Dysferlin Deficiency Results in Myofiber-Type Specific Differences in Abundances of Calcium-Handling and Glycogen Metabolism Proteins
Abstract
1. Introduction
2. Results
2.1. Phenotype
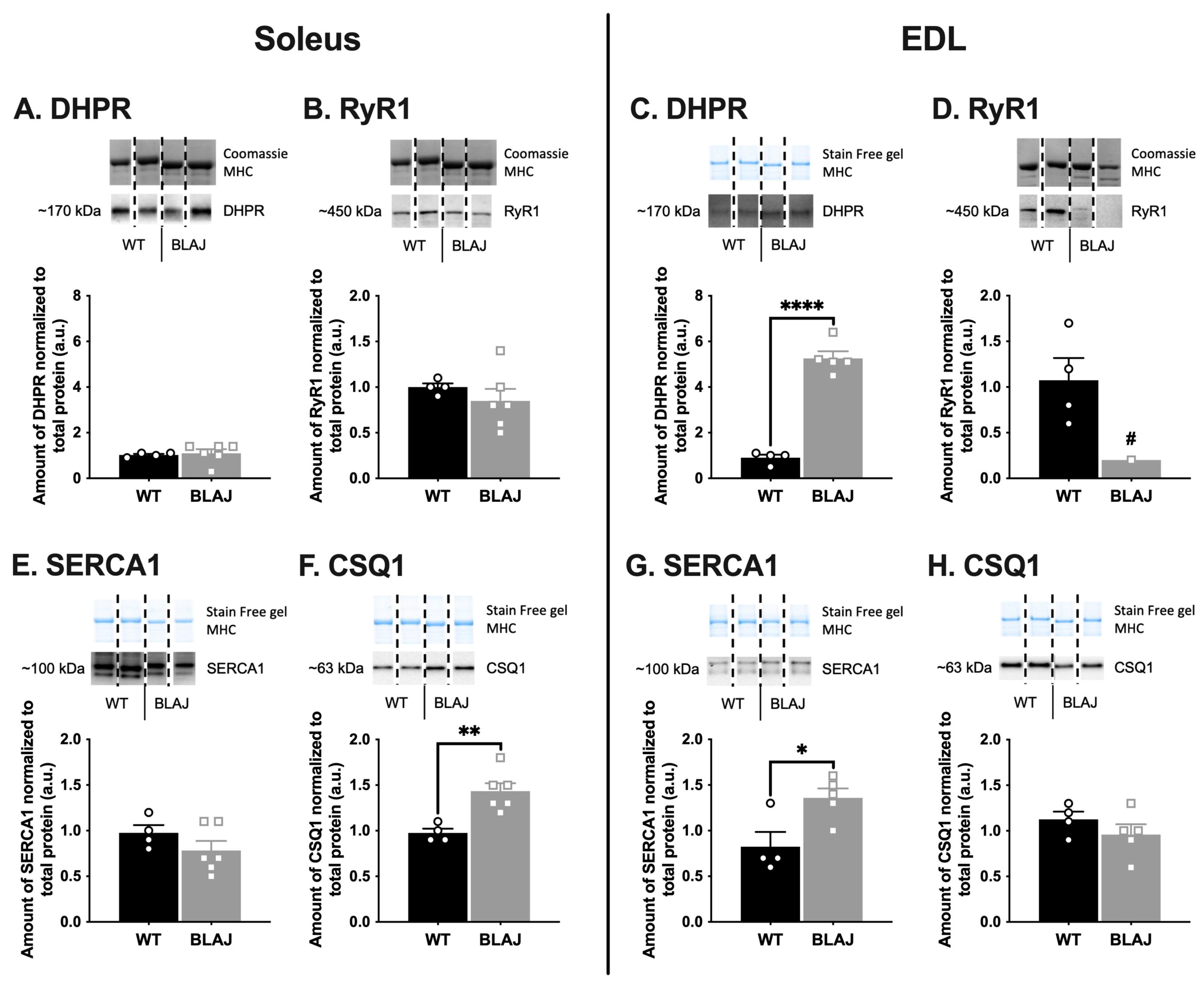
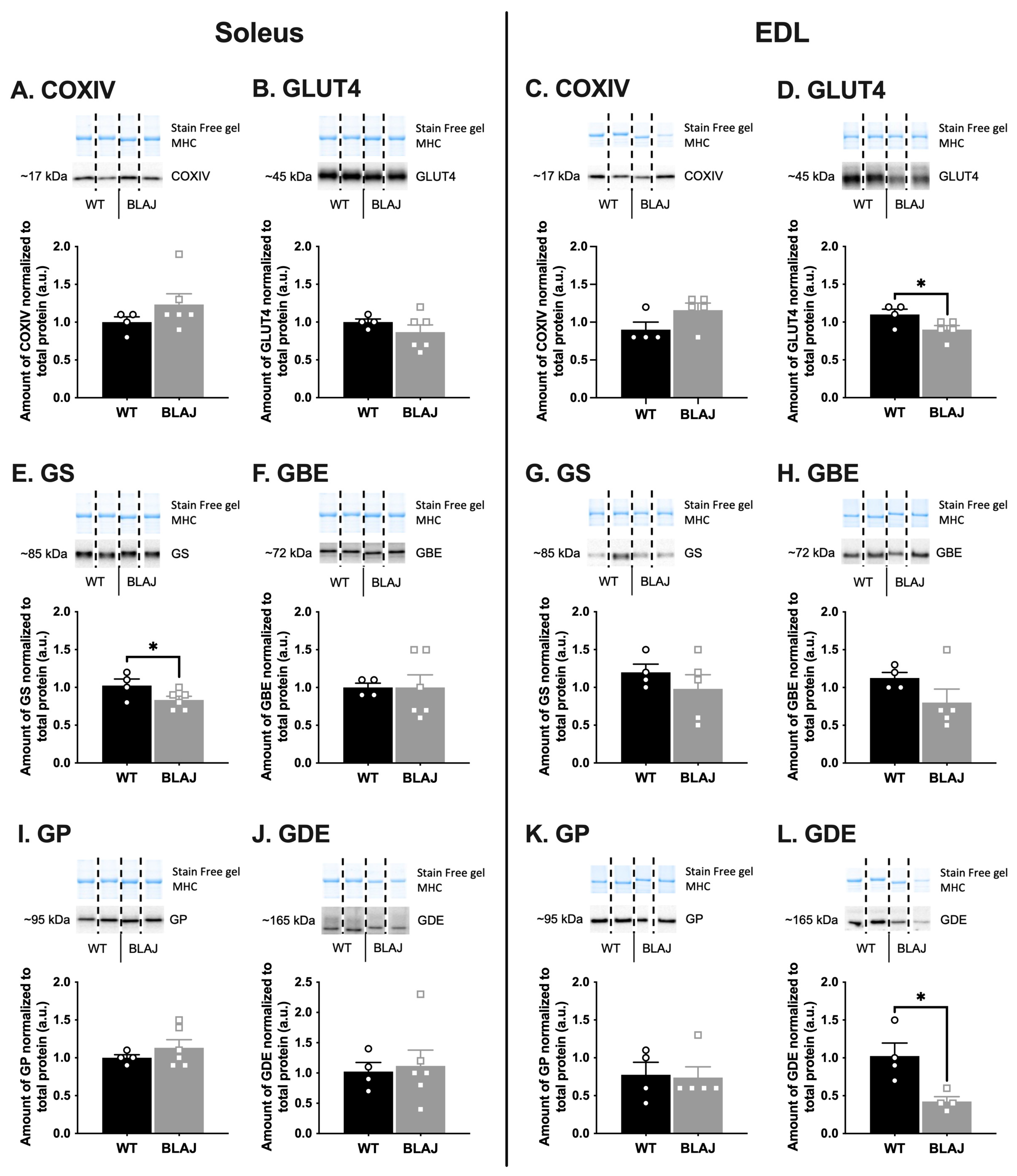
2.2. Quantification of Proteins Related to Ca2+ Handling and Glucose/Glycogen Metabolism in Soleus and EDL Muscles of Mice Aged 10 Months
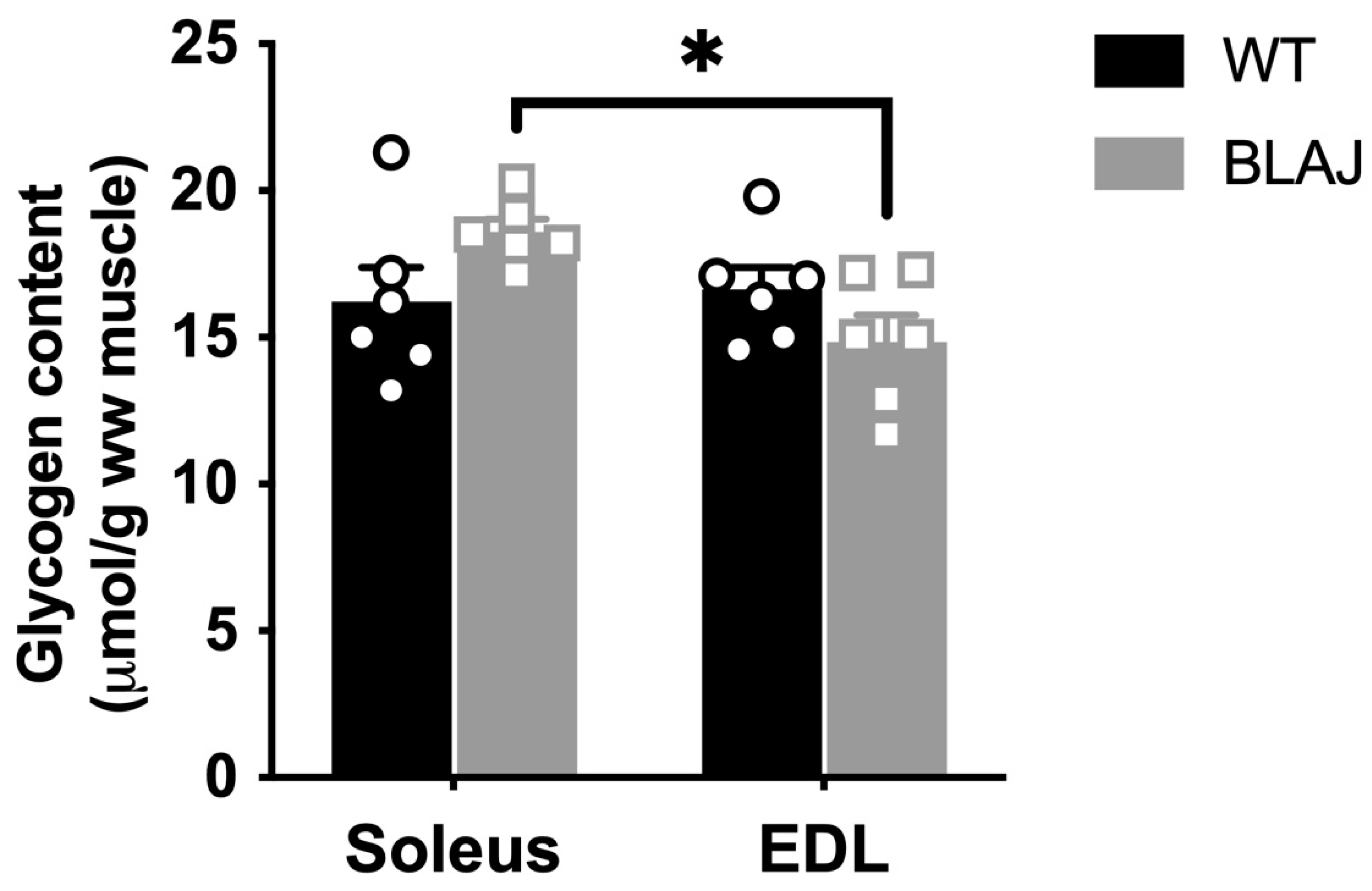
2.3. Glycogen Content
2.4. Ca2+ Activation of the Contractile Apparatus from Individual Slow- and Fast-Twitch Myofibers from Mice Aged Three Months
3. Discussion
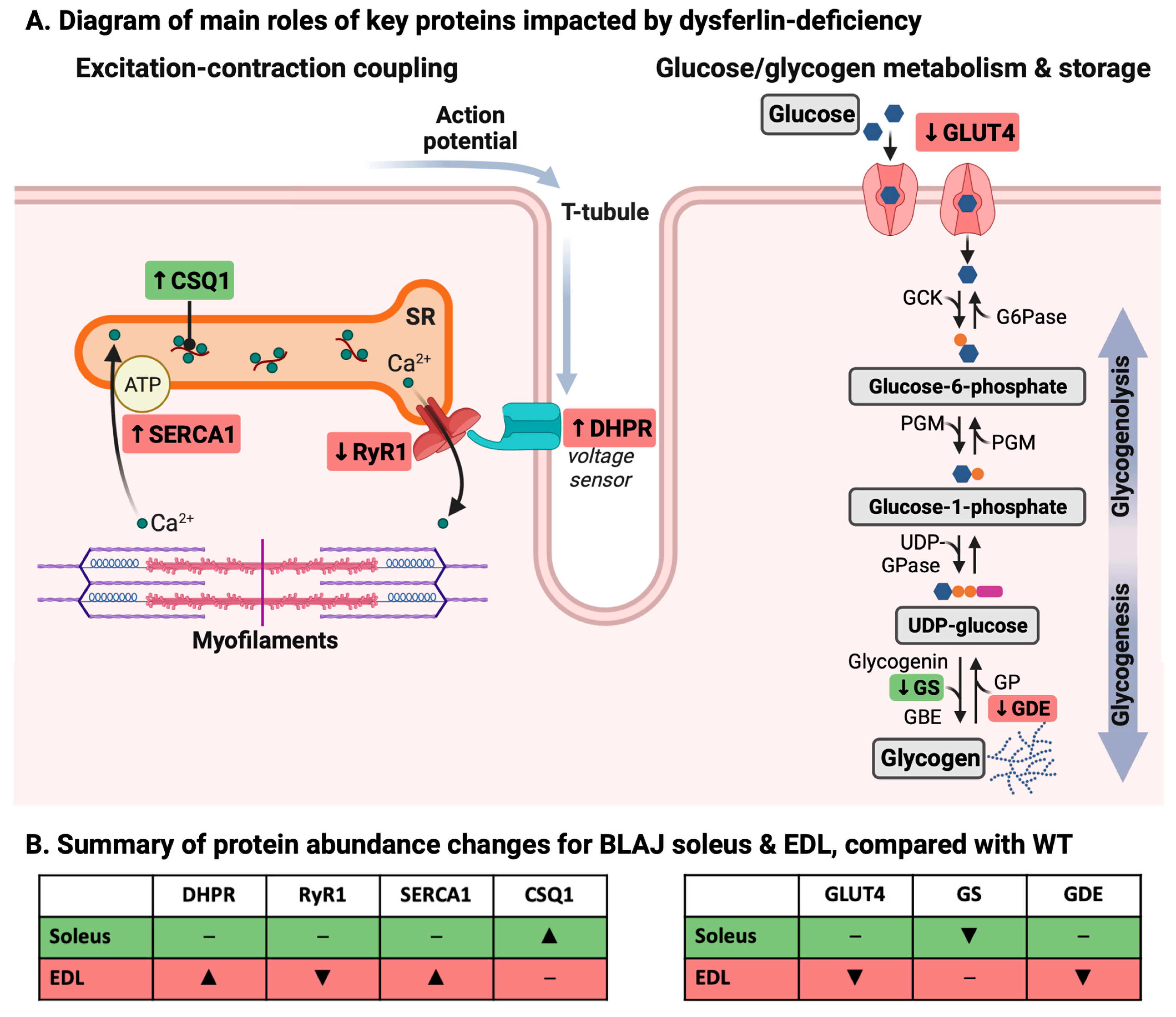
3.1. Dysferlin Deficiency Differentially Alters the Levels of Ca2+ Handling and Glucose/Glycogen Metabolism Proteins in BLAJ Soleus and EDL Muscles at 10 Months of Age
3.1.1. Ca2+-Handling Proteins
3.1.2. Glucose/Glycogen Metabolism Proteins
3.2. Dysferlin Deficiency Does Not Disrupt Contractile Apparatus Ca2+ Activation in Mice at Three Months
4. Materials and Methods
4.1. Animals and Muscle Dissection
4.2. Western Immunoblotting to Quantify Various Proteins in Intact Soleus and EDL Muscles
4.3. Glycogen Assay
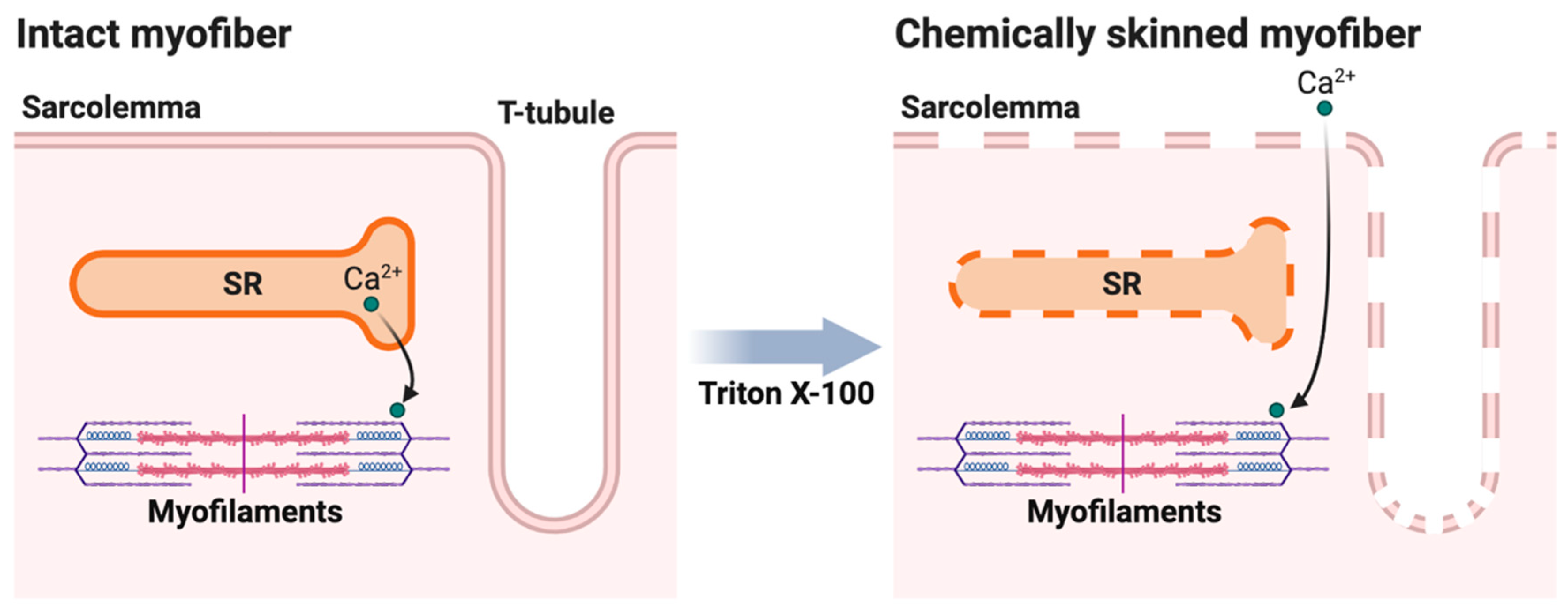
4.4. Measurement of Contractile Apparatus Ca2+-Activation Properties in Isolated Myofibers
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Amato, A.A.; Brown, R.H., Jr. Dysferlinopathies. Handb. Clin. Neurol. 2011, 101, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, A.M.; González-Jamett, A.M.; Cea, L.A.; Bevilacqua, J.A.; Caviedes, P. Dysferlin function in skeletal muscle: Possible pathological mechanisms and therapeutical targets in dysferlinopathies. Exp. Neurol. 2016, 283, 246–254. [Google Scholar] [CrossRef]
