The Sulphur Response in Wheat Grain and Its Implications for Acrylamide Formation and Food Safety
Abstract
1. Introduction
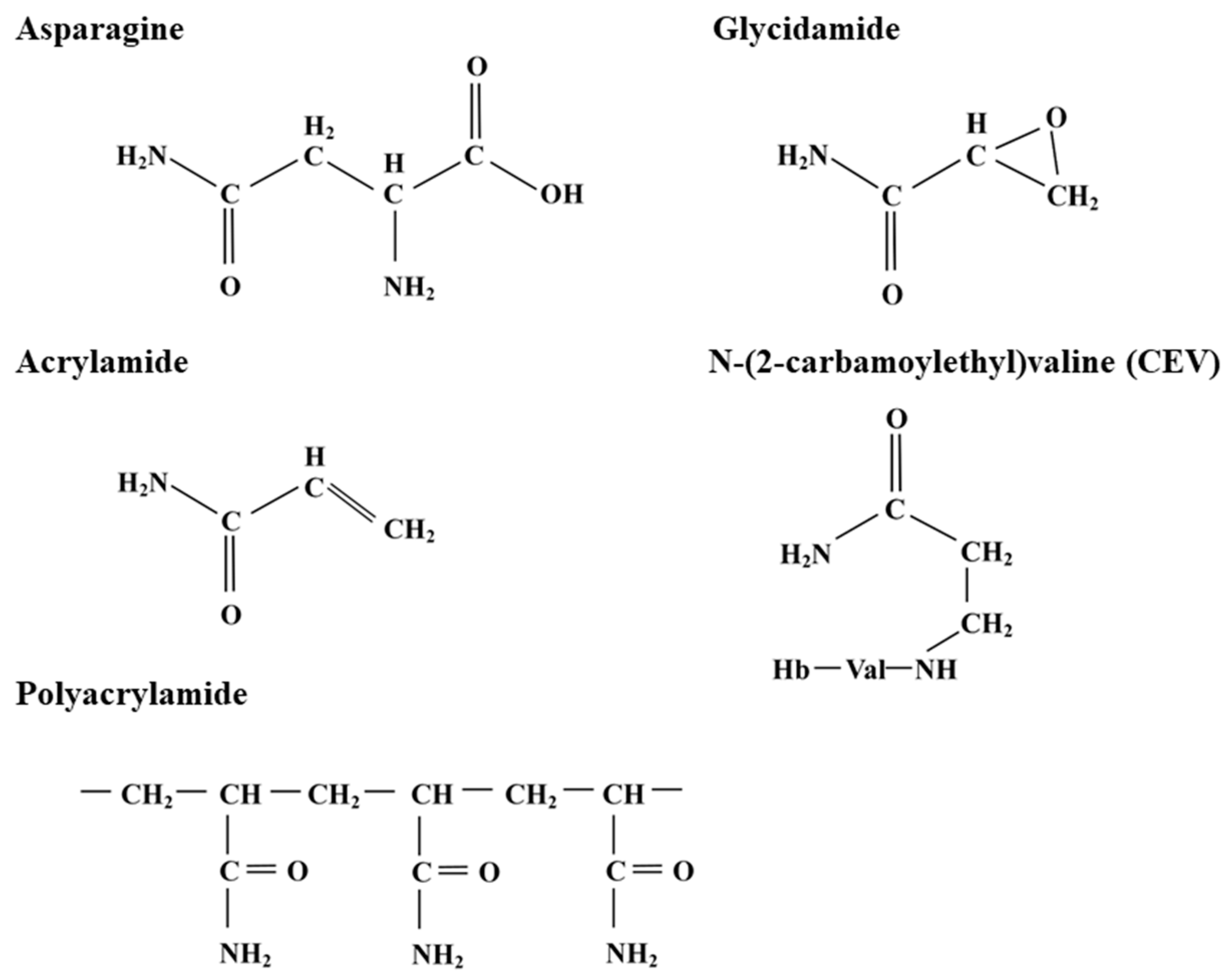
2. The Discovery of Acrylamide in Food
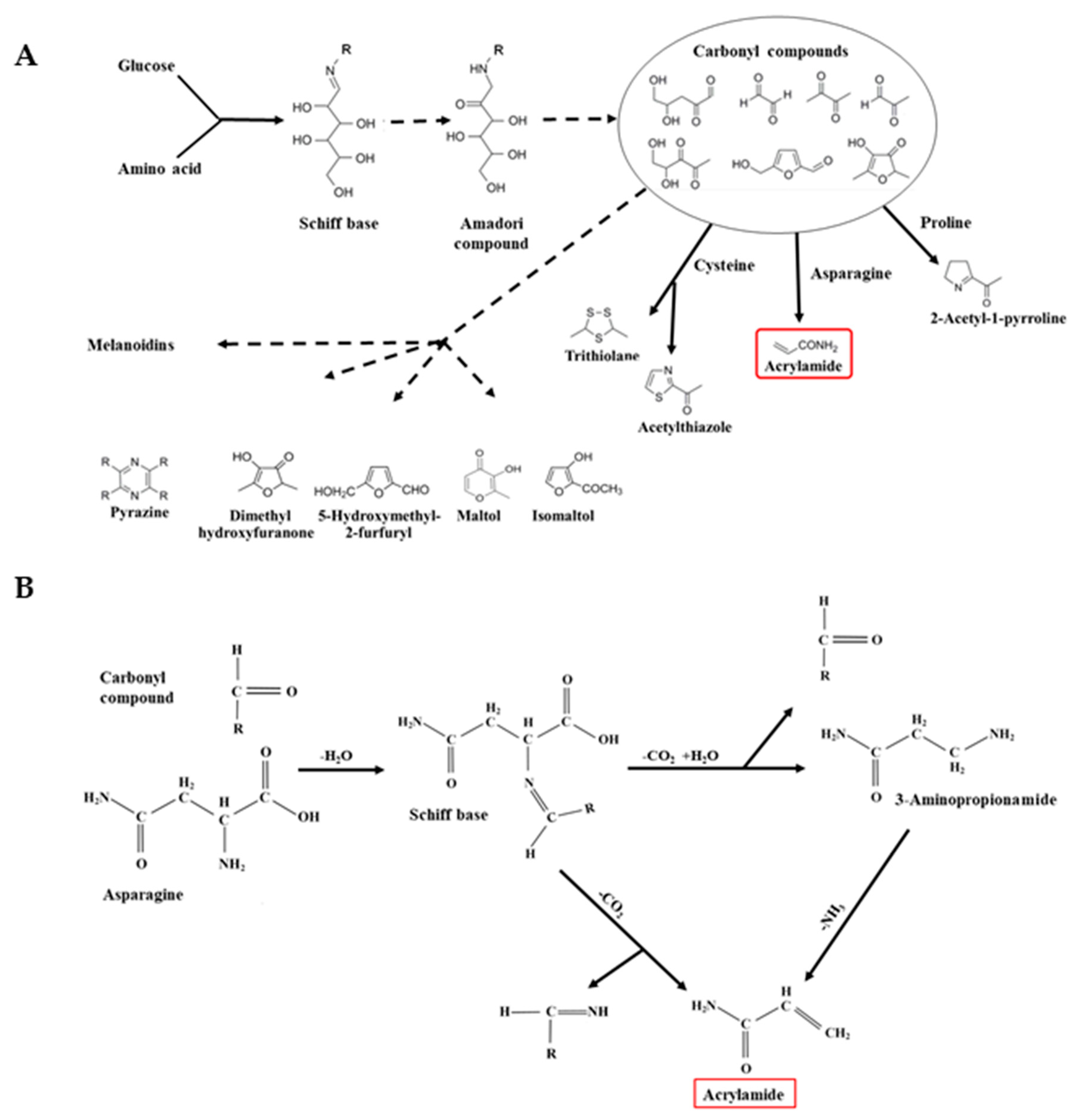
3. Free Asparagine and Acrylamide Formation: The Maillard Reaction
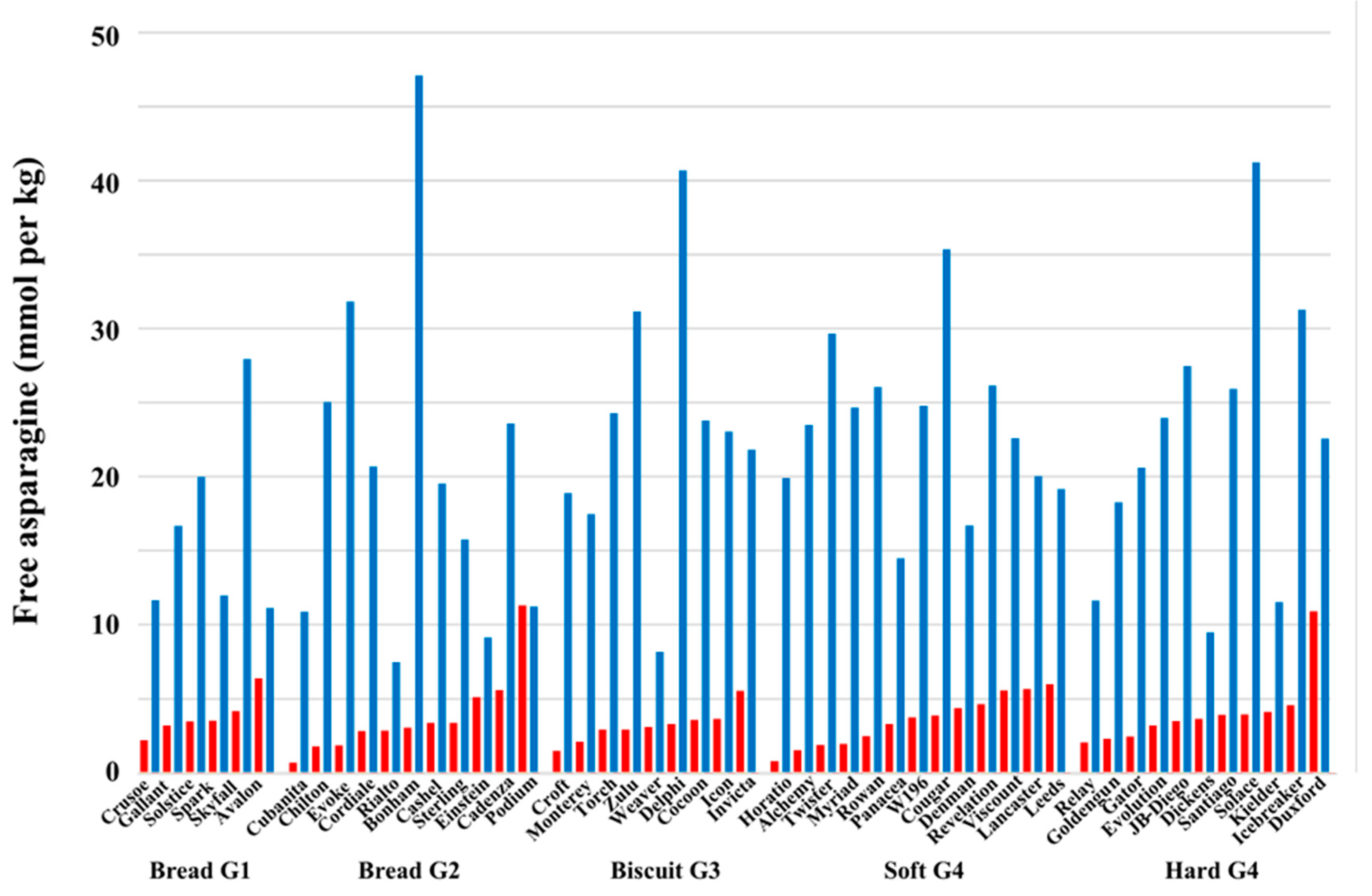
4. The Effect of Sulphur Deficiency on Free Asparagine Concentrations in Wheat Grain
5. Interacting Effects of Sulphur and Nitrogen Fertilization on Free Asparagine Concentrations in Wheat Grain
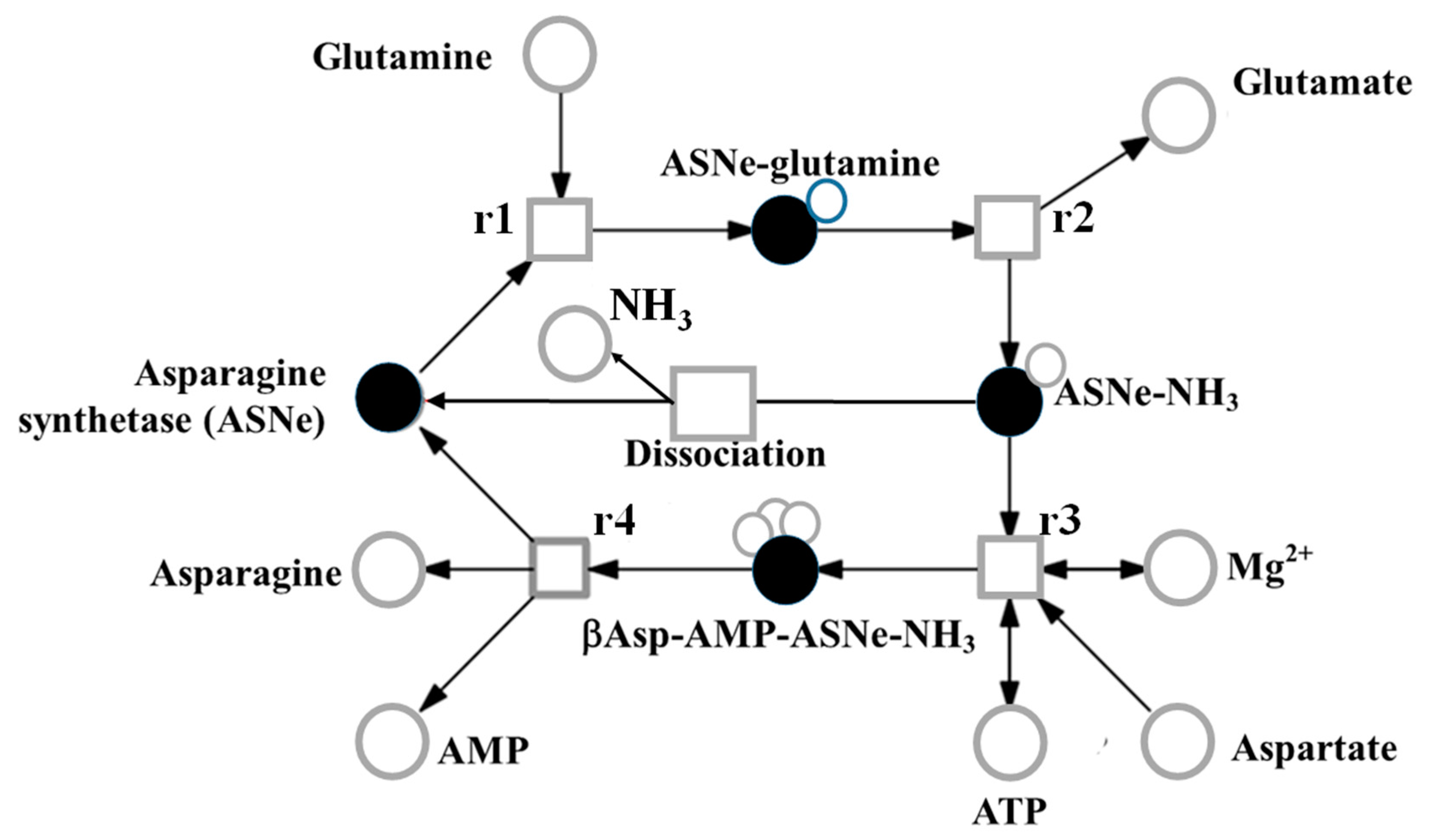
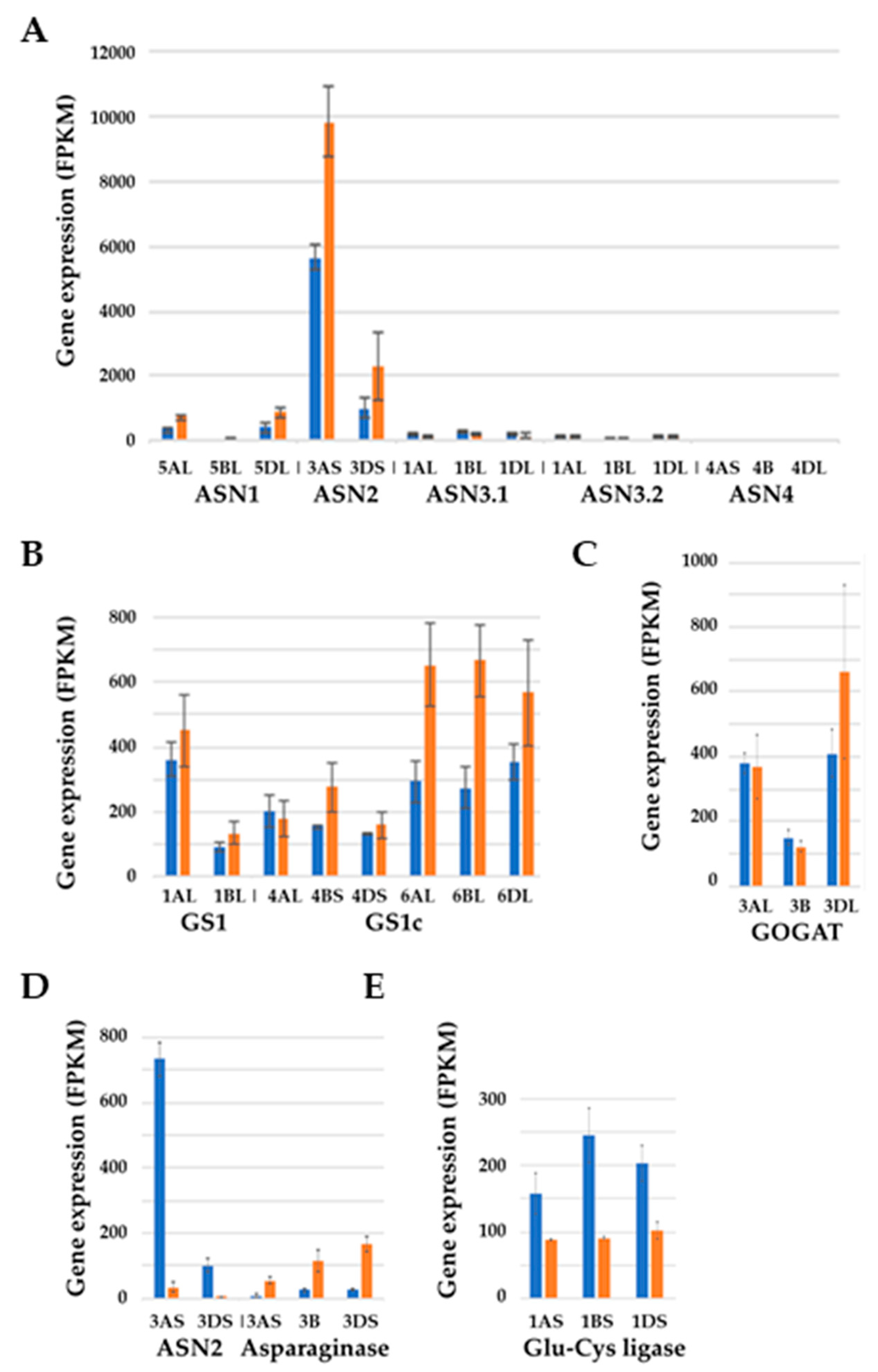
6. Genetic Control of Free Asparagine Concentration in Wheat Grain and its Response to Sulphur
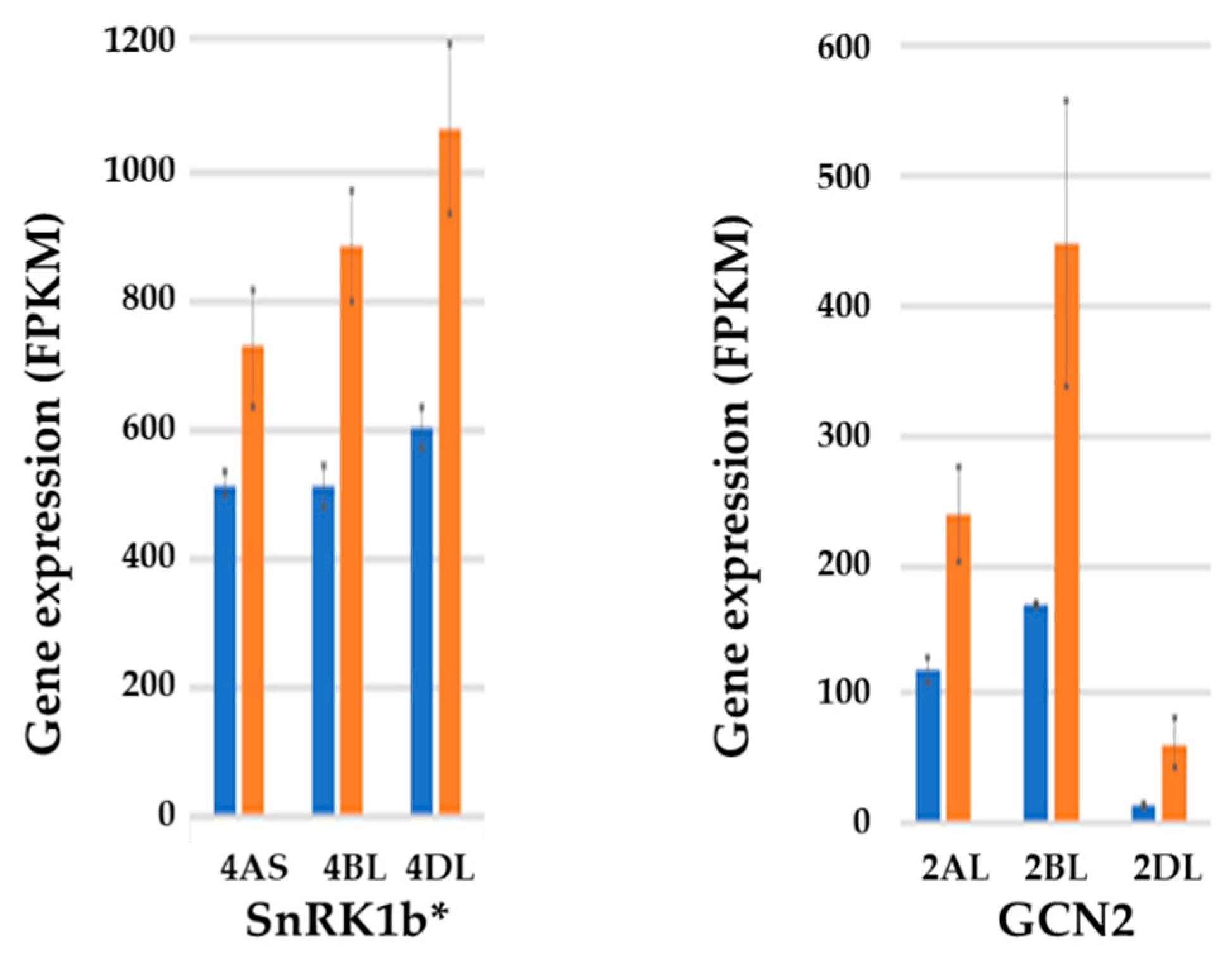
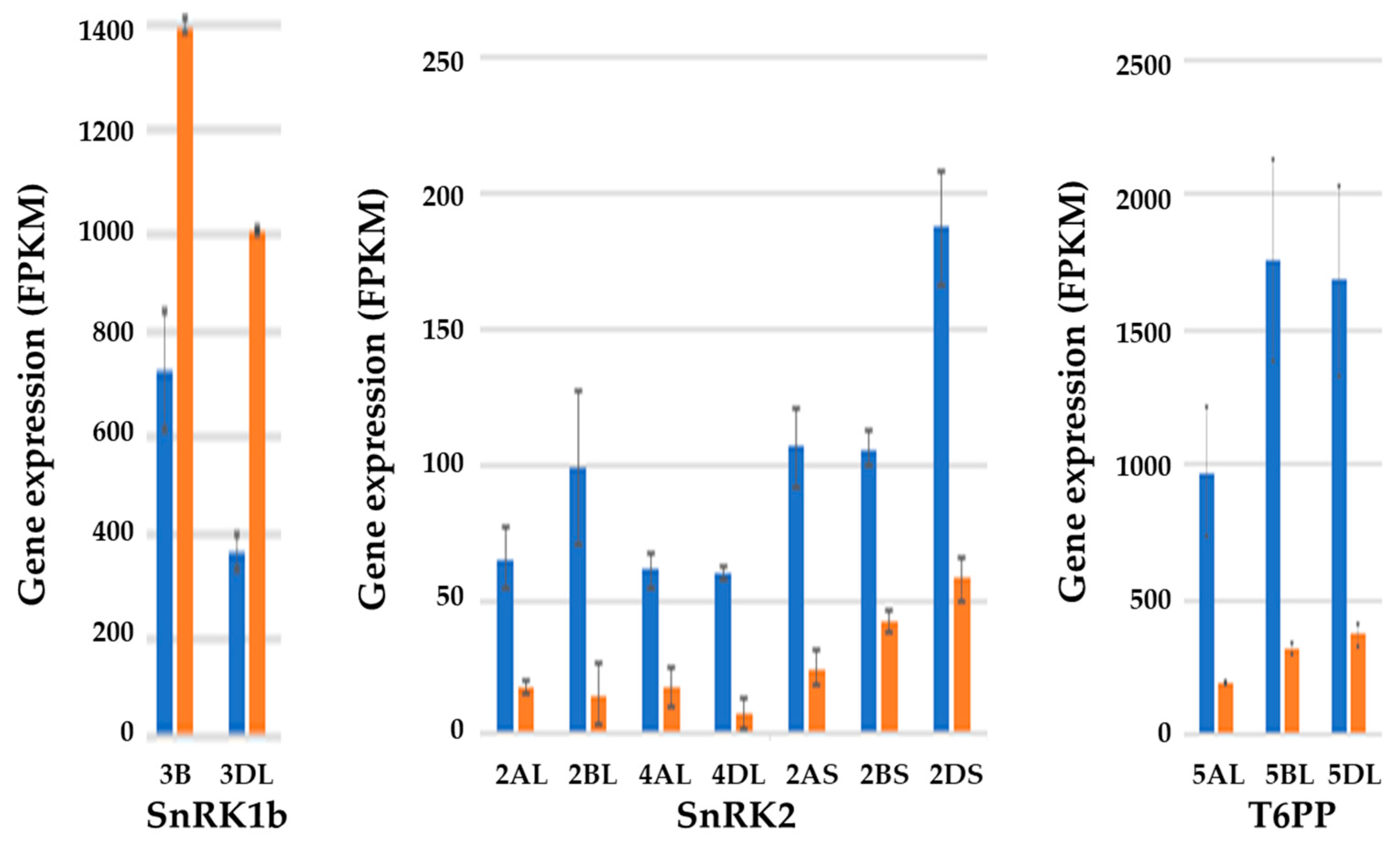
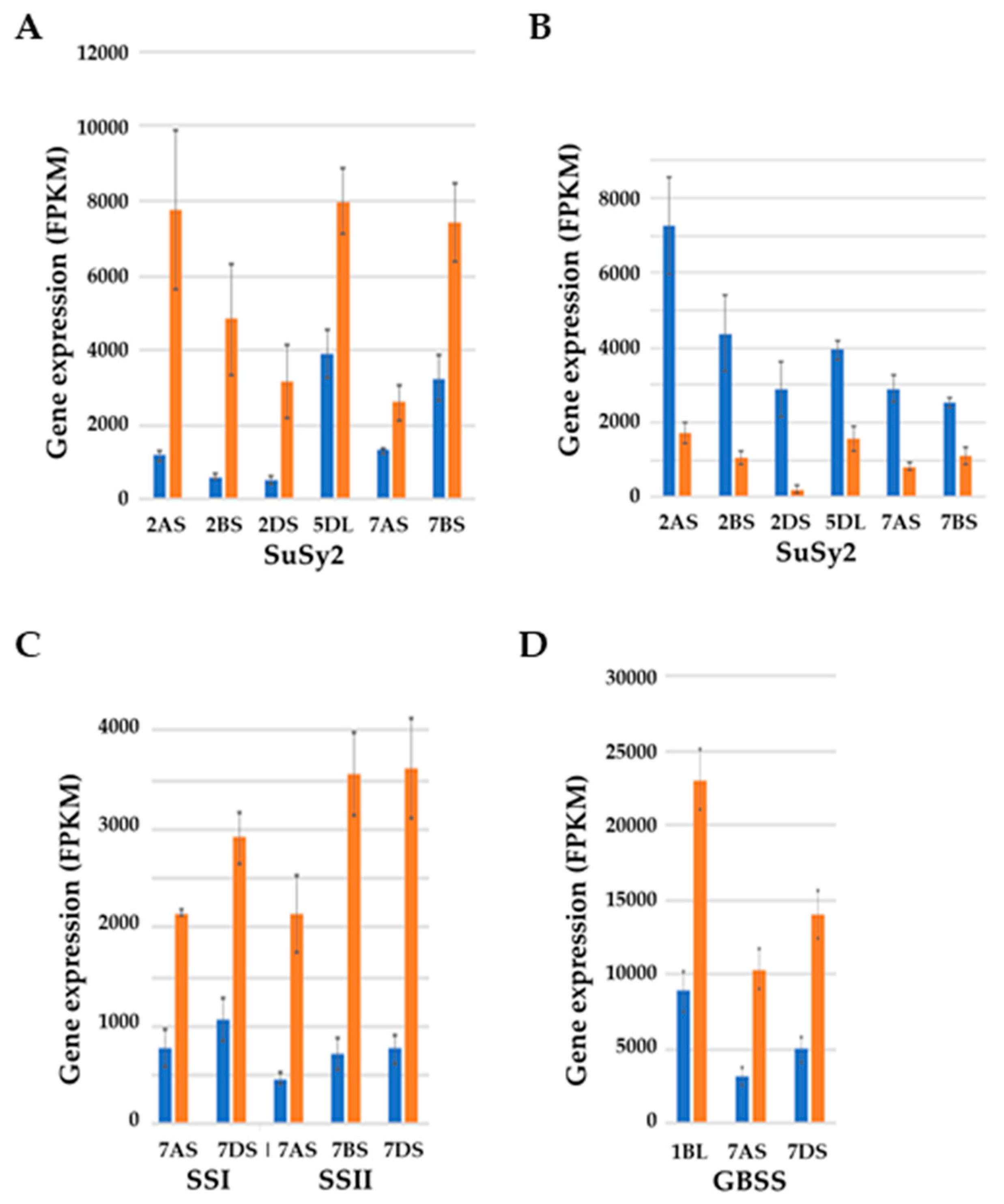
7. Effects of Sulphur Nutrition on Genes Involved in Carbon Metabolism
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shewry, P.R.; Franklin, J.; Parmar, S.; Smith, S.J.; Miflin, B.J. The effects of sulphur starvation on the amino acid and protein compositions of barley grain. J. Cereal Sci. 1983, 1, 21–31. [Google Scholar]
- Zhao, F.J.; Hawkesford, M.J.; McGrath, S.P. Sulphur assimilation and effects on yield and quality of wheat. J. Cereal Sci. 1999, 30, 1–17. [Google Scholar] [CrossRef]
- Blair, G.J. Sulphur fertilisers: A global perspective. In IFS Proceeding No 498; The International Fertiliser Society: York, UK, 2002. [Google Scholar]
- Zhao, F.J.; McGrath, S.P.; Blake-Kalff, M.M.A.; Link, A.; Tucker, M. Crop responses to sulphur fertilisation in Europe. In IFS Proceeding No 504; The International Fertiliser Society: York, UK, 2002. [Google Scholar]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [PubMed]
