Advances in Targeting HPV Infection as Potential Alternative Prophylactic Means
Abstract
1. Introduction
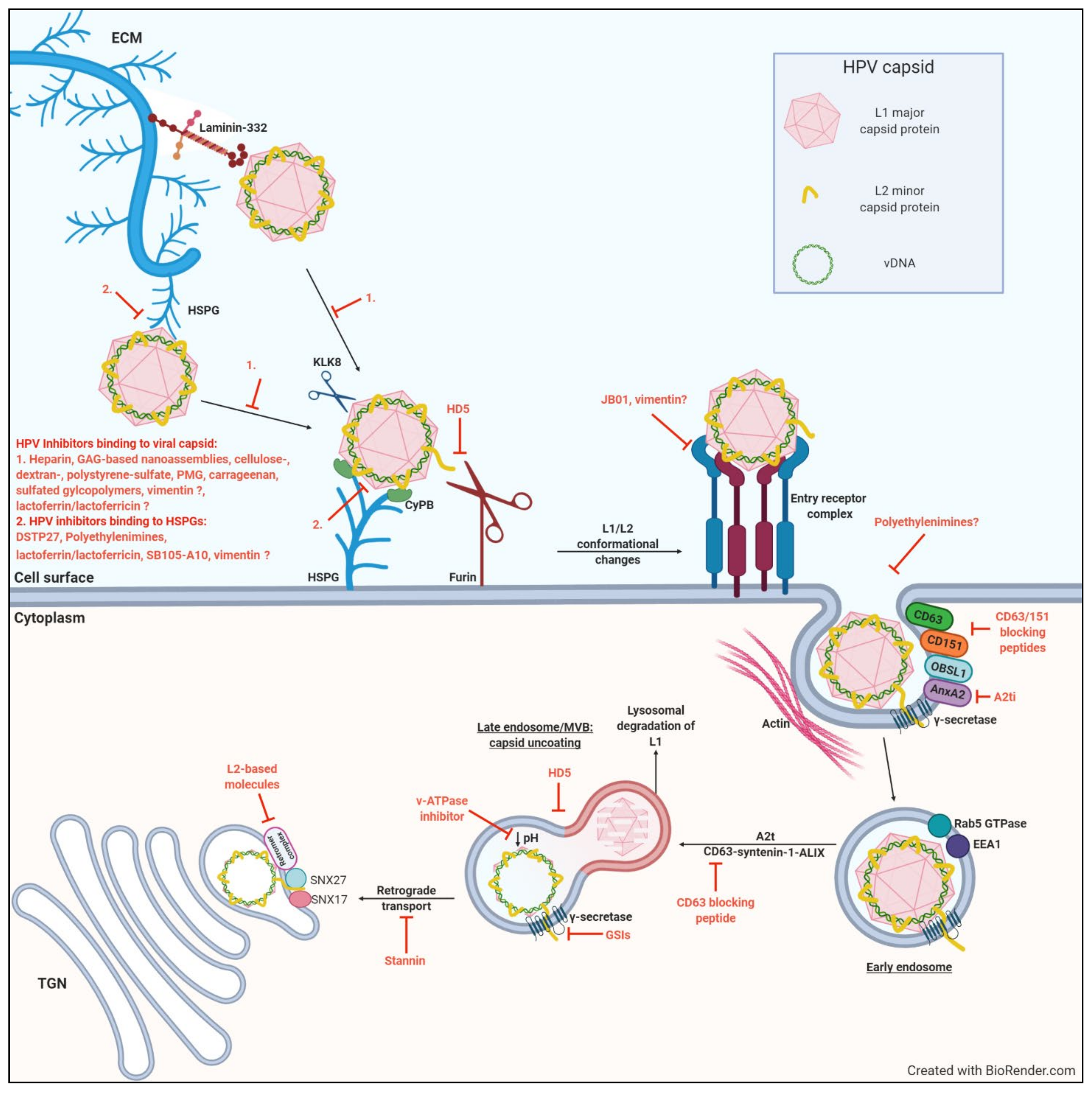
2. Early Stages of HPV Infection
2.1. HSPG Binding at The Cell Surface
2.2. Furin Cleavage and Subsequent Transfer to An Unknown Receptor/Receptor Complex
2.3. Intracellular Trafficking and Transport of The L2/Vdna Complex to The Nucleus
3. Molecular Targets of Early HPV Infection
3.1. Targets of Heparan Sulfate Binding
3.1.1. Heparin and Heparin-Based Molecules
3.1.2. Naturally Derived Sulfated Polysaccharides
3.1.3. Synthetic Sulfated Polysaccharides
3.1.4. Dispirotripiperazine
3.1.5. Polyethylenimines
3.1.6. Lactoferrin/Lactoferricin
3.1.7. Dendrimers
3.2. Targets of HPV Cellular Internalization
3.2.1. Vimentin
3.2.2. Anhydride-modified Protein (JB01)
3.2.3. Annexin A2
3.2.4. Tetraspanin Blocking Peptides (CD63 and CD151)
3.3. Targets of Intracellular Trafficking
3.3.1. v-ATPase Inhibitors
3.3.2. Human α-Defensin 5
3.3.3. γ-Secretase Inhibitors
3.3.4. Stannin
3.3.5. L2-Based Molecules
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; Zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.M.; Baker, C.C. Papillomavirus genome structure, expression, and post-transcriptional regulation. Front. Biosci. 2006, 11, 2286–2302. [Google Scholar] [CrossRef] [PubMed]
- International Human Papillomavirus (HPV) Reference Center Human Reference Clones. Available online: https://www.hpvcenter.se/human_reference_clones/ (accessed on 1 December 2020).
- Bosch, F.X.; Manos, M.M.; Muñoz, N.; Sherman, M.; Jansen, A.M.; Peto, J.; Schiffman, M.H.; Moreno, V.; Kurman, R.; Shan, K.V.; et al. Prevalence of Human Papillomavirus in Cervical Cancer: A Worldwide Perspective. J. Natl. Cancer Inst. 1995, 87, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Munoz, N.; Bosch, F.X.; Castellsague, X.; Diaz, M.; De Sanjose, S.; Hammouda, D.; Shah, K.V.; Meijer, C.J.L.M. Against Which Papillomavirus Types Shall We Vaccinate and Screen? The International Perspective. Int. J. Cancer 2004, 111, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Lacey, C.J.N.; Lowndes, C.M.; Shah, K.V. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6 / 11 disease. Vaccine 2006, 3, 35–41. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. WHO Cervical Cancer: Estimated Incidence, Mortality And prevalence Worldwide in 2012; WHO: Lyon, France, 2012. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- World HPV Information Center Human Papillomavirus and Related Diseases Report-South Africa. ICO HPV Inf. Cent. Rep. 2017, 1–78.
