Molecular Cytogenetic Analysis of the Introgression between Agropyron cristatum P Genome and Wheat Genome
Abstract
:1. Introduction
2. Results
2.1. The Acquisition of Disease Resistance-Related A. cristatum Genes of the Translocation Line WAT655
2.2. The Preliminary Analysis of the Introgression between A. cristatum P Genome and Wheat Genome in the BC5F2 and BC5F2:3 Genetic Populations of the Translocation Line WAT655 Using the Molecular Markers
2.3. The Identification of the Introgression between A. cristatum P Genome and Wheat Genome in the Translocation Line WAT655
2.4. Molecular Cytogenetic Analysis of the Physical Positions of Three Disease Resistance-Related Genes
2.5. Tracing A. cristatum P Genomic Components of Breeding Lines Selected from Progenies of Distant Hybridization between A. cristatum and Wheat
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Molecular Cytogenetic Analysis
4.3. Evaluation of the Agronomic Traits of Wheat–A. cristatum Breeding Lines in the Field
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FISH | Fluorescence in situ hybridization |
| GISH | Genomic in situ hybridization |
| BAC | Bacterial artificial chromosome |
| KASP | Kompetitive allele-specific PCR |
| SD | Standard Deviation |
| Pst | Puccinia striiformis f. sp. tritici |
| TGW | Thousand grain weight |
| GNS | Grain number per spike |
| NCBI | National Center of Biotechnology Information |
| DAPI | 4,6-diamino-2-phenyl indole |
| IT | Infection type |
References
- United States Department of Agriculture Foreign Agricultural Service. World Agricultural Production (WAP). Circular Series. WAP 05-21. 2020. Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf (accessed on 11 May 2021).
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Friebe, B.; Jiang, J.; Raupp, W.J.; McIntosh, R.A.; Gill, B.S. Characterization of wheat-alien translocations conferring resistance to diseases and pests: Current status. Euphytica 1996, 91, 59–87. [Google Scholar] [CrossRef]
- Wellings, C.R. Global status of stripe rust: A review of historical and current threats. Euphytica 2011, 179, 129–141. [Google Scholar] [CrossRef]
- Beddow, J.M.; Pardey, P.G.; Chai, Y.; Hurley, T.M.; Kriticos, D.J.; Braun, H.J.; Park, R.F.; Cuddy, W.S.; Yonow, T. Research investment implications of shifts in the global geography of wheat stripe rust. Nat. Plants 2015, 1, 15132. [Google Scholar] [CrossRef]
- Wan, A.M.; Zhao, Z.H.; Chen, X.M.; He, Z.H.; Jin, S.L.; Jia, Q.Z.; Yao, G.; Yang, J.X.; Wang, B.T.; Li, G.B.; et al. Wheat stripe rust epidemic and virulence of Puccinia striiformis f. sp. tritici in China in 2002. Plant Dis. 2004, 88, 896–904. [Google Scholar]