- Friedman, M. Chemistry, biochemistry and safety of acrylamide. A review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1994; Volume 60. [Google Scholar]
- Bergmark, E.; Calleman, C.J.; He, F.; Costa, L.G. Hemoglobin adducts in humans occupationally exposed to acrylamide. Toxicol. Appl. Pharmacol. 1993, 120, 45–54. [Google Scholar]
- Bergmark, E. Hemoglobin adducts of acrylamide and acrylonitrile in laboratory workers, smokers and nonsmokers. Chem. Res. Toxicol. 1997, 10, 78–84. [Google Scholar]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Acrylamide: A cooking carcinogen? Chem. Res. Toxicol. 2000, 13, 517–522. [Google Scholar]
- Curtis, T.Y.; Postles, J.; Halford, N.G. Reducing the potential for processing contaminant formation in cereal products. J. Cereal Sci. 2014, 59, 382–392. [Google Scholar] [CrossRef]
- Raffan, S.; Halford, N.G. Acrylamide in food: Progress in and prospects for genetic and agronomic solutions. Ann. Appl. Biol. 2019, 175, 259–281. [Google Scholar] [CrossRef]
- Powers, S.J.; Mottram, D.S.; Curtis, A.; Halford, N.G. Acrylamide levels in potato crisps in Europe from 2002 to 2016. Food Addit. Contam. Part. A 2017, 34, 2085–2100. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation, Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food. Off. J. Euro. Union 2017, 304. [Google Scholar]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S. The Maillard reaction: Source of flavor in thermally processed foods. In Flavors and Fragrances: Chemistry, Bioprocessing and Sustainability; Berger, R.G., Ed.; Springer: Berlin, Germany, 2007; pp. 269–284. [Google Scholar]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.