- De Vuyst, H.; Alemany, L.; Lacey, C.; Chibwesha, C.J.; Sahasrabuddhe, V.; Banura, C.; Denny, L.; Parham, G.P. The Burden of Human Papillomavirus Infections and Related Diseases in Sub-Saharan Africa. Vaccine 2013, 31, F32–F46. [Google Scholar] [CrossRef]
- Williamson, A.-L. The Interaction between Human Immunodeficiency Virus and Human Papillomaviruses in Heterosexuals in Africa. J. Clin. Med. 2015, 4, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Cobucci, R.N.O.; Lima, P.H.; de Souza, P.C.; Costa, V.V.; da Conceição de Mesquita Cornetta, M.; Fernandes, J.V.; Gonçalves, A.K. Assessing the impact of HAART on the incidence of defining and non-defining AIDS cancers among patients with HIV/AIDS: A systematic review. J. Infect. Public Health 2015, 8, 1–10. [Google Scholar] [CrossRef]
- Stanley, M.A. Genital human papillomavirus infections: Current and prospective therapies. J. Gen. Virol. 2012, 93, 681–691. [Google Scholar] [CrossRef]
- Siddiqui, M.A.A.; Perry, C.M. Vaccine (Gardasil ®). Drugs 2006, 66, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Tumban, E. Gardasil-9: A global survey of projected efficacy. Antiviral Res. 2016, 130, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Monie, A.; Hung, C.F.; Roden, R.; Wu, T.C. CervarixTM: A vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biol. Targets Ther. 2008, 2, 107–113. [Google Scholar] [CrossRef]
- Stanley, M.; Lowy, D.R.; Frazer, I. Chapter 12: Prophylactic HPV vaccines: Underlying mechanisms. Vaccine 2006, 24, s3106–s3113. [Google Scholar] [CrossRef] [PubMed]
- Heley, S.; Brotherton, J. Abnormal Pap tests after the HPV vaccine. Aust. Fam. Physician 2009, 38, 977–979. [Google Scholar]
- Brown, D.R.; Kjaer, S.K.; Sigurdsson, K.; Iversen, O.E.; Mauricio, H.A.; Wheeler, C.M.; Perez, G.; Koutsky, L.A.; Tay, E.H.; Garcia, P.; et al. The impact of quadrivalent human papillomavirus (HPV; Types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J. Infect. Dis. 2009, 199, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Denny, L. Control of Cancer of the Cervix in Low- and Middle-Income Countries. Ann. Surg. Oncol. 2015, 22, 728–733. [Google Scholar] [CrossRef]
- Tathiah, N.; Chb, M.B.; Man, D.H.I.V.; Epi, M.S.; Phm, M. Human papillomavirus (HPV) vaccination of adolescents in the South African private health sector: Lessons from the HPV demonstration project in KwaZulu-Natal. South Afr. Med. J. 2015, 105, 11–13. [Google Scholar] [CrossRef]
- Christensen, N.D.; Reed, C.A.; Culp, T.D.; Hermonat, P.L.; Howett, M.K.; Anderson, R.A.; Zaneveld, L.J.D.; Hemother, A.N.A.G.C. Papillomavirus Microbicidal Activities of High-Molecular-Weight Cellulose Sulfate, Dextran Sulfate, and Polystyrene Sulfonate. Antimicrob. Agents Chemother. 2001, 45, 3427–3432. [Google Scholar] [CrossRef]
- Christensen, N.D.; Kreider, J.W.; Cladel, N.M.; Patrick, S.D.; Welsh, P.A. Monoclonal Antibody-Mediated Neutralization of Infectious Human Papillomavirus Type 11. J. Virol. 1990, 64, 5678–5681. [Google Scholar] [CrossRef] [PubMed]
- Christensen, N.D.; Cladel, N.M.; Reed, C.A. Postattachment Neutralization of Papillomaviruses by Monoclonal and Polyclonal Antibodies. Virology 1995, 207, 136–142. [Google Scholar] [CrossRef]
- Howett, M.K.; Neely, E.B.; Christensen, N.D.; Wigdahl, B.; Krebs, F.C.; Malamud, D.; Patrick, S.D.; Pickel, M.D.; Welsh, P.A.; Reed, C.A.; et al. A Broad-Spectrum Microbicide with Virucidal Activity against Sexually Transmitted Viruses. Antimicrob. Agents Chemother. 1999, 43, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Howett, M.K.; Wigdahl, B.; Malamud, D.; Christensen, N.D.; Wyrick, P.B.; Krebs, F.C.; Catalone, B.J. Alkyl sulfates: A new family of broad spectrum microbicides. In Proceedings of the XIII International AIDS Conference. Monduzzi Editore, International Proceedings Division (Bologna, Italy), Durban, South Africa, 9–14 July 2000; pp. 707–712. [Google Scholar]
- Raff, A.B.; Woodham, A.W.; Raff, L.M.; Skeate, J.G.; Yan, L.; Da Silva, D.M.; Schelhaas, M.; Kast, W.M. The Evolving Field of Human Papillomavirus Receptor Research: A Review of Binding and Entry. J. Virol. 2013, 87, 6062–6072. [Google Scholar] [CrossRef]
- Day, P.M.; Schiller, J.T. The role of furin in papillomavirus infection. Future Microbiol. 2009, 4, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Siddiqa, A.; Broniarczyk, J.; Banks, L. Papillomaviruses and Endocytic Trafficking. Int. J. Mol. Sci. 2018, 19, 2619. [Google Scholar] [CrossRef]
- Mikuličić, S.; Florin, L. The endocytic trafficking pathway of oncogenic papillomaviruses. Papillomavirus Res. 2019, 7, 135–137. [Google Scholar] [CrossRef]
- Stanley, M.A. Epithelial Cell Responses to Infection with Human Papillomavirus. Clin. Microbiol. Rev. 2012, 25, 215–222. [Google Scholar] [CrossRef]
- Chow, L.T.; Broker, T.R.; Steinberg, B.M. The natural history of human papillomavirus infections of the mucosal epithelia. Authors J. Compil. 2010, 118, 422–449. [Google Scholar] [CrossRef]
- Ozbun, M.A. Extracellular events impacting human papillomavirus infections: Epithelial wounding to cell signaling involved in virus entry. Papillomavirus Res. 2019, 7, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Shope, B.R.E.; Hurst, B.E.W. Infectious Papillomatosis of Rabbits. J. Exp. Med. 1933, 58, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.N.; Buck, C.B.; Thompson, C.D.; Kines, R.; Bernardo, M.; Choyke, P.L.; Lowy, D.R.; Schiller, J.T. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 2007, 13, 857–861. [Google Scholar] [CrossRef]
- Stanley, M. HPV-immune response to infection and vaccination. Infect. Agent. Cancer 2010, 5, 19. [Google Scholar] [CrossRef]
- Kirnbauer, R.; Booy, F.; Cheng, N.; Lowy, D.R.; Schiller, J.T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 1992, 89, 12180–12184. [Google Scholar] [CrossRef]
- Buck, C.B.; Thompson, C.D.; Pang, Y.-Y.S.; Lowy, D.R.; Schiller, J.T. Maturation of Papillomavirus Capsids. J. Virol. 2005, 79, 2839–2846. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.B.; Cheng, N.; Thompson, C.D.; Lowy, D.R.; Steven, A.C.; Schiller, J.T.; Trus, B.L. Arrangement of L2 within the Papillomavirus Capsid. J. Virol. 2008, 82, 5190–5197. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, G.; Graham, L.M.; Lang, D.; Blumenthal, M.J.; Bergant, M.; Katz, A.A. Vimentin modulates infectious internalisation of HPV16 pseudovirions. J. Virol. 2017, 91, 307–317. [Google Scholar] [CrossRef]
- Giroglou, T.; Florin, L.; Schafer, F.; Streeck, R.E.; Sapp, M. Human Papillomavirus Infection Requires Cell Surface Heparan Sulfate. Am. Soc. Microbiol. 2001, 75, 1565–1570. [Google Scholar] [CrossRef]