- Chen, W.Q.; Wu, L.R.; Liu, T.G.; Xu, S.C.; Jin, S.L.; Peng, Y.L.; Wang, B.T. Race dynamics, diversity, and virulence evolution in Puccinia striiformis f. sp. tritici, the causal agent of wheat stripe rust in China from 2003 to 2007. Plant Dis. 2009, 93, 1093–1101. [Google Scholar]
- Dewey, D.R. The genomic system of classification as a guide to intergeneric hybridization with the perennial Triticeae. In Gene manipulation in Plant Improvement; Gustafson, J.P., Ed.; Plenum Press: New York, NY, USA, 1984; pp. 209–279. [Google Scholar]
- Dong, Y.C.; Zhou, R.H.; Xu, S.J.; Li, L.H.; Cauderon, Y.; Wang, R.R.C. Desirable characteristics in perennial Triticeae collected in China for wheat improvement. Hereditas 1992, 116, 175–178. [Google Scholar] [CrossRef]
- Cao, A.Z.; Xing, L.P.; Wang, X.Y.; Yang, X.M.; Wang, W.; Sun, Y.L.; Qian, C.; Ni, J.L.; Chen, Y.P.; Liu, D.J.; et al. Serine/threonine kinase gene Stpk-V, a key member of powdery mildew resistance gene Pm21, confers powdery mildew resistance in wheat. Proc. Natl. Acad. Sci. USA 2011, 108, 7727–7732. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.D.; You, C.F.; Hu, Y.; Chen, S.W.; Zhou, B.; Cao, A.Z.; Wang, X.E. Radiation-induced translocations with reduced Haynaldia villosa chromatin at the Pm21 locus for powdery mildew resistance in wheat. Mol. Breed. 2013, 31, 477–484. [Google Scholar] [CrossRef]
- Xing, L.P.; Hu, P.; Liu, J.Q.; Witek, K.; Zhou, S.; Xu, J.F.; Zhou, W.H.; Gao, L.; Huang, Z.P.; Zhang, R.Q.; et al. Pm21 from Haynaldia villosa encodes a CC-NBS-LRR protein conferring powdery mildew resistance in wheat. Mol. Plant 2018, 11, 874–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerechter-Amitai, Z.K.; Wahl, I.; Vardi, A.; Zohary, D. Transfer of stem rust seedling resistance from wild diploid einkorn to tetraploid durum wheat by means of a triploid hybrid bridge. Euphytica 1971, 20, 281–285. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Dyck, P.L.; The, T.T.; Cusick, J.; Milne, D.L. Cytogenetical studies in wheat XIII. Sr35, a third gene from Triticum monococcum for resistance to Puccinia graminis tritici. Z. Pflanzenzucht. 1984, 92, 1–14. [Google Scholar]