-C.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Zyzak, D.V.; Sanders, R.A.; Stojanovic, M.; Tallmadge, D.H.; Eberhart, B.L.; Ewald, D.K.; Gruber, D.C.; Morsch, T.R.; Strothers, M.A.; Rizzi, G.; et al. Acrylamide formation mechanisms in heated foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [Google Scholar] [CrossRef] [PubMed]
- Maillard, L.C. Action des acides aminés sur les sucres: Formation des mélanoïdines par voie méthodique. Comptes Rendus De L’académie Des Sci. 1912, 154, 66–68. [Google Scholar]
- Hodge, J.E. Chemistry of the browning reaction in model systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Granvogl, M.; Schieberle, P. Thermally generated 3-aminopropionamide as a transient intermediate in the formation of acrylamide. J. Agric. Food Chem. 2006, 54, 5933–5938. [Google Scholar] [CrossRef]
- Food Drink Europe. Acrylamide Toolbox 2019; Food Drink Europe: Brussels, Belgium, 2019. [Google Scholar]
- Claus, A.; Weisz, G.M.; Schieber, A.; Carle, R. Pyrolytic acrylamide formation from purified wheat gluten and gluten-supplemented wheat bread rolls. Molec. Nutr. Food Res. 2006, 50, 87–93. [Google Scholar] [CrossRef]
- Muttucumaru, N.; Halford, N.G.; Elmore, J.S.; Dodson, A.T.; Parry, M.; Shewry, P.R.; Mottram, D.S. The formation of high levels of acrylamide during the processing of flour derived from sulfate-deprived wheat. J. Agric. Food Chem. 2006, 54, 8951–8955. [Google Scholar] [CrossRef]
