Lipids in Atherosclerosis: Pathophysiology and the Role of Calculated Lipid Indices in Assessing Cardiovascular Risk in Patients with Hyperlipidemia
Abstract
1. Introduction
2. Key Role of Lipids in Atherosclerosis
3. Oxidized LDL in Assessing CV Risk and Non-Invasive Lipid Indices
3.1. Non-HDL Cholesterol
3.2. Castelli Index I and II
3.3. Triglyceride-Rich Lipoprotein Cholesterol
3.4. Atherogenic Index of Plasma
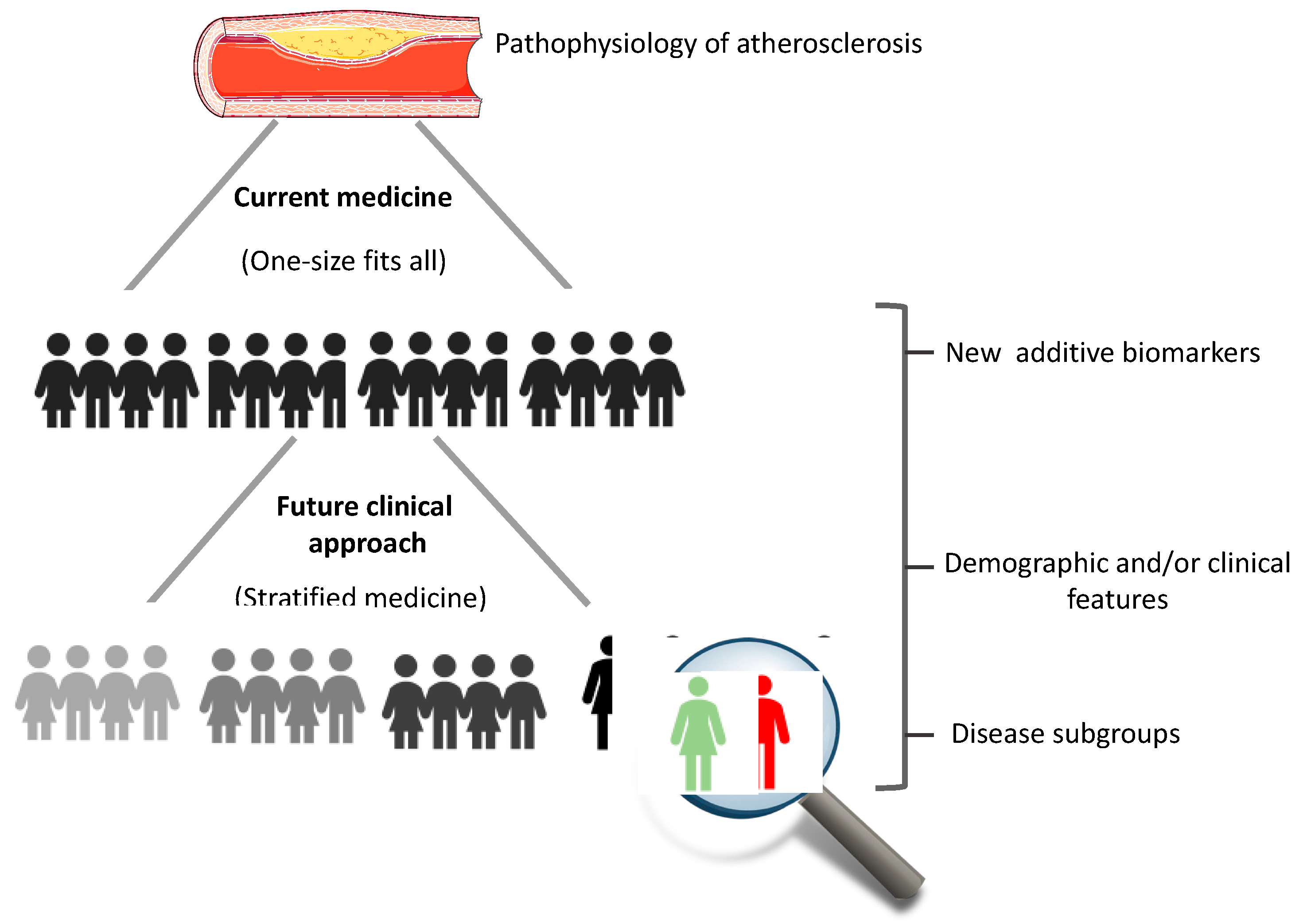
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIP | Atherogenic index of plasma |
| BMI | Body mass index |
| CAD | Coronary artery disease |
| CRI-I | Castelli risk index I |
| CRI-II | Castelli risk index II |
| CV | Cardiovascular |
| EC | Endothelial cell |
| HDL | High-density lipoprotein |
| hsCRP | High-sensitivity C-reactive protein |
| IDL | Intermediate-density lipoprotein |
| IFN-γ | Interferon gamma |
| IL | Interleukin |
| IR | Insulin resistance |
| LDL | Low-density lipoprotein |
| LOX-1 | Lectin-like oxidized low-density lipoprotein receptor-1 |
| MACEs | Major adverse cardiac events |
| MetS | Metabolic syndrome |
| MI | Myocardial infarction |
| NO | Nitric oxide |
| Non-HDL | Non-HDL cholesterol |
| Ox-LDL | Oxidized low-density lipoprotein |
| RCVEs | Recurrent cardiovascular events |
| T2D | Type 2 diabetes |
| TC | Total cholesterol |
| TG | Triglycerides |
| TNF-α | Tumor necrosis factor-alpha |
| TRL-C | Triglyceride-rich lipoprotein cholesterol |
| VLDL | Very low density lipoprotein |
| VSMC | Vascular smooth muscle cells |
References
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.M. The intracellular signaling pathways governing macrophage activation and function in human atherosclerosis. Biochem. Soc. Trans 2022, 50, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Pathogenesis and Clinical Significance of In-Stent Restenosis in Patients with Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 11970. [Google Scholar] [CrossRef] [PubMed]
- Poder, T.G.; Erraji, J.; Coulibaly, L.P.; Koffi, K. Percutaneous coronary intervention with second-generation drug-eluting stent versus bare-metal stent: Systematic review and cost-benefit analysis. PLoS ONE 2017, 12, e0177476. [Google Scholar] [CrossRef]
- Minelli, S.; Minelli, P.; Montinari, M.R. Reflections on Atherosclerosis: Lesson from the Past and Future Research Directions. J. Multidiscip. Healthc. 2020, 13, 621. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Tan, J.; Wei, L.; Wu, D.; Gao, S.; Weng, Y.; Chen, J. Recent Advance in Treatment of Atherosclerosis: Key Targets and Plaque-Positioned Delivery Strategies. J. Tissue Eng. 2022, 13, 20417314221088508. [Google Scholar] [CrossRef]
- Pirillo, A.; Bonacina, F.; Norata, G.D.; Catapano, A.L. The Interplay of Lipids, Lipoproteins, and Immunity in Atherosclerosis. Curr. Atheroscler. Rep. 2018, 20, 1–9. [Google Scholar] [CrossRef]
- Barzi, F.; Patel, A.; Woodward, M.; Lawes, C.M.; Ohkubo, T.; Gu, D.; Lam, T.H.; Ueshima, H.; Asia Pacific Cohort Studies Collaboration. A comparison of lipid variables as predictors of cardiovascular disease in the Asia Pacific region. Ann. Epidemiol. 2005, 15, 405–413. [Google Scholar]
- Fernández-Macías, J.C.; Ochoa-Martínez, A.C.; Varela-Silva, J.A.; Pérez-Maldonado, I.N. Atherogenic Index of Plasma: Novel Predictive Biomarker for Cardiovascular Illnesses. Arch. Med. Res. 2019, 50, 285–294. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Sewell, R.D.; Rafieian-Kopaei, M. Antioxidants and Atherosclerosis: Mechanistic Aspects. Biomolecules 2019, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Stahl, W.; Sevanian, A. Nutritional, Dietary and Postprandial Oxidative Stress. J. Nutr. 2005, 135, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.K.; Anoruo, M.; Jialal, I. Biochemistry, Low Density Lipoprotein. 2018. Available online: https://europepmc.org/article/NBK/nbk500010 (accessed on 15 December 2022).
