Extracellular Vesicles Released from Macrophages Infected with Mycoplasma pneumoniae Stimulate Proinflammatory Response via the TLR2-NF-κB/JNK Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. Establishment and Validation of M. pneumoniae-Infected MΦs Cells
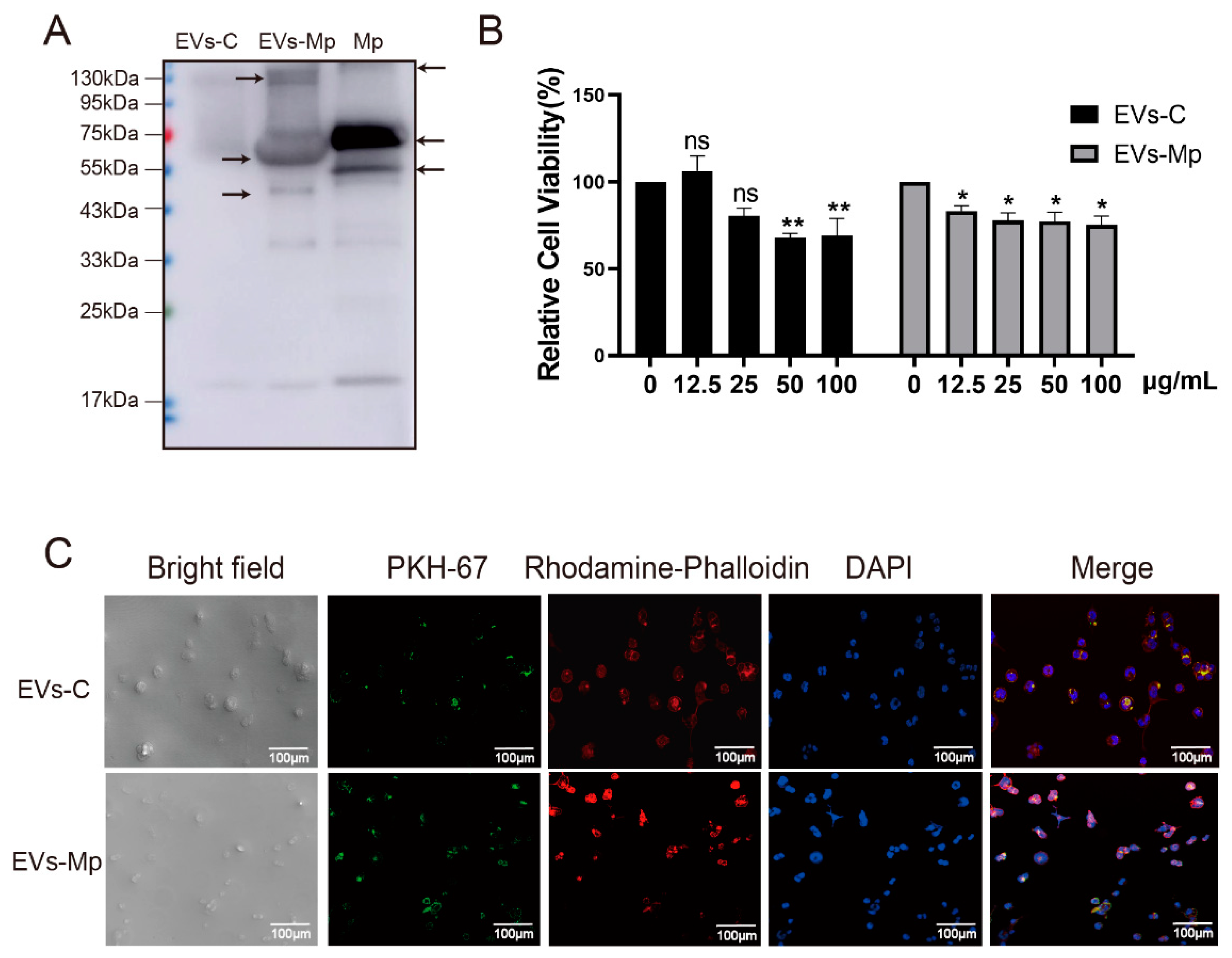
2.2. Isolation and Identification of EVs Derived from M. pneumoniae-Infected THP-1 Macrophages
2.3. Macrophage-Derived EVs Deliver PAMPs of M. pneumoniae to Uninfected Macrophage
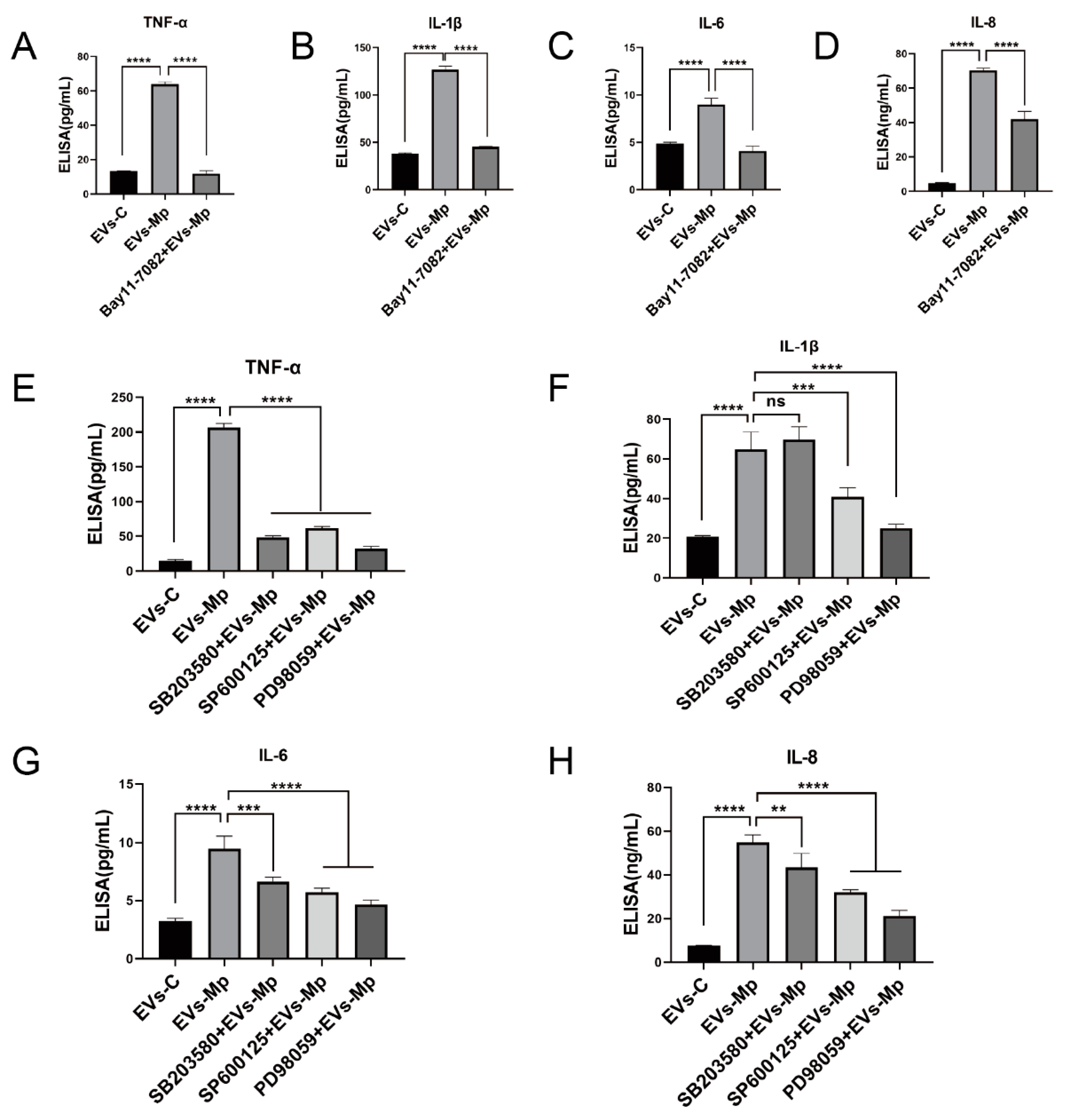
2.4. EVs Released by M. pneumoniae-Infected Macrophages Induce the Production of Inflammatory Cytokines
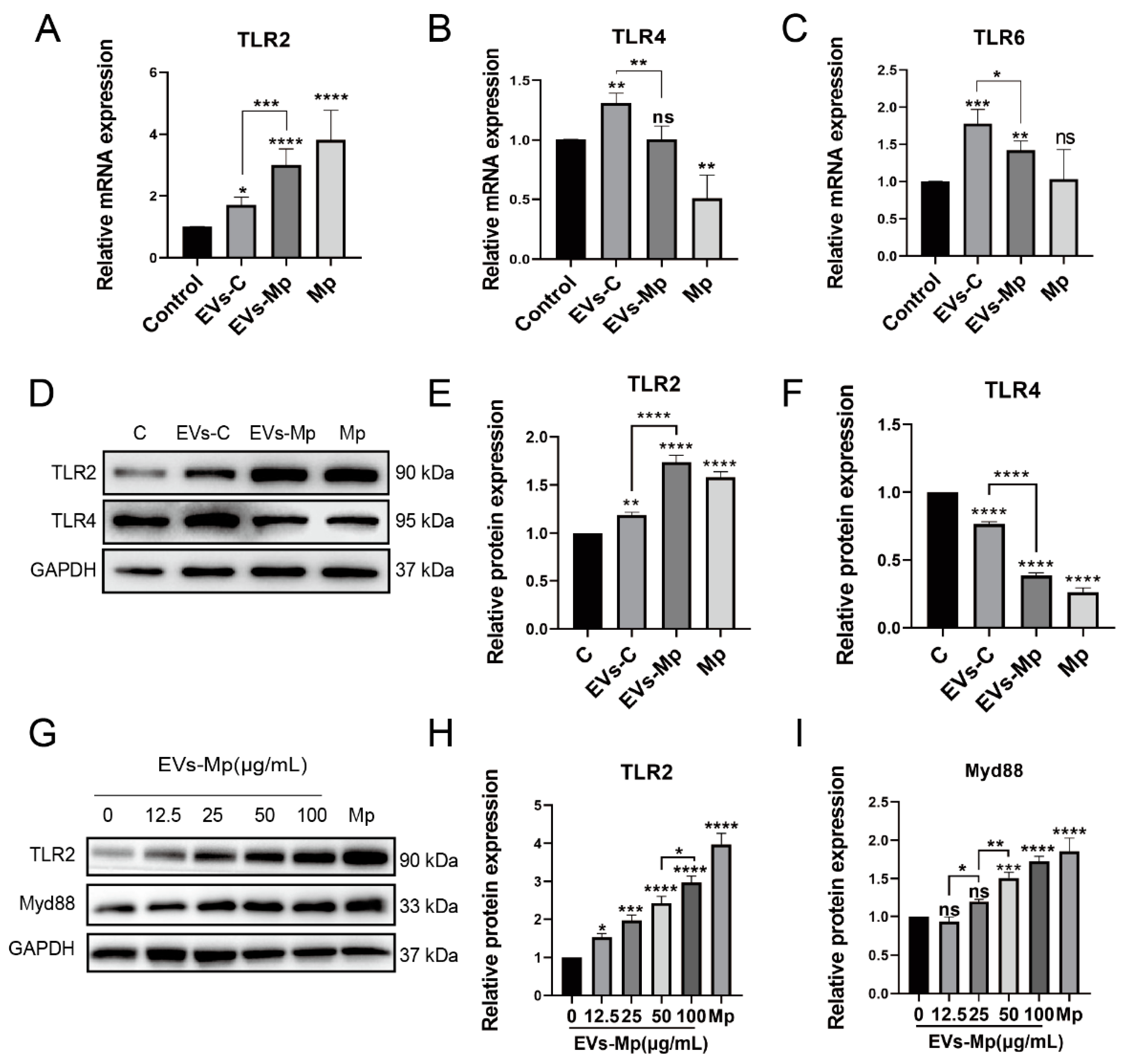
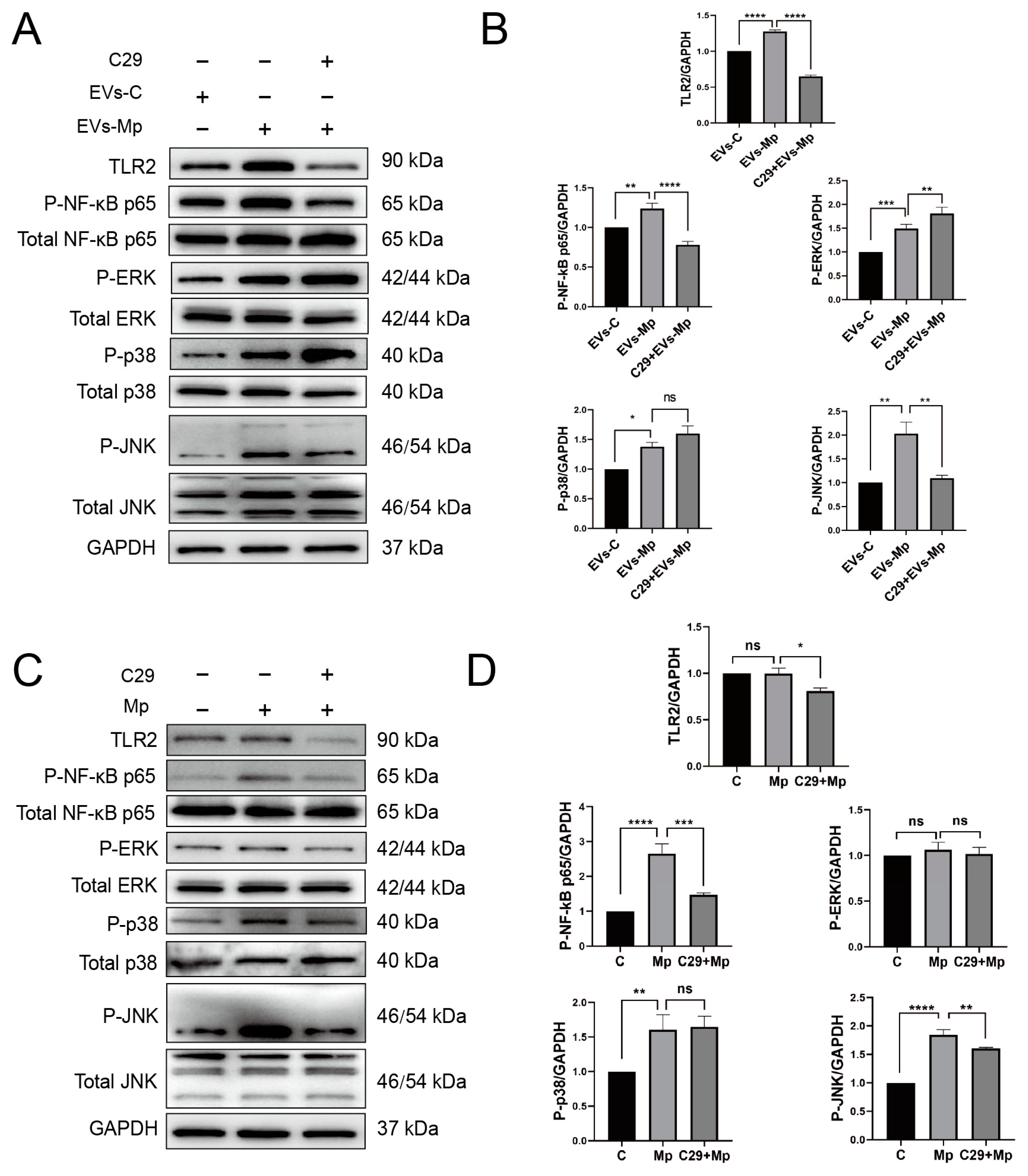
2.5. EVs Released by M. pneumoniae-Infected Macrophages Activate Toll-Like Receptor 2 (TLR2)
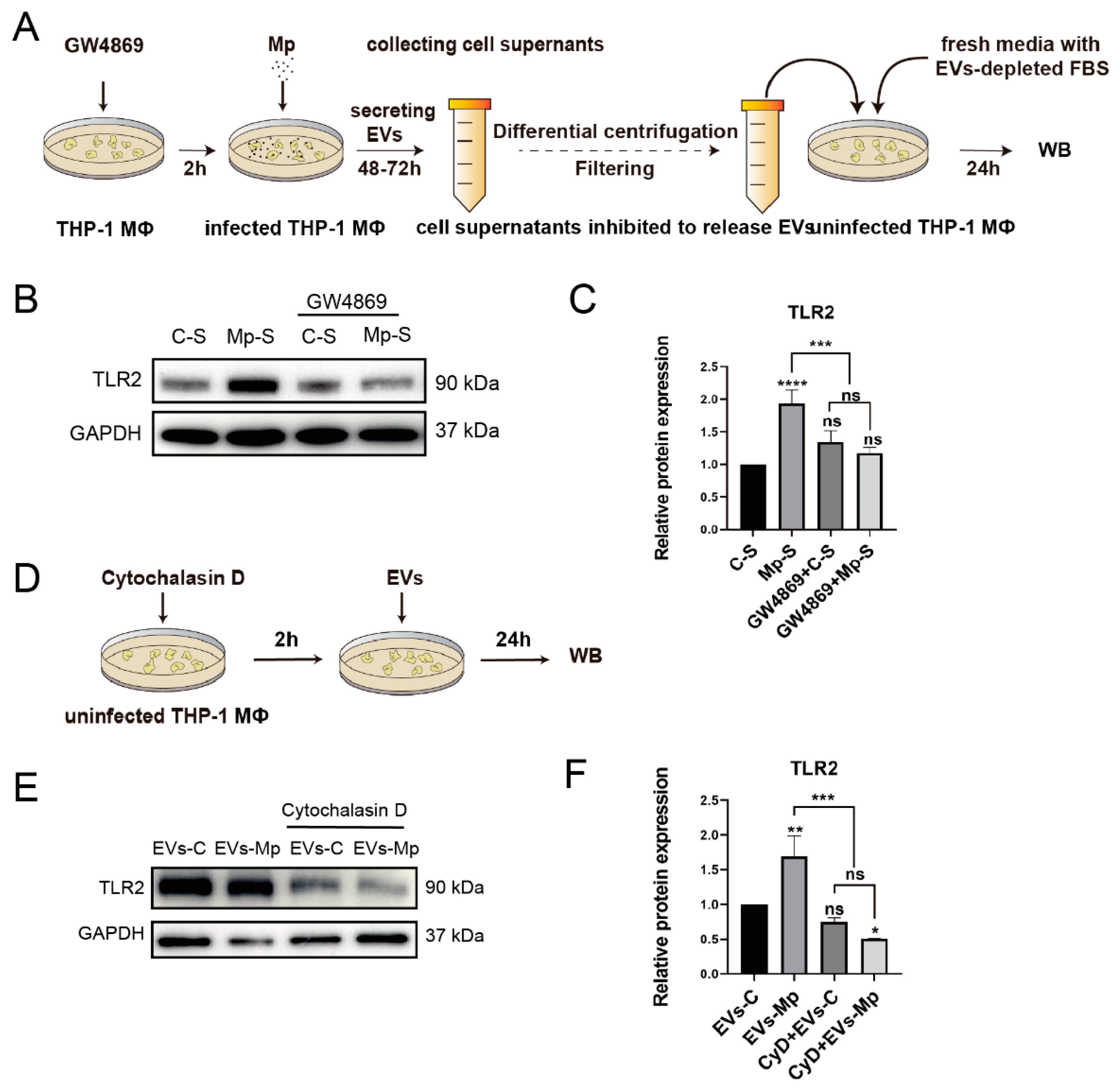
2.6. Inhibition of EVs and Suppression of Recipient Macrophages Phagocytosis Blocks the Activation of TLR2
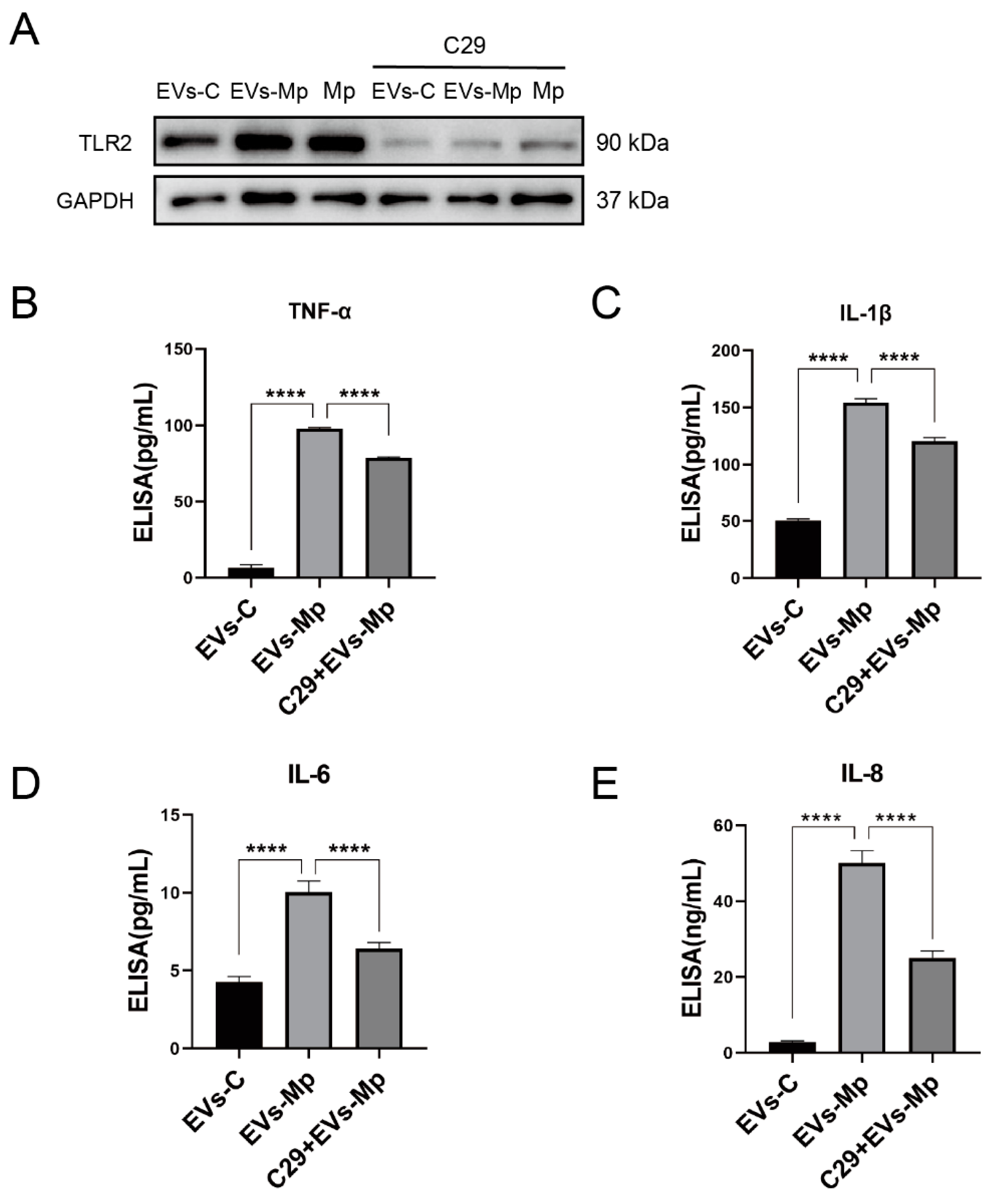
2.7. The Inflammatory Response Induced by EVs Released from M. pneumoniae-Infected Macrophages Is Dependent on TLR2
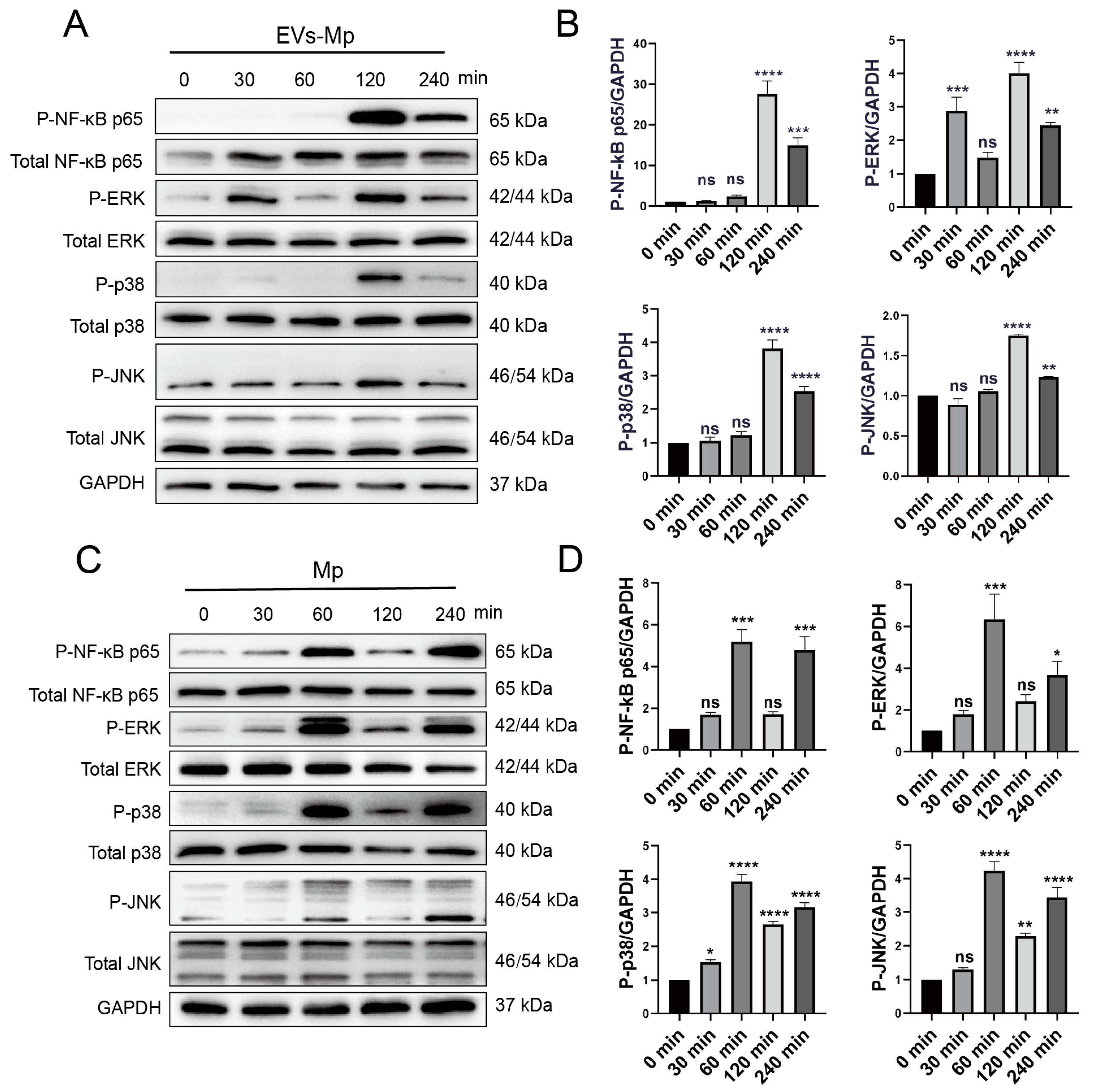
2.8. EVsderived from M. pneumoniae-Infected Macrophages Stimulate the Production of Inflammation Cytokine by Activating NF-κB and MAPK Pathways
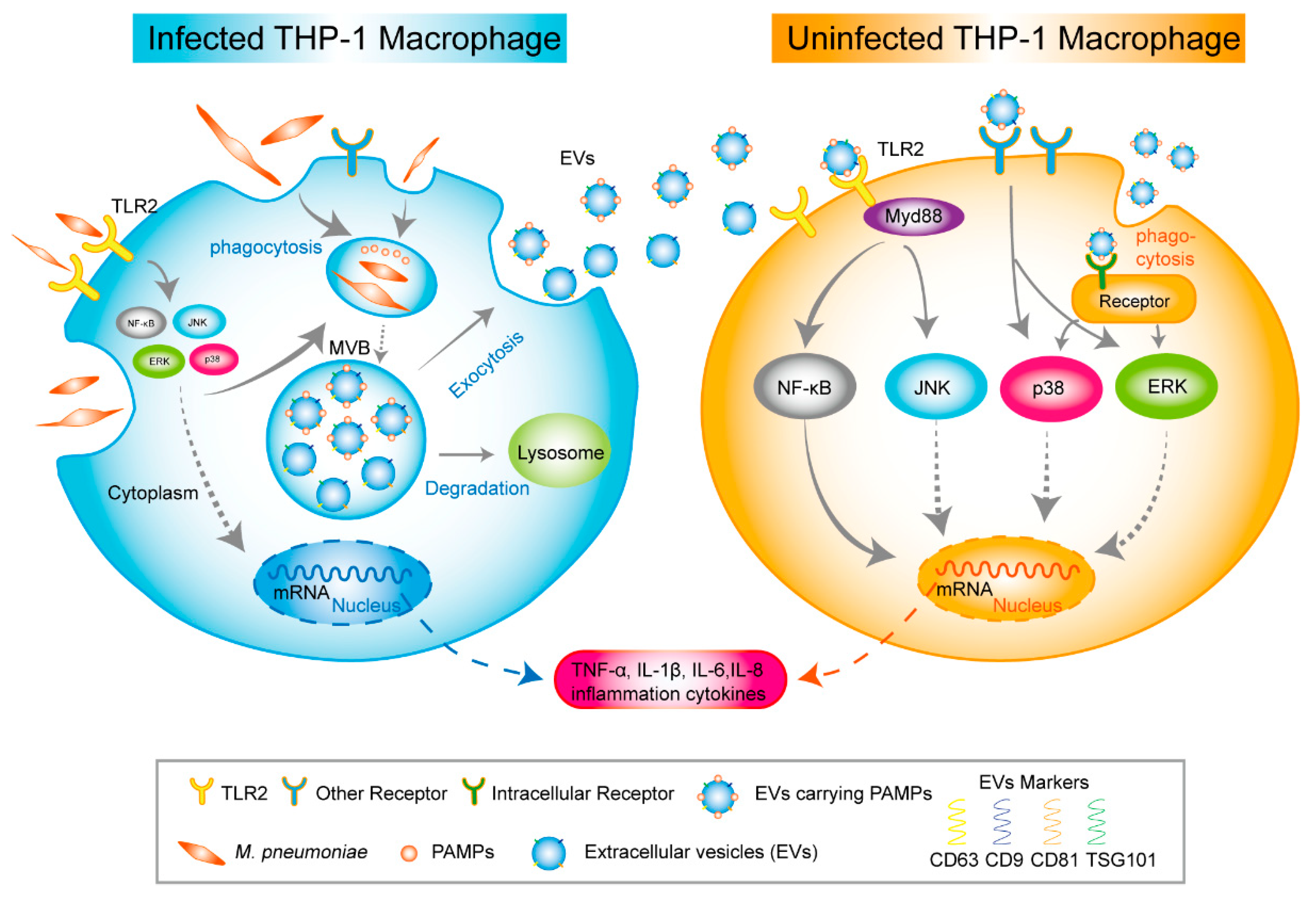
3. Discussion
4. Materials and Methods
4.1. Harvesting and PCR Detection of M. pneumoniae
4.2. Cell Culture and Differentiation
4.3. Cell Viability and Cytotoxicity Assay
4.4. DIO-Labeled M. pneumoniae Survived in Macrophages
4.5. RNA-Seq of the Macrophages Infected with M. pneumoniae
4.6. Macrophage-Derived EVs Isolation
4.7. Transmission Electron Microscopy (TEM)
4.8. Nanoparticle Tracking Analysis (NTA)
4.9. PKH67-Labeled EVs Uptake in Recipient MΦs
4.10. EVs Blocking and Macrophage Phagocytosis Inhibiting Effect
