Morphophysiological and Proteomic Responses on Plants of Irradiation with Electromagnetic Waves
Abstract
1. Introduction
2. Millimeter Waves
2.1. Characteristics
2.2. Morphophysiological Effects
2.3. Proteomic Responses
3. Ultraviolet
3.1. Characteristics
3.2. Morphophysiological Effects
3.3. Proteomic Responses
4. Gamma Rays
4.1. Characteristics
4.2. Morphophysiological Effects
4.3. Proteomic Responses
5. The Effects on Abiotic Stress Tolerance of the Different Irradiation Sources
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Lewandowski, J. Electromagnetic radiation for plant protection. In Physical Control Methods in Plant Protection; Springer: Berlin/Heidelberg, Germany, 2001; pp. 111–113. [Google Scholar]
- Gherardini, L.; Ciuti, G.; Tognarelli, S.; Cinti, C. Searching for the perfect wave: The effect of radiofrequency electromagnetic fields on cells. Int. J. Mol. Sci. 2014, 15, 5366–5387. [Google Scholar] [CrossRef]
- Einstein, A. Electromagnetic waves. In One Scientific Epoch Ended and Another Began with James Clerk Maxwell, Proceedings of the 10th International Workshop on the Electromagnetic Compatibility of Integrated Circuits (EMC Compo), Edinburgh, UK, 10–13 November 2015; IEEE: Piscataway, NJ, USA, 2015. [Google Scholar]
- D’Souza, S. Radiation technology in agriculture. J. Crop. Weed 2014, 10, 1–3. [Google Scholar]
- Hong, M.; Kim, J.; Yoon, Y.; Kim, S.; Ahn, J.; Jeong, I.; Kang, S.; Seo, Y.; Kim, D. The effects of chronic gamma irradiation on oxidative stress response and the expression of anthocyanin biosynthesis-related genes in wheat (Triticum aestivum). Int. J. Radiat. Biol. 2014, 90, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Lim, S.; Kim, Y.; Lee, Y.; Lee, S.; Lee, D.; Park, K.; Sung, J. Diurnal changes in C-N metabolism and response of rice seedlings to UV-B radiation. J. Plant Physiol. 2018, 228, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Sadeghianfar, P.; Nazari, M.; Backes, G. Exposure to ultraviolet (UV-C) radiation increases germination rate of maize (Zea maize L.) and sugar beet (Beta vulgaris) seeds. Plants 2019, 8, 49. [Google Scholar] [CrossRef]
- Mazza, C.; Giménez, P.; Kantolic, A.; Ballaré, C. Beneficial effects of solar UV-B radiation on soybean yield mediated by reduced insect herbivory under field conditions. Physiol. Plant 2013, 147, 307–315. [Google Scholar] [CrossRef]
- Karvansara, P.; Razavi, S. Physiological and biochemical responses of sugar beet (Beta vulgaris L.) to ultraviolet-B radiation. PeerJ 2019, 7, e6790. [Google Scholar] [CrossRef]
- Jamal, M.; Brodie, G.; Gupta, D. The effect of microwave soil treatment on rice production under field conditions. Trans. ASABE 2017, 60, 517–525. [Google Scholar]
- Robson, T.; Klem, K.; Urban, O.; Jansen, M. Re-interpreting plant morphological responses to UV-B radiation. Plant Cell Environ. 2015, 38, 856–866. [Google Scholar] [CrossRef]
- Yasmin, K.; Arulbalachandran, D.; Soundarya, V.; Vanmathi, S. Effects of gamma radiation (γ) on biochemical and antioxidant properties in black gram (Vigna mungo L. Hepper). Int. J. Radiat. Biol. 2019, 95, 1135–1143. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, X.; Ji, Y.; Wang, S.; Chen, Y.; Luo, J.; Shen, Y.; Peng, L. Measurement of metabolite variations and analysis of related gene expression in Chinese liquorice (Glycyrrhiza uralensis) plants under UV-B irradiation. Sci. Rep. 2018, 8, 6144. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Ravishankar, G. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar]
- Mao, B.; Wang, Y.; Zhao, T.; Tian, R.; Wang, W.; Ye, J. Combined effects of elevated O3 concentrations and enhanced UV-B radiation of the biometric and biochemical properties of soybean roots. Front. Plant Sci. 2017, 8, 1568. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Dong, Y.; Yue, X.; Hu, J.; Jiang, S.; Xu, H.; Wang, Y.; Su, M.; Zhang, J.; Zhang, Z.; et al. The B-box zinc finger protein MdBBX20 integrates anthocyanin accumulation in response to ultraviolet radiation and low temperature. Plant Cell Environ. 2019, 42, 2090–2104. [Google Scholar] [CrossRef]
- Vanhaelewyn, L.; Prinsen, E.; van der Straeten, D.; Vandenbussche, F. Hormone-controlled UV-B responses in plants. J. Exp. Bot. 2016, 67, 4469–4482. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.; Datta, S. Light signaling and UV-B-mediated plant growth regulation. J. Integr. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef] [PubMed]
- Nikam, A.; Devarumath, R.; Ahuja, A.; Babu, H.; Shitole, M.; Suprasanna, P. Radiation-induced in vitro mutagenesis system for salt tolerance and other agronomic characters in sugarcane (Saccharum officinarum L.). Crop J. 2015, 3, 46–56. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, V.; Atmaram, C.; Singh, B. Regulated partitioning of fixed carbon (14C), sodium (Na+), potassium (K+) and glycine betaine determined salinity stress tolerance of gamma irradiated pigeonpea (Cajanus cajan L. Millsp). Environ. Sci. Pollut. Res. 2017, 24, 7285–7297. [Google Scholar] [CrossRef]
- Moussa, H. Low dose of gamma irradiation enhanced drought tolerance in soybean. Bulg. J. Agric. Sci. 2011, 17, 63–72. [Google Scholar] [CrossRef]
- Mátai, A.; Nagy, D.; Hideg, É. UV-B strengthens antioxidant responses to drought in Nicotiana benthamiana leaves not only as supplementary irradiation but also as pre-treatment. Plant Physiol. Biochem. 2019, 134, 9–19. [Google Scholar] [CrossRef]
- Yin, X.; Nishimura, M.; Hajika, M.; Komatsu, S. Quantitative proteomics reveals the flooding-tolerance mechanism in mutant and abscisic acid-treated soybean. J. Proteome Res. 2016, 15, 2008–2025. [Google Scholar] [CrossRef]
- Zhong, Z.; Furuya, T.; Ueno, K.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Tani, M.; Tian, J.; Komatsu, S. Proteomic analysis of irradiation with millimeter waves on soybean growth under flooding conditions. Int. J. Mol. Sci. 2020, 21, 486. [Google Scholar] [CrossRef] [PubMed]
- Akandeh, M.; Soufbaf, M.; Kocheili, F.; Rasekh, A. Gamma irradiation on canola seeds affects herbivore-plant and host-parasitoid interactions. Neotrop. Entomol. 2017, 46, 256–263. [Google Scholar] [CrossRef]
