Plant-Derived Smoke Affects Biochemical Mechanism on Plant Growth and Seed Germination
Abstract
1. Introduction
2. Preparation of Plant-Derived Smoke
3. Components in Plant-Derived Smoke
3.1. Karrikins
3.2. Other Components
3.3. Plant-Derived Smoke as Biostimulant
4. Effect of Plant-Derived Smoke on Dormancy Releasing, Seed Germination, and Seedling Growth
4.1. Seed Germination
4.2. Post-Germination Responses of Plant Towards Plant-Derived Smoke
5. Physiological Responses of Plant to a Plant-Derived Smoke
5.1. Pigments
5.2. Phenolic Compounds
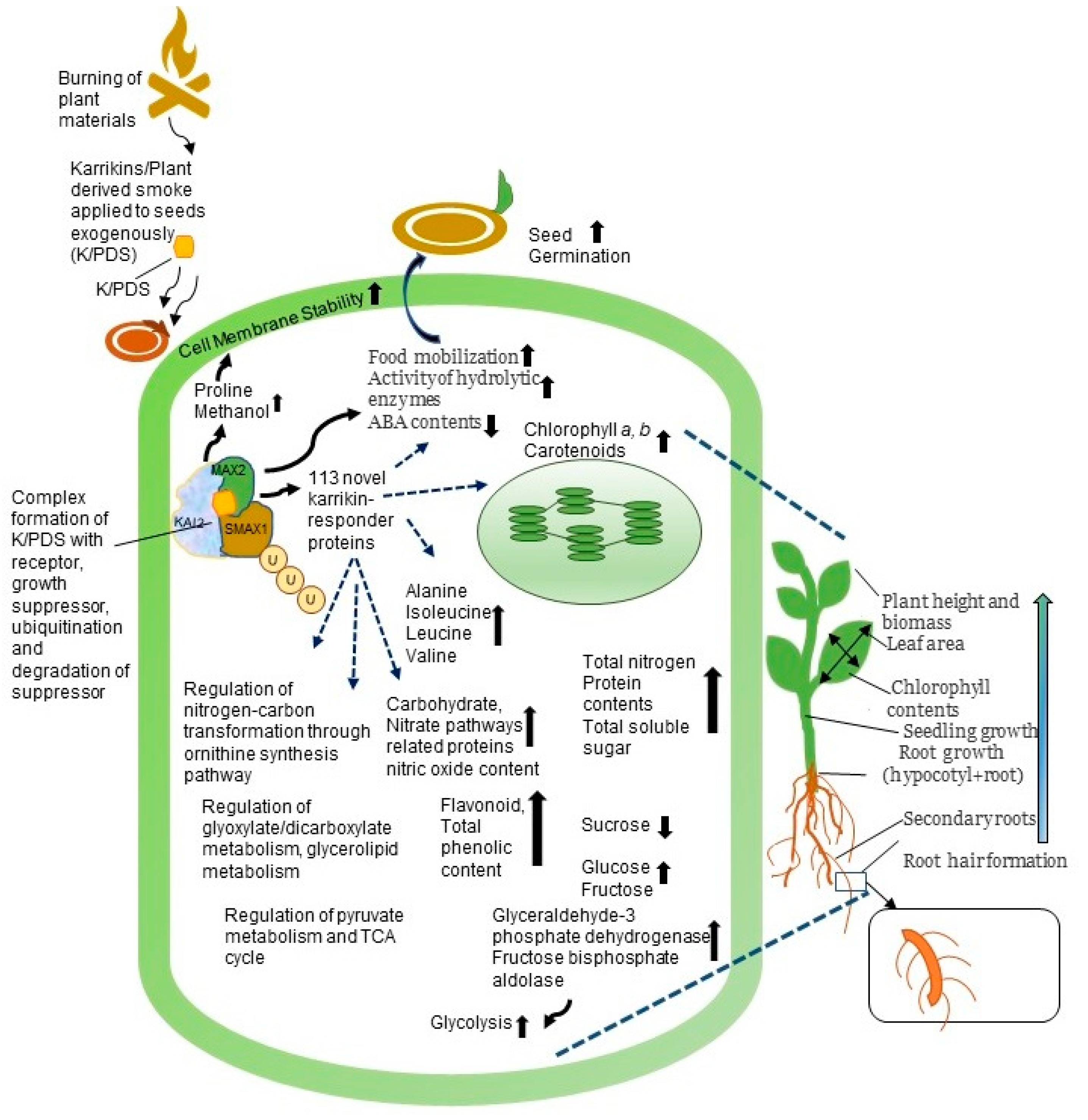
6. Molecular Mechanism in Plant of Plant-Derived Smoke
6.1. Karrikins Signaling and Changes at Transcription Level
6.2. Karrikins Signaling Pathway
6.3. Effect on Proteins in Plants of Plant-Derived Smoke
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| KAR | Karrikins |
References
- De Lange, J.H.; Boucher, C. Auto ecological studies on Audinia capitata (Bruniaceaae), plant-derived smoke as a germination cue. S. Afr. J. Bot. 1990, 56, 188–202. [Google Scholar] [CrossRef]
- Brown, N.A.C. Promotion of germination of fynboss seeds by plant-derived smoke. New Phytol. 1993, 123, 575–583. [Google Scholar] [CrossRef]
- Van Staden, J.; Sparg, S.G.; Kulkarni, M.G.; Light, M.E. Post-germination effects of the smoke-derived compound 3-methyl-2H-furo[2,3-c] pyran-2-one, and its potential as a preconditioning agent. Field Crops Res. 2006, 98, 98–105. [Google Scholar] [CrossRef]
- Paul, B.T.; Charles, E.M.; Tony, D.A. Response surfaces for the combined effects of heat shock and smoke on germination of 16 species forming soil seed bank in South- East Australia. Austral Ecol. 2007, 32, 605–616. [Google Scholar]
- Mandal, K.; Misra, A.; Hati, K.; Bandyopadhyay, K.; Ghosh, P.; Mohanty, M. Rice residue-management options and effects on soil properties and crop productivity. J. Food Agric. Environ. 2004, 2, 224–231. [Google Scholar]
- Todorovic, S.; Giba, Z.; Zivkovic, S.; Grubisic, D.; Konjevic, R. Stimulation of empress tree seed germination by liquid smoke. Plant Growth Regul. 2005, 47, 141–148. [Google Scholar] [CrossRef]
- Light, M.E.; Gardner, M.J.; Jager, A.K.; van Staden, J. Dual regulation of seed germination by smoke solution. Plant Growth Regul. 2002, 37, 135–141. [Google Scholar] [CrossRef]
- Brown, N.A.C.; van Staden, J.; Daws, M.I.; Johnson, T. Patterns in the seed germination response to smoke in plants from the Cape Floristic Region, South Africa. S. Afr. J. Bot. 2003, 69, 514–525. [Google Scholar] [CrossRef]
- Egerton-Warburton, L. A smoke-induced alteration of the sub-testa cuticle in seeds of the post-fire recruiter, Emmenanthe penduliflora Benth. (Hydrophyllaceae). J. Exp. Bot. 1998, 49, 1317–1327. [Google Scholar] [CrossRef]
- Keely, J.E.; Fotheringham, C.J. Smoke-induced seed germination in Californian chaparral. Ecology 1998, 79, 2320–2336. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. A compound from smoke that promotes seed germination. Science 2004, 305, 977. [Google Scholar] [CrossRef]
- Aslam, M.M.; Jamil, M.; Khatoon, A.; Hendawy, S.E.; AL-Suhaibani, N.A.; Malook, I.; Rehman, S.U. Physiological and biochemical responses of maize (Zea mays L.) to plant derived smoke solution. Pak. J. Bot. 2017, 49, 435–443. [Google Scholar]
- Jamil, M.; Kanwal, M.; Aslam, M.M.; Khan, S.S.; Malook, I.; Tu, J.; Rehman, S.U. Effect of plant-derived smoke priming on physiological and biochemical characteristics of rice under salt stress condition. Aust. J. Crop. Sci. 2014, 8, 159–170. [Google Scholar]
- Li, X.; Rehman, S.U.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Yamaguchi, T.; Sunohara, Y.; Matsumoto, H.; Komatsu, S. Proteomic analysis of the effect of plant-derived smoke on soybean during recovery from flooding stress. J. Proteomic. 2018, 181, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Khan, S.; Malook, I.; Rehman, S.U.; Jamil, M. Pb-induced changes in roots of two cultivated rice cultivars grown in lead-contaminated soil mediated by smoke. Environ. Sci. Pollut. Res. 2017, 24, 21298–21310. [Google Scholar] [CrossRef]
- Brown, N.A.C.; van Staden, J. Smoke as a germination cue: A review. Plant Growth Regul. 1997, 22, 115–124. [Google Scholar] [CrossRef]
- Jager, A.K.; Light, M.E.; van Staden, J. Effects of source of plant material and temperature on the production of smoke extracts that promote germination of light-sensitive lettuce seeds. Environ. Exp. Bot. 1996, 36, 421–429. [Google Scholar] [CrossRef]
- Nelson, D.C.; Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Regulation of seed germination and seedling growth by chemical signals from burning vegetation. Ann. Rev. Plant Biol. 2012, 63, 107–130. [Google Scholar] [CrossRef]
- Adriansz, T.D.; Rummey, J.M.; Bennett, I.J. Solid phase extraction and subsequent identification by gas chromatography, Mass-Spectrometry of a germination cue present in smoky water. Anal. Lett. 2000, 33, 2793–2804. [Google Scholar] [CrossRef]
- Light, M.E.; Burger, B.V.; Staerk, D.; Kohout, L.; van Staden, J. Butenolides from plant-derived smoke: Natural plant growth regulators with antagonistic actions on seed germination. J. Nat. Prod. 2010, 73, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Flematti, G.R.; Merritt, D.J.; Piggott, M.J.; Trengove, R.D.; Smith, S.M.; Dixon, K.W.; Ghisalberti, E.L. Burning vegetation produces cyanohydrins that liberate cyanide and promote seed germination. Nat. Commun. 2011, 2, 360. [Google Scholar] [CrossRef]
- Wang, M.; Schoettner, M.; Xu, S.; Paetz, C.; Wilde, J.; Baldwin, I.T.; Groten, K. Catechol, a major component of smoke, influences primary root growth and root hair elongation through reactive oxygen species-mediated redox signaling. New Phytologist. 2017, 213, 1755–1770. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Khan, A.L.; Ali, L.; Hussain, J.; Waqas, M.; Al-Harrasi, A.; Lee, I.J. Hydroquinone; a novel bioactive compound from plant-derived smoke can cue seed germination of lettuce. Front. Chem. 2017, 5, 30. [Google Scholar] [CrossRef]