4.11. RNA Extraction and Real-Time PCR
4.12. ELISA
4.13. Western Blot
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Z.; Li, S.; Zhu, C.; Zhou, R.; Leung, P.H.M. Mycoplasma pneumoniae Infections: Pathogenesis and Vaccine Development. Pathogens 2021, 10, 119. [Google Scholar] [CrossRef]
- Waites, K.B.; Xiao, L.; Liu, Y.; Balish, M.F.; Atkinson, T.P. Mycoplasma pneumoniae from the Respiratory Tract and Beyond. Clin. Microbiol. Rev. 2017, 30, 747–809. [Google Scholar] [CrossRef]
- Meseguer, M.A.; Alvarez, A.; Rejas, M.T.; Sánchez, C.; Pérez-Díaz, J.C.; Baquero, F. Mycoplasma pneumoniae: A reduced-genome intracellular bacterial pathogen. Infect. Genet. Evol. 2003, 3, 47–55. [Google Scholar] [CrossRef]
- Cillóniz, C.; Torres, A.; Niederman, M.; van der Eerden, M.; Chalmers, J.; Welte, T.; Blasi, F. Community-acquired pneumonia related to intracellular pathogens. Intensive Care Med. 2016, 42, 1374–1386. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, D.M.; et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, R.; Wu, R.; Wang, B.; Xu, H.; Zhang, Y.; Li, H.; Zhao, S. Mycoplasma pneumoniae pneumonia associated thrombosis at Beijing Children’s hospital. BMC Infect. Dis. 2020, 20, 51. [Google Scholar] [CrossRef]
- Yavlovich, A.; Tarshis, M.; Rottem, S. Internalization and intracellular survival of Mycoplasma pneumoniae by non-phagocytic cells. FEMS Microbiol. Lett. 2004, 233, 241–246. [Google Scholar] [CrossRef]
- Carrière, J.; Barnich, N.; Nguyen, H.T. Exosomes: From Functions in Host-Pathogen Interactions and Immunity to Diagnostic and Therapeutic Opportunities. Rev. Physiol. Biochem. Pharmacol. 2016, 172, 39–75. [Google Scholar] [CrossRef] [PubMed]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef]
