Relationship of Circulating Irisin with Body Composition, Physical Activity, and Cardiovascular and Metabolic Disorders in the Pediatric Population
Abstract
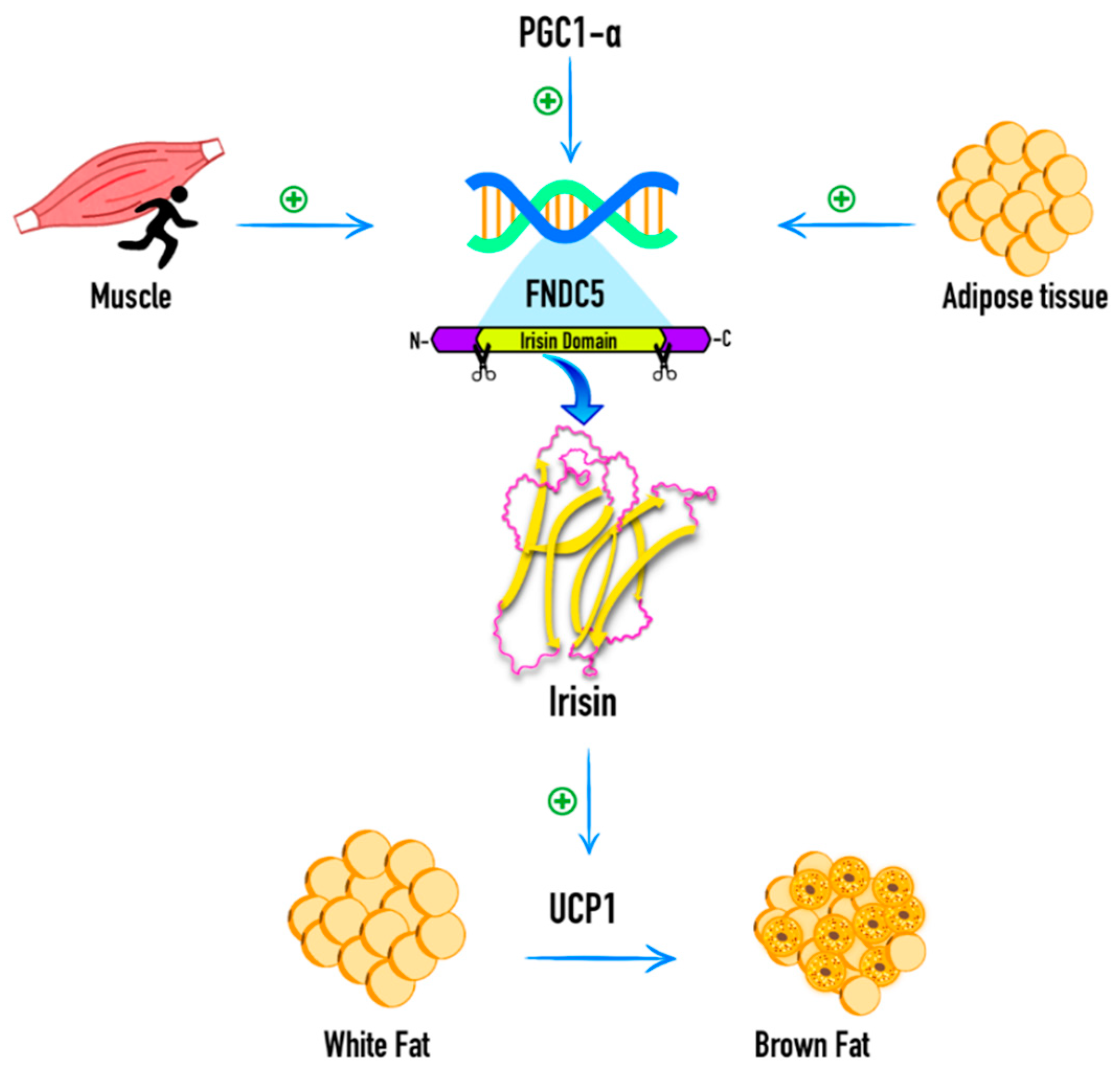
1. Introduction
2. Association of Circulating Irisin with Body Mass Index and Body Composition
2.1. Body Mass Index And Anthropometric Parameters
2.2. Muscle Mass and Fat Free Mass
2.3. Fat Mass
2.4. Bone Mineral Density
3. Association of Circulating Irisin with Physical Activity, Exercise Training and Dietetic Interventions
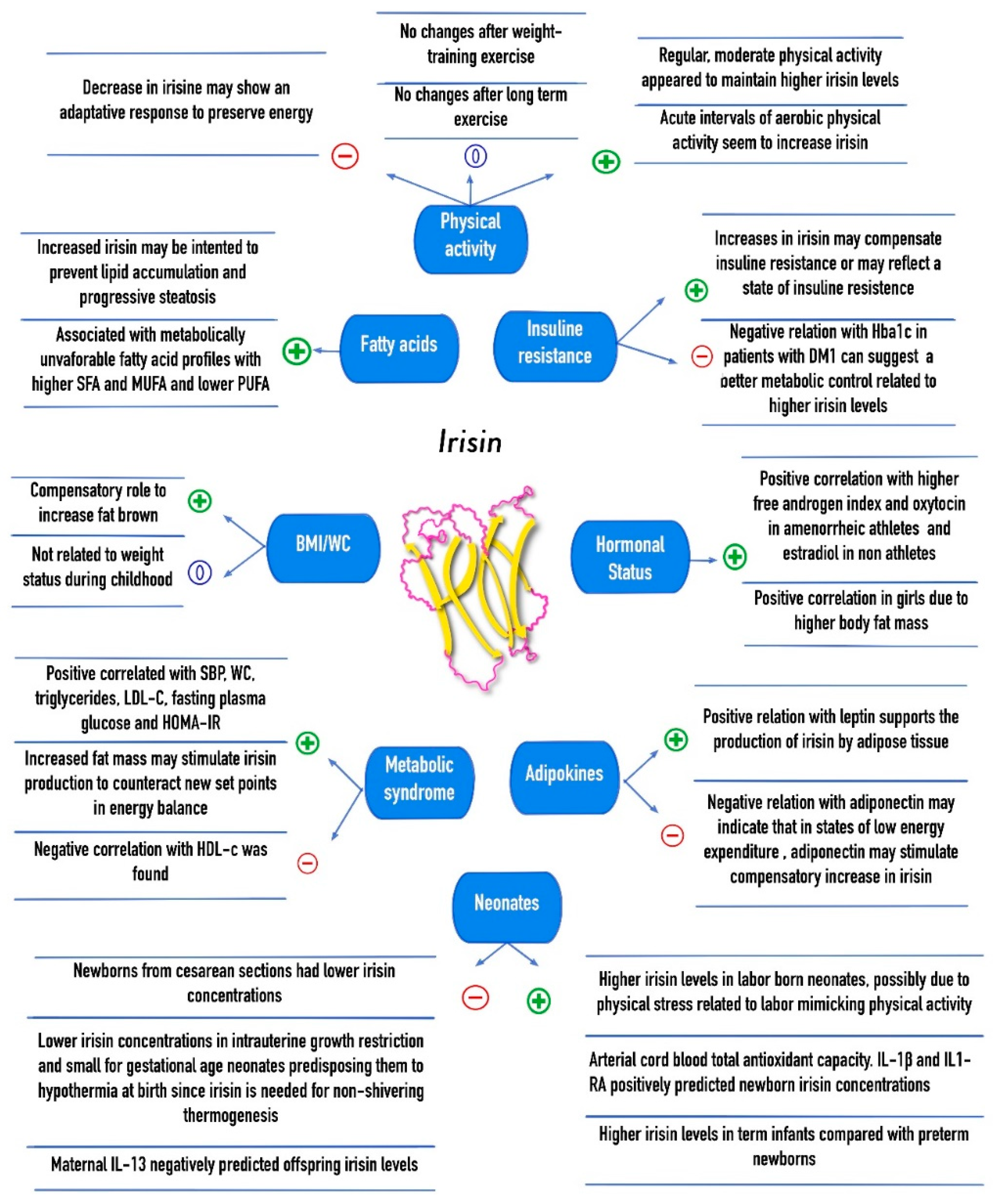
4. Association of Circulating Irisin with Cardiovascular and Metabolic Alterations
4.1. Insulin Resistance and Glucose Regulation
4.2. Cardiovascular Risk Factors and the Metabolic Syndrome
4.3. Adipocytokines
4.4. Fatty Acids Composition
4.5. Energy Intake and Expenditure
5. Association of Circulating Irisin with Gender, Puberty, and Hormonal Status
6. Association of Circulating Irisin with Mother–Offspring Relationship and Gestational Age in Neonates
7. Association of Circulating Irisin and Other Diseases
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| UCP-1 | uncoupling protein 1 |
| FNDC5 | fibronectin type III domain-containing protein 5 |
| VAT | visceral adipose tissue |
| SAT | subcutaneous adipose tissue |
| MS | metabolic syndrome |
| BMI | body mass index |
| WC | waist circumference |
| LDL-c | lipoprotein-c |
| FFM | fat free mass |
| AAs | amenorrheic athletes |
| EAs | eumenorrheic athletes |
| BMD | bone mineral density |
| NAs | non-athletes |
| T1DM | type 1 diabetes mellitus |
| SBP | systolic blood pressure |
| DBP | diastolic blood pressure |
| SGA | small for gestational age |
| AGA | appropriate for gestational age |
| LGA | large for gestational age |
| IUGR | intrauterine growth restriction |
| rhGH | recombinant human growth hormone |
References
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Belen Crujeiras, A.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernandez-Real, J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-Montemayor, L.; Silva-Platas, C.; Torres-Quintanilla, A.; Rodríguez-López, C.; Ruiz-Esparza, G.U.; Reyes-Mendoza, E.; Garcia-Rivas, G. Association of Irisin Plasma Levels with Anthropometric Parameters in Children with Underweight, Normal Weight, Overweight, and Obesity. Biomed. Res. Int. 2017, 2017, 2628968. [Google Scholar] [CrossRef] [PubMed]