- Granvogl, M.; Wieser, H.; Koehler, P.; Von Tucher, S.; Schieberle, P. Influence of sulphur fertilization on the amounts of free amino acids in wheat. Correlation with baking properties as well as with 3-aminopropionamide and acrylamide generation during baking. J. Agric. Food Chem. 2007, 55, 4271–4277. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.Y.; Muttucumaru, N.; Shewry, P.R.; Parry, M.A.; Powers, S.J.; Elmore, J.S.; Mottram, D.S.; Hook, S.; Halford, N.G. Effects of genotype and environment on free amino acid levels in wheat grain: Implications for acrylamide formation during processing. J. Agric. Food Chem. 2009, 57, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.; Halford, N.G.; Powers, S.J.; McGrath, S.P.; Zazzeroni, R. Effect of Sulphur Fertilisation on the Acrylamide-Forming Potential of Wheat; Home Grown Cereals Authority Project Report No. 525; Agriculture and Horticulture Development Board: Kenilworth, UK, 2014; Available online: https://ahdb.org.uk/effect-of-sulphur-fertilisation-on-the-acrylamide-forming-potential-of-wheat (accessed on 28 May 2020).
- Curtis, T.Y.; Powers, S.J.; Wang, R.; Halford, N.G. Effects of variety, year of cultivation and sulphur supply on the accumulation of free asparagine in the grain of commercial wheat varieties. Food Chem. 2018, 239, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.L.; Guttieri, M.J.; Nelson, N.O.; Fritz, A.; Tilley, M. Nitrogen and sulfur effects on hard winter wheat quality and asparagine concentration. J. Cereal Sci. 2020, 93, 102969. [Google Scholar] [CrossRef]
- NABIM. Wheat Guide 2019; National Association of British & Irish Millers: London, UK, 2019; Available online: http://www.nabim.org.uk/sites/0038/uploads/content/nabim-publications/nabim-wheat-guide-2019.pdf?1565708430 (accessed on 28 May 2020).
