Cardiovascular Disease Prevention: The Earlier the Better? A Review of Plant Sterol Metabolism and Implications of Childhood Supplementation
Abstract
:1. Introduction
2. Cardiovascular Diseases (CVDs)
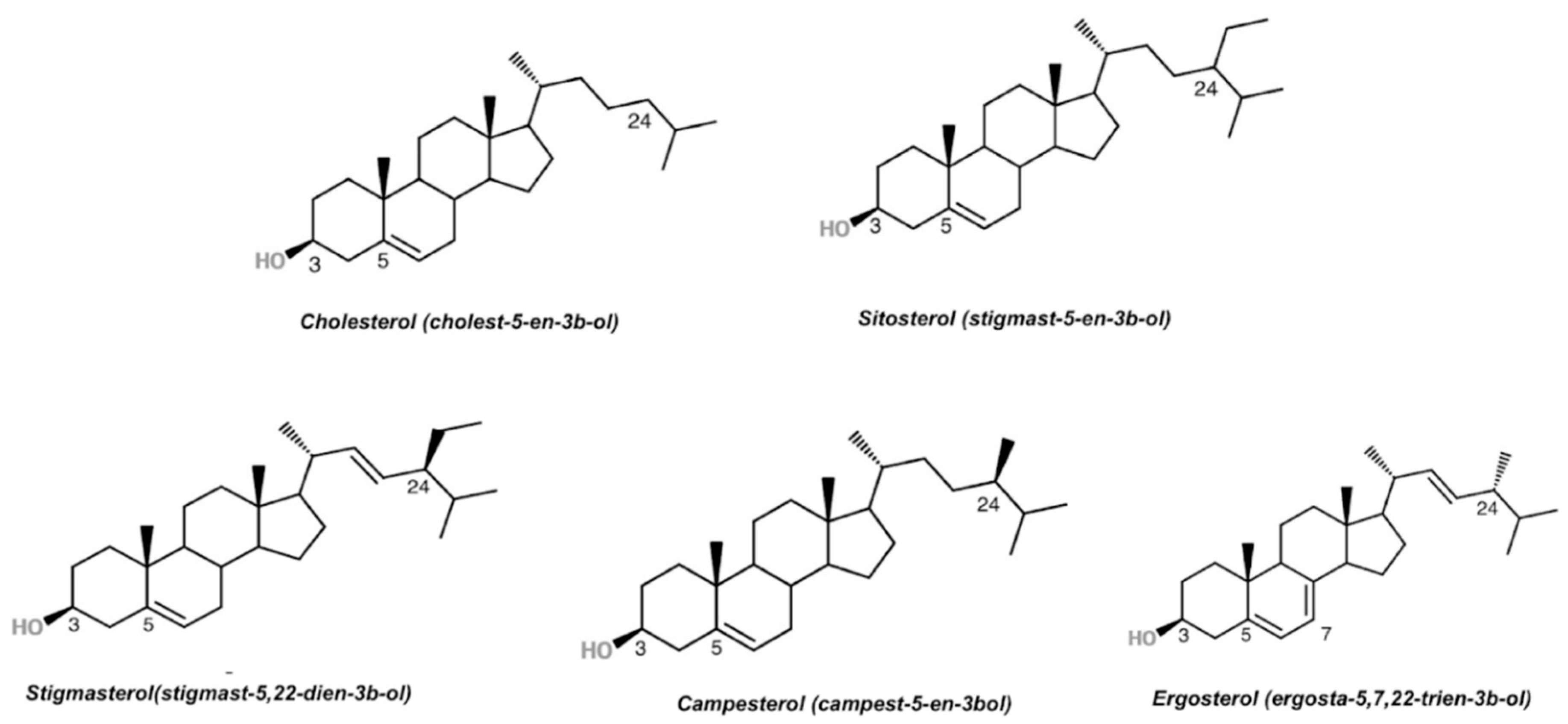
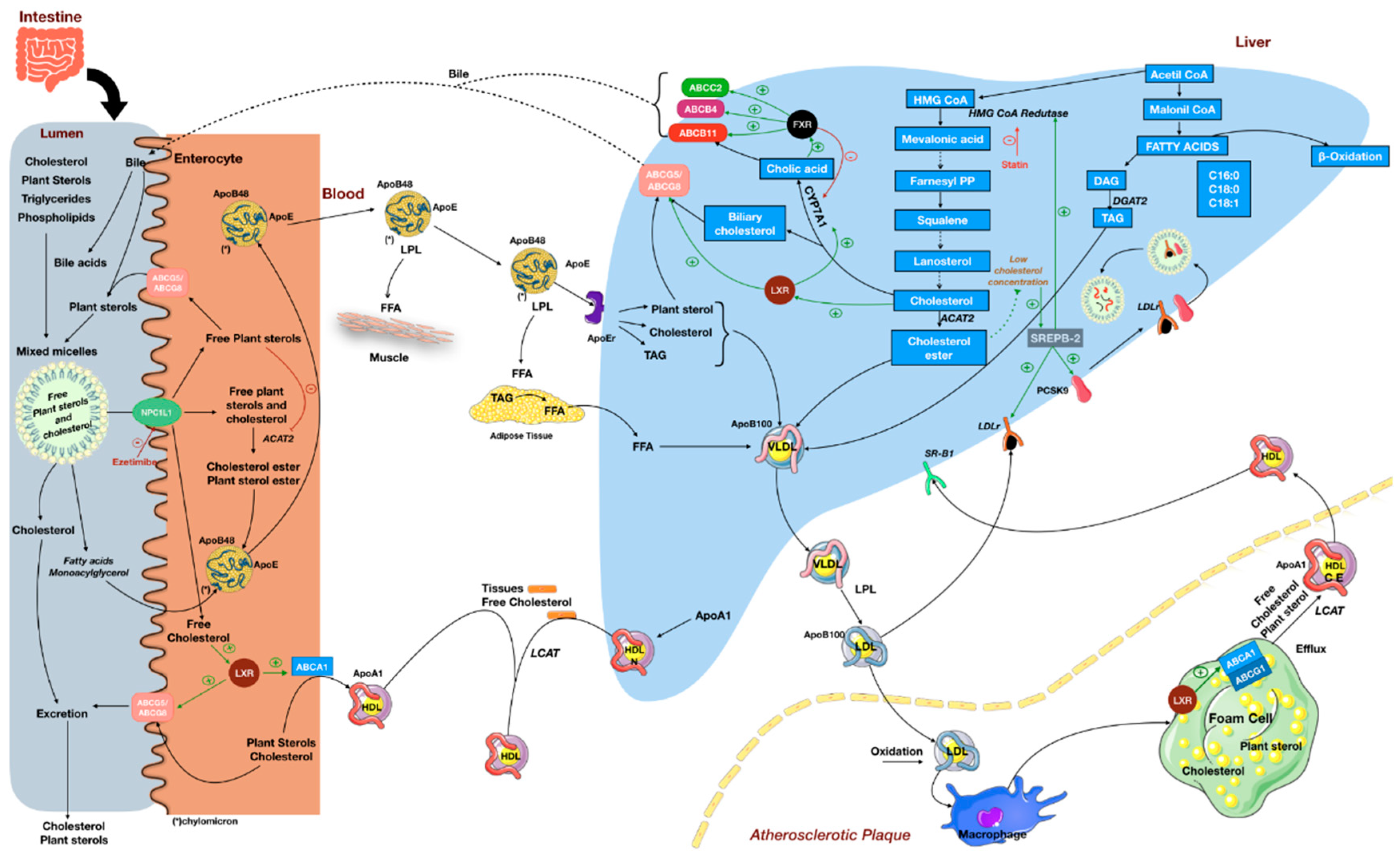
3. Plant Sterols
4. Cholesterol-Lowering Effect of Plant Sterols and Its Implications
5. Evidence from Clinical Trials
6. Plant Sterol Supplementation in Childhood
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette, subfamily A, member 1 |
| Abcb11 | ATP-binding cassette subfamily B member 11 gene |
| Abcc2 | ATP-binding cassette subfamily C member 2 gene |
| ABCG1 | ATP-binding cassette, subfamily G, member 1 |
| ABCG5 | ATP-binding cassette, subfamily G, member 5 |
| ABCG8 | ATP-binding cassette, subfamily G, member 8 |
| ACAT2 | acetyl-CoA acetyltransferase 2 |
| AHA | American Heart Association |
| CHD | coronary heart disease |
| CM | Chylomicron |
| CVD | cardiovascular disease |
| DAG | Diacylglycerol |
| DAMPs | damage-associated molecular patterns |
| DGAT2 | diacylglycerol O-acyltransferase 2 |
| ESC | European Society of Cardiology |
| FDA | Food Drug Administration |
| FFA | free fatty acids |
| FH | familial hypercholesterolemia |
| FXR | farnesoid X receptor |
| HMG CoA-Reductase | 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase |
| HDL | high-density lipoprotein |
| LCAT | lecithin-cholesterol acyltransferase |
| LDL | low-density lipoprotein |
| LDL-C | LDL cholesterol |
| LPL | lipoprotein lipase |
| LXR | liver X receptor |
| MUFA | monounsaturated fatty acids |
| NCD | noncommunicable diseases |
| NCEP | National Cholesterol Education Program |
| NPC1L1 | Niemann–Pick C1 Like 1 |
| PCSK9 | proprotein convertase subtilisin/kexin type 9 |
| PN | parenteral nutrition; PRRs |
| PUFA | polyunsaturated fatty acids |
| SBR1 | scavenger receptor class B member 1 |
| SMCs | smooth muscle cells |
| SO | soybean oil |
| SREBP-2 | sterol regulatory element-binding transcription factor 2 |
| TAG | Triacylglycerol |
| VLDL | very low density lipoprotein |
| WHO | World Health Organization |
References
- Herouvi, D.; Karanasios, E.; Karayianni, C.; Karavanaki, K. Cardiovascular disease in childhood: The role of obesity. Eur. J. Pediatr. 2013, 172, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, K.J.; Maahs, D.M.; Daniels, S.R.; Eckel, R.H. Childhood obesity and cardiovascular disease: Links and prevention strategies. Nat. Rev. Cardiol. 2011, 8, 513–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaikkonen, J.E.; Mikkilä, V.; Magnussen, C.G.; Juonala, M.; Viikari, J.S.A.; Raitakari, O.T. Does childhood nutrition influence adult cardiovascular disease risk?-Insights from the Young Finns Study. Ann. Med. 2013, 45, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, C.G.; Smith, K.J.; Juonala, M. When to prevent cardiovascular disease? As early as possible: Lessons from prospective cohorts beginning in childhood. Curr. Opin. Cardiol. 2013, 28, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Mendis, S.; Nordet, P.; Fernandez-Britto, J.E.; Sternby, N. Atherosclerosis in children and young adults: An overview of the World Health Organization and International Society and Federation of Cardiology study on pathobiological determinants of atherosclerosis in Youth study (1985–1995). Prev. Control 2005, 1, 3–15. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegård, L.; Jessup, W.; Jones, P.J.; Lütjohann, D.; Maerz, W.; Masana, L.; et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef]
- Ference, B.A.; Yoo, W.; Alesh, I.; Mahajan, N.; Mirowska, K.K.; Mewada, A.; Kahn, J.; Afonso, L.; Williams, K.A.; Flack, J.M. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: A Mendelian randomization analysis. J. Am. Coll. Cardiol. 2013, 9, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Everett, B.M. Novel Antiatherosclerotic Therapies. Arterioscler. Thromb. Vasc. Biol. 2019, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals in hypercholesterolaemia: An overview. Br. J. Pharmacol. 2017, 174, 1450–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Jiao, J.; Xu, J.; Zimmermann, D.; Actis-goretta, L.; Guan, L. Effects of plant stanol or sterol- enriched diets on lipid profiles in patients treated with statins: Systematic review and meta- analysis. Sci. Rep. 2016, 6, 31337. [Google Scholar] [CrossRef] [PubMed]