- Komatsu, S.; Nanjo, Y.; Nishimura, M. Proteomic analysis of the flooding tolerance mechanism in mutant soybean. J. Proteom. 2013, 79, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Ragha, L.; Mishra, S.; Ramachandran, V.; Bhatia, M. Effects of low-power microwave fields on seed germination and growth rate. J. Electromagn. Anal. Appl. 2011, 3, 165–171. [Google Scholar] [CrossRef]
- Abu-Elsaoud, A. Effect of microwave electromagnetic radiofrequency on germination and seedling growth consequences of six wheat Triticum aestivum L. cultivar. Adv. Environ. Biol. 2015, 9, 270–280. [Google Scholar]
- Komatsu, S.; Jorrin-Novo, J.V. Plant Proteomic Research 3.0: Challenges and perspectives. Int. J. Mol. Sci. 2021, 22, 766. [Google Scholar] [CrossRef] [PubMed]
- Appleby, R.; Anderton, R.N. Millimeter-wave and submillimeter-wave imaging for security and surveillance. Proc. IEEE 2007, 95, 1683–1690. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Akyel, Y.; Pakhomova, O.N.; Stuck, B.E.; Murphy, M.R. Current state and implications of research on biological effects of millimeter waves: A review of the literature. Bioelectromagnetics 1998, 19, 393–413. [Google Scholar] [CrossRef]
- Marcus, M.; Pattan, B. Millimeter wave propagation: Spectrum management implications. IEEE Microw. Mag. 2005, 6, 54–62. [Google Scholar] [CrossRef]
- Appleby, R.; Gleed, D.G.; Anderton, R.N.; Lettington, A.H. High-performance passive millimeter-wave imaging. Opt. Eng. 1993, 32, 1370. [Google Scholar] [CrossRef]
- Andreev, G.A.; Korbakov, D.A.; Pyatkov, I.I. Technology of orthogonal frequency-division multiplexing combined with interference-resistant coding in millimeter-wave telecommunications systems. J. Commun. Technol. Electron. 2010, 55, 886–892. [Google Scholar] [CrossRef]
- Kolinko, V.G.; Lin, S.H.; Shek, A.; Manning, W.; Martin, C.; Hall, M.; Kirsten, O.; Moore, J.; Wikner, D.A. A passive millimeter-wave imaging system for concealed weapons and explosives detection. In Proceedings of the Optics and Photonics in Global Homeland Security, Orlando, FL, USA, 29 March 2005; pp. 85–92. [Google Scholar]
- Hantscher, S.; Schlenther, B.; Hagelen, M.; Lang, S.A.; Essen, H.; Tessmann, A.; Hulsmann, A.; Leuther, A.; Schlechtweg, M. Security pre-screening of moving persons using a rotating multichannel W-band radar. IEEE Trans. Microw. Theory Tech. 2012, 60, 870–880. [Google Scholar] [CrossRef]
- Di Meo, S.; Svelto, F.; Summers, P.E.; Renne, G.; Preda, L.; Bellomi, M.; Espin-Lopez, P.F.; Martellosio, A.; Pasian, M.; Matrone, G.; et al. On the feasibility of breast cancer imaging systems at millimeter-waves frequencies. IEEE Trans. Microw. Theory Tech. 2017, 65, 1795–1806. [Google Scholar] [CrossRef]
- Niu, Y.; Li, Y.; Jin, D.; Su, L.; Vasilakos, A.V. A survey of millimeter wave communications (mmWave) for 5G: Opportunities and challenges. Wirel. Netw. 2015, 21, 2657–2676. [Google Scholar] [CrossRef]
- Ramundo-Orlando, A. Effects of millimeter waves radiation on cell membrane-a brief review. J. Infrared Millim. Terahertz Waves 2010, 31, 1400–1411. [Google Scholar] [CrossRef]
- Usatii, A.; Molodoi, E.; Rotaru, A.; Moldoveanu, T. The influence of low intensity millimeter waves on the multiplication and biosynthetic activity of Saccharomyces carlsbergensis CNMN-Y-15 yeast. Ann. Oradea Univ.—Biol. Fascicle 2010, 17, 208–212. [Google Scholar]
- Betskii, O.V.; Lebedeva, N.N.; Tambiev, A.H.; Kirikova, N.N.; Slavin, V.E. Millimeter waves in the newest agricultural biotechnologies. J. Sci. Eng. 2007, 23, 236–252. [Google Scholar]
- Sukiasyan, A.; Mikaelyan, Y.; Ayrapetyan, S. Comparative study of non-ionizing and ionizing radiation effect on hydration of winter wheat seeds in metabolic active and inactive states. Environmentalist 2012, 32, 188–192. [Google Scholar] [CrossRef]
- Poghosyan, G.H.; Mukhaelyan, Z.H. The influence of low-intensity EMI treatment on seed germination and early growth of theat. Proc. Yerevan State Univ. Chem. Biol. 2018, 52, 110–115. [Google Scholar]
- Mukhaelyan, Z.H.; Shahinyan, M.A.; Poghosyan, G.H.; Vardevanyan, P.O. Wheat seedlings growth and antioxidant system activity changes as responses to extremely high frequency electromagnetic irradiation. Am. J. Plant Biol. 2016, 2, 1–10. [Google Scholar]
- Mukhaelyan, Z.H. EMI EHF-induced changes of free-radical oxidation processes in Triticum aestivum L. seedlings. Proc. Yerevan State Univ. Chem. Biol. 2017, 51, 31–37. [Google Scholar]
- Seo, D.H.; Kim, M.S.; Choi, H.W.; Sung, J.M.; Park, J.D.; Kum, J.S. Effects of millimeter wave treatment on the germination rate and antioxidant potentials and gamma-aminobutyric acid of the germinated brown rice. Food Sci. Biotechnol. 2016, 25, 111–114. [Google Scholar] [CrossRef]
- Wang, X.; Komatsu, S. Review: Proteomic techniques for the development of flood-tolerant soybean. Int. J. Mol. Sci. 2020, 21, 7497. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Maruyama, J.; Furuya, T.; Yin, X.; Yamaguchi, H.; Hitachi, K.; Miyashita, N.; Tsuchida, K.; Tani, M. Proteomic and biological analyses reveal the effect on growth under flooding stress of chickpea irradiated with millimeter waves. J. Proteome Res. 2021, 20, 4718–4727. [Google Scholar] [CrossRef]
- Calzoni, G.L.; Borghini, F.; del Giudice, E.; Betti, L.; dal Rio, F.; Migliori, M.; Trebbi, G.; Speranza, A. Weak extremely high frequency microwaves affect pollen-tube emergence and growth in kiwifruit: Pollen grain irradiation and water-mediated effects. J. Altern. Complement. Med. 2003, 9, 217–233. [Google Scholar] [CrossRef]
- Mironova, E.A.; Romanovskii, Y.M. Effect of low-intensity infrared and millimeter radiation on higher plants’ biopotentials. Crit. Rev. Biomed. Eng. 2000, 5, 430–439. [Google Scholar]
- Orlacchio, R.; le Page, Y.; le Dréan, Y.; le Guével, R.; Sauleau, R.; Alekseev, S.; Zhadobov, M. Millimeter-wave pulsed heating in vitro: Cell mortality and heat shock response. Sci. Rep. 2019, 9, 15249. [Google Scholar] [CrossRef] [PubMed]