- Saintenac, C.; Zhang, W.J.; Salcedo, A.; Rousse, M.N.; Trick, H.N.; Akhunov, E.; Dubcovsky, J. Identification of wheat gene Sr35 that confers resistance to Ug99 stem rust race group. Science 2013, 341, 783–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steuernagel, B.; Periyannan, S.K.; Hernández-Pinzón, I.; Witek, K.; Rouse, M.N.; Yu, G.T.; Hatta, A.; Ayliffe, M.; Bariana, H.; Jones, J.D.G.; et al. Rapid cloning of disease-resistance genes in plants using mutagenesis and sequence capture. Nat. Biotechnol. 2016, 34, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Zhang, W.J.; Bolus, S.; Rouse, M.N.; Dubcovsky, J. Identification and characterization of wheat stem rust resistance gene Sr21 effective against the Ug99 race group at high temperature. PLoS Genet. 2018, 14, e1007287. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Nelson, J.C.; Sorrells, M.E. Mapping Yr28 and other genes for resistance to stripe rust in wheat. Crop Sci. 2000, 40, 1148–1155. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.Q.; Jia, J.Z.; Yang, H.; Zhang, B.S. SSR mapping of stripe rust resistance gene from Ae. tauschii. Hereditas 2008, 30, 491–494. [Google Scholar] [PubMed]
- Huang, L.; Zhang, L.Q.; Liu, B.L.; Yan, Z.H.; Zhang, B.; Zhang, H.G.; Zheng, Y.L.; Liu, D.C. Molecular tagging of a stripe rust resistance gene in Aegilops tauschii. Euphytica 2011, 179, 313–318. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, C.Z.; Yuan, C.L.; Zhang, L.Q.; Huang, L.; Wu, J.J.; Wang, J.R.; Zheng, Y.L.; Zhang, H.G.; Liu, D.C.; et al. Stripe rust resistance in Aegilops tauschii germplasm. Crop Sci. 2013, 53, 2014–2020. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Singh, R.P.; Lillemo, M.; He, X.Y.; Randhawa, M.S.; Huerta-Espino, J.; Singh, P.K.; Li, Z.K.; Lan, C.X. Two main stripe rust resistance genes identified in synthetic-derived wheat line Soru#1. Phytopathology 2018, 109, 120–126. [Google Scholar]
- Klymiuk, V.; Yaniv, E.; Huang, L.; Raats, D.; Fatiukha, A.; Chen, S.S.; Feng, L.H.; Frenkel, Z.; Krugman, T.; Lidzbarsky, G.; et al. Cloning of the wheat Yr15 resistance gene sheds light on the plant tandem kinase-pseudokinase family. Nat. Commun. 2018, 9, 3735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limin, A.E.; Fowler, D.B. An interspecific hybrid and amphiploid produced from Triticum aestivum crosses with Agropyron cristatum and Agropyron desertorum. Genome 1990, 33, 581–584. [Google Scholar] [CrossRef]
- Li, L.H.; Dong, Y.C.; Zhou, R.H.; Li, X.Q.; Li, P. Cytogenetics and self-fertility of hybrids between Triticum aestivum L. and Agropyron cristatum (L.) Gaertn. Acta Genet. Sin. 1995, 22, 109–114. [Google Scholar]
- Li, L.H.; Li, X.Q.; Li, P.; Dong, Y.C.; Zhao, G.S. Establishment of wheat-Agropyron cristatum alien addition lines. I. Cytology of F3, F2BC1, BC4, and BC3F1 progenies. Acta Genet. Sin. 1997, 24, 154–159. [Google Scholar]