- Patch, C.S.; Tapsell, L.C.; Williams, P.G.; Gordon, M. Plant sterols as dietary adjuvants in the reduction of cardiovascular risk: Theory and evidence. Vasc. Health Risk Manag. 2006, 2, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenfant, C.; Cleeman, J.I.; Ganiats, T.G.; Graham, G.; Kleinman, R.E.; Hixon, P.; Pasternak, R.C. Executive Summary of the Third Report (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar]
- FDA US Food and Drug Administration. Department of Health and Human Services. Food Labeling; Health Claim; Phytosterols and Risk of Coronary Heart Disease. Fed. Regist. 2010, 75. [Google Scholar]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Naghavi, M.; Wang, H.; Lozano, R.; Davis, A.; Liang, X.; Zhou, M.; Vollset, S.E.; Abbasoglu Ozgoren, A.; Abdalla, S.; Abd-Allah, F.; et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Ärnlöv, J.; Afshin, A.; et al. Global, regional, and national age-sex specifc mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [Green Version]
- Andersson, C.; Vasan, R.S. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol. 2018, 15, 230–240. [Google Scholar] [CrossRef]
- WHO About cardiovascular diseases. World Heal. Organ. 2018.
- Karmali, K.N.; Lloyd-Jones, D.M. Adding a life-course perspective to cardiovascular-risk communication. Nat. Rev. Cardiol. 2013, 10, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef]
- Moore, K.J.; Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, A.; Shafiq, N.; Arora, A.; Singh, M.; Kumar, R.; Malhotra, S. Dietary interventions (plant sterols, stanols, omega-3 fatty acids, soy protein and dietary fibers) for familial hypercholesterolaemia. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Sun, Z. Aging, arterial stiffness, and hypertension. Hypertension 2015, 65, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Holzapfel, G.A.A.; Gasser, T.C.C.; Ogden, R.W. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J. Elast. 2000, 61, 1–48. [Google Scholar] [CrossRef]
- Marks, D.; Thorogood, M.; Neil, H.A.W.; Humphries, S.E. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis 2003, 168, 1–14. [Google Scholar] [CrossRef]
- Avis, H.J.; Vissers, M.N.; Stein, E.A.; Wijburg, F.A.; Trip, M.D.; Kastelein, J.J.P.; Hutten, B.A. A systematic review and meta-analysis of statin therapy in children with familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1803–1810. [Google Scholar] [CrossRef] [Green Version]
- Rodenburg, J.; Vissers, M.N.; Wiegman, A.; van Trotsenburg, A.S.P.; van der Graaf, A.; de Groot, E.; Wijburg, F.A.; Kastelein, J.J.P.; Hutten, B.A. Statin Treatment in Children With Familial Hypercholesterolemia: The Younger, the Better. Circulation 2007, 116, 664–668. [Google Scholar] [CrossRef] [Green Version]
- Ostlund, R.E. Phytosterols in human nutrition. Annu. Rev. Nutr. 2002, 22, 533–549. [Google Scholar] [CrossRef]
- Katan, M.B.; Grundy, S.M.; Jones, P.; Law, M.; Miettinen, T.; Paoletti, R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin. proceedings. 2003, 78, 965–978. [Google Scholar] [CrossRef] [Green Version]
- Brufau, G.; Canela, M.A.; Rafecas, M. Phytosterols: Physiologic and metabolic aspects related to cholesterol-lowering properties. Nutr. Res. 2008, 28, 217–225. [Google Scholar] [CrossRef]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018. [Google Scholar] [CrossRef]
- Köhler, J.; Teupser, D.; Elsässer, A.; Weingärtner, O. Plant sterol enriched functional food and atherosclerosis. Br. J. Pharmacol. 2017, 174, 1281–1289. [Google Scholar] [CrossRef]
- Fumeron, F.; Bard, J.M.; Lecerf, J.M. Interindividual variability in the cholesterol-lowering effect of supplementation with plant sterols or stanols. Nutr. Rev. 2017, 75, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Gylling, H.; Simonen, P. Are plant sterols and plant stanols a viable future treatment for dyslipidemia? Expert Rev. Cardiovasc. Ther. 2016, 14, 549–551. [Google Scholar] [CrossRef] [Green Version]