- Burger, B.V.; Postac, M.; Lightb, M.E.; Kulkarni, M.G.; Viviersa, M.Z.; van Staden, J. More butenolides from plant-derived smoke with germination inhibitory activity against karrikinolide. S. Afr. J. Bot. 2018, 115, 256–263. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. Synthesis of the seed germination stimulant 3-methyl-2H-furo[2,3-c] pyran-2-one. Tetrahedron Lett. 2005, 46, 5719–5721. [Google Scholar] [CrossRef]
- Brown, N.A.C.; Botha, P.A. Smoke seed germination studies and a guide to seed propagation of plants from major families of the Cape floristic region, South Africa. S. Afr. J. Bot. 2004, 70, 559–581. [Google Scholar] [CrossRef][Green Version]
- Flematti, G.R.; Goddard-Berg, E.D.; Merritt, D.J.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. Preparation of 3-methyl-2H-furo[2,3-c] pyran-2-one derivatives and evaluation of their germination-promoting activity. J. Agric. Food Chem. 2007, 55, 2189–2194. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D.; Skelton, B.W.; White, A.H. Structural analysis of a potent seed germination stimulant. Aust. J. Chem. 2005, 58, 505–506. [Google Scholar] [CrossRef]
- Flematti, G.R.; Scaffidi, A.; Goddard-Borger, E.D.; Heath, C.H.; Nelson, D.C.; Commander, L.E.; Stick, O.V.R.; Dixon, W.; Smith SMGhisalberti, E.L. Structure-activity relationship of karrikin germination stimulants. J. Agric. Food Chem. 2010, 58, 8612–8617. [Google Scholar] [CrossRef]
- Goddard-Borger, E.D.; Ghisalberti, E.L.; Stick, R.V. Synthesis of the germination stimulant 3-methyle-2H-furo[2,3-c] pyran-2-one and analogous compounds from carbohydrates. Eur. J. Org. Chem. 2007, 2007, 3925–3934. [Google Scholar] [CrossRef]
- Dixon, K.W.; Merritt, D.J.; Flematti, G.R.; Ghisalberti, E.L. Karrikinolide-a phytoreactive compound derived from smoke with applications in horticulture, ecological restoration and agriculture. Acta Hortic. 2009, 813, 155–170. [Google Scholar] [CrossRef]
- Merritt, D.J.; Turner, S.R.; Clark, S.; Dixon, K.W. Seed dormancy and germination stimulation syndromes for Australian temperate species. Aust. J. Bot. 2007, 55, 336–344. [Google Scholar] [CrossRef]
- Iqbal, M.; Asif, S.; Ilyas, N.; Raja, N.I.; Hussain, M.; Sabir, S.; Faz, M.N.A.; Rauf, A. Effect of plant derived smoke on germination and post germination expression of wheat (Triticum aestivum L.). Am. J. Plant Sci. 2016, 7, 806–813. [Google Scholar] [CrossRef]
- Kulkurni, M.G.; Ascough, G.D.; van Staden, J. Smoke-water and smoke-isolated butenolide improve growth and yield of tomatoes under greenhouse conditions. Hortic. Technol. 2008, 18, 449–454. [Google Scholar] [CrossRef]
- Elsadek, M.A.; Yousef, E.A.A. Smoke-water enhances germination and seedling growth of four horticultural crops. Plants 2019, 8, 104. [Google Scholar] [CrossRef]
- Aremu, A.O.; Kulkarni, M.G.; Bair, M.O.; Finnie, J.F.; van Staden, J. Growth stimulation effects of smoke-water and vermin-compost leachate on greenhouse grown-tissue cultured ‘Williams’ bananas. Plant Growth Regul. 2012, 66, 111–118. [Google Scholar] [CrossRef]
- Verschaeve, L.; Maes, J.; Light, M.E.; van Staden, J. Genetic toxicity testing of 3-methyl-2H-furo[2,3-c] pyran-2-one, an important biological active compound from plant-derived smoke. Mutat. Res. Gen. Toxicol. Environ. Mutagen. 2006, 611, 89–95. [Google Scholar] [CrossRef]
- Jain, N.; Kulkarni, M.G.; van Staden, J. A butenolide isolated from smoke, can overcome the detrimental effects of extreme temperature during tomato seed germination. Plant Growth Regul. 2006, 49, 263–267. [Google Scholar] [CrossRef]
- Waheed, M.A.; Jamil, M.; Khan, M.D.; Shakir, S.K.; Rehman, S.U. Effect of plant-derived smoke solutions on physiological and biochemical attributes of maize (Zea mays L.) under salt stress. Pak. J. Bot. 2016, 48, 1763–1774. [Google Scholar]
- Malook, I.; Itlas, A.; Rehman, S.U.; Weng, W.; Jamil, M. Smoke alleviates adverse effects induced by stress on rice. Toxicol. Environ. Chem. 2014, 96, 755–767. [Google Scholar] [CrossRef]
- Oracz, K.; El-Maarouf-Bouteau, H.; Kranner, I.; Bogatek, R.; Corbineau, F.; Bailly, C. The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiol. 2009, 150, 494–505. [Google Scholar] [CrossRef]
- Van Staden, J.; Jager, A.K.; Light, M.E.; Burger, B.V. Isolation of the major germination cue from plant-derived smoke. S. Afr. J. Bot. 2004, 70, 654–659. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Ngoroyemoto, N.; Gupta, S.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Effect of organic biostimulants on the growth and biochemical composition of Amaranthus hybridus L. S. Afr. J. Bot. 2019, 124, 87–93. [Google Scholar] [CrossRef]
- Nemahunguni, N.K.; Gupta, S.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. The effect of biostimulants and light wavelengths on the physiology of Cleome gynandra seeds. Plant Growth Regul. 2019, 90, 467–474. [Google Scholar] [CrossRef]
- Kamran, M.; Imran, Q.M.; Khatoon, A.; Lee, I.J.; Rehman, S.U. Effect of plant extracted smoke and reversion of abscisic acid stress on lettuce. Pak. J. Bot. 2013, 45, 1541–1549. [Google Scholar]
- Raizada, P.; Raghubanshi, A.S. Seed germination behavior of Lantana camara in response to smoke. Trop. Ecol. 2010, 51, 347–352. [Google Scholar]
- Zhou, J.; van Staden, J.; Guo, L.P.; Huang, L.Q. Smoke-water improves shoot growth and indigo accumulation in shoots of Isatis indigotica seedlings. S. Afr. J. Bot. 2011, 77, 787–789. [Google Scholar] [CrossRef][Green Version]
- Tavsanoglu, C. Fire-related cues (heat shock and smoke) and seed germination in a Cistus creticus population in south western Turkey. Ekoloji 2011, 20, 99–104. [Google Scholar]
- Schwilk, D.W.; Zavala, N. Germination response of grassland species to plant-derived smoke. J. Arid Environ. 2012, 79, 111–115. [Google Scholar] [CrossRef]
- Chumpookam, J.; Lin, H.L.; Shiesh, C.C.; Ku, K.L. Effect of smoke-water on seed germination and resistance to Rhizoctonia solani inciting papaya damping-off. Hortic. Sci. 2012, 37, 13–29. [Google Scholar]