- Veerman, R.E.; Güçlüler Akpinar, G.; Eldh, M.; Gabrielsson, S. Immune Cell-Derived Extracellular Vesicles-Functions and Therapeutic Applications. Trends Mol. Med. 2019, 25, 382–394. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, X.; Bao, J.; Wang, Y.; Liu, H.; Tang, L. Exosomes in Pathogen Infections: A Bridge to Deliver Molecules and Link Functions. Front. Immunol. 2018, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chalasani, G.; Ng, Y.H.; Robbins, P.D. Exosomes released from Mycoplasma infected tumor cells activate inhibitory B cells. PLoS ONE 2012, 7, e36138. [Google Scholar] [CrossRef]
- Cronemberger-Andrade, A.; Xander, P.; Soares, R.P.; Pessoa, N.L.; Campos, M.A.; Ellis, C.C.; Grajeda, B.; Ofir-Birin, Y.; Almeida, I.C.; Regev-Rudzki, N.; et al. Trypanosoma cruzi-Infected Human Macrophages Shed Proinflammatory Extracellular Vesicles That Enhance Host-Cell Invasion via Toll-Like Receptor 2. Front. Cell. Infect. Microbiol. 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Shinagawa, K.; Castellino, F.J.; Schorey, J.S. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 2007, 110, 3234–3244. [Google Scholar] [CrossRef]
- Singh, P.P.; Smith, V.L.; Karakousis, P.C.; Schorey, J.S. Exosomes isolated from mycobacteria-infected mice or cultured macrophages can recruit and activate immune cells in vitro and in vivo. J. Immunol. 2012, 189, 777–785. [Google Scholar] [CrossRef]
- Li, D.; Mehta, J.L. Intracellular signaling of LOX-1 in endothelial cell apoptosis. Circ. Res. 2009, 104, 566–568. [Google Scholar] [CrossRef]
- Raucci, A.; Palumbo, R.; Bianchi, M.E. HMGB1: A signal of necrosis. Autoimmunity 2007, 40, 285–289. [Google Scholar] [CrossRef]
- Lee, H.O.; Ferguson, T.A. Biology of FasL. Cytokine Growth Factor Rev. 2003, 14, 325–335. [Google Scholar] [CrossRef]
- Lystad, A.H.; Carlsson, S.R.; Simonsen, A. Toward the function of mammalian ATG12-ATG5-ATG16L1 complex in autophagy and related processes. Autophagy 2019, 15, 1485–1486. [Google Scholar] [CrossRef]