- Wong, A. Chemical and microbiological considerations of phytosterols and their relative efficacies in functional foods for the lowering of serum cholesterol levels in humans: A review. J. Funct. Foods 2014, 6, 60–72. [Google Scholar] [CrossRef]
- Escolà-Gil, J.C.; Quesada, H.; Julve, J.; Martín-Campos, J.M.; Cedó, L.; Blanco-Vaca, F. Sitosterolemia: Diagnosis, Investigation, and Management. Curr. Atheroscler. Rep. 2014, 16, 424. [Google Scholar] [CrossRef]
- Wang, B.; Tontonoz, P. Liver X receptors in lipid signalling and membrane homeostasis. Nat. Rev. Endocrinol. 2018, 14, 452–463. [Google Scholar] [CrossRef]
- Scolaro, B.; Soo Jin Kim, H.; de Castro, I.A. Bioactive compounds as an alternative for drug co-therapy: Overcoming challenges in cardiovascular disease prevention. Crit. Rev. Food Sci. Nutr. 2018, 58, 958–971. [Google Scholar] [CrossRef]
- Massafra, V.; van Mil, S.W.C. Farnesoid X receptor: A “homeostat” for hepatic nutrient metabolism. Biochim. Biophys. Acta - Mol. Basis Dis. 2018, 1864, 45–59. [Google Scholar] [CrossRef]
- Othman, R.A.; Myrie, S.B.; Jones, P.J.H. Non-cholesterol sterols and cholesterol metabolism in sitosterolemia. Atherosclerosis 2013, 231, 291–299. [Google Scholar] [CrossRef]
- Field, F.J.; Born, E.; Mathur, S.N. LXR/RXR ligand activation enhances basolateral efflux of β-sitosterol in CaCo-2 cells. J. Lipid Res. 2004, 45, 905–913. [Google Scholar] [CrossRef] [Green Version]
- Ras, R.T.; Koppenol, W.P.; Garczarek, U.; Otten-Hofman, A.; Fuchs, D.; Wagner, F.; Trautwein, E.A. Increases in plasma plant sterols stabilize within four weeks of plant sterol intake and are independent of cholesterol metabolism. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, I.; Tanabe, Y.; Sugano, M. Effects of sitosterol and sitostanol on micellar solubility of cholesterol. J. Nutr. Sci. Vitaminol. (Tokyo). 1989, 35, 361–369. [Google Scholar] [CrossRef]
- Abumweis, S.S.; Marinangeli, C.P.F.; Frohlich, J.; Jones, P. Implementing Phytosterols into Medical Practice as a Cholesterol-Lowering Strategy: Overview of Efficacy, Effectiveness and Safety. Can. J. Cardiol. 2014, 30, 1225–1232. [Google Scholar] [CrossRef]
- De Smet, E.; Mensink, R.P.; Plat, J. Effects of plant sterols and stanols on intestinal cholesterol metabolism: Suggested mechanisms from past to present. Mol. Nutr. Food Res. 2012, 56, 1058–1072. [Google Scholar] [CrossRef]
- Brufau, G.; Kuipers, F.; Lin, Y.; Trautwein, E.A.; Groen, A.K. A Reappraisal of the mechanism by which plant sterols promote neutral sterol loss in mice. PLoS One 2011, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Plat, J.; Nichols, J.A.; Mensink, R.P. Plant sterols and stanols: Effects on mixed micellar composition and LXR (target gene) activation. J. Lipid Res. 2005, 46, 2468–2476. [Google Scholar] [CrossRef] [Green Version]
- Cedó, L.; Santos, D.; Ludwig, I.A.; Silvennoinen, R.; García-León, A.; Kaipiainen, L.; Carbó, J.M.; Valledor, A.F.; Gylling, H.; Motilva, M.J.; et al. Phytosterol-mediated inhibition of intestinal cholesterol absorption in mice is independent of liver X receptor. Mol. Nutr. Food Res. 2017, 61, 1–11. [Google Scholar] [CrossRef]
- Trautwein, E.A.; Koppenol, W.P.; De Jong, A.; Hiemstra, H.; Vermeer, M.A.; Noakes, M.; Luscombe-marsh, N.D. Plant sterols lower LDL-cholesterol and triglycerides in dyslipidemic individuals with or at risk of developing type 2 diabetes; a randomized, double-blind, placebo-controlled study. Nutr. Diabetes 2018. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.J.H.; Shamloo, M.; Mackay, D.S.; Rideout, T.C.; Myrie, S.B.; Plat, J.; Roullet, J.; Baer, D.J.; Calkins, K.L.; Davis, H.R.; et al. Progress and perspectives in plant sterol and plant stanol research. Nutr. Rev. 2018, 76, 725–746. [Google Scholar] [CrossRef] [Green Version]
- Van Schie, M.C.; Jainandunsing, S.; van Lennep, J.E.R. Monogenetic disorders of the cholesterol metabolism and premature cardiovascular disease. Eur. J. Pharmacol. 2017, 816, 146–153. [Google Scholar] [CrossRef]
- Yoshida, A.; Naito, M.; Miyazaki, K. Japanese Sisters Associated and with Pseudohomozygous Familial Hypercholesterolemia Sitosterolemia. J. Atheroscler. Thromb. 2000, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.B. Recent advances in understanding the STSL locus and ABCG5/ABCG8 biology. Curr. Opin. Lipidol. 2014, 25, 169–175. [Google Scholar] [CrossRef]