- Sypniewska, R.K.; Millenbaugh, N.J.; Kiel, J.L.; Blystone, R.V.; Ringham, H.N.; Mason, P.A.; Witzmann, F.A. Protein changes in macrophages induced by plasma from rats exposed to 35 GHz millimeter waves. Bioelectromagnetics 2010, 31, 656–663. [Google Scholar] [CrossRef]
- Salesse-Smith, C.E.; Sharwood, R.E.; Busch, F.A.; Kromdijk, J.; Bardal, V.; Stern, D.B. Overexpression of Rubisco subunits with RAF1 increases Rubisco content in maize. Nat. Plants 2018, 4, 802–810. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Ogren, W.L. The mechanism of Rubisco activase: Insights from studies of the properties and structure of the enzyme. Photosynth. Res. 1996, 47, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Scafaro, A.P.; Gallé, A.; van Rie, J.; Carmo-Silva, E.; Salvucci, M.E.; Atwell, B.J. Heat tolerance in a wild Oryza species is attributed to maintenance of Rubisco activation by a thermally stable Rubisco activase ortholog. New Phytol. 2016, 211, 899–911. [Google Scholar] [PubMed]
- Aliakbari, M.; Cohen, S.P.; Lindlöf, A.; Shamloo-Dashtpagerdi, R. Rubisco activase A (RcaA) is a central node in overlapping gene network of drought and salinity in Barley (Hordeum vulgare L.) and may contribute to combined stress tolerance. Plant Physiol. Biochem. 2021, 161, 248–258. [Google Scholar] [CrossRef]
- Greenway, H.; Gibbs, J. Review: Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Funct. Plant Biol. 2003, 30, 999. [Google Scholar] [CrossRef]
- Sairam, R.K.; Dharmar, K.; Chinnusamy, V.; Meena, R.C. Waterlogging-induced increase in sugar mobilization, fermentation, and related gene expression in the roots of mung bean (Vigna radiata). J. Plant Physiol. 2009, 166, 602–616. [Google Scholar] [CrossRef]
- Cronin, T.W.; Bok, M.J. Photoreception and vision in the ultraviolet. J. Exp. Biol. 2016, 219, 2790–2801. [Google Scholar] [PubMed]
- Zhang, W.; Jiang, W. UV treatment improved the quality of postharvest fruits and vegetables by inducing resistance. Trends Food Sci. Technol. 2019, 92, 71–80. [Google Scholar] [CrossRef]
- McKenzie, R.L.; Björn, L.O.; Bais, A.; Ilyasad, M. Changes in biologically active ultraviolet radiation reaching the Earth’s surface. Photochem. Photobiol. Sci. 2003, 2, 5–15. [Google Scholar] [CrossRef]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, R.L.; Aucamp, P.J.; Bais, A.F.; Björn, L.O.; Ilyas, M. Changes in biologically-active ultraviolet radiation reaching the Earth’s surface. Photochem. Photobiol. Sci. 2007, 6, 218–231. [Google Scholar]
- Barnes, P.W.; Williamson, C.E.; Lucas, R.M.; Robinson, S.A.; Madronich, S.; Paul, N.D.; Bornman, J.F.; Bais, A.F.; Sulzberger, B.; Wilson, S.R.; et al. Ozone depletion, ultraviolet radiation, climate change and prospects for a sustainable future. Nat. Sustain. 2019, 2, 569–579. [Google Scholar] [CrossRef]
- Ulm, R.; Nagy, F. Signalling and gene regulation in response to ultraviolet light. Curr. Opin. Plant Biol. 2005, 8, 477–482. [Google Scholar] [CrossRef]
- Koutchma, T. Advances in Ultraviolet light technology for non-thermal processing of liquid foods. Food Bioprocess. Technol. 2009, 2, 138–155. [Google Scholar] [CrossRef]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and potential applications of ultraviolet light in the food industry-a critical review. J. Sci. Food Agric. 2000, 80, 637–645. [Google Scholar] [CrossRef]
- Bernado, W.P.; Rakocevic, M.; Santos, A.R.; Ruas, K.F.; Baroni, D.F.; Abraham, A.C.; Pireda, S.; Oliveira, D.; Cunha, M.D.; Ramalho, J.C.; et al. Biomass and Leaf Acclimations to Ultraviolet Solar Radiation in Juvenile Plants of Coffea arabica and C. canephora. Plants 2021, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Sen Mandi, S. Natural UV Radiation in Enhancing Survival Value and Quality of Plants; Springer: New Delhi, India, 2016; pp. 73–123. [Google Scholar]
- Frohnmeyer, H.; Staiger, D. Ultraviolet-B radiation-mediated responses in plants: Balancing damage and protection. Plant Physiol. 2003, 133, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.W.; Shinkle, J.R.; Flint, S.D.; Ryel, R.J. UV-B radiation, photomorphogenesis and plant-plant Interactions. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2005; pp. 313–340. [Google Scholar]
- Shinkle, J.R.; Atkins, A.K.; Humphrey, E.E.; Rodgers, C.W.; Wheeler, S.L.; Barnes, P.W. Growth and morphological responses to different UV wavebands in cucumber (Cucumis sativum) and other dicotyledonous seedlings. Physiol. Plant 2004, 120, 240–248. [Google Scholar] [CrossRef]
- Verdaguer, D.; Jansen, M.A.K.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, Y.; Zhang, Y.; Zou, J.; Yang, Q.; Li, T. Ultraviolet-A radiation stimulates growth of indoor cultivated tomato (Solanum lycopersicum) seedlings. HortScience 2018, 53, 1429–1433. [Google Scholar] [CrossRef]
- Chen, Y.; Li, T.; Yang, Q.; Zhang, Y.; Zou, J.; Bian, Z.; Wen, X. UVA radiation is beneficial for yield and quality of indoor cultivated lettuce. Front. Plant Sci. 2019, 10, 1563. [Google Scholar] [CrossRef]
- Bernal, M.; Verdaguer, D.; Badosa, J.; Abadía, A. Effects of enhanced UV radiation and water availability on performance, biomass production and photoprotective mechanisms of Laurus nobilis seedlings. Environ. Exp. Bot. 2015, 109, 264–275. [Google Scholar] [CrossRef]
- Hader, D.P. Effects of solar radiation on local and German wheat seedlings in a Chilean high mountain station. J. Photochem. Photobiol. B Biol. 1996, 35, 181–187. [Google Scholar] [CrossRef]
- Krizek, D.T.; Mirecki, R.M.; Britz, S.J. Inhibitory effects of ambient levels of solar UV-A and UV-B radiation on growth of cucumber. Physiol. Plant 1997, 4, 886–893. [Google Scholar] [CrossRef]
- Zhang, L.; Allen, L.H.; Vaughan, M.M.; Hauser, B.A.; Boote, K.J. Solar ultraviolet radiation exclusion increases soybean internode lengths and plant height. Agric. For. Meteorol. 2014, 184, 170–178. [Google Scholar] [CrossRef]
- Rizzini, L.; Favory, J.J.; Cloix, C.; Faggionato, D.; O’Hara, A.; Kaiserli, E.; Baumeister, R.; Schafer, E.; Nagy, F.; Jenkins, G.I.; et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef]