- Cenacchi, G.; Fanin, M.; De Giorgi, L.B.; Angelini, C. Ultrastructural changes in dysferlinopathy support defective membrane repair mechanism. J. Clin. Pathol. 2005, 58, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Fanin, M.; Nascimbeni, A.C.; Angelini, C. Muscle atrophy, ubiquitin–proteasome, and autophagic pathways in dysferlinopathy. Muscle Nerve 2014, 50, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Straub, V.; Murphy, A.; Udd, B.; LGMD Workshop Study Group. 229th ENMC international workshop: Limb girdle muscular dystrophies—Nomenclature and reformed classification Naarden, The Netherlands, 17–19 March 2017. Neuromuscul. Disord. 2018, 28, 702–710. [Google Scholar] [CrossRef]
- Liu, J.; Aoki, M.; Illa, I.; Wu, C.; Fardeau, M.; Angelini, C.; Serrano, C.; Urtizberea, J.A.; Hentati, F.; Hamida, M.B.; et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat. Genet. 1998, 20, 31–36. [Google Scholar] [CrossRef]
- Harris, E.; Bladen, C.L.; Mayhew, A.; James, M.; Bettinson, K.; Moore, U.; Smith, F.E.; Rufibach, L.; Cnaan, A.; Bharucha-Goebel, D.X.; et al. The Clinical Outcome Study for dysferlinopathy: An international multicenter study. Neurol. Genet. 2016, 2, e89. [Google Scholar] [CrossRef]
- van Putten, M.; Lloyd, E.M.; de Greef, J.C.; Raz, V.; Willmann, R.; Grounds, M.D. Mouse models for muscular dystrophies: An overview. Dis. Model. Mech. 2020, 13, dmm043562. [Google Scholar] [CrossRef]