- Pirahanchi, Y.; Sinawe, H.; Dimri, M. Biochemistry, LDL Cholesterol. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Gleissner, C.A.; Leitinger, N.; Ley, K. Effects of Native and Modified Low-Density Lipoproteins on Monocyte Recruitment in Atherosclerosis. Hypertension 2007, 50, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, N.; Formoso, G.; Pandolfi, A. Physiology and Pathophysiology of OxLDL Uptake by Vascular Wall Cells in Atherosclerosis. Vascul. Pharmacol. 2016, 84, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zakiev, E.R.; Sukhorukov, V.N.; Melnichenko, A.A.; Sobenin, I.A.; Ivanova, E.A.; Orekhov, A.N. Lipid Composition of Circulating Multiple-Modified Low Density Lipoprotein. Lipids Health Dis. 2016, 15, 1–6. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Ivanova, E.A.; Melnichenko, A.A.; Sobenin, I.A. Circulating Desialylated Low Density Lipoprotein. Cor. Vasa. 2017, 59, e149–e156. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Wu, W.-K.; Melnichenko, A.A.; Wetzker, R.; Sukhorukov, V.; Markin, A.M.; Khotina, V.A.; Orekhov, A.N. Signaling Pathways and Key Genes Involved in Regulation of Foam Cell Formation in Atherosclerosis. Cells 2020, 9, 584. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front. Cardiovasc. Med. 2021, 8, 707529. [Google Scholar] [CrossRef]
- Sánchez-Quesada, J.L.; Villegas, S.; Ordóñez-Llanos, J. Electronegative Low-Density Lipoprotein. A Link between Apolipoprotein B Misfolding, Lipoprotein Aggregation and Proteoglycan Binding. Curr. Opin. Lipidol. 2012, 23, 479–486. [Google Scholar] [CrossRef]
- Maiolino, G.; Rossitto, G.; Caielli, P.; Bisogni, V.; Rossi, G.P.; Calò, L.A. The Role of Oxidized Low-Density Lipoproteins in Atherosclerosis: The Myths and the Facts. Mediat. Inflamm. 2013, 2013, 714653. [Google Scholar] [CrossRef]
- Summerhill, V.I.; Grechko, A.V.; Yet, S.-F.; Sobenin, I.A.; Orekhov, A.N. The Atherogenic Role of Circulating Modified Lipids in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 3561. [Google Scholar] [CrossRef] [PubMed]
- Mofidi, R.; Crotty, T.B.; McCarthy, P.; Sheehan, S.J.; Mehigan, D.; Keaveny, T.V. Association between Plaque Instability, Angiogenesis and Symptomatic Carotid Occlusive Disease. J. Br. Surg. 2001, 88, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Robertson, A.-K.L.; Söderberg-Nauclér, C. Inflammation and Atherosclerosis. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 297–329. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, F.; Hayat, S.M.G.; Bianconi, V.; Johnston, T.P.; Sahebkar, A. Atherosclerosis and Immunity: A Perspective. Trends Cardiovasc. Med. 2019, 29, 363–371. [Google Scholar] [CrossRef]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, OxLDL, and Atherosclerosis. Mediators Inflamm. 2013, 2013, 152786. [Google Scholar] [CrossRef]
- Nickel, T.; Schmauss, D.; Hanssen, H.; Sicic, Z.; Krebs, B.; Jankl, S.; Summo, C.; Fraunberger, P.; Walli, A.K.; Pfeiler, S. OxLDL Uptake by Dendritic Cells Induces Upregulation of Scavenger-Receptors, Maturation and Differentiation. Atherosclerosis 2009, 205, 442–450. [Google Scholar] [CrossRef]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis. Endotext Internet 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK343489/ (accessed on 15 December 2022).