- Ghazanfaria, P.; Abdollahia, M.R.; Moienib, A.; Moosavia, S.S. Effect of plant-derived smoke extract on in vitro plantlet regeneration from rapeseed (Brassica napus L. cv. Topas) microspore-derived embryos. Int. J. Plant Prod. 2012, 6, 309–324. [Google Scholar]
- Tormo, J.; Moreira, B.; Pausas, J.G. Field evidence of smoke-stimulated seedling emergence and establishment in Mediterranean Basin flora. J. Veg. Sci. 2013, 25, 771–777. [Google Scholar] [CrossRef]
- Aremu, A.O.; Masondo, N.A.; van Staden, J. Smoke–water stimulates secondary metabolites during in vitro seedling development in Tulbaghia species. S. Afr. J. Bot. 2014, 91, 49–52. [Google Scholar] [CrossRef]
- Catav, S.S.; Kucukakyuz, K.; Akbas, K.; Tavsanoglu, C. Smoke-enhanced seed germination in Mediterranean Lamiaceae. Seed Sci. Res. 2014, 24, 257. [Google Scholar] [CrossRef]
- Preston, C.A.; Becker, R.; Baldwin, I.T. Is ‘NO’ news good news? Nitrogen oxides are not components of smoke that elicits germination in two smoke-stimulated species, Nicotiana attenuate and Emmenanthe penduliflora. Seed Sci. Res. 2014, 14, 73–79. [Google Scholar] [CrossRef]
- Aslam, M.M.; Akhter, A.; Jamil, M.; Khatoon, A.; Malook, I.; Rehman, S.U. Effect of Plant-derived smoke solution on root of Ipomoea marguerite cuttings under cobalt stress. J. Bio Mol. Sci. 2014, 2, 6–11. [Google Scholar]
- Pirzada, K.; Shafiq, U.R.; Jamil, M.; Irfan, S.; Waheed, M.A.; Aslam, M.M.; Kanwal, M.; Shakir, S.K. Alleviation of boron stress through plant derived smoke extracts in Sorghum bicolor. J. Stress Physiol. Biochem. 2014, 10, 153–165. [Google Scholar]
- Asaf, S.; Imran, Q.M.; Khatoon, A.; Lubna, J.R.; Jung, H.-Y.; Rehman, S.U. Plant derived smoke promotes seed germination and alleviates auxin stress in carrot. J. Agric. Biol. Sci. 2014, 9, 308–314. [Google Scholar]
- Kamran, M.; Khan, A.L.; Waqas, M.; Imran, Q.M.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Kim, M.J.; Lee, I.J. Effects of plant-derived smoke on the growth dynamics of Barnyard Grass (Echinochloa crus-galli). Acta Agric. Scand. Sect. B Soil Plant Sci. 2014, 64, 121–128. [Google Scholar] [CrossRef]
- Abu, Y.; Romo, J.T.; Bai, Y.; Coulman, B. Priming seeds in aqueous smoke solutions to improve seed germination and biomass production of perennial forage species. Can. J. Plant Sci. 2016, 96, 551–563. [Google Scholar] [CrossRef]
- Catav, S.S.; Kuçukakyuz, K.; Tavsanoglu, C.; Akbas, K. Effects of aqueous smoke and nitrate treatments on germination of 12 eastern mediterranean basin plants. Ann. Bot. Fenn. 2015, 52, 93–100. [Google Scholar] [CrossRef]
- Fornwalt, P.J. Does smoke promote seed germination in 10 Interior West Penstemon species? Nat. Plants J. 2015, 16, 5–12. [Google Scholar] [CrossRef]
- Ren, L.; Bai, Y. Smoke originated from different plants has various effects on germination and seedling growth of species in Fescue Prairie. Botany 2016, 94, 1141–1150. [Google Scholar] [CrossRef]
- Martinez-Baniela, M.; Carlon, L.; Diaz, T.E.; Bueno, A.; Fernandez-Pascual, E. Plant-derived smoke and temperature effects on seed germination of five Helianthemum (Cistaceae). Flora 2016, 223, 56–61. [Google Scholar] [CrossRef]
- Naghipour, A.A.; Bashari, H.; Khajeddin, S.J.; Tahmasebi, P.; Iravani, M. Effects of smoke, ash and heat shock on seed germination of seven species from Central Zagros rangelands in the semi-arid region of Iran. Afr. J. Range Forage Sci. 2016, 33, 67–71. [Google Scholar] [CrossRef]
- Mukundamago, M.; Adu-Acheampong, S.; Moshobane, M.C. Impact of gaseous smoke treatment on germination and seedling emergence of the cape flats sand fynbos species. Asian J. Plant Sci. Res. 2017, 7, 50–59. [Google Scholar]
- Cembrowska-Lech, D.; Kępczyńsk, J. Plant-derived smoke induced activity of amylases, DNA replication and β-tubulin accumulation before radicle protrusion of dormant Avena fatua L. caryopses. Acta Physiol. Plant. 2017, 39, 39–50. [Google Scholar] [CrossRef][Green Version]
- Cox, R.D.; Chou, Y.F.; Wester, D.B. Smoke water and heat influence emergence of shortgrass prairie species. Fire Ecol. 2017, 13, 138–148. [Google Scholar] [CrossRef]
- Catav, Ş.S.; Kucukakyuz, K.; Tavşanoglu, C.; Pausas, J.G. Effect of fire-derived chemicals on germination and seedling growth in Mediterranean plant species. Basic Appl. Ecol. 2018, 30, 65–75. [Google Scholar] [CrossRef]
- Moreira, B.; Pausas, J.G. Shedding light through the smoke on the germination of Mediterranean Basin flora. S. Afr. J. Bot. 2018, 115, 244–250. [Google Scholar] [CrossRef]
- Plazek, A.; Dubert, F.; Kopec, P.; Dziurka, M.; Kalandyk, A.; Pastuszak, J.; Wolko, B. Seed hydropriming and smoke water significantly improve low-temperature germination of Lupinus angustifolius L. Int. J. Mol. Sci. 2018, 19, 992. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Rehman, S.U.; Khatoon, A.; Qasim, M.; Itohd, T.; Iwasakid, Y.; Wang, X.; Sunohara, Y.; Matsumoto, H.; Komatsu, S. Proteomic analysis of the promotive effect of plant-derived smoke on plant growth of chickpea. J. Proteom. 2018, 176, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.; Rehman, S.U.; Khatoon, A.; Jamil, M.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Sunohara, Y.; Matsumoto, H.; Komatsu, S. Proteomic analysis of the effect of plant-derived smoke on maize growth. Int. J. Mol. Sci. 2019, 20, 1319. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hrdlička, J.; Ngoroyemoto, N.; Nemahunguni, N.K.; Gucký, T.; Novák, O.; Kulkarni, M.J.; Dolezal, K.; van Staden, J. Preparation and standardisation of smoke-water for seed germination and plant growth stimulation. Plant Growth Regul. 2019, 39, 338–345. [Google Scholar] [CrossRef]
- Roeder, M.; Yang, W.; Tomlinson, K.W. Influence of smoke, heat and fire on germination of woody species occurring in the dry valleys of southwest China. J. Plant Ecol. 2019, 12, 931–940. [Google Scholar] [CrossRef]
- Jamil, M.; Jahangir, M.; Rehman, S.U. Smoke induced physiological, biochemical and molecular changes in germinating rice seeds. Pak. J. Bot. 2020, 52, 865–871. [Google Scholar] [CrossRef]