- Grounds, M.D.; Terrill, J.R.; Radley-Crabb, H.G.; Robertson, T.; Papadimitriou, J.; Spuler, S.; Shavlakadze, T. Lipid accumulation in dysferlin-deficient muscles. Am. J. Pathol. 2014, 184, 1668–1676. [Google Scholar] [CrossRef]
- Demonbreun, A.R.; Rossi, A.E.; Alvarez, M.G.; Swanson, K.E.; Deveaux, H.K.; Earley, J.U.; Hadhazy, M.; Vohra, R.; Walter, G.A.; Pytel, P.; et al. Dysferlin and myoferlin regulate transverse tubule formation and glycerol sensitivity. Am. J. Pathol. 2014, 184, 248–259. [Google Scholar] [CrossRef]
- Fanin, M.; Angelini, C. Progress and challenges in diagnosis of dysferlinopathy. Muscle Nerve 2016, 54, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Haynes, V.R.; Keenan, S.N.; Bayliss, J.; Lloyd, E.M.; Meikle, P.J.; Grounds, M.D.; Watt, M.J. Dysferlin deficiency alters lipid metabolism and remodels the skeletal muscle lipidome in mice. J. Lipid Res. 2019, 60, 1350–1364. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Welc, S.S.; Wehling-Henricks, M. Immunobiology of inherited muscular dystrophies. Compr. Physiol. 2018, 8, 1313–1356. [Google Scholar] [CrossRef]
- Terrill, J.R.; Radley-Crabb, H.G.; Iwasaki, T.; Lemckert, F.A.; Arthur, P.G.; Grounds, M.D. Oxidative stress and pathology in muscular dystrophies: Focus on protein thiol oxidation and dysferlinopathies. FEBS J. 2013, 280, 4149–4164. [Google Scholar] [CrossRef]
- Diaz-Manera, J.; Fernandez-Torron, R.; Llauger, J.; James, M.K.; Mayhew, A.; Smith, F.E.; Moore, U.R.; Blamire, A.M.; Carlier, P.G.; Rufibach, L.; et al. Muscle MRI in patients with dysferlinopathy: Pattern recognition and implications for clinical trials. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1071–1081. [Google Scholar] [CrossRef]