- Millette, E.; Rauch, B.H.; Kenagy, R.D.; Daum, G.; Clowes, A.W. Platelet-Derived Growth Factor-BB Transactivates the Fibroblast Growth Factor Receptor to Induce Proliferation in Human Smooth Muscle Cells. Trends Cardiovasc. Med. 2006, 16, 25–28. [Google Scholar] [CrossRef]
- Nakahara, T.; Dweck, M.R.; Narula, N.; Pisapia, D.; Narula, J.; Strauss, H.W. Coronary Artery Calcification: From Mechanism to Molecular Imaging. JACC Cardiovasc. Imaging 2017, 10, 582–593. [Google Scholar] [CrossRef]
- Clemente, A.; Traghella, I.; Mazzone, A.; Sbrana, S.; Vassalle, C. Vascular and Valvular Calcification Biomarkers. Adv. Clin. Chem. 2020, 95, 73–103. [Google Scholar]
- Lippi, G.; Franchini, M.; Targher, G. Arterial Thrombus Formation in Cardiovascular Disease. Nat. Rev. Cardiol. 2011, 8, 502–512. [Google Scholar] [CrossRef]
- Ouweneel, A.B.; Van Eck, M. Lipoproteins as Modulators of Atherothrombosis: From Endothelial Function to Primary and Secondary Coagulation. Vascul. Pharmacol. 2016, 82, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mućka, S.; Miodońska, M.; Jakubiak, G.K.; Starzak, M.; Cieślar, G.; Stanek, A. Endothelial Function Assessment by Flow-Mediated Dilation Method: A Valuable Tool in the Evaluation of the Cardiovascular System. Int. J. Environ. Res. Public Health 2022, 19, 11242. [Google Scholar] [CrossRef] [PubMed]
- Papagiouvanni, I.; Sarafidis, P.; Theodorakopoulou, M.P.; Sinakos, E.; Goulis, I. Endothelial and microvascular function in liver cirrhosis: An old concept that needs re-evaluation? Ann. Gastroenterol. 2022, 35, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D. ESC National Cardiac Societies; ESC Scientific Document Group. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Goldberg, I.J.; Eckel, R.H.; McPherson, R. Triglycerides and Heart Disease: Still a Hypothesis? Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1716–1725. [Google Scholar] [CrossRef]
- Taskinen, M.-R.; Borén, J. New Insights into the Pathophysiology of Dyslipidemia in Type 2 Diabetes. Atherosclerosis 2015, 239, 483–495. [Google Scholar] [CrossRef]
- Arsenault, B.J.; Rana, J.S.; Stroes, E.S.; Després, J.-P.; Shah, P.K.; Kastelein, J.J.; Wareham, N.J.; Boekholdt, S.M.; Khaw, K.-T. Beyond Low-Density Lipoprotein Cholesterol: Respective Contributions of Non–High-Density Lipoprotein Cholesterol Levels, Triglycerides, and the Total Cholesterol/High-Density Lipoprotein Cholesterol Ratio to Coronary Heart Disease Risk in Apparently Healthy Men and Women. J. Am. Coll. Cardiol. 2009, 55, 35–41. [Google Scholar]
- Płaczkowska, S.; Sołkiewicz, K.; Bednarz-Misa, I.; Kratz, E.M. Atherogenic Plasma Index or Non-High-Density Lipoproteins as Markers Best Reflecting Age-Related High Concentrations of Small Dense Low-Density Lipoproteins. Int. J. Mol. Sci. 2022, 23, 5089. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, S.; Feng, X.; Yang, J.; Qiao, J.; Zhao, Y.; Shi, D.; Zhou, Y. The Sensibility of the New Blood Lipid Indicator Atherogenic Index of Plasma (AIP) in Menopausal Women with Coronary Artery Disease. Lipids Health Dis. 2020, 19, 1–8. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the Special Contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016, 253, 281–344. [Google Scholar]
- Sampson, U.K.; Fazio, S.; Linton, M.F. Residual Cardiovascular Risk despite Optimal LDL Cholesterol Reduction with Statins: The Evidence, Etiology, and Therapeutic Challenges. Curr. Atheroscler. Rep. 2012, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Ogura, S.; Little, P.J.; Xu, S.W.; Sawamura, T. Targeting LOX-1 in atherosclerosis and vasculopathy: Current knowledge and future perspectives. Ann. N. Y. Acad. Sci. 2019, 1443, 34–53. [Google Scholar] [CrossRef]
- Varghese, D.S.; Ali, B.R. Pathological Crosstalk Between Oxidized LDL and ER Stress in Human Diseases: A Comprehensive Review. Front. Cell Dev. Biol. 2021, 9, 674103. [Google Scholar] [CrossRef] [PubMed]
- Zingg, J.M.; Vlad, A.; Ricciarelli, R. Oxidized LDLs as Signaling Molecules. Antioxidants 2021, 10, 1184. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhao, D.; Wang, M.; Zhao, F.; Han, X.; Qi, Y.; Liu, J. Association Between Circulating Oxidized LDL and Atherosclerotic Cardiovascular Disease: A Meta-analysis of Observational Studies. Can. J. Cardiol. 2017, 33, 1624–1632. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, D.; Qi, Y.; Wang, W.; Wang, M.; Sun, J.; Liu, J.; Li, Y.; Liu, J. Circulating Oxidized Low-Density Lipoprotein Levels Independently Predict 10-Year Progression of Subclinical Carotid Atherosclerosis: A Community-Based Cohort Study. J. Atheroscler Thromb. 2018, 25, 1032–1043. [Google Scholar] [CrossRef]