- Çatlı, G.; Küme, T.; Tuhan, H.; Anık, A.; Çalan, Ö.; Böber, E.; Abacı, A. Relation of serum irisin level with metabolic and antropometric parameters in obese children. J. Diabetes Complicat. 2016, 30, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Palacios-González, B.; Vadillo-Ortega, F.; Polo-Oteyza, E.; Sánchez, T.; Ancira-Moreno, M.; Romero-Hidalgo, S.; Meráz, N.; Antuna-Puente, B. Irisin levels before and after physical activity among school-age children with different BMI: A direct relation with leptin. Obesity 2015, 23, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Binay, Ç.; Paketçi, C.; Güzel, S.; Samancı, N. Serum Irisin and Oxytocin Levels as Predictors of Metabolic Parameters in Obese Children. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.B.; Kim, H.J.; Kang, J.H.; Park, S.I.; Park, K.H.; Lee, H.J. Association of circulating irisin levels with metabolic and metabolite profiles of Korean adolescents. Metabolism 2017, 73, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.S.; Kang, M.J.; Yang, S.; Hwang, I.T. Irisin is a biomarker for metabolic syndrome in prepubertal children. Endocr. J. 2018, 65, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; Elfers, C.; Lass, N.; Roth, C.L. Irisin and its relation to insulin resistance and puberty in obese children: A longitudinal analysis. J. Clin. Endocrinol. Metab. 2015, 100, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Löffler, D.; Müller, U.; Scheuermann, K.; Friebe, D.; Gesing, J.; Bielitz, J.; Erbs, S.; Landgraf, K.; Wagner, I.V.; Kiess, W.; et al. Serum irisin levels are regulated by acute strenuous exercise. J. Clin. Endocrinol. Metab. 2015, 100, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Singhal, V.; Lawson, E.A.; Ackerman, K.E.; Fazeli, P.K.; Clarke, H.; Lee, H.; Eddy, K.; Marengi, D.A.; Derrico, N.P.; Bouxsein, M.L.; et al. Irisin levels are lower in young amenorrheic athletes compared with eumenorrheic athletes and non-athletes and are associated with bone density and strength estimates. PLoS ONE 2014, 9, e100218. [Google Scholar] [CrossRef] [PubMed]
- Soininen, S.; Sidoroff, V.; Lindi, V.; Mahonen, A.; Kröger, L.; Kröger, H.; Jääskeläinen, J.; Atalay, M.; Laaksonen, D.E.; Laitinen, T.; et al. Body fat mass, lean body mass and associated biomarkers as determinants of bone mineral density in children 6–8 years of age—The Physical Activity and Nutrition in Children (PANIC) study. Bone 2018, 108, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Brunetti, G.; Sanesi, L.; Colaianni, G.; Celi, M.; Piacente, L.; D’Amato, G.; Schipani, E.; Colucci, S.; Grano, M. High irisin levels are associated with better glycemic control and bone health in children with Type 1 diabetes. Diabetes Res. Clin. Pract. 2018, 141, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Triantafyllou, G.A.; Fernandez-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; Crujeiras, A.B.; Amil, M.; Aguera, Z.; Jimenez-Murcia, S.; Banos, R.; Botella, C.; de la Torre, R.; Estivill, X.; Fagundo, A.B.; et al. Association of irisin with fat mass, resting energy expenditure, and daily activity in conditions of extreme body mass index. Int. J. Endocrinol. 2014, 2014, 857270. [Google Scholar] [CrossRef] [PubMed]
