Abstract
Unhealthy diet and physical inactivity—major risk factors for the main non-communicable diseases—can be addressed by mobile health applications. Using an evidence-based systematic review design, we analysed studies on mobile applications to foster physical activity to determine whether they met the objective of increasing adults’ physical activity. A bibliographic search was conducted in October 2020 using PubMed, Cochrane Library Plus, Biomed Central, Psychology Database, and SpringerLink, retrieving 191 articles. After titles and abstracts were reviewed, 149 articles were excluded, leaving 42 articles for a full-text review, of which 14 met the inclusion criteria. Despite differences in study duration, design, and variables, 13 of the 14 studies reported that applications were effective in increasing physical activity and healthy habits as dietary behaviour. However, further longer-term studies with larger samples are needed to confirm the effectiveness of mobile health applications in increasing physical activity.
1. Introduction
The main risk factors for the most important non-communicable diseases (cardiovascular conditions, type 2 diabetes, and certain types of cancer) are unhealthy eating habits and physical inactivity, which both greatly contribute to the global burden of morbidity, mortality, and disability [1]. Estimates for Europe indicate that more than a third of adults are not sufficiently active. The WHO European Region study entitled “Integrating Diet, Physical Activity and Weight Management Services into Primary Care” [2] confirmed the high European incidence of non-communicable diseases, accounting for 77% of the disease burden and nearly 86% of premature deaths. In 2016 the global prevalence of insufficient physical activity was 27.5%, and in high-income countries was more than double (36.8%) that of low-income countries (16.2%). Furthermore, in high-income countries, the incidence of insufficient physical activity has increased over time (31.6% in 2001) [3]. For the WHO European Region, two recommendations to tackle the epidemic of non-communicable diseases include a wide range of actions aimed at reducing risk factors, with primary care signalled to play “a fundamental role in the provision of services to promote healthy diets, involve people in the practice of physical activity, and help patients in weight management.”
The WHO defines physical activity as “any bodily movement produced by skeletal muscles that requires energy expenditure”, indicating that it is “a fundamental means of improving people’s physical and mental health” [1]. This further indicates that physical activity should not be confused with exercise, as the latter is “a subcategory of planned, structured, and repetitive physical activity”, whereas “physical activity includes exercise as well as other activities which involve bodily movement and are done as part of playing, working, active transportation, house chores, and recreational activities.” Participation in “150 min of moderate-intensity aerobic physical activity throughout the week or do at least 75 min of vigorous intensity aerobic physical activity throughout the week or an equivalent combination of moderate- and vigorous-intensity activity is estimated to reduce the risk of ischemic heart disease by approximately 30%, the risk of diabetes by 27% and the risk of breast and colon cancer by between 21 and 25%”. Mental health also benefits from physical activity, as stress, anxiety, and depression are reduced, and the effects of senile dementia or Alzheimer disease can be delayed [4].
A 2014 study entitled “Nutrition: The Impact of Smartphone Apps on the Nutrition Industry” [5] estimated that the global smartphone market would grow in 2017 to 3.45 billion users, and predicted an upsurge in applications (apps) specialized in nutrition, health, and fitness. A 2019 Spanish report on mobile use in Spain and worldwide indicates that 31.3 million people in Spain are smartphone users and estimates that 67% of all internet connections in the world are made from a smartphone. According to the same report, in 2018, 194 billion apps were downloaded worldwide (9% more than the previous year), 21.9% of smartphone users possessed a smartwatch, and lifestyle applications were the seventh most downloaded apps for iOS, Apple Inc., Cupertino, CA, USA [6].
Mobile apps can be effective in improving health outcomes and may come to be considered a means to developing cost-effective and scalable interventions. However, for interventions to effectively increase physical activity they need to motivate people to undertake behavioural change, offer realistic goals that can be combined with primary care guidance, and provide regular feedback on activity levels [7].
The use of mobile apps aimed at promoting physical activity linked to a healthy diet could further health education and help reduce non-communicable disease rates or, at the very least, enable monitoring and control of these diseases. Numerous reviews have already been carried out to determine the efficacy of health apps for weight reduction [8,9]. However, more interventions are necessary as well as subsequent reviews of apps that, either specifically or within a larger study, monitor physical activity to determine whether this increases/is maintained over time in response to appropriate motivation, given the risk that physical activity levels may taper off once an intervention ends [10,11].