- Jamialahmadi, T.; Baratzadeh, F.; Reiner, Ž.; Mannarino, M.R.; Cardenia, V.; Simental-Mendía, L.E.; Pirro, M.; Watts, G.F.; Sahebkar, A. The Effects of Statin Therapy on Oxidized LDL and Its Antibodies: A Systematic Review and Meta-Analysis. Oxid. Med. Cell Longev. 2022, 2022, 7850659. [Google Scholar] [CrossRef]
- Banerjee, J.; Mishra, N.; Damle, G.; Dhas, Y. Beyond LDL-c: The importance of serum oxidized LDL in predicting risk for type 2 diabetes in the middle-aged Asian Indians. Diabetes Metab. Syndr. 2019, 13, 206–213. [Google Scholar] [CrossRef]
- Meisinger, C.; Baumert, J.; Khuseyinova, N.; Loewel, H.; Koenig, W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation 2005, 112, 651–657. [Google Scholar] [CrossRef]
- Nishi, K.; Itabe, H.; Uno, M.; Kitazato, K.T.; Horiguchi, H.; Shinno, K.; Nagahiro, S. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1649–1654. [Google Scholar] [CrossRef]
- Hartley, A.; Haskard, D.; Khamis, R. Oxidized LDL and anti-oxidized LDL antibodies in atherosclerosis—Novel insights and future directions in diagnosis and therapy. Trends Cardiovasc. Med. 2019, 29, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, G.K.; Cieślar, G.; Stanek, A. Nitrotyrosine, Nitrated Lipoproteins, and Cardio-vascular Dysfunction in Patients with Type 2 Diabetes: What Do We Know and What Remains to Be Explained? Antioxidants 2022, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-K.; Liao, C.-W.; Meng, S.-W.; Wu, W.-K.; Chiang, J.-Y.; Wu, M.-S. Lipids and Lipoproteins in Health and Disease: Focus on Targeting Atherosclerosis. Biomedicines 2021, 9, 985. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Kastelein, J.J.; Ray, K.K.; Ginsberg, H.N.; Chapman, M.J.; Packard, C.J.; Laufs, U.; Oliver-Williams, C.; Wood, A.M.; Butterworth, A.S. Association of Triglyceride-Lowering LPL Variants and LDL–Lowering LDLR Variants with Risk of Coronary Heart Disease. JAMA 2019, 321, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Hegele, R.A.; Ginsberg, H.N.; Chapman, M.J.; Nordestgaard, B.G.; Kuivenhoven, J.A.; Averna, M.; Borén, J.; Bruckert, E.; Catapano, A.L.; Descamps, O.S. European Atherosclerosis Society Consensus Panel. The Polygenic Nature of Hypertriglyceridaemia: Implications for Definition, Diagnosis, and Management. Lancet Diabetes Endocrinol. 2014, 2, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.M.; Wonnerth, A.; Huber, K.; Wojta, J. Cardiovascular Disease Risk Reduction by Raising HDL Cholesterol–Current Therapies and Future Opportunities. Br. J. Pharmacol. 2012, 167, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Brea, A.; Hernández-Mijares, A.; Millán, J.; Ascaso, J.F.; Blasco, M.; Díaz, A.; Mantilla, T.; Pedro-Botet, J.C.; Pintó, X. Non-HDL Cholesterol as a Therapeutic Goal. Clin. Investig. Arterioscler. 2019, 31, 28–33. [Google Scholar]
- Rana, J.S.; Boekholdt, S.M.; Kastelein, J.J.; Shah, P.K. The Role of Non-HDL Cholesterol in Risk Stratification for Coronary Artery Disease. Curr. Atheroscler. Rep. 2012, 14, 130–134. [Google Scholar] [CrossRef]
- Brunner, F.J.; Waldeyer, C.; Ojeda, F.; Salomaa, V.; Kee, F.; Sans, S.; Thorand, B.; Giampaoli, S.; Brambilla, P.; Tunstall-Pedoe, H. Application of Non-HDL Cholesterol for Population-Based Cardiovascular Risk Stratification: Results from the Multinational Cardiovascular Risk Consortium. Lancet 2019, 394, 2173–2183. [Google Scholar] [CrossRef]
- Wang, G.; Jing, J.; Wang, A.; Zhang, X.; Zhao, X.; Li, Z.; Wang, C.; Li, H.; Liu, L.; Wang, Y. Non–High-Density Lipoprotein Cholesterol Predicts Adverse Outcomes in Acute Ischemic Stroke. Stroke 2021, 52, 2035–2042. [Google Scholar] [CrossRef]
- Li, C.; Ford, E.S.; Tsai, J.; Zhao, G.; Balluz, L.S.; Gidding, S.S. Serum Non-High-Density Lipoprotein Cholesterol Concentration and Risk of Death from Cardiovascular Diseases among US Adults with Diagnosed Diabetes: The Third National Health and Nutrition Examination Survey Linked Mortality Study. Cardiovasc. Diabetol. 2011, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Deng, X.; Peng, Y.; Zeng, Q.; Song, Z.; He, W.; Zhang, L.; Gao, G.; Xiao, T.; Yu, X. Meta-Analysis of Serum Non-High-Density Lipoprotein Cholesterol and Risk of Coronary Heart Disease in the General Population. Clin. Chim. Acta 2017, 471, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.S.; Vasan, R.S.; Xanthakis, V. Trajectories of Blood Lipid Concentrations over the Adult Life Course and Risk of Cardiovascular Disease and All-cause Mortality: Observations from the Framingham Study over 35 Years. J. Am. Heart Assoc. 2019, 8, e011433. [Google Scholar] [CrossRef] [PubMed]