- Crujeiras, A.B.; Zulet, M.A.; Lopez-Legarrea, P.; de la Iglesia, R.; Pardo, M.; Carreira, M.C.; Martínez, J.A.; Casanueva, F.F. Association between circulating irisin levels and the promotion of insulin resistance during the weight maintenance period after a dietary weight-lowering program in obese patients. Metabolism 2014, 63, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Hofmann, T.; Goebel-Stengel, M.; Elbelt, U.; Kobelt, P.; Klapp, B.F. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity-correlation with body mass index. Peptides 2013, 39, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Scudiero, O.; Ludovica Monaco, M.; Polito, R.; Schettino, P.; Grandone, A.; Perrone, L.; Miraglia Del Giudice, E.; Daniele, A. Adiponectin profile and Irisin expression in Italian obese children: Association with insulin-resistance. Cytokine 2017, 94, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Gkiomisi, A.; Bisbinas, I.; Katsarou, A.; Filippaios, A.; Mantzoros, C.S. Circulating irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos. Int. 2014, 25, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, H.J.; Guo, W.C.; Yang, J. Low serum concentrations of Irisin are associated with increased risk of hip fracture in Chinese older women. Jt. Bone Spine 2018, 85, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Strollo, R.; Maddaloni, E.; Tuccinardi, D.; D’Onofrio, L.; Briganti, S.I.; Defeudis, G.; De Pascalis, M.; Lazzaro, M.C.; Colleluori, G.; et al. Irisin is associated with osteoporotic fractures independently of bone mineral density, body composition or daily physical activity. Clin. Endocrinol. 2015, 82, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Brychta, R.J.; Collins, M.T.; Linderman, J.; Smith, S.; Herscovitch, P.; Millo, C.; Chen, K.Y.; Celi, F.S. Cold-activated brown adipose tissue is an independent predictor of higher bone mineral density in women. Osteoporos. Int. 2013, 24, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Ponrartana, S.; Aggabao, P.C.; Hu, H.H.; Aldrovandi, G.M.; Wren, T.A.; Gilsanz, V. Brown adipose tissue and its relationship to bone structure in pediatric patients. J. Clin. Endocrinol. Metab. 2012, 97, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Blizzard LeBlanc, D.R.; Rioux, B.V.; Pelech, C.; Moffatt, T.L.; Kimber, D.E.; Duhamel, T.A.; Dolinsky, V.W.; McGavock, J.M.; Senechal, M. Exercise-induced irisin release as a determinant of the metabolic response to exercise training in obese youth: The EXIT trial. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Bluher, S.; Panagiotou, G.; Petroff, D.; Markert, J.; Wagner, A.; Klemm, T.; Filippaios, A.; Keller, A.; Mantzoros, C.S. Effects of a 1-year exercise and lifestyle intervention on irisin, adipokines, and inflammatory markers in obese children. Obesity 2014, 22, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Alkharfy, K.M.; Rahman, S.; Amer, O.E.; Vinodson, B.; Sabico, S.; Piya, M.K.; Harte, A.L.; McTernan, P.G.; Alokail, M.S.; et al. Irisin as a predictor of glucose metabolism in children: Sexually dimorphic effects. Eur. J. Clin. Investig. 2014, 44, 119–124. [Google Scholar] [CrossRef] [PubMed]