- Hansel, B.; Carrié, A.; Brun-Druc, N.; Leclert, G.; Chantepie, S.; Coiffard, A.S.; Kahn, J.F.; Chapman, M.J.; Bruckert, E. Premature atherosclerosis is not systematic in phytosterolemic patients: Severe hypercholesterolemia as a confounding factor in five subjects. Atherosclerosis 2014, 234, 162–168. [Google Scholar] [CrossRef]
- Mymin, D.; Salen, G.; Triggs-Raine, B.; Waggoner, D.J.; Dembinski, T.; Hatch, G.M. The natural history of phytosterolemia: Observations on its homeostasis. Atherosclerosis 2018, 269, 122–128. [Google Scholar] [CrossRef]
- Rideout, T.C.; Harding, S.V.; Mackay, D.; Abumweis, S.S.; Jones, P.J.H. High basal fractional cholesterol synthesis is associated with nonresponse of plasma LDL cholesterol to plant sterol therapy. Am. J. Clin. Nutr. 2010, 92, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Mackay, D.S.; Gebauer, S.K.; Eck, P.K.; Baer, D.J.; Jones, P.J.H. Lathosterol-to-cholesterol ratio in serum predicts cholesterol-lowering response to plant sterol consumption in a dual-center, randomized, single-blind placebo-controlled trial. Am. J. Clin. Nutr. 2015, 101, 432–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scolaro, B.; Nogueira, M.S.; Paiva, A.; Bertolami, A.; Barroso, L.P.; Vaisar, T.; Heffron, S.P.; Fisher, E.A.; Castro, I.A. Statin dose reduction with complementary diet therapy: A pilot study of personalized medicine. Mol. Metab. 2018, 11, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.B. Plant sterols and stanols: Their role in health and disease. J. Clin. Lipidol. 2008, 2, S11–S19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alphonse, P.A.S.; Jones, P.J.H. Revisiting Human Cholesterol Synthesis and Absorption: The Reciprocity Paradigm and its Key Regulators. Lipids 2016, 51, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Alphonse, P.A.S.; Ramprasath, V.; Jones, P.J.H. Effect of dietary cholesterol and plant sterol consumption on plasma lipid responsiveness and cholesterol trafficking in healthy individuals. Br. J. Nutr. 2017, 117, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Demonty, I.; Ras, R.T.; van der Knaap, H.C.; Duchateau, G.S.; Meijer, L.; Zock, P.L.; Geleijnse, J.M.; Trautwein, E.A. Continuous Dose-Response Relationship of the LDL-Cholesterol – Lowering Effect of. J. Nutr. 2009, 139, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Gylling, H.; Halonen, J.; Lindholm, H.; Konttinen, J.; Simonen, P.; Nissinen, M.J.; Savolainen, A.; Talvi, A.; Hallikainen, M. The effects of plant stanol ester consumption on arterial stiffness and endothelial function in adults: A randomised controlled clinical trial. BMC Cardiovasc. Disord. 2013, 13, 50. [Google Scholar] [CrossRef] [Green Version]
- Matvienko, O.A.; Lewis, D.S.; Swanson, M.; Arndt, B.; Rainwater, D.L.; Stewart, J.; Lee Alekel, D. A single daily dose of soybean phytosterols in ground beef decreases serum total cholesterol and LDL cholesterol in young, mildly hypercholesterolemic men. Am. J. Clin. Nutr. 2002, 76, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Mussner, M.J.; Parhofer, K.G.; Von Bergmann, K.; Schwandt, P.; Broedl, U.; Otto, C. Effects of phytosterol ester-enriched margarine on plasma lipoproteins in mild to moderate hypercholesterolemia are related to basal cholesterol and fat intake. Metabolism. 2002, 51, 189–194. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Assmann, G.; Cullen, P.; Erbey, J.; Ramey, D.R.; Kannenberg, F.; Schulte, H. Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: Results of a nested case-control analysis of the Prospective Cardiovascular Münster (PROCAM) study. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 13–21. [Google Scholar] [CrossRef]
- Rajaratnam, R.A.; Gylling, H.; Miettinen, T.A. Independent association of serum squalene and noncholesterol sterols with coronary artery disease in postmenopausal women. J. Am. Coll. Cardiol. 2000, 35, 1185–1191. [Google Scholar] [CrossRef] [Green Version]