- Wu, J.; Yang, X.M.; Wang, H.; Li, H.J.; Li, L.H.; Li, X.Q.; Liu, W.H. The introgression of chromosome 6P specifying for increased numbers of florets and kernels from Agropyron cristatum into wheat. Theor. Appl. Genet. 2006, 114, 13–20. [Google Scholar] [CrossRef]
- Lu, M.J.; Lu, Y.Q.; Li, H.H.; Pan, C.L.; Guo, Y.; Zhang, J.P.; Yang, X.M.; Li, X.Q.; Liu, W.H.; Li, L.H. Transferring desirable genes from Agropyron cristatum 7P chromosome into common wheat. PLoS ONE 2017, 11, e0159577. [Google Scholar] [CrossRef] [Green Version]
- Li, H.H.; Jiang, B.; Wang, J.C.; Lu, Y.Q.; Zhang, J.P.; Pan, C.L.; Yang, X.M.; Li, X.Q.; Liu, W.H.; Li, L.H. Mapping of novel powdery mildew resistance gene(s) from Agropyron cristatum chromosome 2P. Theor. Appl. Genet. 2017, 130, 109–121. [Google Scholar] [CrossRef]
- Said, M.; Parada, A.C.; Gaál, E.; Molnár, I.; Cabrera, A.; Doležel, J.; Vrána, J. Uncovering homeologous relationships between tetraploid Agropyron cristatum and bread wheat genomes using COS markers. Theor. Appl. Genet. 2019, 132, 2881–2898. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Han, H.M.; Liu, W.H.; Song, L.Q.; Zhang, J.P.; Zhou, S.H.; Yang, X.M.; Li, X.Q.; Li, L.H. Deletion mapping and verification of an enhanced-grain number per spike locus from the 6PL chromosome arm of Agropyron cristatum in common wheat. Theor. Appl. Genet. 2019, 132, 2815–2827. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Liu, T.G.; Li, H.H.; Han, H.M.; Li, L.H.; Zhang, J.P.; Yang, X.M.; Zhou, S.H.; Li, X.Q.; Liu, W.H. Physical Mapping of a Novel Locus Conferring Leaf Rust Resistance on the Long Arm of Agropyron cristatum Chromosome 2P. Front. Plant Sci. 2018, 9, 817. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, L.Q.; Han, H.M.; Zhou, S.H.; Zhang, J.P.; Yang, X.M.; Li, X.Q.; Liu, W.H.; Li, L.H. Physical localization of a locus from Agropyron cristatum conferring resistance to stripe rust in common wheat. Int. J. Mol. Sci. 2017, 18, 2403. [Google Scholar] [CrossRef] [Green Version]
- Gessese, M.; Bariana, H.; Wong, D.; Hayden, M.; Bansal, U. Molecular mapping of stripe rust resistance gene Yr81 in a common wheat landrace Aus27430. Plant Dis. 2019, 103, 1166–1171. [Google Scholar] [CrossRef]