- He, M.X.; He, Y.W. CFLAR/c-FLIPL: A star in the autophagy, apoptosis and necroptosis alliance. Autophagy 2013, 9, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Cheng, Y.; Schorey, J.S. Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur. J. Immunol. 2013, 43, 3279–3290. [Google Scholar] [CrossRef]
- Segovia, J.A.; Chang, T.H.; Winter, V.T.; Coalson, J.J.; Cagle, M.P.; Pandranki, L.; Bose, S.; Baseman, J.B.; Kannan, T.R. NLRP3 Is a Critical Regulator of Inflammation and Innate Immune Cell Response during Mycoplasma pneumoniae Infection. Infect. Immun. 2018, 86, e00548-17. [Google Scholar] [CrossRef]
- Shimizu, T. Inflammation-inducing Factors of Mycoplasma pneumoniae. Front. Microbiol. 2016, 7, 414. [Google Scholar] [CrossRef]
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The Role of TLR2 in Infection and Immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef]
- Mukherjee, S.; Karmakar, S.; Babu, S.P. TLR2 and TLR4 mediated host immune responses in major infectious diseases: A review. Braz. J. Infect. Dis. Off. Publ. Braz. Soc. Infect. Dis. 2016, 20, 193–204. [Google Scholar] [CrossRef]
- Shimizu, T.; Kimura, Y.; Kida, Y.; Kuwano, K.; Tachibana, M.; Hashino, M.; Watarai, M. Cytadherence of Mycoplasma pneumoniae induces inflammatory responses through autophagy and toll-like receptor 4. Infect. Immun. 2014, 82, 3076–3086. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Golenbock, D.; Bowie, A.G. The history of Toll-like receptors—Redefining innate immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles 2020, 9, 1703244. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, K.; Yang, L.; Wang, X.; Huang, W.; Qin, D.; Hao, J.; Wang, Y.; Zingarelli, B.; Peng, T.; Fan, G.C. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim. Biophys. Acta 2015, 1852, 2362–2371. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, C.; Xu, S.; Su, W.; Du, C.; Dong, J.; Feng, R.; Huang, C.; Li, J.; Ma, T. Macrophage-derived, LRG1-enriched extracellular vesicles exacerbate aristolochic acid nephropathy in a TGFβR1-dependent manner. Cell Biol. Toxicol. 2021, 38, 629–648. [Google Scholar] [CrossRef]
- Mistry, P.; Laird, M.H.; Schwarz, R.S.; Greene, S.; Dyson, T.; Snyder, G.A.; Xiao, T.S.; Chauhan, J.; Fletcher, S.; Toshchakov, V.Y.; et al. Inhibition of TLR2 signaling by small molecule inhibitors targeting a pocket within the TLR2 TIR domain. Proc. Natl. Acad. Sci. USA 2015, 112, 5455–5460. [Google Scholar] [CrossRef]
- Xia, Z.B.; Meng, F.R.; Fang, Y.X.; Wu, X.; Zhang, C.W.; Liu, Y.; Liu, D.; Li, G.Q.; Feng, F.B.; Qiu, H.Y. Inhibition of NF-κB signaling pathway induces apoptosis and suppresses proliferation and angiogenesis of human fibroblast-like synovial cells in rheumatoid arthritis. Medicine 2018, 97, e10920. [Google Scholar] [CrossRef]
- Kim, K.Y.; Park, K.I.; Kim, S.H.; Yu, S.N.; Park, S.G.; Kim, Y.W.; Seo, Y.K.; Ma, J.Y.; Ahn, S.C. Inhibition of Autophagy Promotes Salinomycin-Induced Apoptosis via Reactive Oxygen Species-Mediated PI3K/AKT/mTOR and ERK/p38 MAPK-Dependent Signaling in Human Prostate Cancer Cells. Int. J. Mol. Sci. 2017, 18, 1088. [Google Scholar] [CrossRef] [PubMed]
- Leelahavanichkul, K.; Amornphimoltham, P.; Molinolo, A.A.; Basile, J.R.; Koontongkaew, S.; Gutkind, J.S. A role for p38 MAPK in head and neck cancer cell growth and tumor-induced angiogenesis and lymphangiogenesis. Mol. Oncol. 2014, 8, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Dai, M.; Xu, G.; Chang, L.; Yang, J.; Liu, A. Inhibition of inflammation by SP600125 in cholestatic liver injury is dependent on the administration-based exposure profile. Int. J. Mol. Med. 2020, 46, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Groot Kormelink, T.; Mol, S.; de Jong, E.C.; Wauben, M.H.M. The role of extracellular vesicles when innate meets adaptive. Semin. Immunopathol. 2018, 40, 439–452. [Google Scholar] [CrossRef]