- Johnson, K.M.; Kines, R.C.; Roberts, J.N.; Lowy, D.R.; Schiller, J.T.; Day, P.M. Role of Heparan Sulfate in Attachment to and Infection of the Murine Female Genital Tract by Human Papillomavirus. J. Virol. 2009, 83, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–33. [Google Scholar] [CrossRef]
- Bernfield, M.; Götte, M.; Park, P.W.; Reizes, O.; Fitzgerald, M.L.; Lincecum, J.; Zako, M. Functions of Cell Surface Heparan Sulfate Proteoglycans. Annu. Rev. Biochem. 1999, 68, 729–777. [Google Scholar] [CrossRef] [PubMed]
- Bernfield, M.; Kokenyesi, R.; Kato, M.; Hinkes, M.; Spring, J.; Gallor, L. Biology of the syndecans: A family of transmembrane heparan sulfate proteoglycans. Annu. Rev. Cell Biol. 1992, 8, 365–393. [Google Scholar] [CrossRef]
- Fransson, L.-Å. Glypicans. Int. J. Biochem. Cell Biol. 2003, 35, 125–129. [Google Scholar] [CrossRef]
- Lembo, D.; Donalisio, M.; Laine, C.; Cagno, V.; Civra, A.; Bianchini, E.P.; Zeghbib, N.; Bouchemal, K. Auto-associative heparin nanoassemblies: A biomimetic platform against the heparan sulfate-dependent viruses HSV-1, HSV-2, HPV-16 and RSV. Eur. J. Pharm. Biopharm. 2014, 88, 275–282. [Google Scholar] [CrossRef]
- Schäfer, G.; Blumenthal, Melissa, J.; Katz, A.A. Interaction of human tumor viruses with host cell surface receptors and cell entry. Viruses 2015, 7, 2592–2617. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, M.; Rusnati, M.; Presta, M.; Giacca, M. Internalization of HIV-1 Tat Requires Cell Surface Heparan Sulfate Proteoglycans. J. Biol. Chem. 2001, 276, 3254–3261. [Google Scholar] [CrossRef]
- Kalia, M.; Chandra, V.; Rahman, S.A.; Sehgal, D.; Jameel, S. Heparan Sulfate Proteoglycans Are Required for Cellular Binding of the Hepatitis E Virus ORF2 Capsid Protein and for Viral Infection. J. Virol. 2009, 83, 12714–12724. [Google Scholar] [CrossRef]
- Xu, Y.; Martinez, P.; Séron, K.; Luo, G.; Allain, F.; Dubuisson, J.; Belouzard, S. Characterization of Hepatitis C Virus Interaction with Heparan Sulfate Proteoglycans. J. Virol. 2015, 89, 3846–3858. [Google Scholar] [CrossRef] [PubMed]
- Summerford, C.; Samulski, R.J. Membrane-Associated Heparan Sulfate Proteoglycan Is a Receptor for Adeno-Associated Virus Type 2 Virions. J. Virol. 1998, 72, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Hilgard, P.; Stockert, R. Heparan sulfate proteoglycans initiate dengue virus infection of hepatocytes. Hepatology 2000, 32, 1069–1077. [Google Scholar] [CrossRef]
- Schulze, A.; Gripon, P.; Urban, S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology 2007, 46, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Shieh, M.T.; WuDunn, D.; Montgomery, R.I.; Esko, J.D.; Spear, P.G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J. Cell Biol. 1992, 116, 1273–1281. [Google Scholar] [CrossRef]
- Culp, T.D.; Budgeon, L.R.; Christensen, N.D. Human papillomaviruses bind a basal extracellular matrix component secreted by keratinocytes which is distinct from a membrane-associated receptor. Virology 2006, 347, 147–159. [Google Scholar] [CrossRef]
- Culp, T.D.; Budgeon, L.R.; Marinkovich, M.P.; Meneguzzi, G.; Christensen, N.D. Keratinocyte-Secreted Laminin 5 Can Function as a Transient Receptor for Human Papillomaviruses by Binding Virions and Transferring Them to Adjacent Cells. J. Virol. 2006, 80, 8940–8950. [Google Scholar] [CrossRef]
- Cerqueira, C.; Ventayol, P.S.; Vogeley, C.; Schelhaas, M. Kallikrein-8 Proteolytically Processes Human Papillomaviruses in the Extracellular Space To Facilitate Entry into Host Cells. J. Virol. 2015, 89, 7038–7052. [Google Scholar] [CrossRef]
- Bienkowska-Haba, M.; Patel, H.D.; Sapp, M. Target Cell Cyclophilins Facilitate Human Papillomavirus Type 16 Infection. Pathogens 2009, 5, 1–11. [Google Scholar] [CrossRef]
- Richards, R.M.; Lowy, D.R.; Schiller, J.T.; Day, P.M. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc. Natl. Acad. Sci. USA 2006, 103, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Selinka, H.-C.; Florin, L.; Patel, H.D.; Freitag, K.; Schmidtke, M.; Makarov, V.A.; Sapp, M. Inhibition of Transfer to Secondary Receptors by Heparan Sulfate-Binding Drug or Antibody Induces Noninfectious Uptake of Human Papillomavirus. J. Virol. 2007, 81, 10970–10980. [Google Scholar] [CrossRef] [PubMed]
- Day, P.M.; Lowy, D.R.; Schiller, J.T. Heparan Sulfate-Independent Cell Binding and Infection with Furin-Precleaved Papillomavirus Capsids. J. Virol. 2008, 82, 12565–12568. [Google Scholar] [CrossRef]
- Schelhaas, M.; Shah, B.; Holzer, M.; Blattmann, P.; Kühling, L.; Day, P.M.; Schiller, J.T.; Helenius, A. Entry of human papillomavirus type 16 by actin-dependent, clathrin- and lipid raft-independent endocytosis. PLoS Pathog. 2012, 8, e1002657. [Google Scholar] [CrossRef] [PubMed]
- Spoden, G.; Freitag, K.; Husmann, M.; Boller, K.; Sapp, M.; Lambert, C.; Florin, L. Clathrin- and Caveolin-Independent Entry of Human Papillomavirus type 16-Involvement of Tetraspanin-Enriched Microdomains (TEMs). PLoS ONE 2008, 3, e3313. [Google Scholar] [CrossRef] [PubMed]
- Bannach, C.; Brinkert, P.; Kühling, L.; Greune, L.; Schmidt, M.A.; Schelhaas, M. Epidermal Growth Factor Receptor and Abl2 Kinase Regulate Distinct Steps of Human Papillomavirus 16 Endocytosis. J. Virol. 2020, 94, e02143-19. [Google Scholar] [CrossRef] [PubMed]
- Fast, L.A.; Mikuličić, S.; Fritzen, A.; Schwickert, J.; Boukhallouk, F.; Hochdorfer, D.; Sinzger, C.; Suarez, H.; Monk, P.N.; Yáñez-Mó, M.; et al. Inhibition of tetraspanin functions impairs human papillomavirus and cytomegalovirus infections. Int. J. Mol. Sci. 2018, 19, 3007. [Google Scholar] [CrossRef] [PubMed]
- Hampe, L.; Boukhallouk, F.; Schneider, M.A.; Spoden, G.A.; Negwer, I.; Koynov, K.; Kast, W.M.; Florin, L. The Cytoskeletal Adaptor Obscurin-Like 1 Interacts with the Human Papillomavirus 16 ( HPV16 ) Capsid Protein L2 and Is Required for. J. Virol. 2016, 90, 10629–10641. [Google Scholar] [CrossRef]
- Dziduszko, A.; Ozbun, M.A. Annexin A2 and S100A10 Regulate Human Papillomavirus Type 16 Entry and Intracellular Trafficking in Human Keratinocytes. J. Virol. 2013, 87, 7502–7515. [Google Scholar] [CrossRef]
- Spoden, G.; Kühling, L.; Cordes, N.; Frenzel, B.; Sapp, M.; Boller, K.; Florin, L. Human Papillomavirus Types 16, 18, and 31 Share Similar Endocytic Requirements for Entry. J. Virol. 2013, 87, 7765–7773. [Google Scholar] [CrossRef]
- Bergant, M.; Ozbun, M.A.; Campos, S.K.; Myers, M.P.; Banks, L. Human Papillomavirus L2 Facilitates Viral Escape from Late Endosomes via Sorting Nexin 17. Traffic 2012, 13, 455–467. [Google Scholar] [CrossRef]
- Smith, J.L.; Campos, S.K.; Wandinger-Ness, A.; Ozbun, M.A. Caveolin-1-Dependent Infectious Entry of Human Papillomavirus Type 31 in Human Keratinocytes Proceeds to the Endosomal Pathway for pH-Dependent Uncoating. J. Virol. 2008, 82, 9505–9512. [Google Scholar] [CrossRef]