- Bulankina, A.V.; Thoms, S. Functions of vertebrate ferlins. Cells 2020, 9, 534. [Google Scholar] [CrossRef]
- Bansal, D.; Miyake, K.; Vogel, S.S.; Groh, S.; Chen, C.C.; Williamson, R.; McNeil, P.L.; Campbell, K.P. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 2003, 423, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Glover, L.; Brown, R.H. Dysferlin in membrane trafficking and patch repair. Traffic 2007, 8, 785–794. [Google Scholar] [CrossRef]
- Ampong, B.N.; Imamura, M.; Matsumiya, T.; Yoshida, M.; Takeda, S. Intracellular localization of dysferlin and its association with the dihydropyridine receptor. Acta Myol. 2005, 24, 134–144. [Google Scholar]
- Roche, J.A.; Ru, L.W.; O’Neill, A.M.; Resneck, W.G.; Lovering, R.M.; Bloch, R.J. Unmasking potential intracellular roles for dysferlin through improved immunolabeling methods. J. Histochem. Cytochem. 2011, 59, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Klinge, L.; Harris, J.; Sewry, C.; Charlton, R.; Anderson, L.; Laval, S.; Chiu, Y.H.; Hornsey, M.; Straub, V.; Barresi, R.; et al. Dysferlin associates with the developing T-tubule system in rodent and human skeletal muscle. Muscle Nerve 2010, 41, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Flix, B.; de la Torre, C.; Castillo, J.; Casal, C.; Illa, I.; Gallardo, E. Dysferlin interacts with calsequestrin-1, myomesin-2 and dynein in human skeletal muscle. Int. J. Biochem. Cell Biol. 2013, 45, 1927–1938. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.P.; Ward, C.W.; Bloch, R.J. Dysferlin at transverse tubules regulates Ca2+ homeostasis in skeletal muscle. Front. Physiol. 2014, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Schoewel, V.; Keller, S.; Kunz, S.; Boschmann, M.; Herrmann, C.; Mastrobuoni, G.; Al-Hasani, H.; Kempa, S.; Spuler, S. The metabolic footprint of dysferlinopathy (P2.048). Neurology 2015, 84. [Google Scholar]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Lloyd, E.M.; Xu, H.Y.; Murphy, R.M.; Grounds, M.D.; Pinniger, G.J. Dysferlin-deficiency has greater impact on function of slow muscles, compared with fast, in aged BLAJ mice. PLoS ONE 2019, 14, e0214908. [Google Scholar] [CrossRef]
- Augusto, V.; Padovani, C.R.; Campos, G.E.R. Skeletal muscle fiber types in C57BL6J mice. J. Morphol. Sci. 2004, 21, 89–94. [Google Scholar]
- Shin, D.W.; Pan, Z.; Kim, E.K.; Lee, J.M.; Bhat, M.B.; Parness, J.; Kim, D.H.; Ma, J.J. A retrograde signal from calsequestrin for the regulation of store-operated Ca2+ entry in skeletal muscle. J. Biol. Chem. 2003, 278, 3286–3292. [Google Scholar] [CrossRef]
- Baylor, S.M.; Hollingworth, S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J. Physiol. 2003, 551, 125–138. [Google Scholar] [CrossRef]
- Delbono, O.; Meissner, G. Sarcoplasmic reticulum Ca2+ release in rat slow- and fast-twitch muscles. J. Membr. Biol. 1996, 151, 123–130. [Google Scholar] [CrossRef]