- Lund, M.E.; To, J.; O’Brien, B.A.; Donnelly, S. The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a pro-inflammatory stimulus. J. Immunol. Methods 2016, 430, 64–70. [Google Scholar] [CrossRef]
- Park, E.K.; Jung, H.S.; Yang, H.I.; Yoo, M.C.; Kim, C.; Kim, K.S. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2007, 56, 45–50. [Google Scholar] [CrossRef]
- Anand, S.; Samuel, M.; Kumar, S.; Mathivanan, S. Ticket to a bubble ride: Cargo sorting into exosomes and extracellular vesicles. Biochim. Et Biophys. Acta. Proteins Proteom. 2019, 1867, 140203. [Google Scholar] [CrossRef]
- Cipriano, M.J.; Hajduk, S.L. Drivers of persistent infection: Pathogen-induced extracellular vesicles. Essays Biochem. 2018, 62, 135–147. [Google Scholar] [CrossRef]
- Guo, B.B.; Bellingham, S.A.; Hill, A.F. Stimulating the Release of Exosomes Increases the Intercellular Transfer of Prions. J. Biol. Chem. 2016, 291, 5128–5137. [Google Scholar] [CrossRef]
- Soto-Serna, L.E.; Diupotex, M.; Zamora-Chimal, J.; Ruiz-Remigio, A.; Delgado-Domínguez, J.; Cervantes-Sarabia, R.B.; Méndez-Bernal, A.; Escalona-Montaño, A.R.; Aguirre-García, M.M.; Becker, I. Leishmania mexicana: Novel Insights of Immune Modulation through Amastigote Exosomes. J. Immunol. Res. 2020, 2020, 8894549. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Clos, J.; Horakova, E.; Wang, A.Y.; Wiesgigl, M.; Kelly, I.; Lynn, M.A.; McMaster, W.R.; Foster, L.J.; Levings, M.K.; et al. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J. Immunol. 2010, 185, 5011–5022. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.P.; Dittmer, D.P. Extracellular vesicles in virus infection and pathogenesis. Curr. Opin. Virol. 2020, 44, 129–138. [Google Scholar] [CrossRef]