- Kataria, S.; Jajoo, A.; Guruprasad, K.N. Impact of increasing ultraviolet-B (UV-B) radiation on photosynthetic processes. J. Photochem. Photobiol. B Biol. 2014, 137, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Prasad, M.N.V.; Agrawal, S.B. Growth and defense metabolism of plants exposed to ultraviolet-B radiation. Sustain. Agric. Rev. 2015, 17, 263–305. [Google Scholar]
- Hideg, É.; Jansen, M.A.K.; Strid, Å. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef]
- Heijde, M.; Ulm, R. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 2012, 17, 230–237. [Google Scholar] [CrossRef]
- Hectors, K.; van Oevelen, S.; Guisez, Y.; Prinsen, E.; Jansen, M.A.K. The phytohormone auxin is a component of the regulatory system that controls UV-mediated accumulation of flavonoids and UV-induced morphogenesis. Physiol. Plant 2012, 145, 594–603. [Google Scholar] [CrossRef]
- Valenta, K.; Dimac-Stohl, K.; Baines, F.; Smith, T.; Piotrowski, G.; Hill, N.; Kuppler, J.; Nevo, O. Ultraviolet radiation changes plant color. BMC Plant Biol. 2020, 20, 253. [Google Scholar] [CrossRef]
- Saxena, S.C.; Joshi, P.K.; Grimm, B.; Arora, S. Alleviation of ultraviolet-C-induced oxidative damage through overexpression of cytosolic ascorbate peroxidase. Biologia 2011, 66, 1052–1059. [Google Scholar] [CrossRef]
- Darras, A.I.; Tsikaloudakis, G.; Lycoskoufis, I.; Dimitriadis, C.; Karamousantas, D. Low doses of UV-C irradiation affects growth, fruit yield and photosynthetic activity of tomato plants. Sci. Hortic. 2020, 267, 109357. [Google Scholar] [CrossRef]
- Sarghein, S.H.; Carapetian, J.; Khara, J. The effects of UV radiation on some structural and ultrastructural parameters in pepper (Capsicum longum A.DC.). Turk. J. Biol. 2011, 35, 69–77. [Google Scholar]
- Xu, Y.; Charles, M.T.; Luo, Z.; Roussel, D.; Rolland, D. Potential link between fruit yield, quality parameters and phytohormonal changes in preharvest UV-C treated strawberry. Plant Physiol. Biochem. 2017, 116, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.M.; Rushing, J.W. Effect of ultraviolet-C light on quality and microbial population of fresh-cut watermelon. Postharvest Biol. Technol. 2006, 40, 256–261. [Google Scholar] [CrossRef]
- Manzocco, L.; da Pieve, S.; Bertolini, A.; Bartolomeoli, I.; Maifreni, M.; Vianello, A.; Nicoli, M.C. Surface decontamination of fresh-cut apple by UV-C light exposure: Effects on structure, colour and sensory properties. Postharvest Biol. Technol. 2011, 61, 165–171. [Google Scholar] [CrossRef]
- Kotilainen, T.; Tegelberg, R.; Julkunen-Titto, R.; Lindfors, A.; Aphalo, P.J. Metabolite specific effects of solar UV-A and UV-B on alder and birch leaf phenolics. Glob. Chang. Biol. 2008, 14, 1294–1304. [Google Scholar] [CrossRef]
- Wang, H.; Gui, M.; Tian, X.; Xin, X.; Wang, T.; Li, J. Effects of UV-B on vitamin C, phenolics, flavonoids and their related enzyme activities in mung bean sprouts (Vigna radiata). Int. J. Food Sci. Technol. 2017, 52, 827–833. [Google Scholar] [CrossRef]
- Sun, M.; Gu, X.; Fu, H.; Zhang, L.; Chen, R.; Cui, L.; Zheng, L.; Zhang, D.; Tian, J. Change of secondary metabolites in leaves of Ginkgo biloba L. in response to UV-B induction. Innov. Food Sci. Emerg. Technol. 2010, 11, 672–676. [Google Scholar] [CrossRef]
- Xu, R.Y.; Nan, P.; Yang, Y.; Pan, H.; Zhou, T.; Chen, J. Ultraviolet irradiation induces accumulation of isoflavonoids and transcription of genes of enzymes involved in the calycosin-7-O-β-d-glucoside pathway in Astragalus membranaceus Bge. var. mongholicus (Bge.) Hsiao. Physiol. Plant 2011, 142, 265–273. [Google Scholar] [CrossRef]
- Ning, W.; Peng, X.; Ma, L.; Cui, L.; Lu, X.; Wang, J.; Tian, J.; Li, X.; Wang, W.; Zhang, L. Enhanced secondary metabolites production and antioxidant activity in postharvest Lonicera japonica Thunb. in response to UV radiation. Innov. Food Sci. Emerg. Technol. 2012, 13, 231–243. [Google Scholar] [CrossRef]
- Pandey, N.; Pandey-Rai, S. Modulations of physiological responses and possible involvement of defense-related secondary metabolites in acclimation of Artemisia annua L. against short-term UV-B radiation. Planta 2014, 240, 611–627. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, B.; Komatsu, S.; Lu, X.; Li, X.; Tian, J. Binary stress induces an increase in indole alkaloid biosynthesis in Catharanthus roseus. Front. Plant Sci. 2015, 6, 582. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Komatsu, S.; Zhu, W.; Zhang, L.; Li, X.; Cui, L.; Tian, J. Response and defense mechanisms of Taxus chinensis leaves under UV-A radiation are revealed using comparative proteomics and metabolomics analyses. Plant Cell Physiol. 2016, 57, 1839–1853. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Guan, Q.; Tian, J.; Komatsu, S. Transcriptomic and proteomic analyses of leaves from Clematis terniflora DC. under high level of ultraviolet-B irradiation followed by dark treatment. J. Proteom. 2017, 150, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, X.; Wang, C.; Tang, L.; Zhang, W.; Qi, W. The response of Achyranthes bidentata Blume to short-term UV-B exposure. Russ. J. Plant Physiol. 2019, 66, 160–170. [Google Scholar] [CrossRef]
- Yin, X.; Fan, H.; Chen, Y.; Li, L.; Song, W.; Fan, Y.; Zhou, W.; Ma, G.; Alolga, R.N.; Li, W.; et al. Integrative omic and transgenic analyses reveal the positive effect of ultraviolet-B irradiation on salvianolic acid biosynthesis through upregulation of SmNAC1. Plant J. 2020, 104, 781–799. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Wang, H.Z.; Wu, K.X.; Guo, X.R.; Mu, L.Q.; Tang, Z.H. Comparison of the global metabolic responses to UV-B radiation between two medicinal Astragalus species: An integrated metabolomics strategy. Environ. Exp. Bot. 2020, 176, 104094. [Google Scholar] [CrossRef]
- Zhong, Z.; Liu, S.; Zhu, W.; Ou, Y.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Tian, J.; Komatsu, S. Phosphoproteomics reveals the biosynthesis of secondary metabolites in Catharanthus roseus under ultraviolet-B radiation. J. Proteome Res. 2019, 18, 3328–3341. [Google Scholar] [CrossRef]