- De Meneck, F.; Victorino de Souza, L.; Oliveira, V.; do Franco, M.C. High irisin levels in overweight/obese children and its positive correlation with metabolic profile, blood pressure, and endothelial progenitor cells. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Viitasalo, A.; Atalay, M.; Pihlajamaki, J.; Jaaskelainen, J.; Korkmaz, A.; Kaminska, D.; Lindi, V.; Lakka, T.A. The 148 M allele of the PNPLA3 is associated with plasma irisin levels in a population sample of Caucasian children: The PANIC Study. Metabolism 2015, 64, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Viitasalo, A.; Agren, J.; Venalainen, T.; Pihlajamaki, J.; Jaaskelainen, J.; Korkmaz, A.; Atalay, M.; Lakka, T.A. Association of plasma fatty acid composition with plasma irisin levels in normal weight and overweight/obese children. Pediatr. Obes. 2016, 11, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Steffen, L.M.; Vessby, B.; Jacobs, D.R., Jr.; Steinberger, J.; Moran, A.; Hong, C.P.; Sinaiko, A.R. Serum phospholipid and cholesteryl ester fatty acids and estimated desaturase activities are related to overweight and cardiovascular risk factors in adolescents. Int. J. Obes. 2008, 32, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.A.; Ackerman, K.E.; Slattery, M.; Marengi, D.A.; Clarke, H.; Misra, M. Oxytocin secretion is related to measures of energy homeostasis in young amenorrheic athletes. J. Clin. Endocrinol. Metab. 2014, 99, E881–E885. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Yousef, M.; Vinodson, B.; Amer, O.E.; Alnaami, A.M.; Sabico, S.; Tripathi, G.; et al. Maternal inheritance of circulating irisin in humans. Clin. Sci. 2014, 126, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Trejo, M.; Garcia-Rivas, G.; Torres-Quintanilla, A.; Laresgoiti-Servitje, E. Relationship between Irisin Concentration and Serum Cytokines in Mother and Newborn. PLoS ONE 2016, 11, e0165229. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Polyzos, S.A.; Newton, D.A.; Wagner, C.L.; Hollis, B.W.; Ouweland, J.V.D.; Dursun, E.; Gezen-Ak, D.; Kotsa, K.; Annweiler, C.; et al. Adiponectin and vitamin D-binding protein are independently associated at birth in both mothers and neonates. Endocrine 2018, 59, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Joung, K.E.; Park, K.H.; Filippaios, A.; Dincer, F.; Christou, H.; Mantzoros, C.S. Cord blood irisin levels are positively correlated with birth weight in newborn infants. Metabolism 2015, 64, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Keles, E.; Turan, F.F. Evaluation of cord blood irisin levels in term newborns with small gestational age and appropriate gestational age. SpringerPlus 2016, 5, 1757. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, T.; Zlamal, F.; Tomandl, J.; Hodicka, Z.; Gulati, S.; Bienertova-Vasku, J. Irisin Maternal Plasma and Cord Blood Levels in Mothers with Spontaneous Preterm and Term Delivery. Dis. Mark. 2018, 2018, 7628957. [Google Scholar] [CrossRef] [PubMed]
- Baka, S.; Malamitsi-Puchner, A.; Boutsikou, T.; Boutsikou, M.; Marmarinos, A.; Hassiakos, D.; Gourgiotis, D.; Briana, D.D. Cord blood irisin at the extremes of fetal growth. Metabolism 2015, 64, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Rigano, S.; Ferrazzi, E.; Beaty, B.L.; Battaglia, F.C.; Galan, H.L. Differences in fat and lean mass proportions in normal and growth-restricted fetuses. Am. J. Obstet. Gynecol. 2004, 191, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Van de Lagemaat, M.; Rotteveel, J.; Lafeber, H.N.; van Weissenbruch, M.M. Lean mass and fat mass accretion between term age and 6 months post-term in growth-restricted preterm infants. Eur. J. Clin. Nutr. 2014, 68, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Verkauskiene, R.; Beltrand, J.; Claris, O.; Chevenne, D.; Deghmoun, S.; Dorgeret, S.; Alison, M.; Gaucherand, P.; Sibony, O.; Levy-Marchal, C. Impact of fetal growth restriction on body composition and hormonal status at birth in infants of small and appropriate weight for gestational age. Eur. J. Endocrinol. 2007, 157, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Ornoy, A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod. Toxicol. 2011, 32, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Bakal, U.; Aydin, S.; Sarac, M.; Kuloglu, T.; Kalayci, M.; Artas, G.; Yardim, M.; Kazez, A. Serum, Saliva, and Urine Irisin with and Without Acute Appendicitis and Abdominal Pain. Biochem. Insights 2016, 9, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Elhady, M.; Youness, E.R.; Gafar, H.S.; Abdel Aziz, A.; Mostafa, R.S.I. Circulating irisin and chemerin levels as predictors of seizure control in children with idiopathic epilepsy. Neurol. Sci. 2018, 39, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.T.; Wang, H.; Zhang, S.; Du, J.; Zhuang, S.; Zhao, T.C. Irisin Ameliorates Hypoxia/Reoxygenation-Induced Injury through Modulation of Histone Deacetylase 4. PLoS ONE 2016, 11, e0166182. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Pochec, E.; Zarawski, M. Anti-Inflammatory Properties of Irisin, Mediator of Physical Activity, Are Connected with TLR4/MyD88 Signaling Pathway Activation. Int. J. Mol. Sci. 2017, 18, 701. [Google Scholar] [CrossRef] [PubMed]