- Posta, M.; Light, M.E.; Papenfus, H.B.; van Staden, J.; Kohout, L. Structure–activity relationships of analogs of 3,4,5- trimethylfuran-2(5H)-one with germination inhibitory activities. J. Plant Physiol. 2013, 170, 1235–1242. [Google Scholar] [CrossRef]
- Ghebrehiwot, H.M.; Kulkarni, M.G.; Szalai, G.; Soos, V.; Balazs, E.; van Staden, J. Karrikinolide residues in grassland soils following fire: Implications on germination activity. S. Afr. J. Bot. 2013, 88, 419–424. [Google Scholar] [CrossRef]
- Baldos, O.C.; DeFrank, J.; Sakamoto, G.S. Germination response of dormant tanglehead (Heteropogon contortus) seeds to smoke-infused water and the smoke-associated stimulatory compounds, karrikinolide and cyanide. HortScience 2015, 50, 421–429. [Google Scholar] [CrossRef]
- Gupta, S.; Plackova, L.; Kulkarni, M.G.; Dolezal, K.; van Staden, J. Role of Smoke Stimulatory and Inhibitory Biomolecules in Phytochrome-Regulated Seed Germination of Lactuca sativa. Plant Physiol. 2019, 181, 458–470. [Google Scholar] [CrossRef]
- Mavi, K.; Light, M.E.; Demir, I.; van Staden, J.; Yasar, F. Positive effect of smoke-derived butenolide priming on melon seedling emergence and growth. N. Z. J. Crop. Hort. 2010, 38, 147–155. [Google Scholar] [CrossRef]
- Downes, K.S.; Lamont, B.B.; Light, M.E.; van Staden, J. The fire ephemeral Tersonia cyathiflora (Gyrostemonaceae) germinates in response to smoke but not the butenolide 3-methyl-2H-furo [2,3-c]pyran-2-one. Ann Bot. 2010, 106, 381–384. [Google Scholar] [CrossRef]
- Long, R.L.; Williams, K.; Griffiths, E.M.; Flematti, G.R.; Merritt, D.J.; Stevens, J.C.; Turner, S.R.; Powles, S.B.; Dixon, K.W. Prior hydration of Brassica tournefortii seeds reduces the stimulatory effect of karrikinolide on germination and increases seed sensitivity to abscisic acid. Ann. Bot. 2010, 105, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Kandari, L.S.; Kulkarni, M.G.; van Staden, J. Effect of nutrients and smoke solutions on seed germination and seedling growth of tropical soda apple (Solanum viarum). Weed Sci. 2011, 59, 470–475. [Google Scholar] [CrossRef]
- Long, R.L.; Stevens, J.C.; Griffiths, E.M.; Adamek, M.; Powles, S.B.; Merritt, D.J. Detecting karrikinolide responses in seeds of the Poaceae. Aust. J. Bot. 2011, 59, 609–619. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Finnie, J.F.; van Staden, J. Stimulatory role of smoke-water and karrikinolide on the photosynthetic pigment and phenolic contents of micro-propagated ‘Williams’ bananas. Plant Growth Regul. 2012, 67, 271–279. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Amoo, S.O.; Kandari, L.S.; van Staden, J. Seed germination and phytochemical evaluation in seedlings of Aloe arborescens Mill. Plant Biosyst. 2013, 148, 460–466. [Google Scholar] [CrossRef]
- Kumari, A.; Papenfus, H.B.; Kulkarni, M.G.; Posta, M.; van Staden, J. Effect of smoke derivatives on in vitro pollen germination and pollen tube elongation of species from different plant families. Plant Biol. 2015, 17, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Okem, A.; Kulkarni, M.G.; van Staden, J. Enhancing Phytoremediation Potential of Pennisetum clandestinum Hochst in cadmium contaminated soil using smoke-water and smoke isolated karrikinolide. Int. J. Phytoremediat. 2015, 17, 1046–1052. [Google Scholar] [CrossRef]
- Papenfus, H.B.; Kulkarni, M.G.; Stirk, W.A.; Rengasamy, K.R.R.; Salomon, M.V.; Piccoli, P.; Bottini, R.; van Staden, J. Interactions between a plant growth-promoting rhizobia bacterium and smoke-derived compounds and their effect on okra growth. J. Plant Nutr. Soil Sci. 2015, 178, 741–747. [Google Scholar] [CrossRef]
- Ren, L.; Bai, Y.; Reaney, M. Evidence of different compounds in smoke derived from legumes and grasses acting on seed germination and seedling emergence. Seed Sci. Res. 2017, 27, 154–164. [Google Scholar] [CrossRef]
- Downes, K.S.; Light, M.E.; Posta, M.; Kohout, L.; van Staden, J. Comparison of germination responses of Anigozanthos flavidus (Haemodoraceae), Gyrostemon racemiger and Gyrostemon ramulosus (Gyrostemonaceae) to smoke-water and the smoke-derived compounds karrikinolide (KAR1) and glyceronitrile. Ann Bot. 2013, 111, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Erickson, T.E.; Merritt, D.J. Seed dormancy regulates germination response to smoke and temperature in a rhizomatous evergreen perennial. AoB Plants 2018, 10, 42. [Google Scholar] [CrossRef]
- Nelson, D.C.; Flematti, G.R.; Riseborough, J.A.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Karrikins enhance light responses during germination and seedling development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 7095–7100. [Google Scholar] [CrossRef]
- Nelson, D.C.; Scaffidi, A.; Dun, E.A.; Waters, M.T.; Flematti, G.R.; Dixon, K.W.; Beveridge, C.A.; Ghisalberti, E.L.; Smith, S.M. F-box protein MAX2 has dual roles in karrikin and strigolactone signaling in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2011, 108, 8897–8902. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.H.U.; Khattak, J.Z.K.; Jamil, M.; Malook, I.; Khan, S.U.; Jan, M.; Din, I.; Saud, S.; Kamran, M.; Alharby, H.; et al. Bacillus safensis with plant-derived smoke stimulates rice growth under saline conditions. Environ. Sci. Pollut. Res. 2017, 24, 23850–23863. [Google Scholar] [CrossRef]
- van Staden, J.; Brown, N.A.C.; Jager, A.K.; Johnson, T.A. Smoke as a germination cue. Plant Spec. Biol. 2000, 15, 167–178. [Google Scholar] [CrossRef]
- Abdollahi, M.R.; Mehrshad, B.; Moosavi, S.S. Effect of method of seed treatment with plant derived smoke solutions on germination and seedling growth of milk thistle (Silybum marianum L.). Seed Sci. Technol. 2011, 39, 225–229. [Google Scholar] [CrossRef]
- Modi, A.T. Indigenous storage method enhances seedling vigour of traditional maize. S. Afr. J. Bot. 2002, 98, 138–139. [Google Scholar]
- Jamil, M.; Malook, I.; Parveen, S.; Naz, T.M.; Ali, A.; Jan, S.U.; Rehman, S.U. Smoke priming, a potent protective agent against salinity: Effect on proline accumulation, elemental uptake, pigmental attributes and protein banding patterns of rice (Oryza sativa). J. Stress Physiol. Biochem. 2013, 9, 169–183. [Google Scholar]