The objective of this study, therefore, was to analyse the effectiveness of interventions based on mobile apps aimed at increasing physical activity.
2. Materials and Methods
We performed a systematic review of randomized clinical trials (RCTs) evaluating app efficacy in increasing physical activity. Electronic databases (PubMed, Cochrane Library Plus, Biomed Central, Psychology Database, and SpringerLink) were searched for articles published up to 23 October 2020. The following keywords and MeSH terms were used for the search: physical activity, physical fitness, cell phone, mobile app, sport, exercise, and smartphone. Boolean operators (AND, OR) were used to expand, exclude, or join keywords in the search. The search only considered RCTs conducted in adult humans whose results reflected changes in physical activity. The review is not restricted to comparing no app use groups and app use groups. The initial search, limited to publications in English and Spanish, was expanded by using the snowball technique to identify relevant articles in the references of retrieved articles [12]. The AMSTAR [13] and PRISMA [14] checklists were used to ensure the quality of the review. The risk of bias was assessed using Consolidated Standards of Reporting Trials (CONSORT) checklist [15] and disagreement regarding bias and the interpretation of results was resolved by consensus discussions.
Study Selection
A total of 191 studies were identified, whose titles and abstracts were reviewed against the inclusion and exclusion criteria (Table 1). After the review of titles and abstracts, 149 articles were excluded, 42 underwent a more detailed review consisting of reading the full text, and 14 articles were considered to meet the inclusion criteria. The main reasons for excluding articles were that the studies were not RCTs, only described study design, had reference populations that were non-adult, were missing data on physical activity variations, referred to improvements to apps, and were exclusively based on a website or SMS delivery (Figure 1). Studies in which participants might have some difficulty performing physical activity were not excluded if the objective of the study was aimed at improving it. To ensure the maximum reliability of the information collected from each article, the analysis was performed by two independent researchers [16]

Table 1.
Inclusion and exclusion criteria.
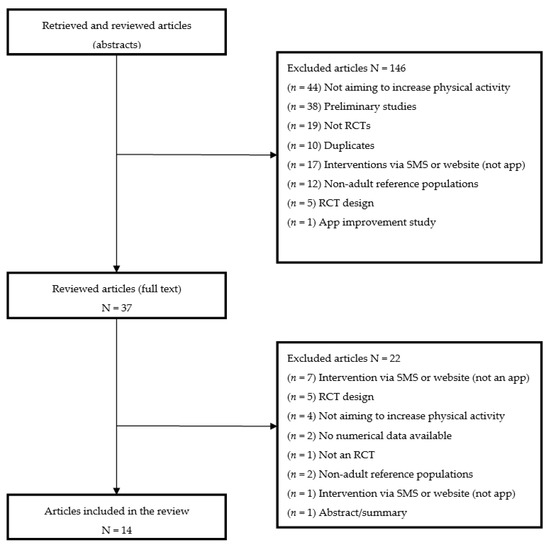
Figure 1.
Search results and selection process.
We used a standardized form for extraction of the following data: study details, population and sample details, objectives, study type, study duration, intervention methodology, measures used, and results summary. Four authors reviewed the 14 articles independently and discrepancies were resolved by discussion with the lead author as necessary. All data were analysed both qualitatively and quantitatively.
3. Results
3.1. Population and Sample
The 14 selected studies (see Table 2 for details) were carried out in the USA (six studies [17,18,19,20,21,22]), Australia (two studies [23,24]), UK (one study [25]), Sweden (one study [26]), Israel (one study [27]), Pakistan (one study [28]), Denmark (one study [29]), and Belgium and Israel simultaneously (one study [30]). Study participant numbers ranged from 40 to 301 and nine of the 14 studies had sample sizes less than 100. Moreover, ten studies included participants of both sexes, although in seven of them women outnumbered men, three studies exclusively included men [23,24,25], and one study exclusively included women [27]. One study each was aimed at people with technical experience interested in healthy living [23], and at people without technical experience [19]. Participants were aged 18 to 69 years. Mean age reported was between 20.63 and 66 years. The body mass index (BMI, in kg/m2) of the individuals varied considerably (from 25.5 to 34.6), three studies did not report BMI [18,25,30], and two studies reported the percentage of overweight and obese participants by group [21,24]. Finally, only two studies provided information on abdominal circumference in women and men [17,27].

Table 2.
Details of the 14 reviewed articles.