- Shaikh, S.A.; Sahoo, S.K.; Periasamy, M. Phospholamban and sarcolipin: Are they functionally redundant or distinct regulators of the Sarco(Endo)Plasmic Reticulum Calcium ATPase? J. Mol. Cell. Cardiol. 2016, 91, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.P.; Ziman, A.P.; Mueller, A.L.; Muriel, J.M.; Kleinhans-Welte, E.; Gumerson, J.D.; Vogel, S.S.; Ward, C.W.; Roche, J.A.; Bloch, R.J. Dysferlin stabilizes stress-induced Ca2+ signaling in the transverse tubule membrane. Proc. Natl. Acad. Sci. USA 2013, 110, 20831–20836. [Google Scholar] [CrossRef] [PubMed]
- Lukyanenko, V.; Muriel, J.M.; Bloch, R.J. Coupling of excitation to Ca2+ release is modulated by dysferlin. J. Physiol. 2017, 595, 5191–5207. [Google Scholar] [CrossRef]
- Rawat, R.; Cohen, T.V.; Ampong, B.; Francia, D.; Henriques-Pons, A.; Hoffman, E.P.; Nagaraju, K. Inflammasome up-regulation and activation in dysferlin-deficient skeletal muscle. Am. J. Pathol. 2010, 176, 2891–2900. [Google Scholar] [CrossRef]
- Han, R. Muscle membrane repair and inflammatory attack in dysferlinopathy. Skelet. Muscle 2011, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Marr, L.; Biswas, D.; Daly, L.A.; Browning, C.; Vial, S.C.M.; Maskell, D.P.; Hudson, C.; Bertrand, J.A.; Pollard, J.; Ranson, N.A.; et al. Mechanism of glycogen synthase inactivation and interaction with glycogenin. Nat. Commun. 2022, 13, 3372. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Tunison, K.; Mitsche, M.A.; McDonald, J.G.; Garg, A. Insights into lipid accumulation in skeletal muscle in dysferlin-deficient mice. J. Lipid Res. 2019, 60, 2057–2073. [Google Scholar] [CrossRef]