- Gräßel, L.; Fast, L.A.; Scheffer, K.D.; Boukhallouk, F.; Overduin, M.; Berditchevski, F.; Florin, L. The CD63-Syntenin-1 Complex Controls Post-Endocytic Trafficking of Oncogenic Human Papillomaviruses. Sci. Rep. 2016, 6, 1–18. [Google Scholar] [CrossRef]
- Taylor, J.R.; Fernandez, D.J.; Thornton, S.M.; Skeate, J.G.; Lühen, K.P.; Da Silva, D.M.; Langen, R.; Kast, W.M. Heterotetrameric annexin A2/S100A10 (A2t) is essential for oncogenic human papillomavirus trafficking and capsid disassembly, and protects virions from lysosomal degradation. Sci. Rep. 2018, 8, 11642. [Google Scholar] [CrossRef]
- Selinka, H.C.; Giroglou, T.; Sapp, M. Analysis of the infectious entry pathway of human papillomavirus type 33 pseudovirions. Virology 2002, 299, 279–287. [Google Scholar] [CrossRef] [PubMed]
- DiGiuseppe, S.; Bienkowska-Haba, M.; Guion, L.G.M.; Keiffer, T.R.; Sapp, M. Human Papillomavirus Major Capsid Protein L1 Remains Associated with the Incoming Viral Genome throughout the Entry Process. J. Virol. 2017, 91, e00537-17. [Google Scholar] [CrossRef]
- Yan, H.; Foo, S.S.; Chen, W.; Yoo, J.S.; Shin, W.J.; Wu, C.; Jung, J.U. Efficient inhibition of human papillomavirus infection by L2 minor capsid-derived lipopeptide. Am. Soc. Microbiol. 2019, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Monteiro da Silva, G.; Deatherage, C.; Burd, C.; DiMaio, D. Cell-Penetrating Peptide Mediates Intracellular Membrane Passage of Human Papillomavirus L2 Protein to Trigger Retrograde Trafficking. Cell 2018, 174, 1465–1476. [Google Scholar] [CrossRef]
- Bronnimann, M.P.; Chapman, J.A.; Park, C.K.; Campos, S.K. A Transmembrane Domain and GxxxG Motifs within L2 Are Essential for Papillomavirus Infection. J. Virol. 2013, 87, 464–473. [Google Scholar] [CrossRef]
- Inoue, T.; Zhang, P.; Zhang, W.; Bingham, K.G.; Dupzyk, A.; Dimaio, D.; Tsai, B. γ-Secretase promotes membrane insertion of the human papillomavirus L2 capsid protein during virus infection. J. Cell Biol. 2018, 217, 3545–3559. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.; Zhang, W.; Harrison, M.S.; Goodner, K.; Kazakov, T.; Goodwin, E.C.; Lipovsky, A.; Burd, C.G.; DiMaio, D. Direct Binding of Retromer to Human Papillomavirus Type 16 Minor Capsid Protein L2 Mediates Endosome Exit during Viral Infection. PLoS Pathog. 2015, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Siddiqa, A.; Massimi, P.; Pim, D.; Broniarczyk, J.; Banks, L. Human Papillomavirus 16 Infection Induces VAP-Dependent Endosomal Tubulation. J. Virol. 2018, 92, e01514-17. [Google Scholar] [CrossRef] [PubMed]
- Pyeon, D.; Pearce, S.M.; Lank, S.M.; Ahlquist, P.; Lambert, P.F. Establishment of Human Papillomavirus Infection Requires Cell Cycle Progression. PLoS Pathog. 2009, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aydin, I.; Weber, S.; Snijder, B.; Samperio Ventayol, P.; Kühbacher, A.; Becker, M.; Day, P.M.; Schiller, J.T.; Kann, M.; Pelkmans, L.; et al. Large Scale RNAi Reveals the Requirement of Nuclear Envelope Breakdown for Nuclear Import of Human Papillomaviruses. PLoS Pathog. 2014, 10, 1–19. [Google Scholar] [CrossRef]
- Calton, C.M.; Bronnimann, M.P.; Manson, A.R.; Li, S.; Chapman, J.A.; Suarez-Berumen, M.; Williamson, T.R.; Molugu, S.K.; Bernal, R.A.; Campos, S.K. Translocation of the papillomavirus L2/vDNA complex across the limiting membrane requires the onset of mitosis. PLoS Pathog. 2017, 13, e1006200. [Google Scholar] [CrossRef]
- Broniarczyk, J.; Massimi, P.; Bergant, M.; Banks, L. Human Papillomavirus Infectious Entry and Trafficking Is a Rapid process. J. Virol. 2015, 89, 8727–8732. [Google Scholar] [CrossRef]
- Schneider, M.A.; Spoden, G.A.; Florin, L.; Lambert, C. Identification of the dynein light chains required for human papillomavirus infection. Cell. Microbiol. 2011, 13, 32–46. [Google Scholar] [CrossRef]
- Aydin, I.; Villalonga-Planells, R.; Greune, L.; Bronnimann, M.P.; Calton, C.M.; Becker, M.; Lai, K.; Campos, S.K.; Schmidt, A.; Schelhaas, M. A central region in the minor capsid protein of papillomaviruses facilitates viral genome tethering and membrane penetration for mitotic nuclear entry. PLoS Pathog. 2017, 13, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Day, P.M.; Baker, C.C.; Lowy, D.R.; Schiller, J.T. Establishment of papillomavirus infection is enhanced by promyelocytic leukemia protein (PML) expression. Proc. Natl. Acad. Sci. USA 2004, 101, 14252–14257. [Google Scholar] [CrossRef]
- Rose, R.C.; Bonnez, W.; Reichman, R.C.; Garcea, R.L. Expression of Human Papillomavirus Type 11 Protein in Insect Cells: In Vivo and In Vitro Assembly of Viruslike Particles. J. Virol. 1993, 67, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, X.; Louis, K.; Frazer, I.H. Interaction of Human Papillomavirus (HPV) Type 16 Capsid Proteins with HPV DNA Requires an Intact L2 N-Terminal Sequence. J. Virol. 1994, 68, 619–625. [Google Scholar] [CrossRef]
- Buck, C.B.; Pastrana, D.V.; Lowy, D.R.; Schiller, J.T. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 2005, 119, 445–462. [Google Scholar] [CrossRef]
- Schäfer, G.; Kabanda, S.; Van Rooyen, B.; Bergant, M.; Banks, L.; Parker, M.I. The role of inflammation in HPV infection of the Oesophagus. BMC Cancer 2013, 13, 185. [Google Scholar] [CrossRef]
- Drobni, P.; Mistry, N.; McMillan, N.; Evander, M. Carboxy-fluorescein diacetate, succinimidyl ester labeled papillomavirus virus-like particles fluoresce after internalization and interact with heparan sulfate for binding and entry. Virology 2003, 310, 163–172. [Google Scholar] [CrossRef]
- Joyce, J.G.; Tung, J.S.; Przysiecki, C.T.; Cook, J.C.; Lehman, E.D.; Sands, J.A.; Jansen, K.U.; Keller, P.M. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 1999, 274, 5810–5822. [Google Scholar] [CrossRef]
- Day, P.M.; Thompson, C.D.; Buck, C.B.; Pang, Y.S.; Lowy, D.R.; Schiller, J.T. Neutralization of Human Papillomavirus with Monoclonal Antibodies Reveals Different Mechanisms of Inhibition . J. Virol. 2007, 81, 8784–8792. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Meyers, C. Differential Dependence on Host Cell Glycosaminoglycans for Infection of Epithelial Cells by High-Risk HPV Types. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef]
- Patterson, N.A.; Smith, J.L.; Ozbun, M.A. Human Papillomavirus Type 31b Infection of Human Keratinocytes Does Not Require Heparan Sulfate. J. Virol. 2005, 79, 6838–6847. [Google Scholar] [CrossRef]
- Cerqueira, C.; Liu, Y.; Kühling, L.; Chai, W.; Hafezi, W.; Van Kuppevelt, T.H.; Kühn, J.E.; Feizi, T.; Schelhaas, M. Heparin increases the infectivity of Human Papillomavirus Type 16 independent of cell surface proteoglycans and induces L1 epitope exposure. Cell. Microbiol. 2013, 15, 1818–1836. [Google Scholar] [CrossRef]