- Shewry, P.R.; Zhao, F.-J.; Gowa, G.B.; Hawkins, N.D.; Ward, J.L.; Beale, M.H.; Halford, N.G.; Parry, M.A.J.; Abécassis, J. Sulphur nutrition differentially affects the distribution of asparagine in wheat grain. J. Cereal Sci. 2009, 50, 407–409. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Sec. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Winkler, U.; Schön, W.J. Amino acid composition of the kernel proteins in barley resulting from nitrogen fertilization at different stages of development. J. Agron. Crop. Sci. 1980, 149, 503–512. [Google Scholar]
- Claus, A.; Schreiter, P.; Weber, A.; Graeff, S.; Herrmann, W.; Claupein, W.; Schieber, A.; Carle, R. Influence of agronomic factors and extraction rate on the acrylamide contents in yeast-leavened breads. J. Agric. Food Chem. 2006, 54, 8968–8976. [Google Scholar] [CrossRef]
- Martinek, P.; Klem, K.; Vanova, M.; Bartackova, V.; Vecerkova, L.; Bucher, P.; Hajslova, J. Effects of nitrogen nutrition, fungicide treatment and wheat genotype on free asparagine and reducing sugars content as precursors of acrylamide formation in bread. Plant Soil Environ. 2009, 55, 187–195. [Google Scholar] [CrossRef]
- Shewry, P.R.; Tatham, A.S.; Halford, N.G. Nutritional control of storage protein synthesis in developing grain of wheat and barley. Plant Growth Reg. 2001, 34, 105–111. [Google Scholar] [CrossRef]
- Beato, V.M.; Rexach, J.; Navarro-Gochicoa, M.T.; Camacho-Cristóbal, J.J.; Herrera-Rodríguez, M.B.; González-Fontes, A. Boron deficiency increases expressions of asparagine synthetase, glutamate dehydrogenase and glutamine synthetase genes in tobacco roots irrespective of the nitrogen source. Soil Sci. Plant Nutr. 2014, 60, 314–324. [Google Scholar]