- Murphy, R.M.; Xu, H.Y.; Latchman, H.; Larkins, N.T.; Gooley, P.R.; Stapleton, D.I. Single fiber analyses of glycogen-related proteins reveal their differential association with glycogen in rat skeletal muscle. Am. J. Physiol.—Cell Physiol. 2012, 303, C1146–C1155. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lamb, G.D.; Murphy, R.M. Changes in contractile and metabolic parameters of skeletal muscle as rats age from 3 to 12 months. J. Muscle Res. Cell Motil. 2017, 38, 405–420. [Google Scholar] [CrossRef]
- Lamboley, C.R.; Pearce, L.; Seng, C.; Meizoso-Huesca, A.; Singh, D.P.; Frankish, B.P.; Kaura, V.; Lo, H.P.; Ferguson, C.; Allen, P.D.; et al. Ryanodine receptor leak triggers fiber Ca2+ redistribution to preserve force and elevate basal metabolism in skeletal muscle. Sci. Adv. 2021, 7, eabi7166. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, D.G.; Williams, D.A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J. Physiol. 1981, 317, 281–302. [Google Scholar] [CrossRef] [PubMed]
- Fink, R.H.A.; Stephenson, D.G.; Williams, D.A. Calcium and strontium activation of single skinned muscle fibres of normal and dystrophic mice. J. Physiol. 1986, 373, 513–525. [Google Scholar] [CrossRef] [PubMed]

| 3 Months | 10 Months | |||
|---|---|---|---|---|
| WT (n = 2) | BLAJ (n = 2) | WT (n = 8) | BLAJ (n = 9) | |
| Body mass (g) | 25.7 ± 0.4 | 24.1 ± 0.9 | 33.3 ± 0.7 | 34.4 ± 0.6 |
| Slow-twitch myofiber CSA (μm2) | 927 ± 46 (3) | 1047 ± 58 (6) | ||
| Fast-twitch myofiber CSA (μm2) | 1205 ± 207 (6) | 922 ± 121 (7) | ||
| Soleus mass (mg/gBM) | 0.33 ± 0.01 | 0.41 ± 0.01 *** | ||
| EDL mass (mg/gBM) | 0.40 ± 0.01 | 0.40 ± 0.01 | ||
| Slow Myofibers | Fast Myofibers | |||
|---|---|---|---|---|
| WT (n = 3) | BLAJ (n = 6) | WT (n = 6) | BLAJ (n = 7) | |