- Navar-Boggan, A.M.; Peterson, E.D.; D’Agostino Sr, R.B.; Neely, B.; Sniderman, A.D.; Pencina, M.J. Hyperlipidemia in Early Adulthood Increases Long-Term Risk of Coronary Heart Disease. Circulation 2015, 131, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Zeng, R.; Zhao, X.; Guo, L.; Zhang, M. Prognostic Value of Non-High-Density Lipoprotein Cholesterol for Mortality in Patients with Coronary Heart Disease: A Systematic Reviewemnant cholesterol and myocardial infarction in normal weight, overweight, and obese individuals from the Copenhagen General Population Study and Meta-Analysis. Int. J. Cardiol. 2017, 227, 950–955. [Google Scholar]
- Boekholdt, S.M.; Arsenault, B.J.; Mora, S.; Pedersen, T.R.; LaRosa, J.C.; Nestel, P.J.; Simes, R.J.; Durrington, P.; Hitman, G.A.; Welch, K.M.A. Association of LDL Cholesterol, Non–HDL Cholesterol, and Apolipoprotein B Levels with Risk of Cardiovascular Events among Patients Treated with Statins: A Meta-Analysis. JAMA 2012, 307, 1302–1309. [Google Scholar] [CrossRef]
- We, J.; Huang, Y.; Lu, Y.; Yuan, H. Associations of non-high-density are effective lipoprotein cholesterol, triglycerides and the total cholesterol/HDL-c ratio with arterial stiffness independent of low-density lipoprotein cholesterol in a Chinese population. Hypertens. Res. 2019, 42, 1223–1230. [Google Scholar]
- Tanaka, F.; Makita, S.; Onoda, T.; Tanno, K.; Ohsawa, M.; Itai, K.; Sakata, K.; Omama, S.; Yoshida, Y.; Ogasawara, K.; et al. Iwate-Kenco Study Group. Predictive value of lipoprotein indices for residual risk of acute myocardial infarction and sudden death in men with low-density lipoprotein cholesterol levels <120 mg/dl. Am J Cardiol 2013, 112, 1063–1068. [Google Scholar]
- Liu, J.; Sempos, C.T.; Donahue, R.P.; Dorn, J.; Trevisan, M.; Grundy, S.M. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their risk predictive values in coronary heart disease. Am. J. Cardiol. 2006, 98, 1363–1368. [Google Scholar] [CrossRef]
- Parhofer, K.G. New targets for treating hypertriglyceridemia. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 106–111. [Google Scholar] [CrossRef]
- Carr, S.S.; Hooper, A.J.; Sullivan, D.R.; Burnett, J.R. Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology 2019, 51, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, R.C.; Ballantyne, C.M. Residual Cardiovascular Risk at Low LDL: Remnants, Lipoprotein(a), and Inflammation. Clin. Chem. 2021, 67, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Soffer, G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease risk: Current status and treatments. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Ballantyne, C.M.; Carmena, R.; Cabezas, M.C.; Chapman, M.J.; Couture, P.; De Graaf, J.; Durrington, P.N.; Faergeman, O.; Frohlich, J. Apo B versus Cholesterol in Estimating Cardiovascular Risk and in Guiding Therapy: Report of the Thirty-person/Ten-country Panel. J. Intern. Med. 2006, 259, 247–258. [Google Scholar] [CrossRef]
- Robinson, J.G.; Wang, S.; Jacobson, T.A. Meta-Analysis of Comparison of Effectiveness of Lowering Apolipoprotein B versus Low-Density Lipoprotein Cholesterol and Nonhigh-Density Lipoprotein Cholesterol for Cardiovascular Risk Reduction in Randomized Trials. Am. J. Cardiol. 2012, 110, 1468–1476. [Google Scholar] [CrossRef]
- Robinson, J.G.; Wang, S.; Smith, B.J.; Jacobson, T.A. Meta-Analysis of the Relationship between Non-High-Density Lipoprotein Cholesterol Reduction and Coronary Heart Disease Risk. J. Am. Coll. Cardiol. 2009, 53, 316–322. [Google Scholar] [CrossRef]
- Hodkinson, A.; Tsimpida, D.; Kontopantelis, E.; Rutter, M.K.; Mamas, M.A.; Panagioti, M. Comparative Effectiveness of Statins on Non-High Density Lipoprotein Cholesterol in People with Diabetes and at Risk of Cardiovascular Disease: Systematic Review and Network Meta-Analysis. BMJ 2022, 376, e067731-74. [Google Scholar] [CrossRef]
- Pencina, K.M.; Thanassoulis, G.; Wilkins, J.T.; Vasan, S.; Navar, A.M.; Peterson, E.D.; Pencina, M.J.; Sniderman, A.D. Trajectories of Non–HDL Cholesterol across Midlife: Implications for Cardiovascular Prevention. J. Am. Coll. Cardiol. 2019, 74, 70–79. [Google Scholar] [CrossRef]
- Armstrong, M.K.; Fraser, B.J.; Hartiala, O.; Buscot, M.J.; Juonala, M.; Wu, F.; Koskinen, J.; Hutri-Kähönen, N.; Kähönen, M.; Laiinen, T.P.; et al. Association of Non-High-Density Lipoprotein Cholesterol Measured in Adolescence, Young Adulthood, and Mid-Adulthood With Coronary Artery Calcification Measured in Mid-Adulthood. JAMA Cardiol. 2021, 6, 661–668. [Google Scholar] [CrossRef]