In the recruitment of participants, frequently taken into account was the level of physical activity (little, moderate or intense) or the need for no additional conditions limiting exercise capacity or non-participation in any other programme (e.g., for weight loss). No studies recruited athletes or physically very active persons, while two studies specifically recruited people with low levels of physical activity [19,20]. Patients with previously diagnosed pathologies were exclusively recruited in seven studies: obesity [17,22], type 2 diabetes [29], colon cancer [21], heart conditions [20], myocardial infarction [26], and Parkinson disease [30].
3.2. Interventions
In six of the studies [17,22,23,24,27,28], the goal of increased physical activity was part of a programme to change habits or lifestyle that also covered eating habits and weight loss. In the remaining eight studies, the main objective was to increase physical activity, although other secondary objectives may have been included, such as assessing quality of life, anxiety levels, or intervention adherence.
Intervention duration was six months or longer in five studies [17,21,22,24,26], two of which included follow-up [21,22], while duration was three months or less in the remaining nine studies.
All the studies required a mobile phone to be able to use the app. In some studies, participants were specifically required to have a smartphone or internet access [17,20,24,26,27,28,29], while a monitoring device was provided in other studies [18,19,21,22,23,25,30].
Of the 14 studies, nine were based on automatic recording of physical activity data (via pedometers and accelerometers), while the remaining five studies required users to manually enter physical activity data in the app [17,18,24,26,27].
Of the 14 studies, six included an intervention via app combined with SMS delivery, either as reminders or as reinforcement [17,18,20,26,27,28], while social interaction was included in the 8 remaining studies [19,21,22,23,24,25,29,30]. With the exception of studies 18 and 24 that included economic incentives linked to the increase in PA, the rest of the studies were based on socio-cognitive or behavioral theories for changing habits, including some kind of feedback (either individual or collective) as a motivational component [17,19,20,23,24,25,30], and offered educational information (face-to-face or through app educational modules) [17,21,23,24,26,27].
3.3. Measurements and Results
All 14 studies measured variation in physical activity from the beginning to the end of the intervention (depending on their duration, some studies included intermediate measures). Physical activity was measured in hours or minutes per day or week, number of sessions, and/or number of daily steps. Data was obtained automatically via an accelerometer or pedometer in 10 studies, or was self-recorded or obtained from the Global Physical Activity Questionnaire (GPAQ) or International Physical Activity Questionnaire (IPAQ) in the remaining four studies. Sedentary lifestyle details were explicitly recorded in one study [19], while perceived barriers were considered in three studies [17,25,29].
Other measures considered were weight loss [17,22,26], quality of diet and dietary habits [23,24,27], BMI [17], wellbeing [23], lifestyle changes and mental health (distress, anxiety, depression, etc.) [21], and health literacy [24]. Frequency of app use, app usability, and participant satisfaction were other measures common to several studies [18,20,23,24,27,29].
In the studies aimed at patients with specific pathologies, variables typical of the condition of interest were also measured. For instance, in their obesity study, Allen et al. [17] measured abdominal circumference. In their study focused on myocardial infarction, Johnson et al. [26] measured adherence to ticagrelor as the primary outcome, and also changes in cardiovascular risk factors, quality of life, and patient satisfaction with the intervention. In relation to Parkinson disease, Ginis et al. [30] focused on gait speed (not only comfortable speed, but also double-task speed), secondary gait, freezing-of-gait severity, balance, and Parkinsonian-related and other cognitive evaluations.
3.4. Analysis
Despite differences in duration, design, and variables, 13 of the 14 included studies reported an increase in physical activity. The only exception was the pilot study by Allen et al. [17], whose results were not statistically significant, probably due to the small sample size and less than robust retention strategies. Although the increases in physical activity were moderate, the studies that included other variables also highlighted improvements in diet [23,24], weight loss [27,28], or adherence to treatment [26]. However, in some cases it was reported that increased physical activity [21] or weight loss [22] were not maintained after the intervention ended at nine or 18 months, respectively. Naimark et al. [27] also demonstrated the positive impact of an app, although they indicated that long-term studies would be necessary to reach more definitive conclusions.
Some characteristics and components of the apps associated with effectiveness in increasing PA seems to be related with setting goals or receiving feedback, rewards, or educational information led to improved motivation and results [18,19,24,25,26,27]. Other findings were that receiving motivational messages was perceived as a positive factor [18], apps adapted to people with little experience of technology were well received [18], people with less technical knowledge had more problems adhering to technology-based interventions [30], and men were possibly less likely to respond to reminders than women [29].