- Matthan, N.R.; Pencina, M.; LaRocque, J.M.; Jacques, P.F.; D’Agostino, R.B.; Schaefer, E.J.; Lichtenstein, A.H. Alterations in cholesterol absorption/synthesis markers characterize Framingham Offspring Study participants with CHD. J. Lipid Res. 2009, 50, 1927–1935. [Google Scholar] [CrossRef] [Green Version]
- Glueck, C.J.; Speirs, J.; Tracy, T.; Streicher, P.; Illig, E.; Vandegrift, J. Relationships of serum plant sterols and cholesterol in 595 hypercholesterolemic subjects, and familial aggregation of phytosterols, cholesterol, and premature coronary heart disease in hyperphytosterolemic probands and their first-degree relatives. Metabolism 1991, 40, 842–848. [Google Scholar] [CrossRef]
- Wilund, K.R.; Yu, L.; Xu, F.; Vega, G.L.; Grundy, S.M.; Cohen, J.C.; Hobbs, H.H. No association between plasma levels of plant sterols and atherosclerosis in mice and men. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2326–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinedo, S.; Vissers, M.N.; von Bergmann, K.; Elharchaoui, K.; Lütjohann, D.; Luben, R.; Wareham, N.J.; Kastelein, J.J.P.; Khaw, K.-T.; Boekholdt, S.M. Plasma levels of plant sterols and the risk of coronary artery disease: The prospective EPIC-Norfolk Population Study. J. Lipid Res. 2007, 48, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Bollella, M.C.; Campanaro, L.; Strobino, B.A.; Boccia, L. Plant Stanol Ester and Bran Fiber in Childhood: Effects on Lipids, Stool Weight and Stool Frequency in Preschool Children. J. Am. Coll. Nutr. 1999, 18, 572–581. [Google Scholar] [CrossRef]
- Guardamagna, O.; Abello, F.; Baracco, V.; Federici, G.; Bertucci, P.; Mozzi, A.; Mannucci, L.; Gnasso, A.; Cortese, C. Primary hyperlipidemias in children: Effect of plant sterol supplementation on plasma lipids and markers of cholesterol synthesis and absorption. Acta Diabetol. 2011, 48, 127–133. [Google Scholar] [CrossRef]
- Becker, M.; Staab, D.; Bergmann, K. Von Treatment of severe familial hypercholesterolemia in childhood with sitosterol and sitostanol. Pediatr. Pharmacol. Ther. 1993, 122, 292–296. [Google Scholar]
- Amundsen, A.L.; Ose, L.; Nenseter, M.S.; Ntanios, F.Y. Plant sterol ester-enriched spread lowers plasma total and LDL cholesterol in children with familial hypercholesterolemia. Am J Clin Nutr 2002, 76, 338–344. [Google Scholar] [CrossRef]
- De Jongh, S.; Vissers, M.N.; Rol, P.; Bakker, H.D.; Kastelein, J.J.P.; Stroes, E.S.G. Plant sterols lower LDL cholesterol without improving endothelial function in prepubertal children with familial hypercholesterolaemia. J. Inherit. Metab. Dis. 2003, 26, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Jakulj, L.; Vissers, M.N.; Rodenburg, J.; Wiegman, A.; Trip, M.D.; Kastelein, J.J.P. Plant stanols do not restore endothelial function in pre-pubertal children with familial hypercholesterolemia despite reduction of low-density lipoprotein cholesterol levels. J. Pediatr. 2006, 148, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Ribas, S.A.; Sichieri, R.; Moreira, A.S.B.; Souza, D.O.; Cabral, C.T.F.; Gianinni, D.T.; Cunha, D.B. Phytosterol-enriched milk lowers LDL-cholesterol levels in Brazilian children and adolescents: Double-blind, cross-over trial. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 971–977. [Google Scholar] [CrossRef] [PubMed]
- NHLBI Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics 2011, 128, S213–S256. [CrossRef] [Green Version]
- Tammi, A.; Rönnemaa, T.; Valsta, L.; Seppänen, R.; Rask-Nissilä, L.; Miettinen, T.A.; Gylling, H.; Viikari, J.; Anttolainen, M.; Simell, O. Dietary plant sterols alter the serum plant sterol concentration but not the cholesterol precursor sterol concentrations in young children (the STRIP Study). J. Nutr. 2001, 131, 1942–1945. [Google Scholar] [CrossRef] [Green Version]
- Vuorio, A.F.; Gylling, H.; Turtola, H.; Kontula, K.; Ketonen, P.; Miettinen, T.A. Stanol Ester Margarine Alone and With Simvastatin Lowers Serum Cholesterol in Families With Familial Hypercholesterolemia Caused by the FH-North Karelia Mutation. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Botelho, P.B.; Guimaraes, J.P.; Mariano, K.R.; Afonso, M.d.S.; Koike, M.K.; Lottenberg, A.M.P.; Castro, I.A. Effect of echium oil combined with phytosterols on biomarkers of atherosclerosis in LDLr-knockout mice: Echium oil is a potential alternative to marine oils for use in functional foods. Eur. J. Lipid Sci. Technol. 2015, 117, 1561–1568. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Hamosh, M.; Chowdhry, P.; Scanlon, J.W.; Hamosh, P. Lecithin-cholesterol acyltransferase in newborn infants: Low activity level in preterm infants. J. Pediatr. 1988, 113, 896–898. [Google Scholar] [CrossRef]