- Castelli, W.P.; Abbott, R.D.; McNamara, P.M. Summary Estimates of Cholesterol Used to Predict Coronary Heart Disease. Circulation 1983, 67, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.; Pintó, X.; Muñoz, A.; Zúñiga, M.; Rubiés-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernández-Mijares, A.; González-Santos, P. Lipoprotein Ratios: Physiological Significance and Clinical Usefulness in Cardiovascular Prevention. Vasc. Health Risk Manag. 2009, 5, 757. [Google Scholar] [PubMed]
- Albrektsen, G.; Heuch, I.; Løchen, M.L.; Thelle, D.S.; Wilsgaard, T.; Njølstad, I.; Bønaa, K.H. Risk of incident myocardial infarction by gender: Interactions with serum lipids, blood pressure and smoking. The Tromsø Study 1979–2012. Atherosclerosis 2017, 261, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Von Mühlen, D.; Langer, R.D.; Barrett-Connor, E. Sex and time differences in the associations of non-high-density lipoprotein cholesterol versus other lipid and lipoprotein factors in the prediction of cardiovascular death (The Rancho Bernardo Study). Am. J. Cardiol. 2003, 91, 1311–1315. [Google Scholar] [CrossRef]
- Kouvari, M.; Panagiotakos, D.B.; Chrysohoou, C.; Georgousopoulou, E.N.; Tousoulis, D.; Pitsavos, C. Sex-Related Differences of the Effect of Lipoproteins and Apolipoproteins on 10-Year Cardiovascular Disease Risk; Insights from the ATTICA Study (2002–2012). Molecules 2020, 25, 1506. [Google Scholar] [CrossRef]
- Vigna, L.; Tirelli, A.S.; Gaggini, M.; Di Piazza, S.; Tomaino, L.; Turolo, S.; Moroncini, G.; Chatzianagnostou, K.; Bamonti, F.; Vassalle, C. Insulin Resistance and Cardiometabolic Indexes: Comparison of Concordance in Working-Age Subjects with Overweight and Obesity. Endocrine 2022, 77, 1–11. [Google Scholar] [CrossRef]
- Lemieux, I.; Lamarche, B.; Couillard, C.; Pascot, A.; Cantin, B.; Bergeron, J.; Dagenais, G.R.; Després, J.-P. Total Cholesterol/HDL Cholesterol Ratio vrequiring extensive reorganization and re-writings LDL Cholesterol/HDL Cholesterol Ratio as Indices of Ischemic Heart Disease Risk in Men: The Quebec Cardiovascular Study. Arch. Intern. Med. 2001, 161, 2685–2692. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Bhattacharjee, J.; Bhatnagar, M.K.; Tyagi, S.; Delhi, N. Atherogenic Index of Plasma, Castelli Risk Index and Atherogenic Coefficient-New Parameters in Assessing Cardiovascular Risk. Int. J. Pharm. Biol. Sci. 2013, 3, 359–364. [Google Scholar]
- Nair, D.; Carrigan, T.P.; Curtin, R.J.; Popovic, Z.B.; Kuzmiak, S.; Schoenhagen, P.; Flamm, S.D.; Desai, M.Y. Association of Total Cholesterol/High-density Lipoprotein Cholesterol Ratio with Proximal Coronary Atherosclerosis Detected by Multislice Computed Tomography. Prev. Cardiol. 2009, 12, 19–26. [Google Scholar] [CrossRef]
- Toth, P.P. Triglyceride-Rich Lipoproteins as a Causal Factor for Cardiovascular Disease. Vasc. Health Risk Manag. 2016, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Castañer, O.; Pintó, X.; Subirana, I.; Amor, A.J.; Ros, E.; Hernáez, Á.; Martínez-González, M.Á.; Corella, D.; Salas-Salvadó, J.; Estruch, R. Remnant Cholesterol, Not LDL Cholesterol, Is Associated with Incident Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 76, 2712–2724. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.H.; Khokhar, A.A.; Massaro, J.M.; Lirette, S.T.; Griswold, M.E.; Martin, S.S.; Blaha, M.J.; Kulkarni, K.R.; Correa, A.; D’Agostino, R.B.; et al. Remnant Lipoprotein Cholesterol and Incident Coronary Heart Disease: The Jackson Heart and Framingham Offspring Cohort Studies. J Am Heart Assoc. 2016, 5, e002765. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Obata, J.; Hirano, M.; Kitta, Y.; Fujioka, D.; Saito, Y.; Kawabata, K.; Watanabe, K.; Watanabe, Y.; Mishina, H. Predictive Value of Remnant Lipoprotein for Cardiovascular Events in Patients with Coronary Artery Disease after Achievement of LDLholesterol Goals. Atherosclerosis 2011, 218, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Varbo, A.; Freiberg, J.J.; Nordestgaard, B.G. Extreme nonfasting remnant cholesterol vs extreme LDL cholesterol as contributors to cardiovascular disease and all-cause mortality in 90000 individuals from the general population. Clin. Chem. 2015, 61, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, A.M.; Langsted, A.; Varbo, A.; Bang, L.E.; Kamstrup, P.R.; Nordestgaard, B.G. Increased Remnant Cholesterol Explains Part of Residual Risk of All-Cause Mortality in 5414 Patients with Ischemic Heart Disease. Clin. Chem. 2016, 62, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-H.; Li, S.; Cao, Y.-X.; Guo, Y.-L.; Zhu, C.-G.; Wu, N.-Q.; Li, J.-J. Association of Triglyceride-Rich Lipoprotein-Cholesterol with Recurrent Cardiovascular Events in Statin-Treated Patients According to Different Inflammatory Status. Atherosclerosis 2021, 330, 29–35. [Google Scholar] [CrossRef]