- Aslam, M.M.; Jamil, M.; Khatoon, A.; El-Hendawy, S.E.; Al-Suhaibani, N.A.; Shakir, S.K.; Malook, I.; Rehman, S.U. Does weeds-derived smoke improve plant growth of wheat? J. Bio Mol. Sci. 2015, 3, 86–96. [Google Scholar]
- Tieu, A.; Dixon, K.W.; Meney, K.A.; Sivasithamparam, K. Interaction of soil burial and smoke on germination patterns in seeds of selected Australian native plants. Seed Sci. Res. 2001, 11, 69–76. [Google Scholar] [CrossRef]
- Thomas, T.H.; van Staden, J. Dormancy break of celery (Apium gravveolens L.) seeds by plant derived smoke extract. Plant Growth Regul. 1995, 17, 195–198. [Google Scholar] [CrossRef]
- Kepczynski, J.; Cembrowska, D.; van Staden, J. Releasing primary dormancy in Avena fatua L. caryopses by smoke-derived butenolide. Plant Growth Regul. 2010, 62, 85–91. [Google Scholar] [CrossRef]
- Soos, V.; Sebestyen, E.; Posta, M.; Kohout, L.; Light, M.E.; van Staden, J.; Balazs, E. Molecular aspects of the antagonistic interaction of smoke-derived butenolides on the germination process of Grand Rapids lettuce (Lactuca sativa) achenes. New Phytol. 2012, 196, 1060–1073. [Google Scholar] [CrossRef]
- Nelson, D.C.; Riseborough, J.A.; Flematti, G.R.; Stevens, J.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Karrikins discovered in smoke trigger Arabidopsis seed germination by a mechanism requiring gibberellic acid synthesis and light. Plant Physiol. 2009, 149, 863–873. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Ascough, G.D.; van Staden, J. Effects of foliar applications of smoke-water and a smoke-isolated butenolide on seedling growth of okra and tomato. HortScience 2007, 42, 179–182. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Sprag, S.G.; van Staden, J. Germination and post germination response of Acacia seeds to smoke-water and butenolide, a smoke derived compound. J. Arid. Environ. 2007, 69, 177–187. [Google Scholar] [CrossRef]
- Taylor, J.L.S.; van Staden, J. Plant-derived smoke solutions stimulate the growth of Lycopersicon esculentum roots in vitro. Plant Growth Regul. 1998, 26, 77–83. [Google Scholar] [CrossRef]
- Taylor, J.L.S.; van Staden, J. Root initiation in Vigna radiates (L.) Wilczek hypocotyls cuttings are stimulated by smoke-derived extracts. Plant Growth Regul. 1996, 18, 165–168. [Google Scholar] [CrossRef]
- Ramos, D.M.; Valls, J.F.M.; Borghetti, F.; Ooi, M.K.J. Fire cues trigger germination and stimulate seedling growth of grass species from Brazilian savannas. Am. J. Bot. 2019, 106, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.B.; Dixon, K.; Flematti, G. Comparative enhancement of germination and vigor in seed and somatic embryos by the smoke chemical 3-methyl-2H-furo[2,3-c] pyran-2-one in Tetraphyllum (Restionaceae). In Vitro Cell. Dev. Biol. Plant. 2006, 42, 306–308. [Google Scholar] [CrossRef]
- Doherty, L.C.; Cohn, M.A. Seed dormancy in rice (Oryza sativa). Commercial liquid smoke elicits germination. Seed Sci. Res. 2000, 10, 415–421. [Google Scholar] [CrossRef]
- Malook, J.; Shah, G.; Jan, M.; Shinwari, K.I.; Aslam, M.M.; Rehman, S.U.; Jamil, M. Smoke priming regulates growth and the expression of myeloblastosis and zinc-finger genes in rice under salt stress. Arab. J. Sci. Eng. 2017, 42, 2207–2215. [Google Scholar] [CrossRef]
- Jain, N.; Soos, V.; Balazs, E.; van Staden, J. Changes in cellular macromolecules (DNA, RNA and protein) during seed germination in tomato, following the use of a butenolide, isolated from plant derived smoke. Plant Growth Regul. 2008, 54, 105–113. [Google Scholar] [CrossRef]
- Zhou, J.; Da-Silva, J.A.T.; Ma, G. Effects of smoke water and karrikin on seed germination of 13 species growing in China. Cent. Eur. J. Biol. 2014, 9, 1108–1116. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Ripley, B.S. The effect of smoke on the photosynthetic gas exchange of Chrysanthemoides monilifera. S. Afr. J. Bot. 2002, 68, 525–531. [Google Scholar] [CrossRef][Green Version]
- Papenfus, H.B.; Kulkarni, M.G.; Jeffrey, M.P.; Finnie, F.; van Staden, J. Smoke-isolated trimethyl butenolide inhibits seed germination of different weed species by reducing amylase activity. Weed Sci. 2015, 63, 312–320. [Google Scholar] [CrossRef]
- Jager, A.K.; van Staden, J. Soluble sugars in light-sensitive Grand Rapids lettuce seeds treated with red light, gibberellic acid and a plant-derived smoke extract. S. Afr. J. Bot. 2002, 68, 404–407. [Google Scholar] [CrossRef]
- Baxter, B.J.M.; van Staden, J. Plant-derived smoke: An effective seed pre-treatment. Plant Growth Regul. 1994, 14, 279–282. [Google Scholar] [CrossRef]
- Akeel, A.; Khan, M.M.A.; Jaleel, H.; Din, U.M. Smoke-saturated water and karrikinolide modulate germination, growth, photosynthesis and nutritional values of carrot (Daucus carota L.). Plant Growth Regul. 2019, 38, 1387–1401. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, F.; Shuai, H.; Luo, X.; Ding, J.; Tang, S.; Liu, J. Karrikins delay soybean seed germination by mediating abscisic acid and gibberellin biogenesis under shaded conditions. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Bieza, K.; Lois, R. An Arabidopsis mutant tolerant to lethal ultraviolet-B levels shows constitutively elevated accumulation of flavonoids and other phenolics. Plant Physiol. 2001, 126, 1105–1115. [Google Scholar] [CrossRef]
- Peer, W.A.; Murphy, A.S. Flavonoids and auxin-transport: Modulators or regulators? Trends Plant Sci. 2007, 12, 556–563. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, G.J.; Guan, H.; Huisman, P.; Marinova, S. Effects of phenolic compounds on adventitious root formation and oxidative decarboxylation of applied indoleacetic acid in Malus ‘Jork9’. Plant Growth Regul. 2011, 63, 175–185. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defense mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Pourmorad, F.; Hosseinimehr, S.J.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006, 5, 1142–1145. [Google Scholar]