- Marchal, C.; Zhang, J.P.; Zhang, P.; Fenwick, P.; Steuernagel, B.; Adamski, N.M.; Boyd, L.; Mclntosh, R.; Wulff, B.B.H.; Berry, S.; et al. BED-domain-containing immune receptors confer diverse resistance spectra to yellow rust. Nat. Plants 2018, 4, 662–668. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Huang, L.; Zhang, H.F.; Hao, Q.Q.; Lyu, B.; Wang, M.N.; Epstein, L.; Liu, M.; Kou, C.L.; Qi, J.; et al. An ancestral NB-LRR with duplicated 3′ UTRs confers stripe rust resistance in wheat and barley. Nat. Commun. 2019, 10, 4023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, D.L.; Uauy, C.; Distelfeld, A.; Blechl, A.; Epstein, L.; Chen, X.M.; Sela, H.; Fahima, T.; Dubcovsky, J. A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science 2009, 323, 1357–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krattinger, S.G.; Keller, B. Molecular genetics and evolution of disease resistance in cereals. New Phytol. 2016, 212, 320–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, S.; Wang, H.; Li, Y.; Kong, Z.S.; Tang, D. The NB-LRR gene Pm60 confers powdery mildew resistance in wheat. New Phytol. 2018, 218, 298–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.M.; Dong, L.L.; Li, B.B.; Wang, Z.Z.; Xie, J.Z.; Qiu, D.; Li, Y.H.; Shi, W.Q.; Yang, L.J.; Wu, Q.H.; et al. A CNL protein in wild emmer wheat confers powdery mildew resistance. New Phytol. 2020, 228, 1027–1037. [Google Scholar] [CrossRef]
- Song, L.Q.; Lu, Y.Q.; Zhang, J.P.; Pan, C.L.; Yang, X.M.; Li, X.Q.; Liu, W.H.; Li, L.H. Cytological and molecular analysis of wheat-Agropyron cristatum translocation lines with 6P chromosome fragments conferring superior agronomic traits in common wheat. Genome 2016, 59, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.H.; Zhang, J.P.; Han, H.M.; Zhang, J.; Ma, H.H.; Zhang, Z.; Lu, Y.Q.; Liu, W.H.; Yang, X.M.; Li, X.Q.; et al. Full-length transcriptome sequences of Agropyron cristatum facilitate the prediction of putative genes for thousand-grain weight in a wheat-A. cristatum translocation line. BMC Genom. 2019, 20, 1025. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Cepas, J.; Forslund, K.; Coelho, L.P.; Szklarczyk, D.; Jensen, L.J.; von Mering, C.; Bork, P. Fast Genome-Wide Functional Annotation through Orthology Assignment by eggNOG-Mapper. Mol. Biol. Evol. 2017, 34, 2115–2122. [Google Scholar] [CrossRef] [Green Version]
- Han, H.M.; Liu, W.H.; Lu, Y.Q.; Zhang, J.P.; Yang, X.M.; Li, X.Q.; Hu, Z.M.; Li, L.H. Isolation and application of P genome-specific DNA sequences of Agropyron Gaertn in Triticeae. Planta 2017, 245, 425–437. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Concepcion, G.T.; Feng, X.W.; Zhang, H.W.; Li, H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat. Methods 2021, 18, 170–175. [Google Scholar] [CrossRef] [PubMed]