| Maximum specific force (mN/mm2) | 371 ± 6 | 318 ± 19 | 218 ± 24 | 312 ± 65 |
| Hillslope | 5.71 ± 0.99 | 5.05 ± 0.47 | 4.62 ± 0.37 | 5.16 ± 0.30 |
| pCa10 | 6.65 ± 0.03 | 6.65 ± 0.03 | 6.45 ± 0.03 | 6.43 ± 0.01 |
| pCa50 | 6.48 ± 0.03 | 6.47 ± 0.01 | 6.24 ± 0.03 | 6.25 ± 0.01 |
| pCa90 | 6.30 ± 0.04 | 6.24 ± 0.01 | 6.02 ± 0.05 | 6.05 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lloyd, E.M.; Pinniger, G.J.; Grounds, M.D.; Murphy, R.M. Dysferlin Deficiency Results in Myofiber-Type Specific Differences in Abundances of Calcium-Handling and Glycogen Metabolism Proteins. Int. J. Mol. Sci. 2023, 24, 76. https://doi.org/10.3390/ijms24010076
Lloyd EM, Pinniger GJ, Grounds MD, Murphy RM. Dysferlin Deficiency Results in Myofiber-Type Specific Differences in Abundances of Calcium-Handling and Glycogen Metabolism Proteins. International Journal of Molecular Sciences. 2023; 24(1):76. https://doi.org/10.3390/ijms24010076
Chicago/Turabian StyleLloyd, Erin M., Gavin J. Pinniger, Miranda D. Grounds, and Robyn M. Murphy. 2023. "Dysferlin Deficiency Results in Myofiber-Type Specific Differences in Abundances of Calcium-Handling and Glycogen Metabolism Proteins" International Journal of Molecular Sciences 24, no. 1: 76. https://doi.org/10.3390/ijms24010076
APA StyleLloyd, E. M., Pinniger, G. J., Grounds, M. D., & Murphy, R. M. (2023). Dysferlin Deficiency Results in Myofiber-Type Specific Differences in Abundances of Calcium-Handling and Glycogen Metabolism Proteins. International Journal of Molecular Sciences, 24(1), 76. https://doi.org/10.3390/ijms24010076