- Savio, L.E.B.; Astarita, L.V.; Santarem, E.R. Secondary metabolism in micropropagated Hypericum perforatum L. grown in non-aerated liquid medium. Plant Cell Tissue Organ Cult. 2011, 108, 465–472. [Google Scholar] [CrossRef]
- Thanos, C.A.; Rundel, P.W. Fire-followers in chaparral: Nitrogenous compounds trigger seed germination. J. Ecol. 1995, 83, 207–216. [Google Scholar] [CrossRef]
- Tieu, A.; Dixon, K.A.; Sivasithamparam, K.; Plummer, J.A. Germination of four species of native Western Australian plant using plant-derived smoke. Aust. J. Bot. 1999, 47, 207–219. [Google Scholar] [CrossRef]
- Duermeyer, L.; Khodapanahi, E.; Yan, D.; Krapp, A.; Rothstein, S.J.; Nambara, E. Regulation of seed dormancy and germination by nitrate. Seed Sci. Res. 2018, 28, 150–157. [Google Scholar] [CrossRef]
- Soos, V.; Juhasz, A.; Light, M.E.; van Staden, J.; Balazs, E. Smoke-water-induced changes of expression pattern in Grand Rapids lettuce achenes. Seed Sci. Res. 2009, 19, 37–49. [Google Scholar] [CrossRef]
- Soos, V.; Sebestyen, E.; Juhasz, A.; Pinter, J.; Light, M.E.; van Staden, J.; Balazs, E. Stress-related genes define essential steps in the response of maize seedlings to smoke-water. Funct. Integr. Genom. 2009, 9, 231–242. [Google Scholar] [CrossRef]
- Soos, V.; Sebestyen, E.; Juhasz, A.; Light, M.E.; Kohout, L.; Szalai, G.; Tandori, J.; van Staden, J.; Balazs, E. Transcriptome analysis of germinating maize kernels exposed to smoke-water and the active compound KAR 1. BMC Plant Biol. 2010, 10, 236. [Google Scholar] [CrossRef]
- Baldrianova, J.; Cerny, M.; Novák, J.; Jedelsky, P.; Brzobohaty, B. Arabidopsis proteome responses to the smoke-derived growth regulator karrikin. J. Proteom. 2015, 120, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Trinh, C.; Gevaert, L.; Kohout, L.; van Staden, J.; Verschaeve, L. Genotoxicity evaluation of two kinds of smoke-water and 3,7-dimethyl-2H-furo[2,3-c] pyran-2-one. J. Appl. Toxicol. 2010, 30, 596–602. [Google Scholar] [CrossRef]
- Zhong, Z.; Kobayashi, T.; Zhu, W.; Imai, H.; Zhao, R.; Ohno, T.; Rehman, S.U.; Uemura, M.; Tian, J.; Komatsu, S. Plant-derived smoke enhances plant growth through ornithine-synthesis pathway and ubiquitin-proteasome pathway in soybean. J. Proteomics 2020, 221, 103781. [Google Scholar] [CrossRef]
- Otori, M.; Murashita, Y.; Rehman, S.U.; Komatsu, S. Proteomic study to understand promotive effects of plant-derived smoke on soybean (Glycine max L.) root growth under flooding stress. Plant Mol. Biol. Rep 2020, 1–10. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, Z.X.; Sun, H.; Guo, L.P. Smoke-isolated karrikins stimulated tanshinones biosynthesis in Salvia miltiorrhiza through endogenous nitric oxide and jasmonic acid. Molecules 2019, 24, 1229. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, Z.; Clair, J.J.L.; Chory, J.; Noel, J.P. Smoke-derived karrikin perception by the α/β-hydrolase KAI2 from Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 8284–8289. [Google Scholar] [CrossRef]
- Waters, M.T.; Nelson, D.C.; Scaffidi, A.; Flematti, G.R.; Sun, Y.M.; Dixon, K.W.; Smith, S.M. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 2012, 139, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Kagiyama, M.; Hirano, Y.; Mori, T.; Kim, S.Y.; Kyozuka, J.; Seto, Y.; Yamaguchi, S.; Hakoshima, T. Structures of D14 and D14L in the strigolactone and karrikin signaling pathways. Genes Cells 2013, 18, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Scaffidi, A.; Sun, Y.K.; Flematti, G.R.; Smith, S.M. The karrikin response system of Arabidopsis. Plant J. 2014, 79, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.J.; Snowden, K.C. Strigolactone and karrikin signal perception: Receptors, enzymes, or both? Front Plant Sci. 2012, 3, 296. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Li, J. Signaling and responses to strigolactones and karrikins. Curr. Opin. Plant Biol. 2014, 21, 23–29. [Google Scholar] [CrossRef]
- Stanga, J.P.; Smith, S.M.; Briggs, W.R.; Nelson, D.C. SUPPRESSOR OF MORE AXILLARY GROWTH2 1 controls seed germination and seedling development in Arabidopsis. Plant Physiol. 2013, 163, 318–330. [Google Scholar] [CrossRef]
- Stanga, J.P.; Morffy, N.; Nelson, D.C. Functional redundancy in the control of seedling growth by the karrikin signaling pathway. Planta 2016, 243, 1397–1406. [Google Scholar] [CrossRef]
- Machin, D.C.; Hamon-Josse, M.; Bennett, T. Fellowship of the rings: A saga of strigolactones and other small signals. New Phytol. 2020, 225, 621–636. [Google Scholar] [CrossRef]
- Yao, J.; Mashiguchi, K.; Scaffidi, A.; Akatsu, T.; Melville, K.T.; Morita, R.; Morimoto, R.; Smith, S.M.; Seto, Y.; Flematti, G.R.; et al. An allelic series at the KARRIKIN INSENSITIVE 2 locus of Arabidopsis thaliana decouples ligand hydrolysis and receptor degradation from downstream signaling. Plant J. 2018, 96, 75–89. [Google Scholar] [CrossRef]
- Wang, L.; Waters, M.T.; Smith, S.M. Karrikin-KAI2 signalling provides Arabidopsis seeds with tolerance to abiotic stress and inhibits germination under conditions unfavorable to seedling establishment. New Phytol. 2018, 219, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Q.; Yu, H.; Ma, H.; Li, X.; Yang, J.; Li, J. Strigolactone and karrikin signaling pathways elicit ubiquitination and proteolysis of SMXL2 to regulate hypocotyl elongation in Arabidopsis. Plant Cell 2020, 32, 2251–2270. [Google Scholar] [CrossRef] [PubMed]


| Plant Source for Plant-Derived Smoke | Identified Compounds | References |
|---|---|---|
| Lettuce | 1,8–cineole | [19] |
| Skilpadbessie; Red oat grass | 3-methyle-2-Hfuro [2,3-C]pyran-2-one (Butenolide) | [11] |
| Skilpadbessie; Red oat grass | 3,4,5-trimethylfuran-2(5H)-one | [20] |
| Red-and-green kangaroo paw | glyceronitrile, cyanohydrin | [21] |
| Coyote tobacco | Catechol | [22] |
| Ginkgo | Hydroquinone | [23] |
| Skilpadbessie | 5,5-dimethylfuran-2(5H)-one | [24] |
| Red oat grass | (5RS)-5-ethylfuran-2(5H)-one | [24] |
| Experimental Plant Species | Major Findings | Ref. |
|---|---|---|