- Piret, J.; Lamontagne, J.; Bestman-smith, J.; Roy, S.; Omar, R.F.; Juha, J.; Gourde, P.; Bergeron, M.G. In Vitro and In Vivo Evaluations of Sodium Lauryl Sulfate and Dextran Sulfate as Microbicides against Herpes Simplex and Human Immunodeficiency Viruses. J. Clin. Microbiol. 2000, 38, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Herold, B.C.; Siston, A.; Bremer, J.; Kirkpatrick, R.; Wilbanks, G.; Fugedi, P.; Peto, C.; Cooper, M. Sulfated Carbohydrate Compounds Prevent Microbial Adherence by Sexually Transmitted Disease Pathogens. Antimicrob. Agents Chemother. 1997, 41, 2776–2780. [Google Scholar] [CrossRef] [PubMed]
- Herold, B.C.; Bourne, N.; Marcellino, D.; Kirkpatrick, R.; Strauss, D.M.; Zaneveld, L.J.D.; Waller, D.P.; Anderson, R.A.; Chany, C.J.; Barham, B.J.; et al. Poly (Sodium 4-Styrene Sulfonate): An Effective Candidate Topical Antimicrobial for the Prevention of Sexually Transmitted Diseases. J. Infect. Dis. 2000, 181, 770–773. [Google Scholar] [CrossRef]
- Lembo, D.; Donalisio, M.; Rusnati, M.; Bugatti, A.; Cornaglia, M.; Cappello, P.; Giovarelli, M.; Oreste, P.; Landolfo, S. Sulfated K5 Escherichia coli Polysaccharide Derivatives as Wide-Range Inhibitors of Genital Types of Human Papillomavirus. Antimicrob. Agents Chemother. 2008, 52, 1374–1381. [Google Scholar] [CrossRef]
- Wang, S.; Lu, Z.; Wang, S.; Liu, W.; Gao, J.; Tian, L.; Wang, L.; Zhang, X.; Zhao, X.; Wang, W.; et al. The inhibitory effects and mechanisms of polymannuroguluronate sulfate against human papillomavirus infection in vitro and in vivo. Carbohydr. Polym. 2020, 241, 116365. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Astani, A.; Ghosh, T.; Schnitzler, P.; Ray, B. Phytochemistry Polysaccharides from Sargassum tenerrimum: Structural features, chemical modification and anti-viral activity. Phytochemistry 2010, 71, 235–242. [Google Scholar] [CrossRef]
- Wu, L.; Wang, W.; Zhang, X.; Zhao, X.; Yu, G. Anti-HBV activity and mechanism of marine-derived polyguluronate sulfate (PGS) in vitro. Carbohydr. Polym. 2016, 143, 139–148. [Google Scholar] [CrossRef]
- Thi, T.; Thuy, T.; Minh, B.; Thi, T.; Van, T.; Van Quang, N.; Cam, H.; Zheng, Y.; Seguin-devaux, C.; Mi, B.; et al. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.B.; Thompson, C.D.; Lowy, D.R.; Roberts, J.N.; Mu, M.; Schiller, J.T. Carrageenan Is a Potent Inhibitor of Papillomavirus Infection. PLoS Pathog. 2006, 2, 671–680. [Google Scholar] [CrossRef]
- Calagna, G.; Maranto, M.; Paola, C.; Capra, G.; Perino, A.; Chiantera, V.; Cucinella, G.; Calagna, G.; Maranto, M.; Paola, C.; et al. ‘Secondary prevention’ against female HPV infection: Literature review of the role of carrageenan. Expert Rev. Anti. Infect. Ther. 2020, 18, 865–874. [Google Scholar] [CrossRef]
- Rodríguez, A.; Kleinbeck, K.; Mizenina, O.; Kizima, L.; Levendosky, K.; Jean-Pierre, N.; Villegas, G.; Ford, B.E.; Cooney, M.L.; Teleshova, N.; et al. In vitro and in vivo evaluation of two carrageenan-based formulations to prevent HPV acquisition. Antivir. Res. 2014, 108, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Novetsky, A.P.; Keller, M.J.; Gradissimo, A.; Chen, Z.; Morgan, S.L.; Xue, X.; Strickler, H.D.; Fernández-romero, J.A.; Burk, R.; Einstein, M.H. Gynecologic Oncology In vitro inhibition of human papillomavirus following use of a carrageenan-containing vaginal gel. Gynecol. Oncol. 2016, 143, 313–318. [Google Scholar] [CrossRef]
- Marais, D.; Gawarecki, D.; Allan, B.; Ahmed, K.; Altini, L.; Cassim, N.; Hoffman, M.; Ramjee, G.; Williamson, A. The effectiveness of Carraguard, a vaginal microbicide, in protecting women against high-risk human papillomavirus infection. Antivir. Ther. 2011, 16, 1219–1226. [Google Scholar] [CrossRef]
- Skoler-Karpoff, S.; Ramjee, G.; Ahmed, K.; Altini, L.; Plagianos, M.G.; Friedland, B.; Govender, S.; Town, C.; Town, C. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1977–1987. [Google Scholar] [CrossRef]
- Magnan, S.; Tota, J.E.; Burchell, A.N.; Schiller, J.T.; Ferenczy, A.; Franco, E.L.; Study, C. Efficacy of a Carrageenan gel Against Transmission of Cervical HPV (CATCH): Interim analysis of a randomized, double-blind, placebo-controlled, phase 2B trial. Clin. Microbiol. Infect. 2019, 25, 210–216. [Google Scholar] [CrossRef]
- Perino, A.; Consiglio, P.; Maranto, M.; Franciscis, P.D.E.; Marci, R. Impact of a new carrageenan-based vaginal microbicide in a female population with genital HPV-infection: First experimental results. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6744–6752. [Google Scholar] [CrossRef] [PubMed]
- Soria-Martinez, L.; Bauer, S.; Giesler, M.; Schelhaas, S.; Materlik, J.; Janus, K.; Pierzyna, P.; Becker, M.; Snyder, N.L.; Hartmann, L.; et al. Prophylactic Antiviral Activity of Sulfated Glycomimetic Oligomers and Polymers. JACS 2020, 142, 5252–5265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ye, X. Synthetic Glycans and Glycomimetics: A Promising Alternative to Natural Polysaccharides. Chemistry (Easton) 2018, 24, 6696–6704. [Google Scholar] [CrossRef]
- Schmidtke, M.; Karger, A.; Meerbach, A.; Egerer, R.; Stelzner, A.; Makarov, V. Binding of a N,N’-bisheteryl derivative of dispirotripiperazine to heparan sulfate residues on the cell surface specifically prevents infection of viruses from different families. Virology 2003, 311, 134–143. [Google Scholar] [CrossRef]
- Spoden, G.A.; Besold, K.; Krauter, S.; Plachter, B.; Hanik, N.; Kilbinger, A.F.M.; Lambert, C.; Florin, L. Polyethylenimine Is a Strong Inhibitor of Human Papillomavirus and Cytomegalovirus Infection. Antimicrob. Agents Chemother. 2012, 56, 75–82. [Google Scholar] [CrossRef]
- Schermant, D.; Demeneixt, B.; Behr, J. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef]
- Bellamy, W.; Wakabayashi, H.; Takase, M.; Kawase, K.; Shimamura, S.; Tomita, M. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med. Microbiol. Imunnol. 1993, 182, 97–105. [Google Scholar] [CrossRef]
- Andersen, J.H.; Jenssen, H.; Gutteberg, T.J. Lactoferrin and lactoferricin inhibit Herpes simplex 1 and 2 infection and exhibit synergy when combined with acyclovir. Antivir. Res. 2003, 58, 209–215. [Google Scholar] [CrossRef]
- Sandvik, K.; Gutteberg, T.J.; Andersen, J.H. Anti-HSV Activity of Lactoferrin and Lactoferricin is Dependent on the Presence of Heparan Sulphate at the Cell Surface. J. Med. Virol. 2004, 74, 262–271. [Google Scholar] [CrossRef]
- Harmsen, M.C.; Swart, P.J.; De Béthune, M.; Pauwels, R.; The, S.; Diseases, I.; Aug, N.; Harmsen, M.C.; Swart, P.J.; Pauwels, R.; et al. Antiviral Effects of Plasma and Milk Proteins: Lactoferrin Shows Potent Activity against Both Human Immunodeficiency Virus and Human Cytomegalovirus Replication in vitro. J. Infect. Dis. 1995, 172, 380–388. [Google Scholar] [CrossRef]