- Wikiera, B.; Zawadzka, K.; Laczmanski, L.; Sloka, N.; Bolanowski, M.; Basiak, A.; Noczynska, A.; Daroszewski, J. Growth Hormone Treatment Increases Plasma Irisin Concentration in Patients with Turner Syndrome. Horm. Metab. Res. 2017, 49, 122–128. [Google Scholar] [CrossRef] [PubMed]
| Author | Sample | BMI%/BMI Z-Score | Body Composition Measurement | FM | MM%/FFM | BFP/BFM | WC/WHR | Others |
|---|---|---|---|---|---|---|---|---|
| Elizondo-Montemayor 2017 [4] | n = 40 (20 boys) Mexico 6–12 y-o. UW (n = 5), NW (n = 5), OW (n = 5), OB (n = 5). | + | Fat mass = [(weight-kg) × (body fat%)]/100 Body muscle= (height-cm) [0.264 + (0.0029 × MUAMA-cm2)] FFM = (weight-kg − (weight-kg × body fat%)) PBF:Bioelectric impedance analysis (TANITA TBF 300) | 0 | MM (−) | 0 | WC (+) | N/A |
| Catli 2016 [5] | n = 66 Turkey 8-15 y-o. OB (n = 20 (20 boys)), NW (n = 30 (16 boys)). | 0 | Bioelectric impedance analysis (TanitaBC-41) | 0 | N/A | + | WC (0) | SBP (0), DBP (0) |
| Palacios-González 2015 [6] | n = 85 (40 boys) —Mexico 8–10 y-o. NW (n = 25), OW (n = 23), OB (n = 37). | + | N/A | N/A | N/A | N/A | N/A | N/A |
| Binay 2017 [7] | n = 120 Turkey 10–18 y-o. OB (n = 90), NW (n = 30). | + | Bioelectrical impedance analysis (BC-418MA Tanita Segmental Body Composition Analyzer) | + | 0 | + | WHR (+) | SBP (+) |
| Jang 2017 [8] | n = 618 (316 boys) Korea 12–15 y-o. NW (n = 370), OB (n = 248). | + | Bioelectrical impedance analysis (BC-418; Tanita) | + | + | + | WC (+) | N/A |
| Shim 2018 [9] | n = 96 (56 boys) Korea 6–10 y-o. NW (n = 54), OW (n = 16), OB (n = 26). | - | N/A | N/A | N/A | N/A | WC (-) | SBP (+), DBP (+) |
| Reinehr 2015 [10] | n = 60 Germany 10–15 y-o NW [n = 20 (10 boys)], OB (n = 40 (20 boys)). | 0 | N/A | N/A | N/A | N/A | N/A | DBP (+) |
| Löffler 2015 [11] | n = 105 (46 boys) Germany NW (n = 20), OW/OB (n = 64) 8–21 y-o and OB (n = 58 (23 boys)) 7–17 y-o. | BMI (+) | Bioimpedance analyses (Nutriguard-MS) Fat free mass and body cell mass (NutriPlus Software) | 0 | FFM (+) adults FFM (−) children | 0 | WHR (+) | SBP (0) DBP (0) |
| Singhal 2014 [12] | n = 85 women (81 Caucasian and Asian, 11 mixed- race, 5 Black) 14–21 y-o. AA (n = 38), EA (n = 24), NA (n = 23). | N/A | Dual energy x-ray absorptiometry (DXA) | 0 | (+) in all athletes (+) in AA (0) in EA | N/A | N/A | Spine BMD Z-score (+) Whole body BMD Z-score (+) Total vBMD (+) Trabecular vBMD (+) |
| Soininen 2018 [13] | n = 472 (245 boys) Finland 6–8 y-o. | N/A | MM, FM, PBF, BMD = Lunar Prodigy Advance DXA device | N/A | N/A | N/A | N/A | BMD (+) all children, not with boys and girls separately |
| Faienza 2018 [14] | n = 127 Italy 6–16 y-o. DM1 [n = 96 (41 boys)] 8–16 y-o, CO (n = 35 (21 boys)) 6–12 y-o. | (+) in patients with SCII | N/A | N/A | N/A | N/A | N/A | (+) with BTT-Z score, PTH, osteocalcin (−) with serum calcium, 25(OH) vitamin D, DKK-1, and sclerostin |