- International Wheat Genome Sequencing Consortium (IWGSC). Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef] [Green Version]
- Zeller, F.J. 1B/1R wheat chromosome substitutions and translocations. In Proceedings of 4th International Wheat Genetic Symptoms; Sears, E.R., Sears, L.M.S., Eds.; University of Missouri: Columbia, SC, USA, 1973; pp. 209–221. [Google Scholar]
- Singh, N.K.; Shepherd, K.W.; McIntosh, R.A. Linkage mapping of genes for resistance to leaf, stem, and stripe rusts and x-secalins on the short arm of rye chromosome 1R. Theor. Appl. Genet. 1990, 80, 609–616. [Google Scholar] [CrossRef]
- Mago, R.; Spielmeyer, W.; Lawrence, G.J.; Lagudah, E.S.; Ellis, J.G.; Pryor, A. Identification and mapping of molecular markers linked to rust resistance genes located on chromosome 1RS of rye using wheat-rye translocation lines. Theor. Appl. Genet. 2002, 104, 1317–1324. [Google Scholar] [CrossRef]
- Ochoa, V.; Madrid, E.; Said, M.; Rubiales, D.; Cabrera, A. Molecular and cytogenetic characterization of a common wheat-Agropyron cristatum chromosome translocation conferring resistance to leaf rust. Euphytica 2015, 201, 89–95. [Google Scholar] [CrossRef]
- Song, L.Q.; Lu, Y.Q.; Zhang, J.P.; Pan, C.L.; Yang, X.M.; Li, X.Q.; Liu, W.H.; Li, L.H. Physical mapping of Agropyron cristatum chromosome 6P using deletion lines in common wheat background. Theor. Appl. Genet. 2016, 129, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.P.; Liu, W.H.; Wu, X.Y.; Yang, X.M.; Li, X.Q.; Lu, Y.Q.; Li, L.H. An intercalary translocation from Agropyron cristatum 6P chromosome into common wheat confers enhanced kernel number per spike. Planta 2016, 244, 853–864. [Google Scholar] [CrossRef]
- Copete, A.; Cabrera, A. Chromosomal location of genes for resistance to powdery mildew in Agropyron cristatum and mapping of conserved orthologous set molecular markers. Euphytica 2017, 213, 189. [Google Scholar] [CrossRef]
- Zhou, S.H.; Zhang, J.P.; Che, Y.H.; Liu, W.H.; Lu, Y.Q.; Yang, X.M.; Li, X.Q.; Jia, J.Z.; Liu, X.; Li, L.H. Construction of Agropyron Gaertn. genetic linkage maps using a wheat 660K SNP array reveals a homoeologous relationship with the wheat genome. Plant Biotechnol. J. 2018, 16, 818–827. [Google Scholar] [CrossRef] [Green Version]
- Han, H.M.; Bai, L.; Su, J.J.; Zhang, J.P.; Song, L.Q.; Gao, A.N.; Yang, X.M.; Li, X.Q.; Liu, W.H.; Li, L.H. Genetic Rearrangements of Six Wheat-Agropyron cristatum 6P Addition Lines Revealed by Molecular Markers. PLoS ONE 2014, 9, e91066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuadrado, A.; Schwarzacher, T.; Jouve, N. Identification of different chromatin classes in wheat using in situ hybridization with simple sequence repeat oligonucleotides. Theor. Appl. Genet. 2000, 101, 711–717. [Google Scholar] [CrossRef]