- Dong, Y.; Silbermann, M.; Speiser, A.; Forieri, I.; Linster, E.; Poschet, G.; Allboje Samami, A.; Wanatabe, M.; Sticht, C.; Teleman, A.A.; et al. Sulfur availability regulates plant growth via glucose-TOR signaling. Nat. Commun. 2017, 8, 1174. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, M.D.; Garcia, M.O.; Treseder, K.K. Amino acid uptake in arbuscular mycorrhizal plants. PLoS ONE 2012, 7, e47643. [Google Scholar] [CrossRef] [PubMed]
- Salvioli, A.; Zouari, I.; Chalot, M.; Bonfante, P. The arbuscular mycorrhizal status has an impact on the transcriptome profile and amino acid composition of tomato fruit. BMC Plant Biol. 2012, 12, 44. [Google Scholar]
- Gaude, N.; Bortfeld, S.; Erban, A.; Kopka, J.; Krajinski, F. Symbiosis dependent accumulation of primary metabolites in arbuscule-containing cells. BMC Plant Biol. 2015, 15, 234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saia, S.; Ruisi, P.; Fileccia, V.; di Miceli, G.; Amato, G.; Martinelli, F. Metabolomics suggests that soil inoculation with arbuscular mycorrhizal fungi decreased free amino acid content in roots of durum wheat grown under N-limited, P-rich field conditions. PLoS ONE 2015, 10, e0129591. [Google Scholar] [CrossRef]
- Gahan, J.; Schmalenberger, A. The role of bacteria and mycorrhiza in plant sulfur supply. Frontiers Plant Sci. 2014, 5, 723. [Google Scholar] [CrossRef] [PubMed]
- Agriculture and Horticulture Development Board. Nutrient Management Guide (RB209); Agriculture and Horticulture Development Board: Kenilworth, UK, 2020; Available online: https://projectblue.blob.core.windows.net/media/Default/Imported%20Publication%20Docs/RB209/RB209_Section4_2020_200306_WEB.pdf (accessed on 28 May 2020).