| Author | Sample | Intervention | Correlation | Results | |
|---|---|---|---|---|---|
| Jang 2017 [8] | n = 618 (316 boys) Korea 12–15 y-o. NW (n = 370), OB (n = 248) | No Intervention | + | In NW girls | |
| + | In NW active | ||||
| Elizondo-Montemayor 2017 [4] | n = 40 (20 boys) Mexico 6–12 y-o. UW (n = 5), NW (n = 5), OW (n = 5), OB (n = 5). | Reported aerobic exercise (days per week and hours per day) | − | With days per week | |
| - | With hours per day | ||||
| Löffler 2015 [11] | n = 29 (11 boys) Germany 8–21 y-o. OB (n = 10 (2 boys)). | 15-min maximum cycle ergometer | + | In all subjects | |
| n = 58 OB (23 boys) Germany 7–17 y-o. | 4–6 weeks of nutritional and aerobic training | +/− | In boys | ||
| n = 88 Germany 11–12 y-o. CO [n = 29 (12 boys)], IN (n = 34 (20 boys)), CS (n = 25 (16 boys)) | Intervention group increased one unit of sports activities for 3 years | 0 | No difference among groups | ||
| Blizzard LeBlanc 2017 [26] | n = 11 OB (6 boys) Canada 15–16 y-o. | Acute bouts of exercise: | Aerobic: 45 min at 60% HRR | + | In all subjects |
| Resistance: 45 min at 60–65% 1RM 12–15 reps | 0 | In all subjects | |||
| Palacios-González 2015 [6] | n = 85 (40 boys) Mexico 8–10 y-o. NW (n = 25), OW (n = 23), OB (n = 37). | 5-min warm-up and 25-min aerobic activity at 75% HRMax, 8 months, 5 days/week | − | In all subjects | |
| Blüher 2014 [27] | n = 65 Germany OW/OB (35 boys) 7–18 y-o. | 39 session over 1 year of 150 min/week of combined endurance and resistance exercise plus diet counseling | + | In all subjects | |
| Reinehr 2015 [10] | n = 60 Germany 10-15 y-o NW (n = 20 (10 boys)), OB (n = 40 (20 boys)). | Exercise sessions once per week plus nutrition education for 4–6 weeks | 0 | In OB children who lost weight | |
| + | In OB children who did not lose weight | ||||
| Singhal 2014 [12] | n = 85 women (81 Caucasian and Asian, 11 mixed-race, 5 Black) 14–21 y-o. AA (n = 38), EA (n = 24), NA (n = 23). | No intervention | − | In AA | |
| 0 | In EA and NA | ||||
| Author | Sample | Insulin | HOMA | Glucose | TG | HDL-c | LDL-c | TC | MS | Leptin | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Löffler 2015 [11] | n = 105 (46 boys) Germany NW (n = 20), OW/OB (n = 64) 8–21 y-o and OB (n = 58 (23 boys)) 7–17 y-o. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | N/A | N/A | N/A |
| Al-Daghri 2014 [28] | n = 133 (76 boys) Saudi Arabia 9–15 years y-o. OB (n = 30). | 0 | 0 | − | 0 | 0 | 0 | 0 | N/A | 0 | (+) with ANG II |
| Reinehr 2015 [10] | n = 60 Germany 10–15 y-o NW (n = 20 (10 boys)), OB (n = 40 (20 boys)) | + | + | 0 | + | − | + | N/A | N/A | N/A | (+) with 2-h OGTT and DBP |