- Quah, B.J.; O’Neill, H.C. Mycoplasma contaminants present in exosome preparations induce polyclonal B cell responses. J. Leukoc. Biol. 2007, 82, 1070–1082. [Google Scholar] [CrossRef]
- Luo, H.; He, J.; Qin, L.; Chen, Y.; Chen, L.; Li, R.; Zeng, Y.; Zhu, C.; You, X.; Wu, Y. Mycoplasma pneumoniae lipids license TLR-4 for activation of NLRP3 inflammasome and autophagy to evoke a proinflammatory response. Clin. Exp. Immunol. 2021, 203, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chu, C.; Li, Y.; Li, G.; Lei, X.; Zhou, W.; Chen, Z. High expression of HMGB1 in children with refractory Mycoplasma pneumoniae pneumonia. BMC Infect. Dis. 2018, 18, 439. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Hu, F.; Zou, M.; Luo, R.; Sun, Y.; Wang, T.; Guo, Q.; Peng, X. Extracellular HMGB1 as Inflammatory Mediator in the Progression of Mycoplasma Gallisepticum Infection. Cells 2022, 11, 2817. [Google Scholar] [CrossRef]
- Zakharova, E.; Grandhi, J.; Wewers, M.D.; Gavrilin, M.A. Mycoplasma suppression of THP-1 Cell TLR responses is corrected with antibiotics. PLoS ONE 2010, 5, e9900. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, I.; David, M. MicroRNAs in the immune response. Cytokine 2008, 43, 391–394. [Google Scholar] [CrossRef]
- Luo, H.; Wu, X.; Xu, Z.; Hao, X.; Wang, Y.; Li, M. NOD2/c-Jun NH2-Terminal Kinase Triggers Mycoplasma ovipneumoniae-Induced Macrophage Autophagy. J. Bacteriol. 2020, 202, e00689-19. [Google Scholar] [CrossRef]
- Jiang, D.; Nelson, M.L.; Gally, F.; Smith, S.; Wu, Q.; Minor, M.; Case, S.; Thaikoottathil, J.; Chu, H.W. Airway epithelial NF-κB activation promotes Mycoplasma pneumoniae clearance in mice. PLoS ONE 2012, 7, e52969. [Google Scholar] [CrossRef] [PubMed]