- Asayama, K.; Miyao, A.; Kato, K. High-density lipoprotein (HDL), HDL2, and HDL3 cholesterol concentrations determined in serum of newborns, infants, children, adolescents, and adults by use of a micromethod for combined precipitation ultracentrifugation. Clin. Chem. 1990, 36, 129–131. [Google Scholar]
- Savini, S.; Correani, A.; Pupillo, D.; Ascenzo, R.D.; Biagetti, C.; Pompilio, A.; Simonato, M.; Verlato, G.; Esterified, E. Phytosterol Esterification is Markedly Decreased in Preterm Infants Receiving Routine Parenteral Nutrition. Lipids 2016, 51, 1353–1361. [Google Scholar] [CrossRef]
- Hovingh, G.K.; Hutten, B.A.; Holleboom, A.G.; Petersen, W.; Rol, P.; Stalenhoef, A.; Zwinderman, A.H.; De Groot, E.; Kastelein, J.J.P.; Kuivenhoven, J.A. Compromised LCAT function is associated with increased atherosclerosis. Circulation 2005, 112, 879–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forte, T.M.; Subbanagounder, G.; Berliner, J.A.; Blanche, P.J.; Clermont, A.O.; Jia, Z.; Oda, M.N.; Krauss, R.M.; Bielicki, J.K. Altered activities of anti-atherogenic enzymes LCAT, paraoxonase, and platelet-activating factor acetylhydrolase in atherosclerosis-susceptible mice. J. Lipid Res. 2002, 43, 477–485. [Google Scholar] [PubMed]
- Iyer, K.R.; Spitz, L.; Clayton, P. New insight into mechanisms of parenteral nutrition-associated cholestasis: Role of plant sterols. J. Pediatr. Surg. 1998, 33, 1–6. [Google Scholar] [CrossRef]
- Mutanen, A.; Nissinen, M.J.; Lohi, J.; Heikkilä, P.; Gylling, H.; Pakarinen, M.P. Serum plant sterols, cholestanol, and cholesterol precursors associate with histological liver injury in pediatric onset intestinal failure. Am. J. Clin. Nutr. 2014, 100, 1085–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulet, O. Intravenous lipid emulsions in pediatric patients with intestinal failure. 2017, 22, 142–148. Curr. Opin. Organ Transplant. 2017, 22, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Clayton, P.T.; Whitfield, P.; Iyer, K. The role of phytosterols in the pathogenesis of liver complications of pediatric parenteral nutrition. Nutrition 1998, 14, 158–164. [Google Scholar] [CrossRef]
- Carter, B.A.; Taylor, O.A.; Prendergast, D.R.; Zimmerman, T.L.; Furstenberg, R.V.O.N.; Moore, D.D.; Karpen, S.J.; Gastroenterology, P. Stigmasterol, a Soy Lipid-Derived Phytosterol, Is an Antagonist of the Bile Acid Nuclear Receptor FXR. Pediatr. Res. 2007, 62, 301–306. [Google Scholar] [CrossRef] [Green Version]
- El Kasmi, K.C.; Anderson, A.L.; Devereaux, M.W.; Vue, P.M.; Zhang, W.; Setchell, K.D.R.; Karpen, S.J.; Sokol, R.J. Phytosterols Promote Liver Injury and Kupffer Cell Activation in Parenteral Nutrition-Associated Liver Disease. Sci. Transl. Med. 2013, 5, 206ra137. [Google Scholar] [CrossRef] [Green Version]
- Nghiem-Rao, T.H.; Tunc, I.; Mavis, A.M.; Cao, Y.; Polzin, E.M.; Firary, M.F.; Wang, X.; Simpson, P.M.; Patel, S.B. Kinetics of phytosterol metabolism in neonates receiving parenteral nutrition. Pediatr. Res. 2015, 78, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Kurvinen, A.; Nissinen, M.J.; Andersson, S.; Korhonen, P.; Ruuska, T.; Taimisto, M.; Kalliomäki, M.; Lehtonen, L.; Sankilampi, U.; Arikoski, P.; et al. Parenteral Plant Sterols and Intestinal Failure–associated Liver Disease in Neonates. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 803–811. [Google Scholar] [CrossRef]
- Mellies, M.; Glueck, C.J.; Sweeney, C.; Fallat, R.W.; Tsang, R.C.; Ishikawa, T.T. Plasma and dietary phytosterols in children. Pediatrics 1976, 57, 60–67. [Google Scholar]
- Salen, G.; Ahrens, E.H.; Grundy, S.M. Metabolism of β-sitosterol in man. J. Clin. Invest. 1970, 49, 952–967. [Google Scholar] [CrossRef] [PubMed]
- NCEP National Cholesterol Education Program (NCEP): Highlights of the Report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics 1992, 89, 495–501.