- Ikeda, M.; Sugiyama, K.; Tanaka, T.; Tanaka, K.; Sekihara, H.; Shimotohno, K.; Kato, N. Lactoferrin Markedly Inhibits Hepatitis C Virus Infection in Cultured Human Hepatocytes. Biochemical 1998, 245, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Longhi, G.; Pietropaolo, V.; Mischitelli, M.; Longhi, C.; Pia, M.; Marchetti, M.; Tinari, A.; Valenti, P.; Marta, A.; Seganti, L.; et al. Lactoferrin inhibits early steps of human BK polyomavirus infection. Antivir. Res. 2006, 72, 145–152. [Google Scholar] [CrossRef]
- Drobni, P.; Näslund, J.; Evander, M. Lactoferrin inhibits human papillomavirus binding and uptake in vitro. Antivir. Res. 2004, 64, 63–68. [Google Scholar] [CrossRef]
- Mistry, N.; Drobni, P.; Näslund, J.; Sunkari, V.G.; Jenssen, H.; Evander, M. The anti-papillomavirus activity of human and bovine lactoferricin. Antivir. Res. 2007, 75, 258–265. [Google Scholar] [CrossRef]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005, 62, 2588–2598. [Google Scholar] [CrossRef]
- Wu, H.; Monroe, D.M.; Church, F.C. Characterization of the Glycosaminoglycan-Binding Region of Lactoferrin. Arch. Biochem. Biophys. 1995, 317, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Superti, F.; Grazia, M.; Paola, A.; Rossi, P. Inhibition of poliovirus type 1 infection by iron-, manganese- and zinc-saturated lactoferrin. Med. Microbiol. Imunnol. 1999, 187, 199–204. [Google Scholar] [CrossRef]
- Bourne, N.; Stanberry, L.R.; Kern, E.R.; Holan, G.; Matthews, B. Dendrimers, a New Class of Candidate Topical Microbicides with Activity against Herpes Simplex Virus Infection. Antimicrob. Agents Chemother. 2000, 44, 2471–2474. [Google Scholar] [CrossRef] [PubMed]
- Donalisio, M.; Rusnati, M.; Civra, A.; Bugatti, A.; Allemand, D.; Pirri, G.; Giuliani, A.; Landolfo, S.; Lembo, D. Identification of a dendrimeric heparan sulfate-binding peptide that inhibits infectivity of genital types of human papillomaviruses. Antimicrob. Agents Chemother. 2010, 54, 4290–4299. [Google Scholar] [CrossRef]
- Virtanen, I.; Lehto, V.; Lehtonen, E.; Vartio, T.; Stenman, S.; Kurki, P.; Wager, O.; Small, J.V.; Dahl, D.; Badley, R.A. Expression of intermediate filaments in cultured cells. J. Cell Sci. 1981, 50, 45–63. [Google Scholar] [PubMed]
- Brown, M.J.; Hallam, J.A.; Colucci-Guyon, E.; Shaw, S. Rigidity of circulating lymphocytes is primarily conferred by vimentin intermediate filaments. J. Immunol. 2001, 166, 6640–6646. [Google Scholar] [CrossRef] [PubMed]
- Azumi, N.; Battifora, H. The distribution of vimentin and keratin in epithelial and nonepithelial neoplasms. A comprehensive immunohistochemical study on formalin- and alcohol-fixed tumors. Am. J. Clin. Pathol. 1987, 88, 286–296. [Google Scholar] [CrossRef]
- Herrmann, H.; Bär, H.; Kreplak, L.; Strelkov, S.V.; Aebi, U. Intermediate filaments: From cell architecture to nanomechanics. Nat. Rev. Mol. Cell Biol. 2007, 8, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, J.; Pallari, H.M.; Nevo, J.; Eriksson, J.E. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res. 2007, 313, 2050–2062. [Google Scholar] [CrossRef]
- Moisan, E.; Girard, D. Cell surface expression of intermediate lament proteins vimentin and lamin B1 in human neutrophil spontaneous apoptosis. J. Leukoc. Biol. 2006, 79, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Gonzalez, A.M.; DeBiase, P.J.; Trejo, H.E.; Goldman, R.D.; Flitney, F.W.; Jones, J.C.R. Recruitment of vimentin to the cell surface by β3 integrin and plectin mediates adhesion strength. J. Cell Sci. 2009, 122, 1390–1400. [Google Scholar] [CrossRef]
- Mor-Vaknin, N.; Punturieri, A.; Sitwala, K.; Markovitz, D.M. Vimentin is secreted by activated macrophages. Nat. Cell Biol. 2003, 5, 59–63. [Google Scholar] [CrossRef]
- Shigyo, M.; Tohda, C. Extracellular vimentin is a novel axonal growth facilitator for functional recovery in spinal cord-injured mice. Sci. Rep. 2016, 6, 28293. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ravi, V.; Desai, A. Japanese encephalitis virus interacts with vimentin to facilitate its entry into porcine kidney cell line. Virus Res. 2011, 160, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yu, C.; Liao, C.; Lin, Y. Vimentin binding is critical for infection by the virulent strain of Japanese encephalitis virus. Cell. Microbiol. 2011, 13, 1358–1370. [Google Scholar] [CrossRef]
- Miller, M.S.; Hertel, L. Onset of Human Cytomegalovirus Replication in Fibroblasts Requires the Presence of an Intact Vimentin Cytoskeleton. J. Virol. 2009, 83, 7015–7028. [Google Scholar] [CrossRef]
- Du, N.; Cong, H.; Tian, H.; Zhang, H.; Zhang, W.; Song, L.; Tien, P. Cell Surface Vimentin Is an Attachment Receptor for Enterovirus 71. J. Virol. 2014, 88, 5816–5833. [Google Scholar] [CrossRef] [PubMed]
- Kokuba, H.; Aurelian, L.; Neurath, A.R. 3-Hydroxyphthaloyl β-lactoglobulin. IV. Antiviral activity in the mouse model of genital herpesvirus infection. Antivir. Chem. Chemother. 1998, 9, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Neurath, A.R.; Strick, N.; Li, Y.Y. 3-Hydroxyphthaloyl β-lactoglobulin. III. Antiviral activity against herpesviruses. Antivir. Chem. Chemother. 1998, 9, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, L.; Tan, S.; Guo, X.; Lu, H.; Qi, Z.; Pan, C.; An, X.; Jiang, S.; Liu, S. 3-hydroxyphthalic anhydride-modified chicken ovalbumin exhibits potent and broad anti-HIV-1 activity: A potential microbicide for preventing sexual transmission of HIV-1. Antimicrob. Agents Chemother. 2010, 54, 1700–1711. [Google Scholar] [CrossRef]
- Lu, L.; Yang, X.; Li, Y.; Jiang, S. Chemically modified bovine beta-lactoglobulin inhibits human papillomavirus infection. Microbes Infect. 2013, 15, 506–510. [Google Scholar] [CrossRef]
- Hua, C.; Zhu, Y.; Wu, C.; Si, L.; Wang, Q.; Sui, L.; Jiang, S. The underlying mechanism of 3-hydroxyphthalic anhydride-modified bovine beta-lactoglobulin to block human papillomavirus entry into the host cell. Front. Microbiol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Qiu, L.; Wang, Y.; Wang, Y.; Wang, Q.; Song, L.; Li, Y.; Huang, K.; Du, X.; Fan, W.; et al. A randomized open-label clinical trial of an anti-HPV biological dressing (JB01-BD) administered intravaginally to treat high-risk HPV infection. Microbes Infect. 2016, 18, 148–152. [Google Scholar] [CrossRef]
- Woodham, A.W.; da Silva, D.M.; Skeate, J.G.; Raff, A.B.; Ambroso, M.R.; Brand, H.E.; Isas, J.M.; Langen, R.; Kast, W.M. The S100A10 subunit of the annexin A2 heterotetramer facilitates L2-mediated human papillomavirus infection. PLoS ONE 2012, 7, e43519. [Google Scholar] [CrossRef] [PubMed]