- Thaikoottathil, J.; Chu, H.W. MAPK/AP-1 activation mediates TLR2 agonist-induced SPLUNC1 expression in human lung epithelial cells. Mol. Immunol. 2011, 49, 415–422. [Google Scholar] [CrossRef]
- Sakurai, H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol. Sci. 2012, 33, 522–530. [Google Scholar] [CrossRef]
- Smith, P.F. The biology of Mycoplasmas; Academic Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. In Current Protocols in Cell Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; Chapter 3, Unit 3.22. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Hao, X.; Gao, L.; Wang, Y.; Shi, J.; Luo, H.; Li, M. Extracellular Vesicles Released from Macrophages Infected with Mycoplasma pneumoniae Stimulate Proinflammatory Response via the TLR2-NF-κB/JNK Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 8588. https://doi.org/10.3390/ijms24108588
Ma C, Hao X, Gao L, Wang Y, Shi J, Luo H, Li M. Extracellular Vesicles Released from Macrophages Infected with Mycoplasma pneumoniae Stimulate Proinflammatory Response via the TLR2-NF-κB/JNK Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(10):8588. https://doi.org/10.3390/ijms24108588
Chicago/Turabian StyleMa, Chunji, Xiujing Hao, Liyang Gao, Yongyu Wang, Juan Shi, Haixia Luo, and Min Li. 2023. "Extracellular Vesicles Released from Macrophages Infected with Mycoplasma pneumoniae Stimulate Proinflammatory Response via the TLR2-NF-κB/JNK Signaling Pathway" International Journal of Molecular Sciences 24, no. 10: 8588. https://doi.org/10.3390/ijms24108588
APA StyleMa, C., Hao, X., Gao, L., Wang, Y., Shi, J., Luo, H., & Li, M. (2023). Extracellular Vesicles Released from Macrophages Infected with Mycoplasma pneumoniae Stimulate Proinflammatory Response via the TLR2-NF-κB/JNK Signaling Pathway. International Journal of Molecular Sciences, 24(10), 8588. https://doi.org/10.3390/ijms24108588