- Kaspar, S.; Matros, A.; Mock, H.P. Proteome and flavonoid analysis reveals distinct responses of epidermal tissue and whole leaves upon UV-B radiation of barley (Hordeum vulgare L.) seedlings. J. Proteome Res. 2010, 9, 2402–2411. [Google Scholar] [CrossRef]
- Ktitorova, I.N.; Skobeleva, O.V.; Kanash, E.V.; Bilova, T.E.; Sharova, E.I. Causes of root growth retardation induced by ultraviolet-B irradiation of shoots in barley seedlings. Russ. J. Plant Physiol. 2006, 53, 85–95. [Google Scholar] [CrossRef]
- Lavola, A.; Julkunen-Tiitto, R.; Aphalo, P.; de la Rosa, T.; Lehto, T. The effect of u.v.-B radiation on u.v.-absorbing secondary metabolites in birch seedlings grown under simulated forest soil conditions. New Phytol. 1997, 137, 617–621. [Google Scholar] [CrossRef]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: Induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef]
- Bidel, L.P.R.; Chomicki, G.; Bonini, F.; Mondolot, L.; Soulé, J.; Coumans, M.; la Fisca, P.; Baissac, Y.; Petit, V.; Loiseau, A.; et al. Dynamics of flavonol accumulation in leaf tissues under different UV-B regimes in Centella asiatica (Apiaceae). Planta 2015, 242, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, X.; Gao, C.; Chen, M.; Guan, Q.; Tian, J.; Komatsu, S. Proteomic and metabolomic analyses of leaf from Clematis terniflora DC. exposed to high-level ultraviolet-B irradiation with dark treatment. J. Proteome Res. 2016, 15, 2643–2657. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakabayashi, R.; Ogata, Y.; Sakurai, N.; Tokimatsu, T.; Goto, S.; Suzuki, M.; Jasinski, M.; Martinoia, E.; Otagaki, S.; et al. Multiomics in grape berry skin revealed specific induction of the stilbene synthetic pathway by ultraviolet-C irradiation. Plant Physiol. 2015, 168, 47–59. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Wu, X.; Wang, Y.; Lin, Y.; Wu, D.; Zhang, H.; Qin, J. Molecular analysis of UV-C induced resveratrol accumulation in Polygonum cuspidatum leaves. Int. J. Mol. Sci. 2019, 20, 6185. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, N.C.; Menguer, P.K.; Sperotto, R.A.; de Almeida, M.R.; Fett-Neto, A.G. Early changes in gene expression induced by acute UV exposure in leaves of Psychotria brachyceras, a bioactive alkaloid accumulating plant. Mol. Biotechnol. 2013, 54, 79–91. [Google Scholar] [CrossRef]
- Zhang, X.; Su, N.; Jia, L.; Tian, J.; Li, H.; Huang, L.; Shen, Z.; Cui, J. Transcriptome analysis of radish sprouts hypocotyls reveals the regulatory role of hydrogen-rich water in anthocyanin biosynthesis under UV-A. BMC Plant Biol. 2018, 18, 227. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Tarpley, L. Morphological and physiological responses of nine southern U.S. rice cultivars differing in their tolerance to enhanced ultraviolet-B radiation. Environ. Exp. Bot. 2011, 70, 174–184. [Google Scholar] [CrossRef]
- Cho, M.H.; Lee, S.W. Phenolic phytoalexins in rice: Biological functions and biosynthesis. Int. J. Mol. Sci. 2015, 16, 29120–29133. [Google Scholar] [CrossRef] [PubMed]
- Tegelberg, R.; Julkunen-Tiitto, R. Quantitative changes in secondary metabolites of dark-leaved willow (Salix myrsinifolia) exposed to enhanced ultraviolet-B radiation. Physiol. Plant 2001, 113, 541–547. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zheng, W.; Fu, Z.; Li, W.; Ma, L.; Li, K.; Sun, L.; Tian, J. Proteomics analysis of UV-irradiated Lonicera japonica Thunb. with bioactive metabolites enhancement. Proteomics 2013, 13, 3508–3522. [Google Scholar] [CrossRef]
- Falvo, S.; Acquadro, A.; Albo, A.G.; America, T.; Lanteri, S. Proteomic analysis of PEG-fractionated UV-C stress-response proteins in globe artichoke. Plant Mol. Biol. Report. 2012, 30, 111–122. [Google Scholar] [CrossRef]
- Falvo, S.; di Carli, M.; Desiderio, A.; Benvenuto, E.; Moglia, A.; America, T.; Lanteri, S.; Acquadro, A. 2-D DIGE analysis of UV-C radiation-responsive proteins in globe artichoke leaves. Proteomics 2012, 12, 448–460. [Google Scholar] [CrossRef]
- Lyu, G.; Li, D.; Xiong, H.; Xiao, L.; Tong, J.; Ning, C.; Wang, P.; Li, S. Quantitative proteomic analyses identify STO/BBX24 -related proteins induced by UV-B. Int. J. Mol. Sci. 2020, 21, 2496. [Google Scholar] [CrossRef]
- Zhong, Z.; Liu, S.; Han, S.; Li, Y.; Tao, M.; Liu, A.; He, Q.; Chen, S.; Dufresne, C.; Zhu, W.; et al. Integrative omic analysis reveals the improvement of alkaloid accumulation by ultraviolet-B radiation and its upstream regulation in Catharanthus roseus. Ind. Crop. Prod. 2021, 166, 113448. [Google Scholar] [CrossRef]
- Du, H.; Liang, Y.; Pei, K.; Ma, K. UV radiation-responsive proteins in rice leaves: A proteomic analysis. Plant Cell Physiol. 2011, 52, 306–316. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, L.; Jiang, H.; Zhang, Y.; Zhang, S. Different proteome profiles between male and female Populus cathayana exposed to UV-B radiation. Front. Plant Sci. 2017, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Sullivan, J.H.; Garrett, W.M.; Caperna, T.J.; Natarajan, S. Impact of solar ultraviolet-B on the proteome in soybean lines differing in flavonoid contents. Phytochemistry 2008, 69, 38–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiao, C.; Gu, Z. iTRAQ-based proteomic analysis reveals changes in response to UV-B treatment in soybean sprouts. Food Chem. 2019, 275, 467–473. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Wang, M.; Fang, X.; Cai, X. Comparative proteomic analysis of latex from Euphorbia kansui laticifers at different development stages with and without UV-B treatment via iTRAQ-coupled two-dimensional liquid chromatography-MS/MS. Funct. Plant Biol. 2020, 47, 67. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, Z. Dis-RUP for COP1 role-switch under UV-B light. Trends Plant Sci. 2019, 24, 569–571. [Google Scholar] [CrossRef]