| Smoke solution for application | ||
| Lantana | Seed germination, germination velocity index and vigor index increased | [47] |
| Dyer’s woad | Seedling mass increased | [48] |
| Rock rose | Germination percentage increased | [49] |
| Edible banana | Seedling length, seedling mass, number of shoots, number of roots, number of leaves, leaf area increased | [36] |
| Plant species from grassland | Seed germination increased | [50] |
| Pawpaw | Seed germination rate, seedling length and vigor, and number of leaves increased | [51] |
| Canola | Plant regeneration, seedling length increased | [52] |
| Mediterranean Basin flora | Seedling emergence was promoted | [53] |
| Society garlic., Wild garlic | Seed germination, seedling mass, root length and root number increased | [54] |
| Lavandula stoechas,Origanum onites, Phlomis bourgaei, Stachys cretica, Satureja thymbra, Teucrium lamiifolium | Seed germination increased | [55] |
| Coyote tobacco., Whispering bells | Germination percentage increased | [56] |
| Sweet potato | Number of adventitious roots, length of adventitious roots and length of lateral roots increased | [57] |
| Millet | Seed germination, seedling length and seedling mass were enhanced | [58] |
| Carrot | Seed germination, seedling length increased | [59] |
| Barnyard grass | Germination percentage, relative root elongation, seedling length and seedling mass were promoted | [60] |
| Perennial forage species | Seed germination increased | [61] |
| 12 eastern Mediterranean basin plants | Seed germination increased | [62] |
| 10 Interior West Penstemon species | Seed germination increased | [63] |
| Canadian horseweed | Seed germination, seedling growth increased | [64] |
| Wheat | Germination percentage, germination index, seedling vigor index and seedling length increased | [33] |
| Helianthemum tinetense | Seed germination increased | [65] |
| Astragalus verus, Bromus tectorum | Seed germination increased | [66] |
| Mediterranean Basin flora | Seed germination increased | [67] |
| Wild oat | Germination percentage and per unit weight water content increased, coat rupturing was stimulated | [68] |
| Shortgrass Prairie species | Seed germination increased | [69] |
| Mediterranean plant species | Seed germination and seedling length were enhanced | [70] |
| Rice | Root length and root fresh/dry weights increased | [15] |
| Cape flats sand Fynbos species | Seedling length and seed germination were promoted | [71] |
| Wheat | Root/shoot length, root fresh/dry weight, shoot fresh/dry weight and leaf area increased | [33] |
| Lupinus angustifolius | Seed germination increased | [72] |
| Chickpea | Seed germination, seedling length and mass increased | [73] |
| Maize | Seed germination, seedling length and mass increased | [74] |
| Tomato, Cucumber, Pot marigold, Sword lily | Seed germination percentage/rate, seedling length and fresh weight increased | [35] |
| Lettuce | Seed germination percentage was promoted | [75] |
| Calotropis gigantea | Seed germination increased | [76] |
| Rice | Seed water uptake and germination percentage were enhanced | [77] |
| Trimethylebutenolide analogs and butenolide solution | ||
| Lettuce., Whispering bells., Tomato bush | Germination percentage increased | [29] |
| Lettuce | Germination percentage increased | [78] |
| Lettuce | Germination percentage increased | [79] |
| Tangle head | Germination percentage increased | [80] |
| Lettuce | Seed germination percentage was promoted | [81] |
| Smoke and butenolide solution | ||
| onion | Number of leaves, leaf length, leaf weight, bulb diameter and bulb weight increased | [35] |
| Melon | Seedling mass increased | [82] |
| Button creeper | Seed germination increased, and seed dormancy broke | [83] |
| Asian mustard | Germination percentage increased | [84] |
| Tropical soda apple | Seed germination, seedling length and mass increased | [85] |
| Wild oat, Wimmera ryegrass, Weeping lovegrass, Little seed canary grass, Barley grass, Perrenial veldgrass, Ripgut brome | Germination percentage increased | [86] |
| Edible banana | Leaves number, branching, seedling length, seedling weight and root number increased | [87] |
| Tree aloe | Seed germination and seedling growth increased | [88] |
| Torch lily, Opal flower | Pollen germination and pollen tube growth was enhanced | [89] |
| Kikuyu grass | Seedling vigor, seedling mass, and leaf number increased | [90] |
| Okra | Seedling length increased | [91] |
| Lettuce | Seed germination and radicle length increased | [92] |
| Glyceronitrile and smoke/butanolide solution | ||
| Kangaroo paw., Gyrostemon, Racemigerus, Gyrostemon ramulosus | Seed germination and seedling length were enhanced | [93] |
| Kangaroo paw | Seed germination and embryo growth increased | [94] |
| Mediterranean plant species | Seed germination and seedling length were enhanced | [70] |
| Aerosol smoke and smoke solution | ||
| Arabidopsis | Seed germination and hypocotyl length increased; and seed dormancy was broken | [95,96] |
| Smoke and PGPR solution | ||
| Rice | Seed germination and shoot/root lengths increased | [97] |
| Mode of Smoke/Smoke Compounds Application | Major Findings | Ref. |
|---|---|---|
| Tomato | ||
| Seed imbibition in Butenoloides solution | Total soluble proteins in embryo, cotyledons and seedlings increased | [116] |
| Smoke and butenolide solution | Ascorbic acid, b-carotene, lycopene and total soluble solids increased | [34] |
| Smoke solution prepared from different plants species | α-amylase activity and abscisic acid content, N, P, and K ion contents, chlorophyll contents increased | [35] |
| Rice | ||
| Seed priming in smoke solution | Proline contents, photosynthetic pigments increased | [101] |
| Seed priming in smoke solution | Ion contents, cell membrane stability, protein contents, total nitrogen contents increased | [115] |
| Wheat | ||
| Smoke solution | Electrolyte (Na+2, Ca+2, K+) contents, nitrogen and protein contents, total soluble sugar, total soluble proteins, proline contents, glycine betaine, antioxidant enzymes increased | [33] |