- Fujioka, Y.; Ishikawa, Y. Remnant lipoproteins as strong key particles to atherogenesis. J. Atheroscler. Thromb. 2009, 16, 145–154. [Google Scholar] [CrossRef]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef]
- Su, X.; Kong, Y.; Peng, D. Evidence for Changing Lipid Management Strategy to Focus on Non-High Density Lipoprotein Cholesterol. Lipids Health Dis. 2019, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Kao, T.W.; Chang, P.K.; Chen, W.L.; Wu, L.W. Atherogenic index of plasma as predictors for metabolic syndrome, hy-pertension and diabetes mellitus in Taiwan citizens: A 9-year longitudinal study. Sci. Rep. 2021, 11, 9900. [Google Scholar] [CrossRef]
- Khakurel, G.; Kayastha, R.; Chalise, S.; Karki, P.K. Atherogenic Index of Plasma in Postmenopausal Women. J. Nepal. Health Res. Counc. 2018, 16, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Shi, G.; Xue, S.; Lu, W. The atherogenic index of plasma is a strong and independent predictor for coronary artery dis-ease in the Chinese Han population. Medicine (Baltimore) 2017, 96, e8058. [Google Scholar] [CrossRef] [PubMed]
- Hartopo, A.B.; Arso, I.A.; Setianto, B.Y. Low Plasma Atherogenic Index Associated with Poor Prognosis in Hospitalized Patients with Acute Myocardial Infarction. Acta Medica Indones. 2016, 48, 106–113. [Google Scholar]
- Shin, H.R.; Song, S.; Cho, J.A.; Ly, S.Y. Atherogenic Index of Plasma and Its Association with Risk Factors of Coronary Artery Disease and Nutrient Intake in Korean Adult Men: The 2013–2014 KNHANES. Nutrients 2022, 14, 1071. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, Q.; Sun, L.; Bao, T.; Dai, Z. Atherogenic Index in Type 2 Diabetes and Its Relationship with Chronic Microvascular Complications. Int. J. Endocrinol. 2018, 2018, 1765835. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zhou, K.; Li, Y.; Cheng, W.; Wang, Z.; Wang, J.; Gao, F.; Yang, L.; Xu, Y.; Wu, Y.; et al. The atherogenic index of plasma plays an important role in predicting the prognosis of type 2 diabetic subjects undergoing percutaneous coronary intervention: Results from an observational cohort study in China. Cardiovasc. Diabetol. 2020, 19, 23. [Google Scholar] [CrossRef]
- Zhou, K.; Qin, Z.; Tian, J.; Cui, K.; Yan, Y.; Lyu, S. The Atherogenic Index of Plasma: A Powerful and Reliable Predictor for Coronary Artery Disease in Patients with Type 2 Diabetes. Angiology 2021, 72, 934–941. [Google Scholar] [CrossRef]
- Mangalesh, S.; Yadav, P.; Dudani, S.; Mahesh, N.K. Atherogenic index of plasma predicts coronary artery disease severity and major adverse cardiac events in absence of conventional risk factors. Coron. Artery Dis. 2022, 33, 523–530. [Google Scholar] [CrossRef]
- Shui, X.; Chen, Z.; Wen, Z.; Tang, L.; Tang, W.; Liao, Y.; Wu, Z.; Chen, L. Association of Atherogenic Index of Plasma With Angiographic Progression in Patients With Suspected Coronary Artery Disease. Angiology 2022, 1, 33197221080911. [Google Scholar] [CrossRef]
- Zhan, Y.; Xu, T.; Tan, X. Two parameters reflect lipid-driven inflammatory state in acute coronary syndrome: Atherogenic index of plasma, neutrophil-lymphocyte ratio. BMC Cardiovasc. Disord. 2016, 16, 96. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Williams, K.; Contois, J.H.; Monroe, H.M.; McQueen, M.J.; de Graaf, J.; Furberg, C.D. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Varbo, A.; Freiberg, J.J.; Nordestgaard, B.G. Remnant cholesterol and myocardial infarction in normal weight, overweight, and obese individuals from the Copenhagen General Population Study. Clin. Chem. 2018, 64, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Bittner, V.; Hardison, R.; Kelsey, S.F.; Weiner, B.H.; Jacobs, A.K.; Sopko, G. Non–High-Density Lipoprotein Cholesterol Levels Predict Five-Year Outcome in the Bypass Angioplasty Revascularization Investigation (BARI). Circulation 2002, 106, 2537–2542. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Reaven, G.; Abbasi, F.; Lamendola, C.; Saad, M.; Waters, D.; Simon, J.; Krauss, R.M. Is There a Simple Way to Identify Insulin-Resistant Individuals at Increased Risk of Cardiovascular Disease? Am. J. Cardiol. 2005, 96, 399–404. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Calabresi, L.; Franceschini, G.; Baldassarre, D.; Amato, M.; Johansson, J.; Salvetti, M.; Monteduro, C.; Zulli, R.; Muiesan, M.L.; et al. Cardiovascular status of carriers of the apolipoprotein A-I(Milano) mutant: The Limone sul Garda study. Circulation 2001, 103, 1949–1954. [Google Scholar] [CrossRef]