- Khudyakova, A.Y.; Kreslavski, V.D.; Shmarev, A.N.; Lyubimov, V.Y.; Shirshikova, G.N.; Pashkovskiy, P.P.; Kuznetsov, V.V.; Allakhverdiev, S.I. Impact of UV-B radiation on the photosystem II activity, pro-/antioxidant balance and expression of light-activated genes in Arabidopsis thaliana hy4 mutants grown under light of different spectral composition. J. Photochem. Photobiol. B Biol. 2019, 194, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Bashandy, T.; Taconnat, L.; Renou, J.P.; Meyer, Y.; Reichheld, J.P. Accumulation of flavonoids in an ntra ntrb mutant leads to tolerance to UV-C. Mol. Plant 2009, 2, 249–258. [Google Scholar] [CrossRef]
- Zhu, W.; Zheng, W.; Hu, X.; Xu, X.; Zhang, L.; Tian, J. Variations of metabolites and proteome in Lonicera japonica Thunb. buds and flowers under UV radiation. BBA-Proteins Proteom. 2017, 1865, 404–413. [Google Scholar] [CrossRef]
- Zhu, W.; Han, H.; Liu, A.; Guan, Q.; Kang, J.; David, L.; Dufresne, C.; Chen, S.; Tian, J. Combined ultraviolet and darkness regulation of medicinal metabolites in Mahonia bealei revealed by proteomics and metabolomics. J. Proteom. 2021, 233, 104081. [Google Scholar] [CrossRef]
- Gao, C.; Yang, B.; Zhang, D.; Chen, M.; Tian, J. Enhanced metabolic process to indole alkaloids in Clematis terniflora DC. after exposure to high level of UV-B irradiation followed by the dark. BMC Plant Biol. 2016, 16, 231. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, W.; Zhang, Y.; Yang, B.; Fu, Z.; Li, X.; Tian, J. Proteomics analysis of Mahonia bealei leaves with induction of alkaloids via combinatorial peptide ligand libraries. J. Proteom. 2014, 110, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Maraschi, L.; Treves, A. gamma Rays from close binaries in the quiet stage. Nature 1979, 31, 401–402. [Google Scholar] [CrossRef]
- Dermer, C.D.; Schlickeiser, R. Quasars, blazars, and gamma rays. Science 1992, 257, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Blaufox, M.D. Becquerel and the discovery of radioactivity: Early concepts. Semin. Nucl. Med. 1996, 26, 145–154. [Google Scholar] [CrossRef]
- Chen, H.Y.; Holz, D.E. Gamma-ray-burst beaming and gravitational-wave observations. Phys. Rev. Lett. 2013, 111, 181101. [Google Scholar] [CrossRef]
- Kovacs, E.; Keresztes, Á. Effect of gamma and UV-B/C radiation on plant cell. Micron 2002, 33, 199–210. [Google Scholar] [CrossRef]
- Kim, J.H.; Baek, M.H.; Chung, B.Y.; Wi, S.G.; Kim, J.S. Alterationsin the photosynthic pigments and antioxidant machineries of red pepper (Capsicum annuum L.) seedlings from gamma-irradiated seeds. J. Plant Biol. 2004, 47, 314–321. [Google Scholar] [CrossRef]
- Wi, S.G.; Chung, B.Y.; Kim, J.S.; Kim, J.H.; Baek, M.H.; Lee, J.W.; Kim, Y.S. Effects of gamma irradiation on morphological changes and biological responses in plants. Micron 2007, 38, 553–564. [Google Scholar] [CrossRef]
- Bolsunovsky, A.; Trofimova, E.; Dementye, D.; Petrichenkov, M. The long-term effects of γ-radiation on the growth of Allium cepa plants. Int. J. Radiat. Biol. 2021, 97, 276–281. [Google Scholar] [CrossRef]
- Kim, S.H.; Jo, Y.D.; Ryu, J.; Hong, M.J.; Kang, B.C.; Kim, J.B. Effects of the total dose and duration of γ-irradiation on the growth responses and induced SNPs of a Cymbidium hybrid. Int. J. Radiat. Biol. 2020, 96, 545–551. [Google Scholar] [CrossRef]
- Jombo, T.Z.; Minnaar, A.; Taylor, J.R. Effects of gamma-irradiation on cotyledon cell separation and pectin solubilisation in hard-to-cook cowpeas. J. Sci. Food Agric. 2018, 98, 1725–1733. [Google Scholar] [CrossRef]
- Beyaz, R. Impact of gamma irradiation pretreatment on the growth of common vetch (Vicia sativa L.) seedlings grown under salt and drought stress. Int. J. Radiat. Biol. 2020, 96, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, M.; Nanjo, T.; Yoshida, K. The effects of gamma irradiation on growth and expression of genes encoding DNA repair-related proteins in Lombardy poplar (Populus nigra var. italica). J. Environ. Radioact. 2012, 109, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Melki, M.; Dahmani, T. Gamma irradiation effects on durum wheat (Triticum durum Desf.) under various conditions. Pak. J. Biol. Sci. 2009, 12, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Marcu, D.; Damian, G.; Cosma, C.; Cristea, V. Gamma radiation effects on seed germination, growth and pigment content, and ESR study of induced free radicals in maize (Zea mays). J. Biol. Phys. 2013, 39, 625–634. [Google Scholar] [CrossRef]
- Beyaz, R.; Kahramanogullari, C.T.; Yildiz, C.; Darcin, E.S.; Yildiz, M. The effect of gamma radiation on seed germination and seedling growth of Lathyrus chrysanthus Boiss. under in vitro conditions. J. Environ. Radioact. 2016, 162–163, 129–133. [Google Scholar] [CrossRef]
- Song, K.E.; Lee, S.H.; Jung, J.G.; Choi, J.E.; Jun, W.; Chung, J.W.; Hong, S.H.; Shim, S. Hormesis effects of gamma radiation on growth of quinoa (Chenopodium quinoa). Int. J. Radiat. Biol. 2021, 97, 906–915. [Google Scholar] [CrossRef]
- Hayashi, G.; Moro, C.F.; Rohila, J.S.; Shibato, J.; Kubo, A.; Imanaka, T.; Kimura, S.; Ozawa, S.; Fukutani, S.; Endo, S.; et al. 2D-DIGE-based proteome expression changes in leaves of rice seedlings exposed to low-level gamma radiation at Iitate village, Fukushima. Plant Signal. Behav. 2015, 10, e1103406. [Google Scholar] [CrossRef]
- Baek, J.; Choi, J.I.; Park, H.; Lim, S.; Park, S.J. Isolation and proteomic analysis of a Chlamydomonas reinhardtii mutant with enhanced lipid production by the gamma irradiation method. J. Microbiol. Biotechnol. 2016, 26, 2066–2075. [Google Scholar] [CrossRef]
- Kogan, G.L.; Gvozdev, V.A. Multifunctional nascent polypeptide-associated complex [NAC]. Mol. Biol. 2014, 48, 189–196. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.R.; Valpuesta, J.M. Hsp70 chaperone: A master player in protein homeostasis. F1000Research 2018, 7, 1497. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, X.B.; Yang, H.; Long, G.Y.; Wang, Z.; Jin, D.C. Effects of abiotic stress on the expression of Hsp70 genes in Sogatella furcifera (Horváth). Cell Stress Chaperones 2020, 25, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Hiraga, S.; Hajika, M.; Nishimura, M.; Komatsu, S. Transcriptomic analysis reveals the flooding tolerant mechanism in flooding tolerant line and abscisic acid treated soybean. Plant Mol. Biol. 2017, 93, 479–496. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, W.; Hashiguchi, A.; Nishimura, M.; Tian, J.; Komatsu, S. Metabolic profiles of flooding-tolerant mechanism in early-stage soybean responding to initial stress. Plant Mol. Biol. 2017, 9, 669–685. [Google Scholar] [CrossRef]