- Tang, Z.X.; Yang, Z.J.; Fu, S.L. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J. Appl. Genet. 2014, 55, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.; Langridge, P. Identification of the entire chromosome complement of bread wheat by two-colour FISH. Genome 1997, 40, 589–593. [Google Scholar] [CrossRef]
- Line, R.F.; Qayoum, A. Collecting and Evaluating Rust Samples for Virulence. In Virulence, Aggressiveness, Evolution, and Distribution of Races of Puccinia Striiformis (the Cause of Stripe Rust of Wheat) in North America, 1968–1987; United States Department of Agriculture: Washington, DC, USA, 1992; pp. 4–9. [Google Scholar]
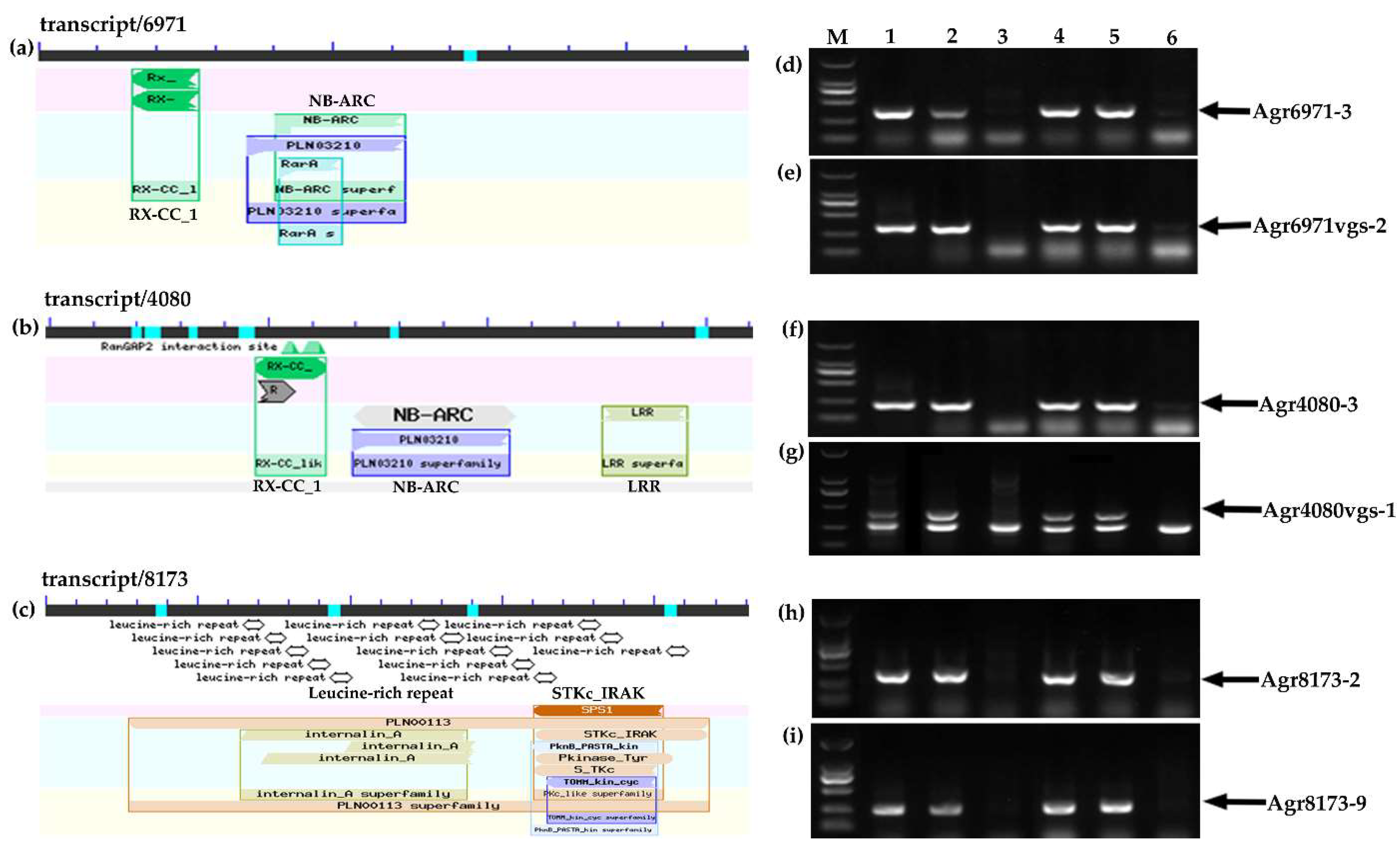


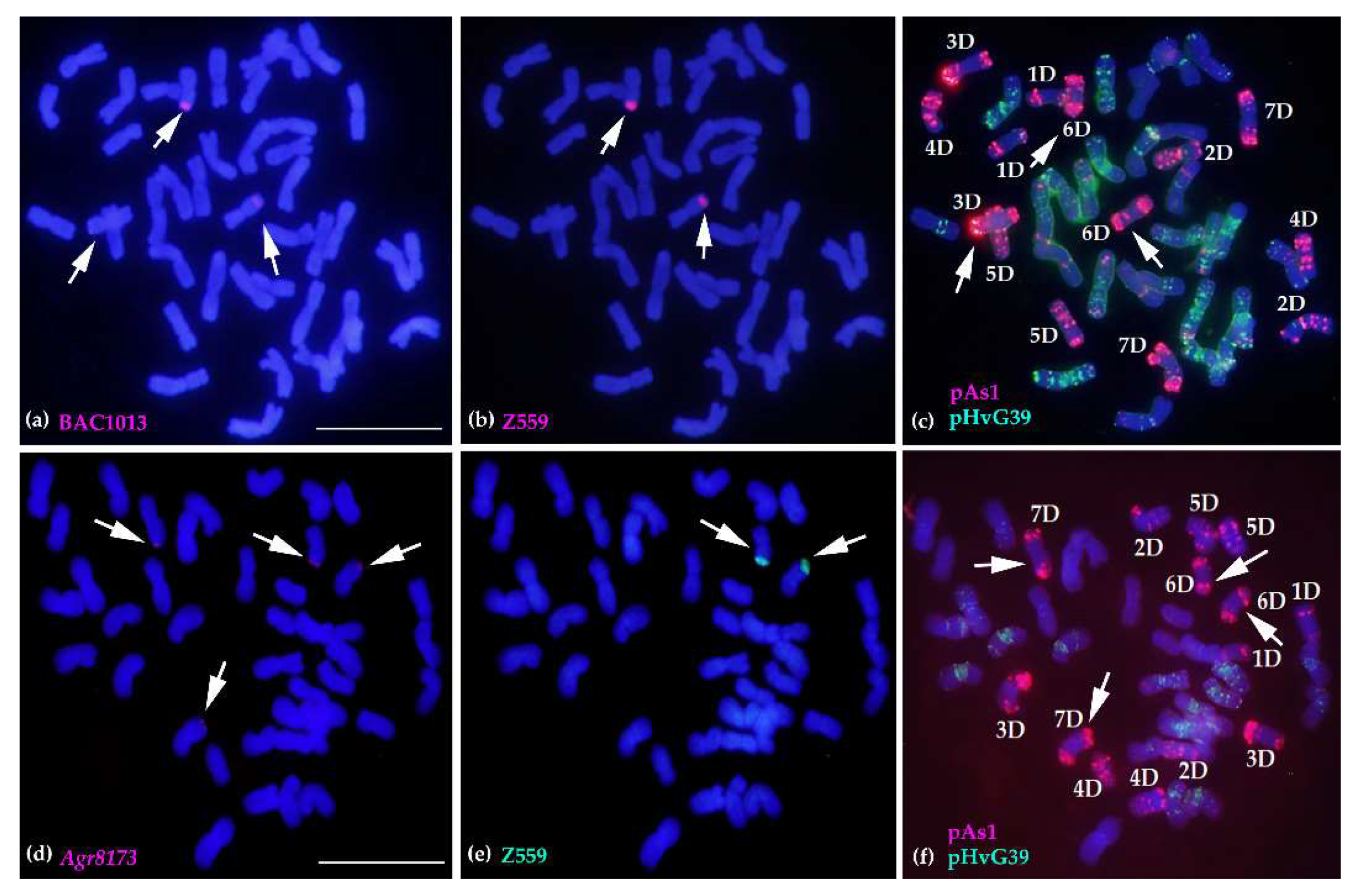
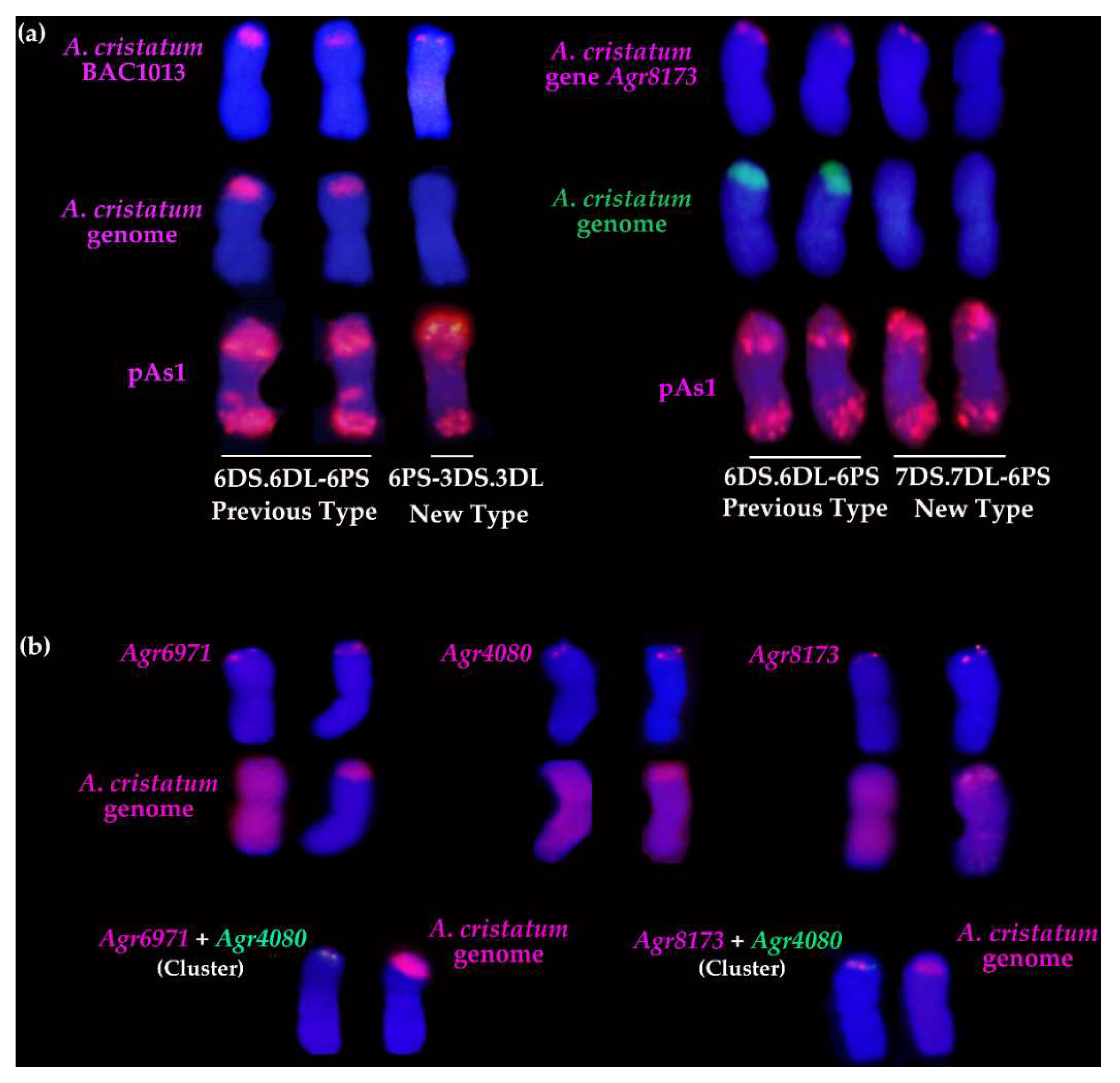


| Transcript Id | Gene Name | BAC Clone | Primer Name | Left Primer (5′–3′) | Right Primer (5′–3′) | Annealing Temperature |
|---|---|---|---|---|---|---|
| transcript/6971 | Agr6971 | BAC1013 | Agr6971-3 | TCACCAAAGATCGAGCTCCT | ACCCGTCTGCAGATTGTACC | 60 °C |
| Agr6971vgs-2 | GACCAGCTAGACAACCAGGT | TGCTGTTTGGGACCATCTCT | 60 °C | |||
| transcript/4080 | Agr4080 | BAC700 | Agr4080-3 | GGGCGGTTTTACTTCACAAA | ACTTGCAGCTGTCAATGTGC | 60 °C |
| Agr4080-4 | TGAAACTGGATGGACGATGA | GTGCTGCTGTGGTGTTGACT | 60 °C | |||
| Agr4080vgs-1 | CCGAGGTGGCGAATGAACTTGT | AGAGATGGAGGCTGTGGTGACT | 60 °C | |||
| Agr4080cds-6 | TTGTCGGACTAATAATAGCGC | TGCCGAGATAATGGGGTT | 60 °C | |||
| transcript/8173 | Agr8173 | BAC940 | Agr8173-2 | GGTCCCATACCTCCCAGTTT | TCGATGAAAGGTCCAGTTCC | 60 °C |
| Agr8173-9 | ATCGTTGGCCAATTCGATAG | CAGCAGAAGGAGCATGTTGA | 60 °C | |||
| Agr8173-6 | TGATCATTGTGGAGACCGGA | CACCCTCTTTTGGCAACCTT | 60 °C |
| Materials | Plant Height (cm) | Plot Yields (kg) | Thousand Grain Weight (g) | Stripe Rust Response |
|---|---|---|---|---|
| Jimai22 | 93.06 ± 1.41 a | 7.52 ± 0.24 c | 41.85 ± 0.28 c | S |
| Zhoumai18 | 86.32 ± 2.15 c | 6.78 ± 0.26 e | 44.61 ± 0.42 b | S |
| WAg20 | 77.25 ± 1.82 e | 6.09 ± 0.18 g | \ | R |
| WAg37 | 80.12 ± 2.65 d | 7.16 ± 0.38 d | 46.75 ± 0.45 a | R |
| WAg38 | 80.50 ± 2.37 d | 6.59 ± 0.16 ef | \ | R |
| WAg60 | 90.61 ± 3.56 b | 8.40 ± 0.27 a | 43.10 ± 0.32 b | MR |
| WAg115 | 83.21 ± 2.73 cd | 6.77 ± 0.23 e | \ | MR |
| WAg130 | 85.36 ± 4.01 c | 8.04 ± 0.26 b | 42.23 ± 0.24 bc | MR |
| WAg165 | 80.58 ± 2.13 d | 7.49 ± 0.14 c | \ | R |