- Xu, H.; Curtis, T.Y.; Powers, S.J.; Raffan, S.; Gao, R.; Huang, J.; Heiner, M.; Gilbert, D.; Halford, N.G. Genomic, biochemical and modelling analyses of asparagine synthetases from wheat. Front. Plant. Sci. 2018, 8, 2237. [Google Scholar] [CrossRef]
- Todd, J.; Screen, S.; Crowley, J.; Peng, J.; Andersen, S.; Brown, T.; Qi, Q.; Fabbri, B.; Duff, S.M.G. Identification and characterization of four distinct asparagine synthetase (AsnS) genes in maize (Zea mays L.). Plant Sci. 2008, 175, 799–808. [Google Scholar] [CrossRef]
- Duff, S.M.G.; Qi, Q.; Reich, T.; Wu, X.Y.; Brown, T.; Crowley, J.H.; Fabbri, B. A kinetic comparison of asparagine synthetase isozymes from higher plants. Plant Physiol. Biochem. 2011, 49, 251–256. [Google Scholar] [CrossRef]
- Gaufichon, L.; Reisdorf-Crena, M.; Rothstein, S.J.; Chardona, F.; Suzuki, A. Biological functions of asparagine synthetase in plants. Plant Sci. 2010, 179, 141–153. [Google Scholar] [CrossRef]
- Gao, R.; Curtis, T.Y.; Powers, S.J.; Xu, H.; Huang, J.; Halford, N.G. Food safety: Structure and expression of the asparagine synthetase gene family of wheat. J. Cereal Sci. 2016, 68, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Raffan, S.; Halford, N.G. Cereal asparagine synthetase genes. Manuscript submitted.
- Curtis, T.Y.; Raffan, S.; Wan, Y.; King, R.; Gonzalez-Uriarte, A.; Halford, N.G. Contrasting gene expression patterns in grain of high and low asparagine wheat genotypes in response to sulphur supply. BMC Genom. 2019, 20, 628. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.Y.; Bo, V.; Tucker, A.; Halford, N.G. Construction of a network describing asparagine metabolism in plants and its application to the identification of genes affecting asparagine metabolism in wheat under drought and nutritional stress. Food Energy Sec. 2018, 7, e00126. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.M.; Habash, D.Z. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 2009, 182, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Lunde, C.; Zygadlo, A.; Simonsen, H.T.; Nielsen, P.L.; Blennow, A.; Haldrup, A. Sulfur starvation in rice: The effect on photosynthesis, carbohydrate metabolism, and oxidative stress protective pathways. Physiol. Plant 2008, 134, 508–521. [Google Scholar]
- Baena-González, E.; Rolland, F.; Thevelein, J.M.; Sheen, J. A central integrator of transcription networks in plant stress and energy signalling. Nature 2007, 448, 938–942. [Google Scholar]
- Byrne, E.H.; Prosser, I.; Muttucumaru, N.; Curtis, T.Y.; Wingler, A.; Powers, S.; Halford, N.G. Over-expression of GCN2-type protein kinase in wheat has profound effects on free amino acid concentration and gene expression. Plant Biotech. J. 2012, 10, 328–340. [Google Scholar]
- Albani, D.; Hammond-Kosack, M.C.U.; Smith, C.; Conlan, S.; Colot, V.; Holdsworth, M.; Bevan, M.W. The wheat transcriptional activator SPA: A seed-specific bZIP protein that recognizes the GCN4-like motif in the bifactorial endosperm box of prolamin genes. Plant Cell 1997, 9, 171–184. [Google Scholar]
- Shewry, P.R.; Halford, N.G.; Lafiandra, D. The genetics of wheat gluten proteins. Adv. Genet. 2003, 49, 111–184. [Google Scholar] [PubMed]
- Müller, M.; Knudsen, S. The nitrogen response of a barley C-hordein promoter is controlled by positive and negative regulation of the GCN4 and endosperm box. Plant J. 1993, 4, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Curci, P.L.; Bergès, H.; Marande, W.; Maccaferri, M.; Tuberosa, R.; Sonnante, G. Asparagine synthetase genes (AsnS1 and AsnS2) in durum wheat: Structural analysis and expression under nitrogen stress. Euphytica 2018, 214, 36. [Google Scholar] [CrossRef]
- Wang, H.; Liu, D.; Sun, J.; Zhang, A. Asparagine synthetase gene TaASN1 from wheat is up-regulated by salt stress, osmotic stress and ABA. J. Plant Physiol. 2005, 162, 81–89. [Google Scholar] [CrossRef] [PubMed]
- McKibbin, R.S.; Muttucumaru, N.; Paul, M.J.; Powers, S.J.; Burrell, M.M.; Coates, S.; Purcell, P.C.; Tiessen, A.; Geigenberger, P.; Halford, N.G. Production of high starch, low glucose potatoes through over-expression of the metabolic regulator, SnRK1. Plant Biotech. J. 2006, 4, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Tiessen, A.; Prescha, K.; Branscheid, A.; Palacios, N.; McKibbin, R.; Halford, N.G.; Geigenberger, P. Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox activation of ADP-glucose pyrophosphorylase in potato tubers. Plant J. 2003, 35, 490–500. [Google Scholar] [PubMed]
- Kanegae, H.; Miyoshi, K.; Hirose, T.; Tsuchimoto, S.; Mori, M.; Nagato, Y.; Takano, M. Expressions of rice sucrose non-fermenting-1 related protein kinase 1 genes are differently regulated during the caryopsis development. Plant Physiol. Biochem. 2005, 43, 669–679. [Google Scholar] [PubMed]
- Jain, M.; Li, Q.-B.; Chourey, P.S. Cloning and expression analyses of sucrose non-fermenting-1-related kinase 1 (SnRK1b) gene during development of sorghum and maize endosperm and its implicated role in sugar-to-starch metabolic transition. Physiol. Plant 2008, 134, 161–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Shewry, P.R.; Jones, H.; Barcelo, P.; Lazzeri, P.A.; Halford, N.G. Expression of antisense SnRK1 protein kinase sequence causes abnormal pollen developpment and male sterility in transgenic barley. Plant J. 2001, 28, 431–442. [Google Scholar]