| Smoke and PGPR solution | Photosynthetic pigments, Electrolyte (Na+2, Ca+2, K+) content, semyzne tnadixoitna, enilorp, stnetnoc nietorp, ragus elbulos latotdesaercni | [102] |
| Smoke solution | Carbohydrate, protein and lipid analysis, macro and micro elements concentrations increased | [33] |
| Wild garlic | ||
| Smoke solution | Flavonoids, total phenolics, condensed tannins were regulated | [54] |
| Dyer’s woad | ||
| Smoke solution | Indigo concentration increased | [48] |
| Smoke solution | Photosynthetic yield, chlorophyll fluorescence increased | [117] |
| Kikuyu grass | ||
| Smoke and butenolide solution | Cd uptake decreased | [90] |
| Pawpaw | ||
| Smoke solution | Nitrogen, Ion contents, Fe, Zn, Cu, chlorophyll content increased | [51] |
| Tree aloe | ||
| Smoke and butenolide solution | Flavonoids, total phenolics were regulated | [88] |
| Bone seed | ||
| Aerosol smoke solution application | Stomatal conductance, CO2 assimilation rate and intercellular CO2 levels increased | [118] |
| Edible banana | ||
| Smoke and karrikinolide (butenolide) solution | Photosynthetic pigments, total phenolics, total flavonoids, proanthocyanidins increased | [36] |
| Ear-leaf nightshade, Talinum, Asthma-weed, Catsear, Akmella | ||
| Smoke, butanolide and trimethylebutanolide solution | α amylase activity increased | [119] |
| Okra | ||
| Smoke, butanolide and trimethylebutenolide solution | α amylase activity and bacterial abundance increased | [119] |
| Maize | ||
| Smoke solution priming | Ion contents, photosynthetic pigments and antioxidant enzymes increased | [39] |
| Smoke solution | Chlorophyll pigments and total soluble proteins increased | [12] |
| Lettuce | ||
| Smoke solution | Total soluble sugar increased | [120] |
| Smoke/butenolide/ trimethylebutanolide solution | α- amylase activity, starch, sugar, protein contents, lipase activity and lipid contents increased | [81] |
| Barnyard grass | ||
| Smoke solution | α amylase and abscisic acid contents increased | [60] |
| Sword lily, Cucumber, Pot marigold | ||
| Smoke solution prepared from different plants species | α-amylase activity and abscisic acid content, N/P/K ion contents, and chlorophyll contents increased | [80] |
| Wild oat | ||
| Smoke solution | α/β-amylase activities, starch contents, β tubulin accumulation increased | [68] |
| Chickpea | ||
| Smoke solution | Total soluble sugar, total soluble proteins, number of rhizobia increased | [73] |
| Smoke Compounds | Major Findings | Ref. |
|---|---|---|
| Lettuce | ||
| Smoke solution | Genes related to germination, cell wall expansion, translation, cell division cycle, carbohydrate metabolism and abscisic acid regulation were regulated | [133] |
| KAR1, trimethylbutenolide | Abscisic acid, seed maturation and dormancy-related transcripts were up-regulated by trimethylbutenolide and suppressed by KAR1 | [106] |
| Maize | ||
| Smoke solution | Stress- and abscisic acid-related genes were up-regulated | [134] |
| Smoke solution, KAR1 | Smoke-water enhanced the ubiquitination of proteins and activated protein-degradation-related genes. KAR1 up regulated aquaporin gene | [135] |
| Smoke solution | Sucrose synthase-, nucleotides-, signaling-, and glutathione-related proteins increased; cell wall-, lipid-, photosynthesis-, and amino acid degradation-related proteins decreased | [74] |
| Arabidopsis | ||
| Karrikins | Karrikin signaling is F-box protein (MAX2) dependent. Seed germination and seedling photomorphogenesis was triggered by karrikin | [96] |
| Karrikins | Photosynthesis, carbohydrate metabolism, redox homeostasis, transcription control, protein transport, processing, protein degradation were regulated | [136] |
| Salmonella typhimurium | ||
| Smoke solution, 3,7-dimethyl-2H-furo[2,3-c] pyran-2-one | No genotoxicity from smoke solution and smoke isolated compounds | [137] |
| Tomato | ||
| Butenolides | Butenolides changed the DNA, RNA and protein profiles, no effect on integrity of DNA | [116] |
| Chickpea | ||
| Smoke solution | Signaling-, nitrate pathway-, and transport-related proteins increased. Protein metabolism-, cell-, and cell wall-related proteins decreased | [73] |
| Soybean | ||
| Smoke solution | Protein abundance and gene expression of O-fucosyltransferase family proteins increased, while that of peptidyl-prolyl cis-trans isomerase and Bowman-Birk proteinase isoinhibitor D-II decreased, sucrose/starch metabolism and glycolysis were suppressed | [14] |
| Smoke solution | Proteins related to protein synthesis, arginine metabolism and ubiquitin-proteasome pathway were regulated; metabolites related to amino acid, carboxylic acids, and sugars were mostly altered | [138] |
| Smoke solution | Protein metabolism-, stress-, redox-, and mitochondrial electron transport chain-related proteins were regulated | [139] |
| Red sage | ||
| KAR1 | Production of tanshinone-I increased | [140] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatoon, A.; Rehman, S.U.; Aslam, M.M.; Jamil, M.; Komatsu, S. Plant-Derived Smoke Affects Biochemical Mechanism on Plant Growth and Seed Germination. Int. J. Mol. Sci. 2020, 21, 7760. https://doi.org/10.3390/ijms21207760
Khatoon A, Rehman SU, Aslam MM, Jamil M, Komatsu S. Plant-Derived Smoke Affects Biochemical Mechanism on Plant Growth and Seed Germination. International Journal of Molecular Sciences. 2020; 21(20):7760. https://doi.org/10.3390/ijms21207760
Chicago/Turabian StyleKhatoon, Amana, Shafiq Ur Rehman, Muhammad Mudasar Aslam, Muhammad Jamil, and Setsuko Komatsu. 2020. "Plant-Derived Smoke Affects Biochemical Mechanism on Plant Growth and Seed Germination" International Journal of Molecular Sciences 21, no. 20: 7760. https://doi.org/10.3390/ijms21207760
APA StyleKhatoon, A., Rehman, S. U., Aslam, M. M., Jamil, M., & Komatsu, S. (2020). Plant-Derived Smoke Affects Biochemical Mechanism on Plant Growth and Seed Germination. International Journal of Molecular Sciences, 21(20), 7760. https://doi.org/10.3390/ijms21207760