Regarding acceptance and satisfaction with the interventions, study participants valued very positively personal or group sessions and the use of mobile apps. Users who did not use an app considered that a data recording device would have enhanced their motivation [17]. Although the participants stated that they remained motivated and eager to continue with the intervention [20,29,30], or intended to continue using the app [27], in several studies (especially the longer ones) a decrease was observed in the use of the apps over time [18,21,22,24,28].
4. Discussion
Our review of different studies of mobile apps used to promote physical activity suggests that such interventions are well accepted and can facilitate success in achieving moderate or intense physical activity levels. Although the analysed studies are generally of short duration and the increases in physical activity are modest, the accessibility and flexibility offered by the applications could be useful to increase the number of people who adhere to the recommended levels of PA and thus reduce the risk of non-communicable diseases such as coronary heart disease, obesity or type 2 diabetes mellitus [31]. Although the increase in physical activity seems to be greater in those applications aimed only at increasing PA, it is important to consider that the studies that also include other healthy behaviors have positive results as well [23,24,25,26,27,28]. Therefore, it could be interesting to work in conjunction to promote healthy lifestyles and thus have a positive impact on reducing costs for the health system.
Around a quarter of the participants in the study by Allen et al. [17] considered the use of a smartphone app the most useful aspect of the programme, while all the participants considered that a tracking device would enhance their motivation to increase physical activity levels. Likewise, several studies reported that users were interested in continuing with the programme and using the app, and would recommend it to friends [27,29,30]. This would suggest that health apps may not only help users maintain a healthy lifestyle but that their use could be transmitted at the social level.
Regarding the association between greater use and goal achievement, Naimark et al. [27] showed that heavier device users were more oriented towards a healthy lifestyle and tended to obtain better results (measured as the number of steps). Adherence to app-based treatment is an important issue in greater frequency of use [32], while it has been suggested than an automatic step-counting system could improve the acceptance of strategies to increase physical activity [17]. Message delivery [20] and individual and social feedback [18] were reported to be key factors in the success of some interventions.
Our review has some limitations that need to be taken into account in future studies. The number of participants in the different studies, as well as study duration, would suggest a need to carry out longer-term studies with larger samples to determine if interventions would continue to retain user interest and whether they would be sustained over the long-term [17,19,27,30]. Given that several studies link sedentary lifestyles with mortality, King et al. [19], for instance, strongly recommended expanding RCTs.
Another limitation was that only some of the included studies included post-intervention follow-up [21,22,30].
Regarding sampling, a possible limitation to interpreting results is that groups need to be balanced in number and sex [27]. The study by Valentiner et al. [29] suggests that men may show less involvement than women in responding to messages. Other studies confirm the greater predisposition of women to actively participate in programmes compared to men [33,34]. King et al. [19] suggested, in relation to behavioural interventions like that of their study, that subgroup analyses were necessary to identify tailored interventions for particular individuals and circumstances, as this would “improve the personalization of e-health interventions and optimize success”. Harries et al. [25], for instance, whose study recruited only men, considered that women’s clothing might mean they would not always carry their smartphone with them. This would suggest that, in designing effective intervention programmes, it is essential to understand psychological determinants underlying self-management of a change in habits [27].
For interventions involving automatic data monitoring devices, the choice of accelerometer or pedometer is important in terms of ensuring reliable data collection, as initial testing is often necessary to calibrate the sensitivity of devices [10]. According to King et al. [19], the fact of needing to have the device constantly to hand could represent a limitation, with the same authors suggesting that the challenge is to develop monitoring devices capable of accurately capturing not only physical activity, but also sedentary and sleep habits 24 h a day. Continuous monitoring that provides immediate feedback is a motivator for change, as is manual recording (once it becomes a habit) and dynamic information on progress, as such motivations improve the efficiency of a programme [25,35].
The ethical issues regarding the collection of personal data need to be taken into account. Beyond the need to respect data protection legislation, the user must also consent to provide data. In the case of the Spanish population, very concerned regarding the presence and use of their data online, 47% admit that they did not fully review permissions for installed apps, and 21% do not do so for newly installed apps [6].
Martin et al. [20] considers that not having a professional to motivate participants through personal interviews is a possible limitation, despite the fact that their study obtained good results without such a professional. Spring et al. [22], however, demonstrated that a coach interacting with the user during the programme improved effectiveness for the intervention group compared to the control group.