- Wong, W.W.; Hachey, D.L.; Insull, W.; Opekun, A.R.; Klein, P.D. Effect of dietary cholesterol on cholesterol synthesis in breast-fed and formula-fed infants. J. Lipid Res. 1993, 34, 1403–1411. [Google Scholar] [PubMed]
- Mellies, M.J.; Ishikawa, T.T.; Glueck, C.J.; Bove, K.; Morrison, J. Phytosterols in aortic tissue in adults and infants. J Lab Clin Med 1976, 88, 914–921. [Google Scholar] [PubMed]
- Plat, J.; Baumgartner, S.; Vreugdenhil, A.C.E.; Konings, M.C.J.M.; Calkins, K.L.; Mensink, R.P. Modifying Serum Plant Sterol Concentrations: Effects on Markers for Whole Body Cholesterol Metabolism in Children Receiving Parenteral Nutrition and Intravenous Lipids. Nutrients 2019, 11, 120. [Google Scholar] [CrossRef] [Green Version]
- Babawale, E.A.; Jones, P.J.H.; Mercer, K.E.; Lin, H.; Yeruva, L.; Yoseph, F.B.; Rutherfurd, S.M. Modulating Sterol Concentrations in Infant Formula Influences Cholesterol Absorption and Synthesis in the Neonatal Piglet. Nutrients 2018, 10, 1848. [Google Scholar] [CrossRef] [Green Version]
- Gylling, H.; Korhonen, M.; Mutanen, A.; Nissinen, M.J.; Pakarinen, M. Serum non-cholesterol sterols and cholesterol metabolism in childhood and adolescence. Atherosclerosis 2018, 278, 91–96. [Google Scholar] [CrossRef] [Green Version]
| Reference | Study Type | Objectives | Main Results |
|---|---|---|---|
| Demonty et al. (2009) [66] | Meta-analysis of randomized controlled trials in adults treated with plant sterols without a co-intervention. Consumption of plant sterol-enriched foods or supplements could not be isolated | Establish a continuous dose–response relationship that would allow predicting the LDL-C-lowering efficacy of different plant sterol doses. | The dose–response equation predicts an LDL-C-lowering effect of 9% for the recommended 2 g/day dose of plant sterols. The continuous dose–response relationship for the LDL-C-lowering effect and plant sterol intake achieved a plateau when it came to approximately 3 g/day. |
| Gylling et al. (2013) [67] | Randomized, controlled, double-blind, parallel trial including 92 asymptomatic subjects (35 men and 57 women, mean age of 50.8 ± 1.0). The subjects consumed 3 g of plant stanols daily through rapeseed oil-based enriched spread for 6 months. | Evaluate the effects of plant stanol esters on arterial stiffness and endothelial function in adults without lipid medication. | LDL-C decrease of 10% and reduction of arterial stiffness in small arteries and marker of subclinical atherosclerosis (cardio-ankle vascular index—CAVI) |
| Ras et al. (2014) [12] | Meta-analysis of randomized controlled studies in adults. In total, 124 human studies with a total of 201 study arms were included. Plant sterols and stanols were administered in 129 and 59 study arms, respectively; in the remaining 13 study arms, a mix of plant sterols and stanols was administered. | To investigate the combined and isolated effects of plant sterols and stanols by evaluating different dose ranges. | The average phytosterol (comprising plant sterols and plant stanols) dose 2.1–3.3 g/day were found to gradually reduce LDL-C concentrations by 6%–12%. |
| Matvienko et al. (2002) [68] | Triple-blind, 34 male college students with elevated total plasma cholesterol (TC), LDL-C, and TC:HDL-C. Randomized: control (ground beef alone) or treatment (ground beef with 2.7 g of plant sterols) group. | Test the hypothesis that a single daily dose of soybean plant sterols added to ground beef would lower TC and LDL-C concentrations in mildly hypercholesterolemic young men. | TC, LDL-C, and TC:HDL-C were reduced from baseline by 9.3%, 14.6%, and 9.1%, respectively. |
| Assmann et al. (2006) [71] | Case–control study using stored samples from male participants in the Prospective Cardiovascular Münster (PROCAM) | Evaluate if modest sitosterol elevations observed in the general population is associated with the occurrence of coronary events. | Among men with an absolute coronary risk ≥20% in 10 years, high sitosterol concentrations were associated with an additional 3-fold increase in the incidence of coronary events; a similar, significant relationship was observed between a high sitosterol/cholesterol ratio and coronary risk |
| Mussner et al. (2002) [69] | Randomized, double-blind, placebo-controlled, cross-over study including 63 healthy subjects (38 women, 25 men, mean age of 42 years old, LDL-C of 130 mg/dL) | Comparison of effects from the intake of a plant sterol-enriched margarine and a control margarine. | Plant sterol ester-enriched margarine significantly changed TC, LDL-C HDL-C, apolipoprotein B, and the LDL-C/HDL-C ratio compared to the control margarine |
| Wilund et al. (2004) [75] | Human subjects from the Dallas Heart Study, 2542 subjects aged 30 to 67 years, were included. Wild-type hypercholesterolemic female mice were also studied. | Determine whether elevated plasma levels of plant sterols were associated with coronary atherosclerosis humans and mice. | Plasma levels of cholesterol, but not of plant sterols, were significantly higher in subjects with coronary atherosclerosis. |