- Bielicki, J.K.; Oda, M.N. Apolipoprotein A-I(Milano) and apolipoprotein A-I(Paris) exhibit an antioxidant activity distinct from that of wild-type apolipoprotein A-I. Biochemistry 2002, 41, 2089–2096. [Google Scholar] [CrossRef]
- Zhang, P.; Su, Q.; Ye, X.; Guan, P.; Chen, C.; Hang, Y.; Dong, J.; Xu, Z.; Hu, W. Trends in LDL and Non-HDL Levels with Age. Aging Dis. 2020, 11, 1046–1057. [Google Scholar] [CrossRef]
- Langlois, M.R.; Chapman, M.J.; Cobbaert, C.; Mora, S.; Remaley, A.T.; Ros, E.; Watts, G.F.; Borén, J.; Baum, H.; Bruckert, E.; et al. Quantifying Atherogenic Lipoproteins: Current and Future Challenges in the Era of Personalized Medicine and Very Low Concentrations of LDL Cholesterol. A Consensus Statement from EAS and EFLM. Clin. Chem. 2018, 64, 1006–1033. [Google Scholar]
| Endothelial Dysfunction | Fatty Streak | Fibrous Plaque | Vulnerable Plaque | Plaque Erosion, Rupture, Thrombosis |
|---|---|---|---|---|
| - Endothelial activation - Upregulation of adhesion molecules - Increased vascular permeability - Monocyte and other cell recruitment and infiltration - Impaired vasodilation (reduced NO) - ROS and inflammatory mediators - LDL intake/accumulation in the arterial intima | - VSMC proliferation - Lipid accumulation - Foam cell development - T lymphocyte infiltration - Platelet aggregation - Macrophage activation - Inflammation | - Deposition of extracellular connective tissue matrix - Fibrous cap formation - Lipid-laden foam cell core containing lipid necrotic debris and calcium - Vascular remodeling - Luminal narrowing - Flow abnormalities - Reduced oxygen supply inflammatory mediated | - Proteolytic enzymes (MMPs) - Intraplaque hemorrhage - Thinning of the fibrous cap - Microcalcification - Necrotic-rich lipid core (apoptosis and necrosis) - Inflammatory mediators | - Platelet aggregation - Fibrin polymerization - Inflammatory coagulation and proteolysis mediators - Thrombosis - Acute coronary syndrome |
| Calcific plaque Dense calcification deposition |
| Index (Acronym) | Formula | Threshold Values (mg/dL) |
|---|---|---|
| Non-HDL Cholesterol (Non-HDL-C) | Total cholesterol (mg/dL)–High-density lipoproteins (mg/dL) | <130 |
| Castelli risk index 1 (CRI-I) | Total cholesterol (mg/dL)/High-density lipoproteins (mg/dL) | Males: <5; females <4.5 |
| Castelli risk index 2 (CRI-II) | Low-density lipoproteins (mg/dL)/High-density lipoproteins (mg/dL) | Males: <3.5; females <3 |
| Triglyceride-Rich Lipoprotein Cholesterol (TRL-C) | Non-HDL–Low-density lipoproteins (mg/dL) | <30 |
| Atherogenic index of plasma (AIP) | Log (Triglycerides (mg/dL)/High-density lipoproteins (mg/dL) | ≤0.11 |
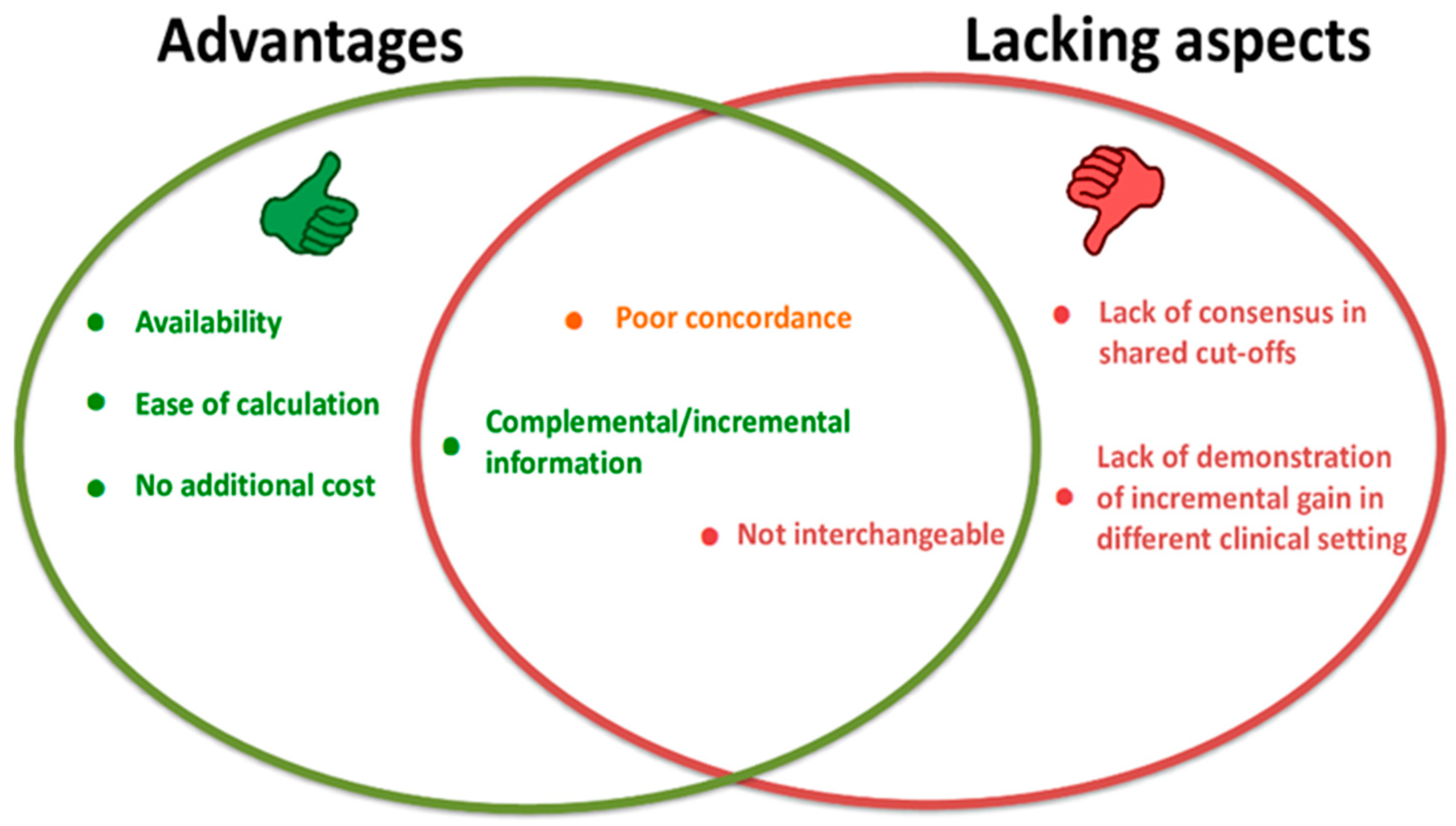
| Strengths | Shortcomings |
|---|---|
| Independence from TG levels | Lack of distinction between remnant-C and LDL |
| Easily available, high throughput, and fast turnaround time | Arbitrary risk cut-offs |
| Calculation in the nonfasting state | Dependency from HDL measurement errors in hypertriglyceridemia, which may influence the calculation of non-HDL |
| Inclusion of remnant-C | Better identification of confounding factors, interferences |
| No additional cost above conventional lipid testing in terms of time and assay | Lack of familiarity for most practitioners |
| Additive utility beyond existing markers (residual risk) | |
| Advised when LDL is low or TG are increased | |
| Associations with CV risk and treatment target (risk reduction proportional to the degree of non-HDL lowering) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaggini, M.; Gorini, F.; Vassalle, C. Lipids in Atherosclerosis: Pathophysiology and the Role of Calculated Lipid Indices in Assessing Cardiovascular Risk in Patients with Hyperlipidemia. Int. J. Mol. Sci. 2023, 24, 75. https://doi.org/10.3390/ijms24010075
Gaggini M, Gorini F, Vassalle C. Lipids in Atherosclerosis: Pathophysiology and the Role of Calculated Lipid Indices in Assessing Cardiovascular Risk in Patients with Hyperlipidemia. International Journal of Molecular Sciences. 2023; 24(1):75. https://doi.org/10.3390/ijms24010075
Chicago/Turabian StyleGaggini, Melania, Francesca Gorini, and Cristina Vassalle. 2023. "Lipids in Atherosclerosis: Pathophysiology and the Role of Calculated Lipid Indices in Assessing Cardiovascular Risk in Patients with Hyperlipidemia" International Journal of Molecular Sciences 24, no. 1: 75. https://doi.org/10.3390/ijms24010075
APA StyleGaggini, M., Gorini, F., & Vassalle, C. (2023). Lipids in Atherosclerosis: Pathophysiology and the Role of Calculated Lipid Indices in Assessing Cardiovascular Risk in Patients with Hyperlipidemia. International Journal of Molecular Sciences, 24(1), 75. https://doi.org/10.3390/ijms24010075