5. Conclusions
People are increasingly using smartphones routinely in more areas of their personal and professional lives. In the health field, apps are becoming an increasingly used means not only to speed up medical consultations, but also to promote education in healthy lifestyles, proper diet, and physical activity.
Mobile apps aimed at increasing physical activity can be effective in helping people acquire healthy habits. Our review of 14 articles, despite differences in study duration, design and variables, and despite the single exception of the pilot study by Allen et al. [17], would broadly suggest a clear trend – that the use of apps increases physical activity. Many of those studies also suggest that users would be willing to continue using the apps and would even recommend them, thus expanding the scope of health interventions.
However, the success and effectiveness of health interventions and apps relies on their adaptation to the target, attractiveness, ease of use, and adherence. Personalized interventions and apps are key to achieving success with manageable goals adapted to individuals, their circumstances, and their progress.
Because motivation is essential to making sustainable changes in behaviours and lifestyles that persist beyond the end of an intervention, attention must be paid to the success factors as highlighted by many of the studies that analysed support and reminders, health education, and feedback on goals and results. Social interactions, whether with other people using the app or with a health professional, also seem to play a key role in increasing the effectiveness and sustainability of interventions. Interpersonal contact would therefore seem to be an important issue to take into account when developing mobile health apps.
A crucial aspect of developing and implementing mobile apps is that they must comply with ethical and health criteria and with data protection legislation, and that they must obtain the user’s consent to the use of their data. Likewise, users would need to thoroughly understand the app to ensure that it is used efficiently and safely.
Further longer-term studies with larger samples are needed to confirm the effectiveness of apps in increasing and maintaining physical activity levels over the long run. Going beyond usability and retention analyses, research is also needed concerning ethical and privacy aspects with a view to conducting exhaustive analyses of interventions aimed at enhancing the efficacy and safety of the growing number of mobile health apps.
Author Contributions
Conception and design, A.A.-M. and F.S.-R.; data acquisition, L.P.-C., J.J.P.-R., L.E. and A.A.-M.; analysis and interpretation of findings, L.P.-C., C.L.-R., A.B.-F. and A.A.-M.; drafting the work and revising it critically for important intellectual content, L.P.-C., C.L.-R., J.J.P.-R., L.E., A.B.-F., A.A.-M., F.S.-R. and F.X.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Fact Sheets. Physical Activity. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 4 December 2019).
- World Health Organization (Regional Office for Europe). Integrating Diet, Physical Activity and Weight Management Services into Primary Care. 2016. Available online: https://www.euro.who.int/__data/assets/pdf_file/0016/324304/Integrating-diet-physical-activity-weight-management-services-primary-care.pdf (accessed on 31 August 2020).
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob. Health 2018. [Google Scholar] [CrossRef]
- World Health Organization. Obesity. Data and Statistics. 2018. Available online: http://www.euro.who.int/en/health-topics/disease-prevention/physical-activity/data-and-statistics (accessed on 4 December 2019).
- Research2Guidance. mNutrition. The Impact of Smartphone Apps on the Nutrition Industry. 2015. Available online: https://research2guidance.com/wp-content/uploads/2015/08/mNutrition-2014-Preview.pdf (accessed on 4 December 2019).
- Ditrendia (Digital Marketing Trends). Informe Mobile en España y en el Mundo 2019. Available online: https://mktefa.ditrendia.es/hubfs/Ditrendia-Informe%20Mobile%202019.pdf?utm_campaign=Informe%20Mobile%202019&utm_medium=email&_hsmi=77268109&_hsenc=p2ANqtz-8vyoNQYMsV6iNVrECCOxqAvVOVBKZl_t2nIyAL1D1zWimz3OB3pK5bggQ-jwFWEv9Jt_kwsAc-_7MbgXy2jY4Wb3Mxgw&utm_content=77268109&utm_source=hs_automation (accessed on 3 July 2020).