- Woodham, A.W.; Taylor, J.R.; Jimenez, A.I.; Skeate, J.G.; Schmidt, T.; Brand, H.E.; Da Silva, D.M.; Martin Kast, W. Small molecule inhibitors of the annexin A2 heterotetramer prevent human papillomavirus type 16 infection. J. Antimicrob. Chemother. 2014, 70, 1686–1690. [Google Scholar] [CrossRef] [PubMed]
- Latysheva, N.; Muratov, G.; Rajesh, S.; Padgett, M.; Hotchin, N.A.; Overduin, M.; Berditchevski, F. Syntenin-1 Is a New Component of Tetraspanin-Enriched Microdomains: Mechanisms and Consequences of the Interaction of Syntenin-1 with CD63. Mol. Cell. Biol. 2006, 26, 7707–7718. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, K.D.; Berditchevski, F.; Florin, L. The Tetraspanin CD151 in Papillomavirus Infection. Viruses 2014, 6, 893–908. [Google Scholar] [CrossRef]
- Lipovsky, A.; Erden, A.; Kanaya, E.; Zhang, W.; Crite, M.; Bradfield, C.; Macmicking, J.; Dimaio, D.; Schoggins, J.W.; Iwasaki, A. The cellular endosomal protein stannin inhibits intracellular trafficking of human papillomavirus during virus entry. J. Gen. Virol. 2017, 98, 2821–2836. [Google Scholar] [CrossRef]
- Müller, K.H.; Spoden, G.A.; Scheffer, K.D.; Brunnhöfer, R.; De Brabander, J.K.; Maier, M.E.; Florin, L.; Muller, C.P. Inhibition by cellular vacuolar atpase impairs human papillomavirus uncoating and infection. Antimicrob. Agents Chemother. 2014, 58, 2905–2911. [Google Scholar] [CrossRef]
- Forgac, M. Structure and Properties of the Vacuolar (H+)-ATPases. J. Biol. Chem. 1999, 274, 12951–12954. [Google Scholar] [CrossRef]
- Lebreton, S.; Jaunbergs, J.; Roth, M.G.; Ferguson, D.A.; De Brabander, J.K. Evaluating the potential of Vacuolar ATPase inhibitors as anticancer agents and multigram synthesis of the potent salicylihalamide analog saliphenylhalamide. Bioorg. Med. Chem. Lett. 2008, 18, 5879–5883. [Google Scholar] [CrossRef]
- Buck, C.B.; Day, P.M.; Thompson, C.D.; Lubkowski, J.; Lu, W.; Lowy, D.R.; Schiller, J.T. Human α-defensins block papillomavirus infection. Proc. Natl. Acad. Sci. USA 2006, 103, 1516–1521. [Google Scholar] [CrossRef]
- Wiens, M.E.; Smith, J.G. Papillomavirus 16 L2 To Block Infection. Virology 2015, 89, 2866–2874. [Google Scholar] [CrossRef] [PubMed]
- Karanam, B.; Peng, S.; Li, T.; Buck, C.; Day, P.M.; Roden, R.B.S. Papillomavirus Infection Requires γ Secretase. J. Virol. 2010, 84, 10661–10670. [Google Scholar] [CrossRef]
- Zhang, W.; Kazakov, T.; Popa, A.; DiMaio, D. Vesicular Trafficking of Incoming Human Papillomavirus 16 to the Golgi Apparatus and Endoplasmic Reticulum Requires γ-Secretase Activity. MBio 2014, 5, e01777-14. [Google Scholar] [CrossRef]
- Huang, H.S.; Buck, C.B.; Lambert, P.F. Inhibition of gamma secretase blocks HPV infection. Virology 2010, 407, 391–396. [Google Scholar] [CrossRef]
- Kwak, K.; Jiang, R.; Wang, J.W.; Jagu, S.; Kirnbauer, R.; Roden, R.B.S. Impact of Inhibitors and L2 Antibodies upon the Infectivity of Diverse Alpha and Beta Human Papillomavirus Types. PLoS ONE 2014, 9, e97232. [Google Scholar] [CrossRef]
- Zhang, P.; Moreno, R.; Lambert, P.F.; DiMaio, D. Cell-penetrating peptide inhibits retromer-mediated human papillomavirus trafficking during virus entry. Proc. Natl. Acad. Sci. USA 2020, 117, 6121–6128. [Google Scholar] [CrossRef]
- Ito, M.; Baba, M.; Sato, A.; Pauwels, R.; De Clercq, E.; Shigeta, S. Inhibitory effect of dextran sulfate and heparin on the replication of human immunodeficiency virus (HIV) in vitro. Antivir. Res. 1987, 7, 361–367. [Google Scholar] [CrossRef]
- Rider, C.C. The potential for heparin and its derivatives in the therapy and prevention of HIV-1 infection. Glycoconj. J. 1997, 14, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Thompson III, G.; Lawrence, V.A.; Crawford, G.E. HIV/AIDS HIV Infection Increases the Risk of Heparin-Induced Thrombocytopenia. Clin. Infect. Dis. 2007, 45, 1393–1396. [Google Scholar] [CrossRef]
- Groveman, M.D.S. Inhibition of HIV-1 infectivity by low molecular weight heparin: Results of in vitro studies and a pilot clinical trial in patients with advanced AIDS. Int. J. Clin. Lab. Res. 1996, 26, 124–131. [Google Scholar] [CrossRef]
- Compton, T.; Nowlin, D.M.; Cooper, N.R. Initiation of Human Cytomegalovirus Infection Requires Initial Interaction with Cell Surface Heparan Sulfate. Virology 1993, 193, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Feathergill, K.; Diao, X.; Cooper, M.; Kirkpatrick, R.; Spear, P.; Waller, D.P.; Chany, C.; Doncel, G.F.; Herold, B.; et al. Evaluation of Poly(Styrene-4-Sulfonate) as a Preventive Agent for Conception and Sexually Transmitted Diseases. J. Androl. 2000, 21, 862–875. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ogamo, A.; Saito, T.; Watanabe, J.; Uchiyama, H. Preparation and anti-HIV activity of low- molecular-weight carrageenans and their sulfated derivatives. Carbohydr. Polym. 1997, 32, 51–55. [Google Scholar] [CrossRef]
- Phillips, D.M. Vaginal Formulations of Carrageenan Protect Mice from Herpes Simplex Virus Infection. Clin. Diagn. Lab. Immunol. 1997, 4, 465–468. [Google Scholar]
- Anderson, J.; Osbakk, S.; Vorland, L.; T, T.; TJ, G. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antivir. Res. 2001, 51, 141–149. [Google Scholar] [CrossRef]
- Van der Strate, B.; Beljaars, L.; Molema, G.; Harmsen, M.; Meijer, D. Antiviral activities of lactoferrin. Antivir. Res. 2001, 52, 225–239. [Google Scholar] [CrossRef]
- Hara, K.; Ikeda, M.; Saito, S.; Matsumoto, S.; Numata, K.; Kato, N.; Tanaka, K.; Sekihara, H. Lactoferrin inhibits hepatitis B virus infection in cultured human hepatocytes. Hepatol. Res. 2002, 24, 228–235. [Google Scholar] [CrossRef]
- Bon, I.; Lembo, D.; Rusnati, M.; Clò, A.; Morini, S.; Miserocchi, A.; Bugatti, A.; Girogolon, S.; Musumeci, G.; Landolfo, S.; et al. Peptide-Derivatized SB105-A10 Dendrimer Inhibits the Infectivity of R5 and X4 HIV-1 Strains in Primary PBMCs and Cervicovaginal Histocultures. PLoS ONE 2013, 8, e76482. [Google Scholar] [CrossRef]
- Luganini, A.; Giuliani, A.; Pirri, G.; Pizzuto, L.; Landolfo, S.; Gribaudo, G. Peptide-derivatized dendrimers inhibit human cytomegalovirus infection by blocking virus binding to cell surface heparan sulfate. Antivir. Res. 2010, 85, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Sep, D.; Ce, R.; Jim, L. Mechanistic Studies of Viral Entry: An Overview of Dendrimer-Based Microbicides As Entry Inhibitors Against Both HIV and HSV-2 Overlapped Infections. Med. Recearch Rev. 2017, 37, 149–179. [Google Scholar] [CrossRef]
- Luganini, A.; Nicoletto, S.F.; Pizzuto, L.; Pirri, G.; Giuliani, A.; Landolfo, S.; Gribaudo, G. Inhibition of Herpes Simplex Virus Type 1 and Type 2 Infections by Peptide-Derivatized Dendrimers. Antimicrob. Agents Chemother. 2011, 55, 3231–3239. [Google Scholar] [CrossRef]