- Halford, N.G.; Vicente-Carbajosa, J.; Sabelli, P.A.; Shewry, P.R.; Hannappel, U.; Kreis, M. Molecular analyses of a barley multigene family homologous to the yeast protein kinase gene SNF1. Plant J. 1992, 2, 791–797. [Google Scholar] [CrossRef]
- Hey, S.J.; Byrne, E.; Halford, N.G. The interface between metabolic and stress signalling. Ann. Bot. 2010, 105, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Murata, M.; Minami, H.; Yamamoto, S.; Kagaya, Y.; Hobo, T.; Yamamoto, A.; Hattori, T. Abscisic acid-activated SnRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J. 2005, 44, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.R.; Rodriguez, P.L.; Finklestein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Ann. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Anderberg, R.J.; Walker-Simmons, M.K. Isolation of a wheat cDNA clone for an abscisic acid-inducible transcript with homology to protein kinases. Proc. Natl. Acad. Sci. USA 1992, 89, 10183–10187. [Google Scholar] [CrossRef]
- Holappa, L.D.; Walker-Simmons, M.K. The wheat abscisic acid-responsive protein kinase mRNA, PKABA1, is up-regulated by dehydration, cold temperature, and osmotic Stress. Plant Physiol. 1995, 108, 1203–1210. [Google Scholar] [CrossRef]
- Lou, D.; Wang, H.; Liang, G.; Yu, D. OsSAPK2 confers abscisic acid sensitivity and tolerance to drought stress in rice. Front. Plant Sci. 2017, 8, 993. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Davies, W.J. ABA-based chemical signalling: The co-ordination of responses to stress in plants. Plant Cell Env. 2002, 25, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, N.; Radchuk, V.; Strickert, M.; Miersch, O.; Weschke, W.; Wobus, U. Gene expression patterns reveal tissue-specific signalling networks controlling programmed cell death and ABA-regulated maturation in developing barley seeds. Plant J. 2006, 47, 310–327. [Google Scholar] [CrossRef] [PubMed]
- Coello, P.; Hirano, E.; Hey, S.J.; Muttucumaru, N.; Martinez-Barajas, E.; Parry, M.J.; Halford, N.G. Evidence that ABA promotes degradation of SNF1-related protein kinase (SnRK) 1 in wheat and activation of a putative calcium-dependent SnRK2. J. Exp. Bot. 2012, 63, 913–924. [Google Scholar] [CrossRef]
- Zhang, Y.; Primavesi, L.F.; Jhurreea, D.; Andralojc, P.J.; Mitchell, R.A.C.; Powers, S.J.; Schluepmann, H.; Delatte, T.; Wingler, A.; Paul, M.J. Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol. 2009, 149, 1860–1871. [Google Scholar] [CrossRef]
- Martinez de Ilarduya, O.; Vicente-Carbajosa, J.; Sanchez de la Hoz, P.; Carbonero, P. Sucrose synthase genes in barley. cDNA cloning of the Ss2 type and tissue-specific expression of Ss1 and Ss2. FEBS Lett. 1993, 320, 177–181. [Google Scholar] [CrossRef]
- Jiang, Q.; Hou, J.; Hao, C.; Wang, L.; Ge, H.; Dong, Y.; Zhang, X. The wheat (T. aestivum) sucrose synthase 2 gene (TaSus2) active in endosperm development is associated with yield traits. Funct. Integr. Genom. 2011, 11, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Volpicella, M.; Fanizza, I.; Leoni, C.; Gadaleta, A.; Nigro, D.; Gattulli, B.; Mangini, G.; Blanco, A.; Ceci, L.R. Identification and characterization of the sucrose synthase 2 gene (Sus2) in durum wheat. Front. Plant Sci. 2016, 7, 266. [Google Scholar] [CrossRef] [PubMed]
- Maraña, C.; García-Olmedo, F.; Carbonero, P. Differential expression of two types of sucrose synthase-encoding genes in wheat in response to anaerobiosis, cold shock and light. Gene 1990, 88, 167–172. [Google Scholar]
- Hou, J.; Jiang, Q.; Hao, C.; Wang, Y.; Zhang, H.; Zhang, X. Global selection on sucrose synthase haplotypes during a century of wheat breeding. Plant Physiol. 2014, 164, 1918–1929. [Google Scholar] [PubMed]
- Denyer, K.; Hylton, C.M.; Jenner, C.F.; Smith, A.M. Identification of multiple isoforms of soluble and granule-bound starch synthase in developing wheat endosperm. Planta 1995, 196, 256–265. [Google Scholar]
- Curtis, T.Y.; Powers, S.J.; Halford, N.G. Effects of fungicide treatment on free amino acid concentration and acrylamide-forming potential in wheat. J. Agric. Food Chem. 2016, 64, 9689–9696. [Google Scholar] [CrossRef]
- Perochon, A.; Váry, Z.; Malla, K.B.; Halford, N.G.; Paul, M.J.; Doohan, F.M. The wheat SnRK1α family and its contribution to Fusarium toxin tolerance. Plant Sci. 2019, 288, 110217. [Google Scholar]
- Jobe, T.O.; Zenzen, I.; Rahimzadeh Karvansara, P.; Kopriva, S. Integration of sulfate assimilation with carbon and nitrogen metabolism in transition from C3 to C4 photosynthesis. J. Exp. Bot. 2019, 70, 4211–4221. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raffan, S.; Oddy, J.; Halford, N.G. The Sulphur Response in Wheat Grain and Its Implications for Acrylamide Formation and Food Safety. Int. J. Mol. Sci. 2020, 21, 3876. https://doi.org/10.3390/ijms21113876
Raffan S, Oddy J, Halford NG. The Sulphur Response in Wheat Grain and Its Implications for Acrylamide Formation and Food Safety. International Journal of Molecular Sciences. 2020; 21(11):3876. https://doi.org/10.3390/ijms21113876
Chicago/Turabian StyleRaffan, Sarah, Joseph Oddy, and Nigel G. Halford. 2020. "The Sulphur Response in Wheat Grain and Its Implications for Acrylamide Formation and Food Safety" International Journal of Molecular Sciences 21, no. 11: 3876. https://doi.org/10.3390/ijms21113876
APA StyleRaffan, S., Oddy, J., & Halford, N. G. (2020). The Sulphur Response in Wheat Grain and Its Implications for Acrylamide Formation and Food Safety. International Journal of Molecular Sciences, 21(11), 3876. https://doi.org/10.3390/ijms21113876