- Gill, D.P.; Blunt, W.; Bartol, C.; Pulford, R.W.; De Cruz, A.; Simmavong, P.K.; Gavarkovs, A.; Newhouse, I.; Pearson, E.; Ostenfeldt, B.; et al. HealtheStepsTM Study Protocol: A pragmatic randomized controlled trial promoting active living and healthy lifestyles in at-risk Canadian adults delivered in primary care and community-based clinics. BMC Public Health 2017. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mateo, G.; Granado-Font, E.; Ferré-Grau, C.; Montaña-Carreras, X. Mobile Phone Apps to Promote Weight Loss and Increase Physical Activity: A Systematic Review and Meta-Analysis. J. Med. Internet Res. 2015, 17, e253. Available online:https://www.jmir.org/2015/11/e253 (accessed on 25 October 2020). [CrossRef] [PubMed]
- Puigdomènech, E.; Saigí, F.; Zamora, A.; Moharra, M.; Paluzié, G.; Balfegó, M.; Cuatrecasas, G.; Garcia-Lorda, P.; Carrion, C. Assessment of the Efficacy, Safety, and Effectiveness of Weight Control and Obesity Management Mobile Health Interventions: Systematic Review. JMIR Mhealth Uhealth 2019, 7, e12612. [Google Scholar] [CrossRef]
- Glynn, L.G.; Hayes, P.S.; Casey, M.; Glynn, F.; Alvarez-Iglesias, A.; Newell, J.; OLaighin, G.; Heaney, D.; O’Donell, M.; Murphy, A.W. Effectiveness of a smartphone application to promote physical activity in primary care: The SMART MOVE randomised controlled trial. Br. J. Gen. Pract. 2014. [Google Scholar] [CrossRef]
- Romeo, A.; Edney, S.; Plotnikoff, R.; Curtis, R.; Ryan, J.; Sanders, I.; Crozier, A.; Maher, C. Can Smartphone Apps Increase Physical Activity? Systematic Review and Meta-Analysis. J. Med. Internet Res. 2019, 21, e12053. Available online:https://www.jmir.org/2019/3/e12053 (accessed on 25 October 2020). [CrossRef] [PubMed]
- Greenhalgh, T.; Peacock, R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: Audit of primary sources. Br. Med. J. 2005, 331, 1064–1065. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Grimshaw, J.M.; Wells, G.A.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.C.; Tugwell, P.; Moher, D.; Bouter, L.M. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007, 7, 10. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8. [Google Scholar] [CrossRef] [PubMed]
- Ferreira González, I.; Urrútia, G.; Alonso-Coello, P. Revisiones sistemáticas y metaanálisis: Bases conceptuales e interpretación. Rev. Española Cardiol. 2011, 64, 688–696. [Google Scholar] [CrossRef]
- Allen, J.K.; Stephens, J.; Dennison Himmelfarb, C.R.; Stewart, K.J.; Hauck, S. Randomized controlled pilot study testing use of smartphone technology for obesity treatment. J. Obes. 2013. [Google Scholar] [CrossRef]
- Fanning, J.; Roberts, S.; Hillman, C.H.; Mullen, S.P.; Ritterband, L.; McAuley, E. A smartphone “app”-delivered randomized factorial trial targeting physical activity in adults. J. Behav. Med. 2017. [Google Scholar] [CrossRef]
- King, A.C.; Hekler, E.B.; Grieco, L.A.; Winter, S.J.; Sheats, J.L.; Buman, M.P.; Banerjee, B.; Robinson, T.N.; Cirimele, J. Effects of three motivationally targeted mobile device applications on initial physical activity and sedentary behavior change in midlife and older adults: A randomized trial. PLoS ONE 2016. [Google Scholar] [CrossRef]
- Martin, S.S.; Feldman, D.I.; Blumenthal, R.S.; Jones, S.R.; Post, W.S.; McKibben, R.A.; Michos, E.D.; Ndumele, C.E.; Ratchford, E.V.; Coresh, J.; et al. mActive: A randomized clinical trial of an automated mHealth intervention for physical activity promotion. J. Am. Heart Assoc. 2015. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.K.; Landucci, G.; Awoyinka, L.; Atwood, A.K.; Carmack, C.L. Demark-Wahnefried, W. McTavish, F.; Gustafson, D. SurvivorCHESS to increase physical activity in colon cancer survivors: Can we get them moving? J. Cancer Surviv. 2018. [Google Scholar] [CrossRef] [PubMed]