- Neurath, A.R.; Debnath, A.K.; Strick, N.; Li, Y.-Y.; Lin, K.; Jiang, S. Blocking of CD4 cell receptors for the human immunodeficiency virus type 1 (HIV-1) by chemically modified bovine milk proteins: Potential for AIDS prophylaxis. J. Mol. Recognit. 1995, 8, 304–316. [Google Scholar] [CrossRef]
- Hazrati, E.; Galen, B.; Lu, W.; Wang, W.; Ouyang, Y.; Keller, M.J.; Lehrer, R.I.; Herold, B.C. Human α- and β-Defensins Block Multiple Steps in Herpes Simplex Virus Infection. J. Immunol. 2006, 177, 8658–8666. [Google Scholar] [CrossRef] [PubMed]
- Wiens, M.E.; Smith, J.G. alpha-Defensin HD5 Inhibits Human Papillomavirus 16 Infection via Capsid Stabilization and Redirection to the Lysosome. Am. Soc. Microbiol. 2017, 8, 1–14. [Google Scholar] [CrossRef]
| Potential Inhibitory Molecule | Proposed Mechanism of Inhibition | Affected HPV Type | Experimental System Used, Stage of Clinical Trial if Applicable | Inhibitor of Other Sexually Transmitted Infections | Caveats/Challenges | Ref |
|---|---|---|---|---|---|---|
| Heparin | Competitive inhibitor, binds to viral capsids and prevents capsid binding to HSPGs | 11 VLP | In vitro: HaCaT, CHO-K1, pgsA-745 cells | HIV-1 [168,169,170,171] HSV-1/2 [56] HCMV [172] | Use of PsV vs. organotypic derived HPV led conflicting results; HPV16-PsV interaction with heparin shown to aid infection in the absence of cell-surface HSPGs | [95] |
| 16, 33 PsV | In vitro: COS-7, HeLa, DG75 cells | [42] | ||||
| 16 PsV | In vitro: HaCaT cells | [96] | ||||
| 18 NV (* 16, 31, 45 NV) | In vitro: HaCaT, CHO par, pgsA-745, primary human keratinocytes derived from newborn foreskin | [97] | ||||
| GAG-based nanoassemblies: O-palmitoyl-heparin (OPH) | 16 PsV | In vitro: 293TT cells | HSV-1/2 [48] | [48] | ||
| Cellulose, dextran, polystyrene sulfate | 11, 40 NV | In vitro: A431 cells | HIV [100,173] HSV-1/2 [100,102,173] Chlamydia [101,102] Neisseria gonorrhoeae [101,102] | [23] | ||
| Alginate (PMG) | 16, 18, 45 PsV | In vitro: 293FT, HeLa, HaCaT cells In vivo: cutaneous PsV infection in mice | HIV [107] HSV [105] HBV [106] | [104] | ||
| Carrageenan | 6, 16, 18, 31, 45 PsV | In vitro: HeLa, HaCaT, 293TT, C127 cells | HIV [174] HSV [175] | [108] | ||
| 18, 31 NV (* 16, 45 NV) | In vitro: COS-7, HeLa, DG75 cells | [97] | ||||
| 16 PsV | Human samples used in vitro: 293TT cells | [111] | ||||
| 16 PsV | In vivo: mouse cervicovaginal challenge model | [36] | ||||
| 16, 18, 45 PsV | In vivo: mouse cervicovaginal challenge model | [110] | ||||
| 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68 detected | Phase III clinical trial | [112] | ||||
| low oncogenic risk: 6, 11, 40, 42, 44, 54 high and intermediate oncogenic risk: 16, 18, 26, 31, 33, 34, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70, 73, 82 commensal types: 61, 62, 71, 72, 81, 83, 84, 89 | Phase 2B clinical trial | No indication of heterogeneity of effects when stratifying by validated vaccination status. No indication of a dose-response relationship with the estimated cumulative compliance. | [114] | |||
| 6, 11, 16, 18, 31, 33, 39, 40, 42, 45, 51, 52, 53, 55, 56, 58, 59, 61, 62, 66, 73, 81, 84 | Prospective observational clinical study | Single-centered study without randomization of patients. Effectiveness on clearance of a specific papillomavirus genotype no evaluated. Short study observation period. | [115] | |||
| Sulfated glycopolymers | 16 PsV | In vitro HeLa cells: In vivo: mouse vaginal challenge model | HSV-1 [116] | [116] | ||
| Vimentin | Inhibitor of virus attachment | 16 PsV | In vitro: HeLa, HaCaT, CHO-K1, pgsD-677, NIKS cells | [41] | ||
| Dispirotripiperazine (DSTP27) | Binds to HSPGs, blocking capsid binding to the cell surface | 16, 18 PsV | In vitro: 293TT, CHO-K1, pgsA-745, HaCaT cells | HSV-1/2 [118] HIV-1 [118] HCMV [118] | [62] | |
| Polyethylenimines | 16, 18, 31 PsV | In vitro: HeLa, Cos7, HaCaT, 293TT, pgsA-745, CHO-K1 cells | HCMV [119] | [119] | ||
| Lactoferrin/lactoferricin | 16 VLP | In vitro: HaCaT cells | HSV-1/2 [122] HCMV [176] HIV-1 [177] HBV [178] | [127] | ||
| 5, 16 PsV | In vitro: HaCaT, C33A cells | [128] | ||||
| Dendrimers (SB105-A10) | 6, 16, 18 PsV | In vitro: SiHa, HeLa, C33A, HL3T1, 293TT, CHO-K1 cells | HIV-1 [179] HCMV [180] HSV-1/2 [181,182] | [133] | ||
| Anhydride modified protein (JB01) | Inhibits viral entry, binds to L1 | 6, 16, 18 PsV | In vitro: 293FT cells | HIV [183] HSV-1/2 [147,148] | [150] | |
| 16, 58 PsV | In vitro: HeLa, HaCaT cells | [151] | ||||
| 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68 | Phase I/IIa clinical trial | [152] | ||||
| Annexin 2 heterotetramer inhibitor (A2ti) | Inhibits viral entry, binds to A2ti | 16 PsV | In vitro: HeLa, HaCaT | [154] | ||
| Tetraspanin blocking peptides (CD63 and CD151) | Inhibits viral entry, blocks tetraspanin functions | 16 PsV | In vitro: HeLa, HaCaT cells | HCMV [67] | [67] | |
| v-ATPase inhibitor (SaliPhe) | Inhibition of Lysosomal acidification, viral uncoating targeted | 6, 11, 16, 18 PsV | In vitro: HeLa HaCaT, 293TT, NHEK cells | [158] | ||
| Human α-defensin 5 (HD5) | Inhibits furin mediated cleavage; Disrupts capsid dissociation | 16 PsV | In vitro: HeLa, HaCaT, 293TT, C127 fibroblast cells | HSV-1/2 [184] | [161] | |
| 16 PsV | In vitro: HeLa, HaCaT cells | [185] | ||||
| γ-secretase inhibitors (GSIs) | Prevents L2/vDNA from reaching the TGN | 11, 16, 31 PsV | In vitro: HaCaT, C127, HeLa cells In vivo: mouse cervicovaginal challenge model | [165] | ||
| Stannin | Blocks virus entry into TGN | 16 PsV | In vitro: HeLa, HaCaT cells | [157] | ||
| L2-based molecules | Block TGN trafficking | 16 PsV | In vitro: HeLa, CHO-K1, 293T, Huh7, U251, pgsA-745 cells | [77] | ||
| 16 PsV | In vitro: HeLa, HaCaT cells | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carse, S.; Bergant, M.; Schäfer, G. Advances in Targeting HPV Infection as Potential Alternative Prophylactic Means. Int. J. Mol. Sci. 2021, 22, 2201. https://doi.org/10.3390/ijms22042201
Carse S, Bergant M, Schäfer G. Advances in Targeting HPV Infection as Potential Alternative Prophylactic Means. International Journal of Molecular Sciences. 2021; 22(4):2201. https://doi.org/10.3390/ijms22042201
Chicago/Turabian StyleCarse, Sinead, Martina Bergant, and Georgia Schäfer. 2021. "Advances in Targeting HPV Infection as Potential Alternative Prophylactic Means" International Journal of Molecular Sciences 22, no. 4: 2201. https://doi.org/10.3390/ijms22042201
APA StyleCarse, S., Bergant, M., & Schäfer, G. (2021). Advances in Targeting HPV Infection as Potential Alternative Prophylactic Means. International Journal of Molecular Sciences, 22(4), 2201. https://doi.org/10.3390/ijms22042201







