. https://doi.org/10.3390/ijms24108596
APA StyleShen, H., Gong, Y., Chen, W., Wei, X., Li, P., & Cheng, C. (2023). Anion Exchange Membrane Based on BPPO/PECH with Net Structure for Acid Recovery via Diffusion Dialysis. International Journal of Molecular Sciences, 24(10), 8596. https://doi.org/10.3390/ijms24108596