| WAg220 | 75.42 ± 2.87 e | 7.63 ± 0.20 c | \ | R |
| WAg270 | 76.09 ± 3.25 e | 7.35 ± 0.17 cd | \ | R |
| WAg274 | 82.20 ± 3.35 d | 6.46 ± 0.18 f | \ | R |
| WAg301 | 85.23 ± 3.89 c | 8.37 ± 0.26 a | 40.84 ± 0.25 c | MR |
| WAg308 | 85.25 ± 2.10 c | 7.07 ± 0.35 d | \ | MR |
| WAg314 | 80.45 ± 2.34 d | 7.09 ± 0.19 d | \ | MR |
| WAg317 | 85.69 ± 3.14 c | 7.25 ± 0.15 d | \ | R |
| WAg322 | 87.57 ± 2.69 c | 8.32 ± 0.38 a | 39.73 ± 0.27 cd | MR |
| WAg330 | 82.63 ± 1.82 d | 8.11 ± 0.20 ab | 37.89 ± 0.38 d | MR |
| WAg335 | 80.33 ± 1.95 d | 7.52 ± 0.27 c | 38.96 ± 0.46 d | R |
| WAg337 | 83.28 ± 2.74 cd | 8.22 ± 0.24 a | 41.08 ± 0.47 c | MR |
| WAg339 | 80.29 ± 2.58 d | 7.13 ± 0.16 d | 38.66 ± 0.61 d | R |
| WAg409 | 81.35 ± 3.46 d | 7.08 ± 0.13 d | 39.32 ± 0.39 d | MR |
| WAg457 | 82.24 ± 2.09 d | 7.84 ± 0.23 b | 43.80 ± 0.46 b | R |
| WAg466 | 75.49 ± 2.51 e | 7.45 ± 0.34 c | 38.58 ± 0.52 d | MR |
| WAg475 | 85.41 ± 2.59 c | 8.24 ± 0.16 a | 45.94 ± 0.56 a | R |
| WAg480 | 85.17 ± 2.49 c | 7.96 ± 0.30 b | 41.84 ± 0.23 c | R |
| Materials | Zygosity | Progeny | Type | 6P Segment Size | Chromosome Constitution | Reference |
|---|---|---|---|---|---|---|
| 4844-12-1 | Homozygous | disomic substitution line | 6P | 42 (6P/6D) | ||
| 4844-12 | Homozygous | disomic addition line | 6P | 44 | Wu et al. [27] | |
| WAT638b | Homozygous | BC2F6 | 6AS•6PL | 6PL arm | 42 | Zhang et al. [33]; Song et al. [41] |
| WAT638a | Homozygous | BC2F6 | 6PS•6AL | 6PS arm | 42 | |
| WAT655 | Homozygous | BC2F6 | 6DS•6DL-6PS | 6PS (0.81–1.00) | 42 | |
| Heterozygous | BC5F2, BC5F2:3 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhou, S.; Liu, W.; Song, L.; Zhang, J.; Han, H.; Yang, X.; Lin, Y.; Li, X.; Li, L. Molecular Cytogenetic Analysis of the Introgression between Agropyron cristatum P Genome and Wheat Genome. Int. J. Mol. Sci. 2021, 22, 11208. https://doi.org/10.3390/ijms222011208
Zhang Z, Zhou S, Liu W, Song L, Zhang J, Han H, Yang X, Lin Y, Li X, Li L. Molecular Cytogenetic Analysis of the Introgression between Agropyron cristatum P Genome and Wheat Genome. International Journal of Molecular Sciences. 2021; 22(20):11208. https://doi.org/10.3390/ijms222011208
Chicago/Turabian StyleZhang, Zhi, Shenghui Zhou, Weihua Liu, Liqiang Song, Jinpeng Zhang, Haiming Han, Xinming Yang, Yida Lin, Xiuquan Li, and Lihui Li. 2021. "Molecular Cytogenetic Analysis of the Introgression between Agropyron cristatum P Genome and Wheat Genome" International Journal of Molecular Sciences 22, no. 20: 11208. https://doi.org/10.3390/ijms222011208