- Spring, B.; Pellegrini, C.A.; Pfammatter, A.; Duncan, J.M.; Pictor, A.; McFadden, H.G.; Siddique, J.; Hedeker, D. Effects of an abbreviated obesity intervention supported by mobile technology: The ENGAGED randomized clinical trial. Obesity 2017. [Google Scholar] [CrossRef]
- Ashton, L.M.; Morgan, P.J.; Hutchesson, M.J.; Rollo, M.E.; Collins, C.E. Feasibility and preliminary efficacy of the “HEYMAN” healthy lifestyle program for young men: A pilot randomised controlled trial. Nutr. J. 2017. [Google Scholar] [CrossRef]
- Duncan, M.; Vandelanotte, C.; Kolt, G.S.; Rosenkranz, R.R.; Caperchione, C.M.; George, E.S.; Ding, H.; Hooker, C.; Karunanithi, M.; Maeder, A.J.; et al. Effectiveness of a web- and mobile phone-based intervention to promote physical activity and healthy eating in middle-Aged males: Randomized controlled trial of the manup study. J. Med. Internet Res. 2014, 21, b2700. [Google Scholar] [CrossRef]
- Harries, T.; Eslambolchilar, P.; Rettie, R.; Stride, C.; Walton, S.; Van Woerden, H.C. Effectiveness of a smartphone app in increasing physical activity amongst male adults: A randomised controlled trial. BMC Public Health 2016. [Google Scholar] [CrossRef]
- Johnston, N.; Bodegard, J.; Jerström, S.; Åkesson, J.; Brorsson, H.; Alfredsson, J.; Albertsson, P.A.; Karlsson, J.E.; Varenhorst, C. Effects of interactive patient smartphone support app on drug adherence and lifestyle changes in myocardial infarction patients: A randomized study. Am. Heart J. 2016. [Google Scholar] [CrossRef]
- Naimark, J.S.; Madar, Z.; Shahar, D.R. The impact of a Web-based app (eBalance) in promoting healthy lifestyles: Randomized controlled trial. J. Med. Internet Res. 2015. [Google Scholar] [CrossRef]
- Memon, A.R.; Masood, T.; Awan, W.A.; Waqas, A. The effectiveness of an incentivized physical activity programme (Active Student) among female medical students in Pakistan: A Randomized Controlled Trial. J. Pak. Med. Assoc. 2018, 68, 1438–1445. [Google Scholar]
- Valentiner, L.S.; Thorsen, I.K.; Kongstad, M.B.; Brinkløv, C.F.; Larsen, R.T.; Karstoft, K.; Nielsen, J.S.; Pedersen, B.K.; Langberg, H.; Ried-Larsen, M. Effect of ecological momentary assessment, goal-setting and personalized phone-calls on adherence to interval walking training using the InterWalk application among patients with type 2 diabetes-A pilot randomized controlled trial. PLoS ONE 2019, 14, e0208181. [Google Scholar] [CrossRef]
- Ginis, P.; Nieuwboer, A.; Dorfman, M.; Ferrari, A.; Gazit, E.; Canning, C.G.; Rocchi, L.; Chiari, L.; Hausdorff, J.M.; Mirelman, A. Feasibility and effects of home-based smartphone-delivered automated feedback training for gait in people with Parkinson’s disease: A pilot randomized controlled trial. Park Relat. Disord. 2016. [Google Scholar] [CrossRef]
- Reiner, M.; Niermann, C.; Jekauc, D.; Woll, A. Long-term health benefits of physical activity--a systematic review of longitudinal studies. BMC Public Health. 2013, 13, 813. [Google Scholar] [CrossRef]
- Aguilar-Martínez, A.; Solé-Sedeño, J.M.; Mancebo-Moreno, G.; Medina, F.X.; Saigí-Rubió, F. Use of mobile phones as a tool for weight loss: A systematic review. J. Telemed. Telecare 2014, 20, 339–349. [Google Scholar] [CrossRef]
- Stevens, W.; Hillsdon, M.; Thorogood, M.; McArdle, D. Cost-effectiveness of a primary care based physical activity intervention in 45–74 year old men and women: A randomised controlled trial. Br. J. Sports Med. 1998, 32, 236–241. [Google Scholar] [CrossRef]
- Robroek, S.J.; van Lenthe, F.J.; van Empelen, P.; Burdorf, A. Determinants of participation in worksite health promotion programmes: A systematic review. Int. J. Behav. Nutr. Phys. Act 2009, 6, 26. [Google Scholar] [CrossRef]
- Greaves, C.J.; Sheppard, K.E.; Abraham, C.; Greaves, C.J.; Sheppard, K.E.; Abraham, C.; Hardeman, W.; Roden, M.; Evans, P.H.; Schwarz, P.; et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011, 11. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).