| Pinedo et al. (2007) [76] | Case–control study among participants of the EPIC-Norfolk Study. Only individuals who did not report a history of heart attack or stroke at the baseline clinic visit were considered. | Evaluate the relationship between plant sterol levels and coronary artery disease risk | Higher levels of plant sterols are unlikely to confer increased risk of coronary artery disease in healthy adults. |
| Williams et al. (1999) [77] | Open cross-over randomized study lasting 13 weeks; eligible children started either with the diet phase A (plant stanol ester) or B (wheat bran fiber). The first diet phase lasted 4 weeks, and then they went under a two-week wash-out followed by a cross-over to the other diet for 4 weeks. | Evaluate the effects of plant stanol ester in healthy two- to five-year-old preschool children. | Reductions in TC and in LDL-C by 12.4% and 15.5%, respectively, from baseline were observed. There were no significant changes in HDL-C or triglyceride levels. |
| Guardamagna et al. (2011) [78] | Interventional study using plant sterol-enriched yoghurt for 12 weeks in 32 children with heterozygous familial hypercholesterolemia (FH), 13 children with familial combined hyperlipidemia (FCH), and 13 children with undefined hypercholesterolemia (UH). | To access the efficacy, tolerability, and safety of plant sterol supplementation in children with primary hyperlipidemia. | LDL-C was significantly reduced in the three groups of different forms of primary hyperlipidemia (10.7%, 14.2%, and 16.0% in FH, FCH, and UH, respectively). High tolerability to the diet was observed. |
| Becker et al. (1993) [79] | Interventional study in 9 children with severe familial hypercholesterolemia. Firstly, there was a 3-month strict diet, followed by the intake sitosterol pastilles (2 g three times a day) for 3 months, and then a 7-month course of sitostanol (0.5 g three times a day). | Set the efficacy difference between sitostanol, a nonabsorbable plant sterol, and sitosterol to reduce serum levels of lipids in children with severe familial hypercholesterolemia. | Sitostanol was significantly more effective in reducing elevated levels of LDL-C than sitosterol (32%). |
| Amundsen et al. (2002) [80] | Randomized, double-blind crossover study with 38 children (aged 7–12 years) with familial hypercholesterolemia (FH) consuming plant sterol ester enriched spread or a control spread. | Access the effects of plant sterol ester enriched spread intake on serum lipids in children with FH. | Compared to the control group, a consumption of 1.6 g of plant sterol esters promoted a 10.2% reduction in LDL-C concentrations. |
| de Jongh et al. (2003) [81] | Double-blind crossover trial using plant sterol enriched spreads and a placebo spread. Forty-one children (aged 5–12 years) with familial hypercholesterolemia (FH) were included in this study. | Evaluate the effect of plant sterols on cholesterol levels and vascular function in prepubertal children with FH. | Compared to the placebo group, the intake of 2.3 g plant sterols per day decreased 11% of TC and 14% of LDL-C. |
| Jakulj et al. (2006) [82] | Double-blind crossover trial testing low-fat yogurt enriched with plant stanols and low-fat placebo yogurt for 4 weeks. The study enrolled 42 prepubertal children with familial hypercholesterolemia (FH). | Evaluate the effects of plant stanols on lipids and endothelial function in prepubertal children with FH. | The group that consumed plant stanols showed a reduction of 9.2% in LDL-C levels without changes in endothelial function. |
| Ribas et al. (2017) [83] | Randomized, double-blind, cross-over trial using phytosterol-enriched milk and skim milk. Twenty-eight dyslipidemic children (aged 6-9 years) were included in this study. | Investigate the effects of daily consumption of a phytosterol-enriched milk on the lipid profiles of children with dyslipidemia. | The concentrations of TC and LDL-C were significantly reduced in the phytosterol-enriched milk group as compared to the skim milk group, with reductions of 5.9% and 10.2%, respectively. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scolaro, B.; Andrade, L.F.S.d.; Castro, I.A. Cardiovascular Disease Prevention: The Earlier the Better? A Review of Plant Sterol Metabolism and Implications of Childhood Supplementation. Int. J. Mol. Sci. 2020, 21, 128. https://doi.org/10.3390/ijms21010128
Scolaro B, Andrade LFSd, Castro IA. Cardiovascular Disease Prevention: The Earlier the Better? A Review of Plant Sterol Metabolism and Implications of Childhood Supplementation. International Journal of Molecular Sciences. 2020; 21(1):128. https://doi.org/10.3390/ijms21010128
Chicago/Turabian StyleScolaro, Bianca, Leticia F.S. de Andrade, and Inar A. Castro. 2020. "Cardiovascular Disease Prevention: The Earlier the Better? A Review of Plant Sterol Metabolism and Implications of Childhood Supplementation" International Journal of Molecular Sciences 21, no. 1: 128. https://doi.org/10.3390/ijms21010128