- Wang, X.; Sakata, K.; Komatsu, S. An integrated approach of proteomics and computational genetic modification effectiveness analysis to uncover the mechanisms of flood tolerance in soybeans. Int. J. Mol. Sci. 2018, 19, 1301. [Google Scholar] [CrossRef]
- Komatsu, S.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Kono, Y.; Nishimura, M. Proteomic and biochemical analyses of the mechanism of tolerance in mutant soybean responding to flooding stress. Int. J. Mol. Sci. 2021, 22, 9046. [Google Scholar] [CrossRef]
- Chen, Y.P.; Jia, J.F.; Wang, Y.J. Weak microwave can enhance tolerance of wheat seedlings to salt stress. J. Plant Growth Regul. 2009, 28, 381–385. [Google Scholar] [CrossRef]
- Chen, Y.P.; Jia, J.F.; Han, X.L. Weak microwave can alleviate water deficit induced by osmotic stress in wheat seedlings. Planta 2009, 229, 291–298. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, J.; Zhang, Y.; Bi, Z.; Wei, H. Microwave pretreatment can enhance tolerance of wheat seedlings to CdCl2 stress. Ecotoxicol. Environ. Saf. 2011, 74, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.R.; Silva, L.R.; Fernandes, L.; Salgado, L.D.; Silva de Assis, H.C.; Firak, D.S.; Bach, L.; Santos-Filho, R.; Voigt, C.L.; Barros, A.C.; et al. Visible-light reduced silver nanoparticles’ toxicity in Allium cepa test system. Environ. Pollut. 2020, 257, 113551. [Google Scholar] [CrossRef] [PubMed]

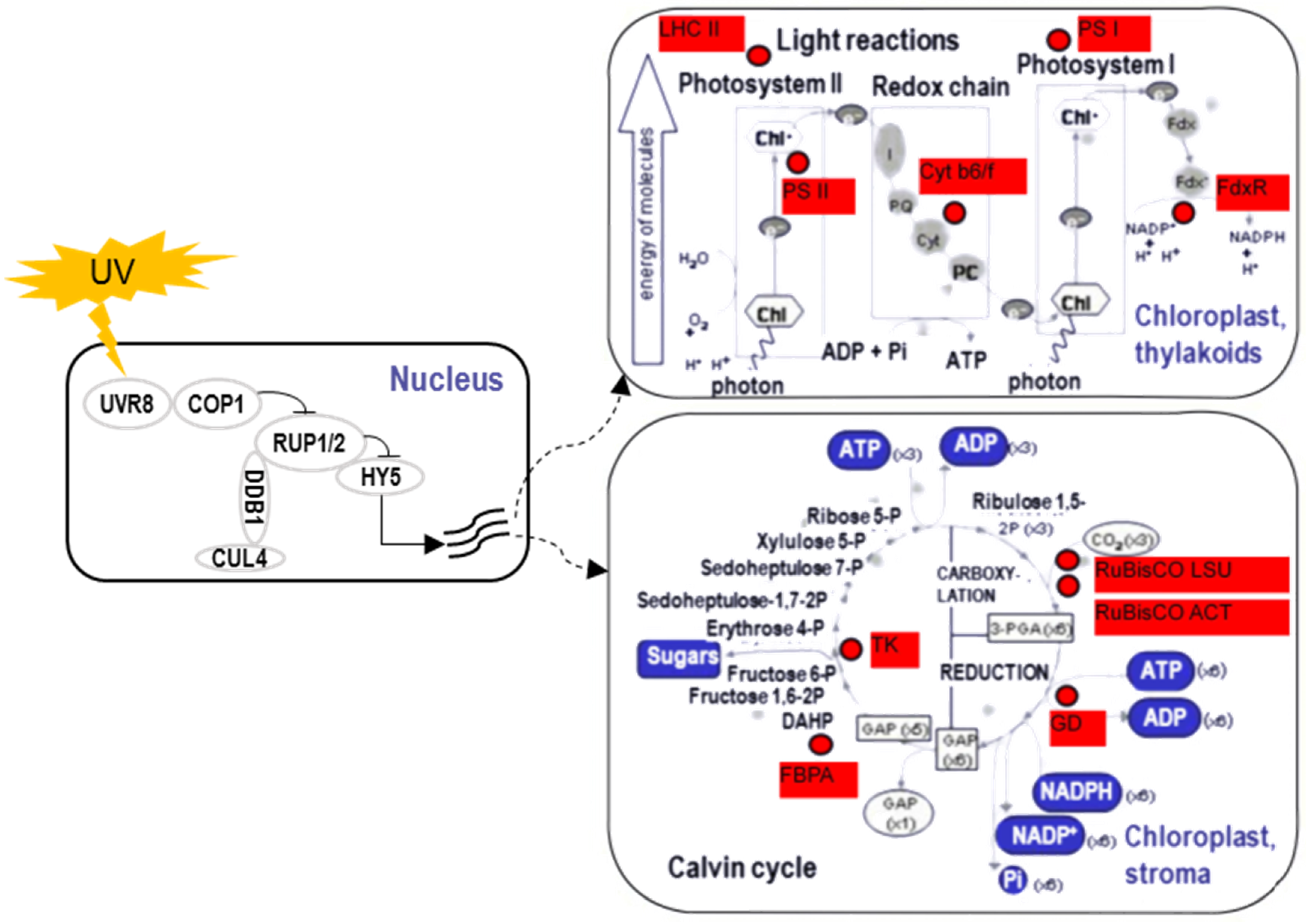
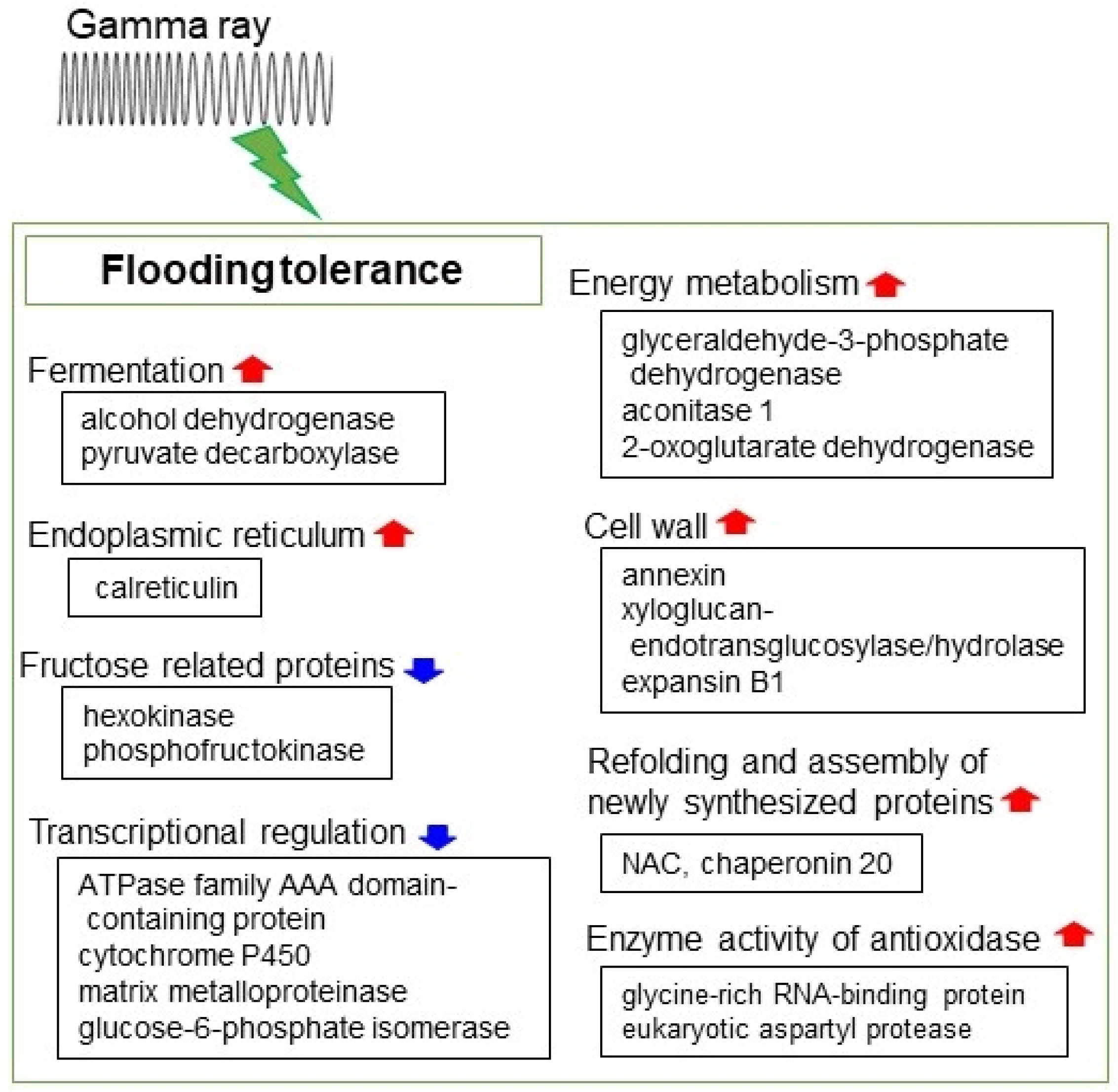
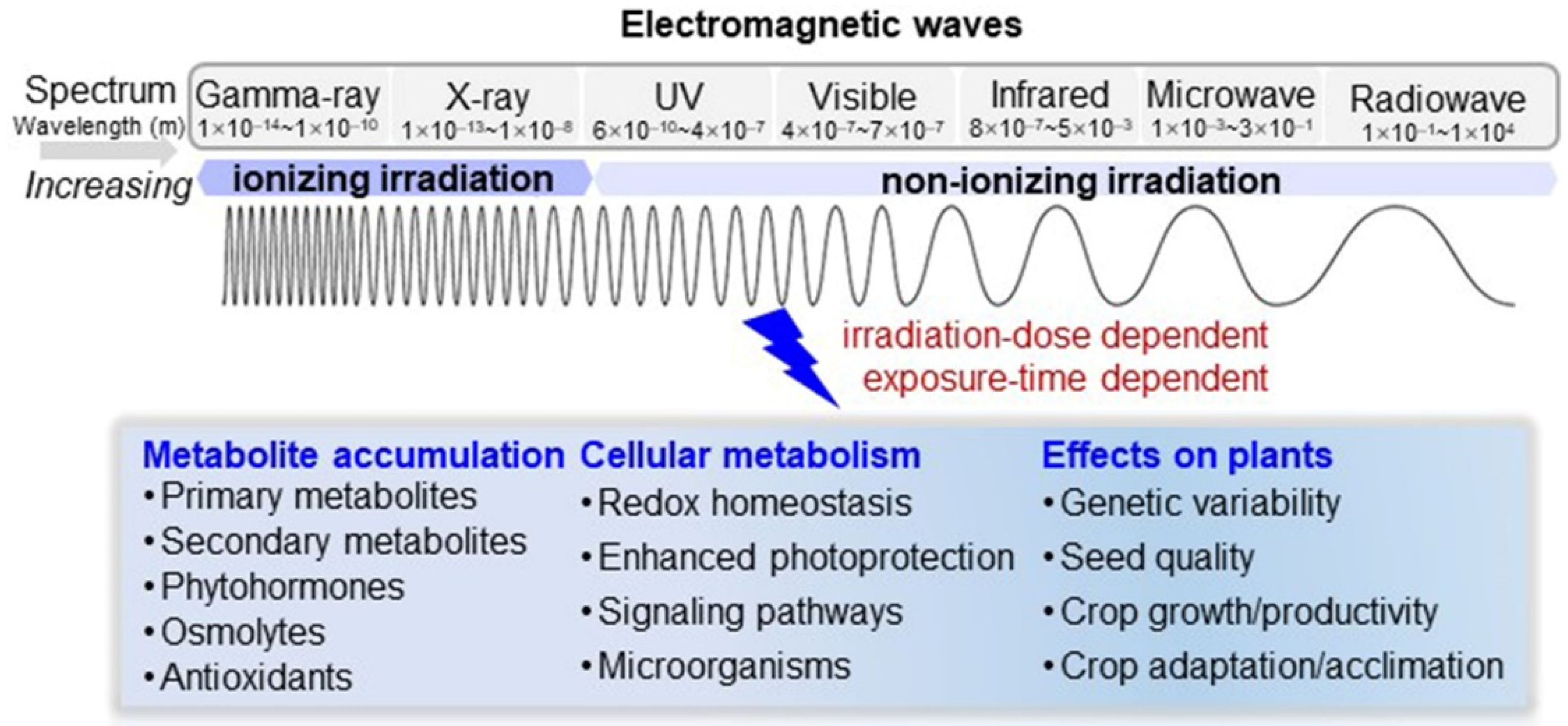
| Electromagnetic Spectrum | Wavelength (m) a | Frequency (Hz) a | Source to Emit Spectra b | Radioactive Categories |
|---|---|---|---|---|
| audio/radio waves | 1 × 10−1–1 × 104 | 3 × 104–3 × 109 | obtained with a ferro or piezoelectric transducer | non-ionizing irradiation |
| microwaves | 1 × 10−3–3 × 10−1 | 1 × 109–3 × 1011 | emitted by a magnetron or a klystron | |
| infrared | 8 × 10−7–5 × 10−3 | 6 × 1010–4 × 1014 | emitted by an incandescent object | |
| visible light | 4 × 10−7–7 × 10−7 | 4 × 1014–7 × 1014 | emitted by an electric light bulb | |
| ultraviolet | 6 × 10−10–4 × 10−7 | 7 × 1014–5 × 1017 | radiated with deuterium or mercury vapor lamps | |
| X-ray | 1 × 10−13–1 × 10−8 | 1 × 1016–3 × 1021 | emitted when electrons collide on a metal plate | ionizing irradiation |
| gamma ray | 1 × 10−14–1 × 10−10 | 3 × 1018–3 × 1022 | emitted by radioactive elements |
| Plant Species | Morphophysiological Effects | Ref b |
|---|---|---|
| Soybean | increased hypocotyl length/weight and main root length | [24] |
| Wheat | increased fresh weight, shoot height, length of main ear, number of grains in an ear, grain weight in an ear, lipid-peroxidation rate, catalase activity, malondialdehyde content, and flood tolerance; improved germination rate and germination potential; altered water absorption during germination; shortened phenophase | [41,42,43,44,45] |
| Brown rice | stimulated germination; increased polyphenol content and DPPH a radical scavenging activity; decreased gamma-aminobutyric acid content | [46] |
| Chickpea | increased leaf length/weight, root length/weight, and flood tolerance; decreased cell death under flooding | [48] |
| Plant Species | UV-Subtype | Morphophysiological Effects | Accumulated Secondary Metabolites | Ref b |
|---|---|---|---|---|
| Mung bean | UV-B | increased activities of phenyl alanine ammonia-lyase, L-galactono-1, 4-lactone dehydrogenase, and chalcone isomerase | vitamin C; total phenolics; total flavonoids | [94] |
| Ginkgo biloba | UV-B | unknown | total flavonoids; quercetin; kaempferol | [95] |
| Astragalus membranaceus Bge. | n.s. a | decreased chlorophyll content, stomatal conductance, and net photosynthesis rate; increased activities of superoxide dismutase, catalase, and ascorbate peroxidase | calycosin-7-O-beta-D-glucoside; daidzein; calycosin | [96,104] |
| Lonicera japonica Thunb. | UV-A, UV-B | increased antioxidant activity | chlorogenic acid; 3,4-di-O-caffeoylquinic acid; 3,5-di-O-caffeoylquinic acid; 4,5-di-O-caffeoylquinic acid; secologanic acid; secoxyloganin; secologanin; (E)-aldosecologanin | [97] |
| Artemisia annua | UV-B | decreased contents of chlorophyll/carotenoid, photosynthetic rate, stomatal conductance, and transpiration rate; increased activities of RuBisCO | essential oils | [98] |
| Catharanthus roseus | UV-B | increased ATP content in leaves | strictosidine; vindoline; catharanthine; ajmalicine | [99,105] |
| Taxus chinensis | UV-A | damaged structures of chloroplasts and mitochondria | paclitaxel; 10-deacetylbaccatin III; baccatin III | [100] |
| Achyranthes bidentata Blume | UV-B | decreased plant height, root length, fresh weight of aerial parts/roots, and contents of photosynthetic pigments; increased activities of superoxide dismutase and peroxidase | oleanolic acid; ecdysterone | [102] |
| Salvia miltiorrhiza Bunge | UV-B | unknown | salvianolic acid B; rosmarinic acid; danshensu | [103] |
| Barley | UV-B | decreased elongation rate of primary roots and root osmotic pressure; increased modulus of elasticity of roots and cell wall rigidity | saponarin | [106,107] |
| Birch | UV-B | unaffected leaf morphology | quercitrin; myricetin-3-galactoside; chlorogenic acid | [108] |
| Broccoli | UV-B | increased resistance against insect feeding | kaempferol; quercetin; glucosinolates | [109] |
| Centella asiatica | UV-B | decreased content of chlorophyll; increased absorbance of adaxial epidermises at 375 nm, and necrotic spots on the epidermises | kaempferol-3-O-beta-d-glucuronopyranoside; quercetin-3-O-beta-d-glucuronopyranoside | [110] |
| Clematis terniflora | UV-B | decreased leaf area and biomass; increased occurrences of burned patches and crispation in leaves | luteolin 7-O-beta-D-glucosiduronic acid; rutin; kaempferol 3-O-rutinose | [111] |