| Binay 2017 [7] | n = 120 Turkey 10–18 y-o NW (n =30), OB (n = 90). | + | + | + | N/A | N/A | N/A | N/A | N/A | N/A | (+) with SBP in OB |
| Catli 2016 [5] | n = 66 Turkey 8–15 y-o. OB (n = 20 (20 boys)), NW (n = 30 (16 boys)). | + | + | 0 | 0 | − | 0 | 0 | N/A | 0 | N/A |
| Nigro 2017 [20] | n = 27 (19 boys) OB Italy 4–13 y-o, NW (n = 13 (4 boys)). | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (−) with adiponectin |
| Blüher 2014 [27] | n = 65 OB (35 boys) 7–18 y-o. | 0 | 0 | 0 | N/A | N/A | N/A | N/A | N/A | N/A | No relation with adiponectin, leptin, or resistin |
| De Meneck 2018 [29] | n = 87 (47 boys) Brazi l6–12 y-o. NW (n = 63), OW/OB (n = 24) | + | + | + | + | − | N/A | 0 | N/A | N/A | (+) with SBP and DBP in the entire cohort, (+) with EPCs |
| Viitasalo 2015 [30] | n = 444 (247 boys) Finland 6–9 y-o. NW (n = 388), OW/OB (n = 55). | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (+) with unfavorable fatty acid profile |
| Shim 2018 [9] | n = 96 (56 boys) Korea 6 to 10 y-o. NW (n = 54), OW (n = 16), OB (n = 26). | N/A | N/A | − | (−) in OW/OB | 0 | 0 | 0 | − | N/A | (+) with SBP and DBP in OB/OW |
| Palacios-González 2015 [6] | n = 85 (40 boys) Mexico 8–10 y-o. NW (n = 25), OW (n = 23), OB (n = 37). | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | + | N/A |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elizondo-Montemayor, L.; Mendoza-Lara, G.; Gutierrez-DelBosque, G.; Peschard-Franco, M.; Nieblas, B.; Garcia-Rivas, G. Relationship of Circulating Irisin with Body Composition, Physical Activity, and Cardiovascular and Metabolic Disorders in the Pediatric Population. Int. J. Mol. Sci. 2018, 19, 3727. https://doi.org/10.3390/ijms19123727
Elizondo-Montemayor L, Mendoza-Lara G, Gutierrez-DelBosque G, Peschard-Franco M, Nieblas B, Garcia-Rivas G. Relationship of Circulating Irisin with Body Composition, Physical Activity, and Cardiovascular and Metabolic Disorders in the Pediatric Population. International Journal of Molecular Sciences. 2018; 19(12):3727. https://doi.org/10.3390/ijms19123727
Chicago/Turabian StyleElizondo-Montemayor, Leticia, Gerardo Mendoza-Lara, Gustavo Gutierrez-DelBosque, Mariana Peschard-Franco, Bianca Nieblas, and Gerardo Garcia-Rivas. 2018. "Relationship of Circulating Irisin with Body Composition, Physical Activity, and Cardiovascular and Metabolic Disorders in the Pediatric Population" International Journal of Molecular Sciences 19, no. 12: 3727. https://doi.org/10.3390/ijms19123727
APA StyleElizondo-Montemayor, L., Mendoza-Lara, G., Gutierrez-DelBosque, G., Peschard-Franco, M., Nieblas, B., & Garcia-Rivas, G. (2018). Relationship of Circulating Irisin with Body Composition, Physical Activity, and Cardiovascular and Metabolic Disorders in the Pediatric Population. International Journal of Molecular Sciences, 19(12), 3727. https://doi.org/10.3390/ijms19123727