| Grape berry | UV-C | increased relative mass of skins; unaffected berry weight and berry caliber | trans-resveratrol; piceid; viniferin | [112] |
| Polygonum cuspidatum | UV-C | unknown | resveratrol | [113] |
| Psychotria brachyceras | UV-B | unknown | brachycerine | [114] |
| Radish | UV-A | decreased plant height;increased release of hydrogen | anthocyanin | [115] |
| Rice | n.s. a | decreased leaf photosynthetic rate, pollen germination, spikelet fertility, and yield; increased spikelet abortion | N-trans-cinnamoyltryptamine; N-(p-coumaroyl) serotonin; N-cinnamoyltyramine | [116,117] |
| Willow | UV-B | increased shoot biomass | luteolin-7-glucoside; monomethyl-monocoumaryl-luteolin-7-glucoside; myricetin derivative; apigenin-7-glucuronide; p-hydroxycinnamic acid derivative | [118] |
| Plant Species | Treatment of Gamma Irradiation | Effects | Ref b |
|---|---|---|---|
| Soybean | Seeds were irradiated with 200 Gy of gamma rays for 20 h. | Root growth was not suppressed even after being exposed to flooding stress for 4 days. | [26] |
| Onion | Seedlings were irradiated at doses ranging from 0.1 to 10 Gy of a 137Cs gamma source for 6 and 10 days. a | The growth of root and shoot was inhibited after 6 days exposure at all doses, including the low dose (0.1 Gy). At a later point in time (day 10), root and shoot inhibition was observed after irradiation at high doses (above 5 Gy). | [144] |
| Cymbidium hybrid | Cymbidium hybrid RB001 protocorm-like bodies were irradiated in a time course and dose-dependent manner (1 h, 16.1 Gy; 4 h, 23.6 Gy; 8 h, 37.9 Gy; 16 h, 37.9 Gy; and 24 h, 40.0 Gy) of gamma rays. | Based on survival rate of the plant, the estimated optimal doses were duration-dependent at irradiation durations shorter than 8 h. | [145] |
| Cowpea | Seeds were irradiated by 60Co source with dose of 11 kGy and the actual dose delivered was an average of 11.2 kGy at a dose rate of 1.7 kGy h−1. | Irradiation led to decrease in wall thickness, increase of cell size, and intercellular spaces in cotyledon. | [146] |
| Common vetch | Seeds were irradiated with 100 Gy of gamma irradiation. | Irradiation pretreatment (100 Gy), alone or in combination with salt stress and drought stress, led to significant increases in dry matter accumulation, catalase/superoxide dismutase/ascorbate peroxidase activities, and proline contents. However, gamma-irradiation pretreatment alone increased chlorophyll contents while decreasing malondialdehyde contents. | [147] |
| Poplar | Plantlets were concomitantly irradiated at doses of 10, 20, 50, 100, 200, and 300 Gy, respectively (dose rates ranged from 0.5 to 15 Gy h−1), for 20 h in 60Co. | Acute irradiation with a dose of 100 Gy greatly reduced height, stem diameter, and biomass of poplar plantlets. After receiving doses of 200 and 300 Gy, all plantlets stopped growing, and most of them withered after 4–10 weeks of irradiation. | [148] |
| Wheat | Seeds were irradiated at doses of 0, 10, 20, and 30 Gy. | The 20 Gy dose improved seed germination capacity compared with non-irradiated ones. | [149] |
| Maize | Seeds were irradiated at doses ranging from 0.1 to 1 kGy of gamma rays. | Germination potential and physiological parameters of maize seedlings decreased by increasing irradiation dose. Plants derived from seeds exposed at higher doses (0.5 kGy) did not survive more than 10 days. | [150] |
| Lathyrus chrysanthus | Seeds were irradiated with different doses (0, 50, 100, 150, 200, and 250 Gy) of 60Co at 0.8 kGy h−1. | Low dose irradiation stimulated germination and shoot growth initiation; however, high level irradiation inhibited seed germination and seedling growth. | [151] |
| Quinoa | Seeds were irradiated at 50, 100, and 200 Gy emitted by 60Co. | Plant height and biomass increased in quinoa treated with a low dose (50 Gy) compared to the control. | [152] |
| Plant/ Organs | Treatment | Stress | Finding | Ref b |
|---|---|---|---|---|
| Wheat/ Seeds | microwave irradiation at 2.45 Ghz for 10 s | Salt | Low energy microwave irradiation pretreatment of seeds for 10 s protected seedlings from salt stress by enhanced enzyme activities of nitric oxide synthase, catalase, peroxidase, superoxidase dismutase, and glutathione reductase. | [162] |
| Wheat/ Seeds | microwave irradiation at 2.45 Ghz for 10 s | Osmotic | Microwave irradiation of seeds for 10 s conferred plant tolerance to osmotic stress by enhancing nitric oxide signaling and antioxidant defense system. | [163] |
| Wheat/ Seeds | microwave irradiation at 2.45 Ghz for 5, 10, and 15 s | Cd | Seeds pretreated with microwave irradiation for 5 or 10 s ameliorated plant growth under Cd stress by decreasing lipid peroxidation and hydrogen peroxide accumulation. | [164] |
| Onion/ Seeds | fluorescent lamp exposure with 32 w for 8 h | AgNPs a | Light exposure reduced genotoxicity and cytotoxicity of AgNPs by reducing uptake of NPs by plant cells. | [165] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Z.; Wang, X.; Yin, X.; Tian, J.; Komatsu, S. Morphophysiological and Proteomic Responses on Plants of Irradiation with Electromagnetic Waves. Int. J. Mol. Sci. 2021, 22, 12239. https://doi.org/10.3390/ijms222212239
Zhong Z, Wang X, Yin X, Tian J, Komatsu S. Morphophysiological and Proteomic Responses on Plants of Irradiation with Electromagnetic Waves. International Journal of Molecular Sciences. 2021; 22(22):12239. https://doi.org/10.3390/ijms222212239
Chicago/Turabian StyleZhong, Zhuoheng, Xin Wang, Xiaojian Yin, Jingkui Tian, and Setsuko Komatsu. 2021. "Morphophysiological and Proteomic Responses on Plants of Irradiation with Electromagnetic Waves" International Journal of Molecular Sciences 22, no. 22: 12239. https://doi.org/10.3390/ijms222212239
APA StyleZhong, Z., Wang, X., Yin, X., Tian, J., & Komatsu, S. (2021). Morphophysiological and Proteomic Responses on Plants of Irradiation with Electromagnetic Waves. International Journal of Molecular Sciences, 22(22), 12239. https://doi.org/10.3390/ijms222212239







