Changes in Lipoinflammation Markers in People with Obesity after a Concurrent Training Program: A Comparison between Men and Women
Abstract
:1. Introduction
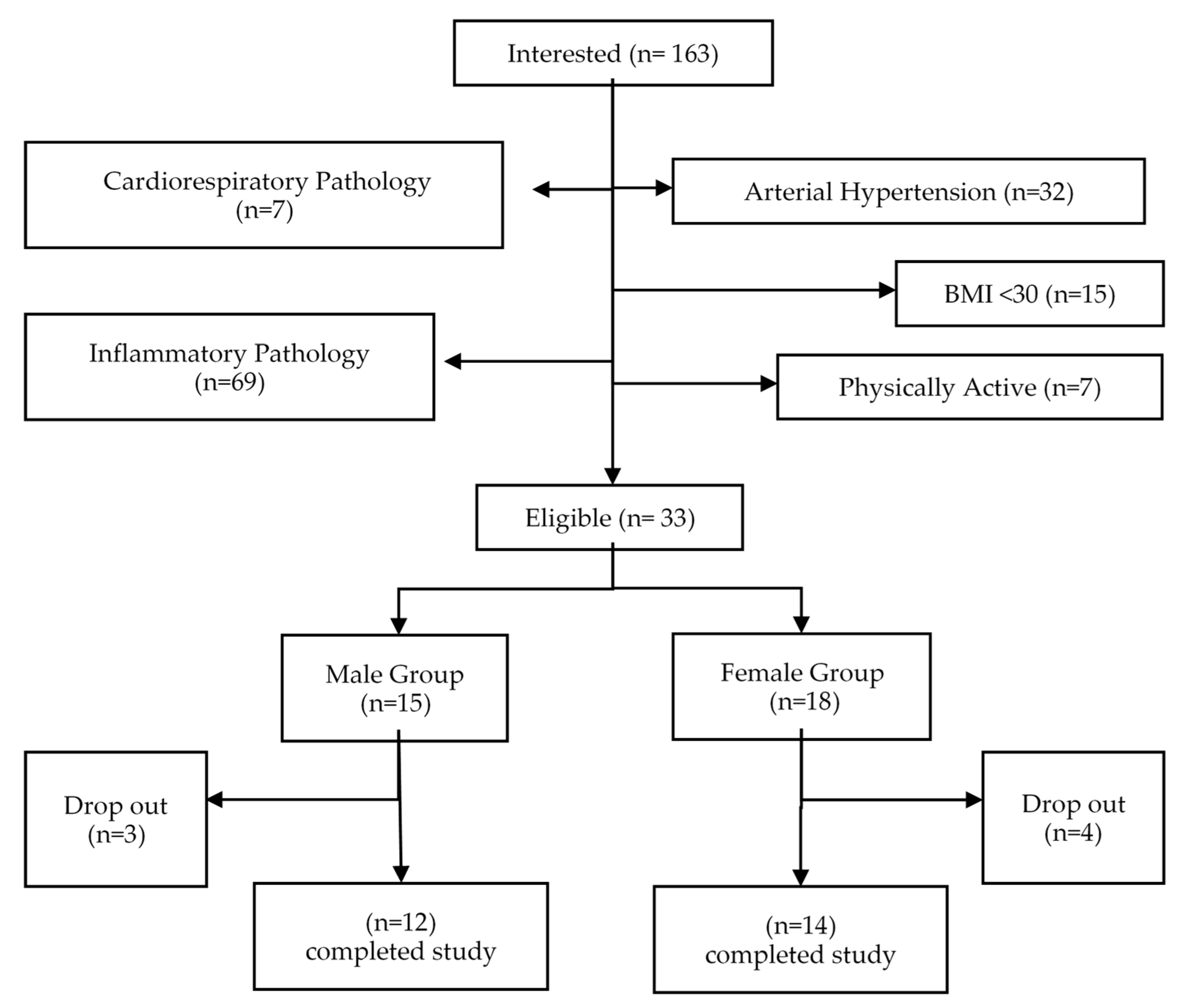
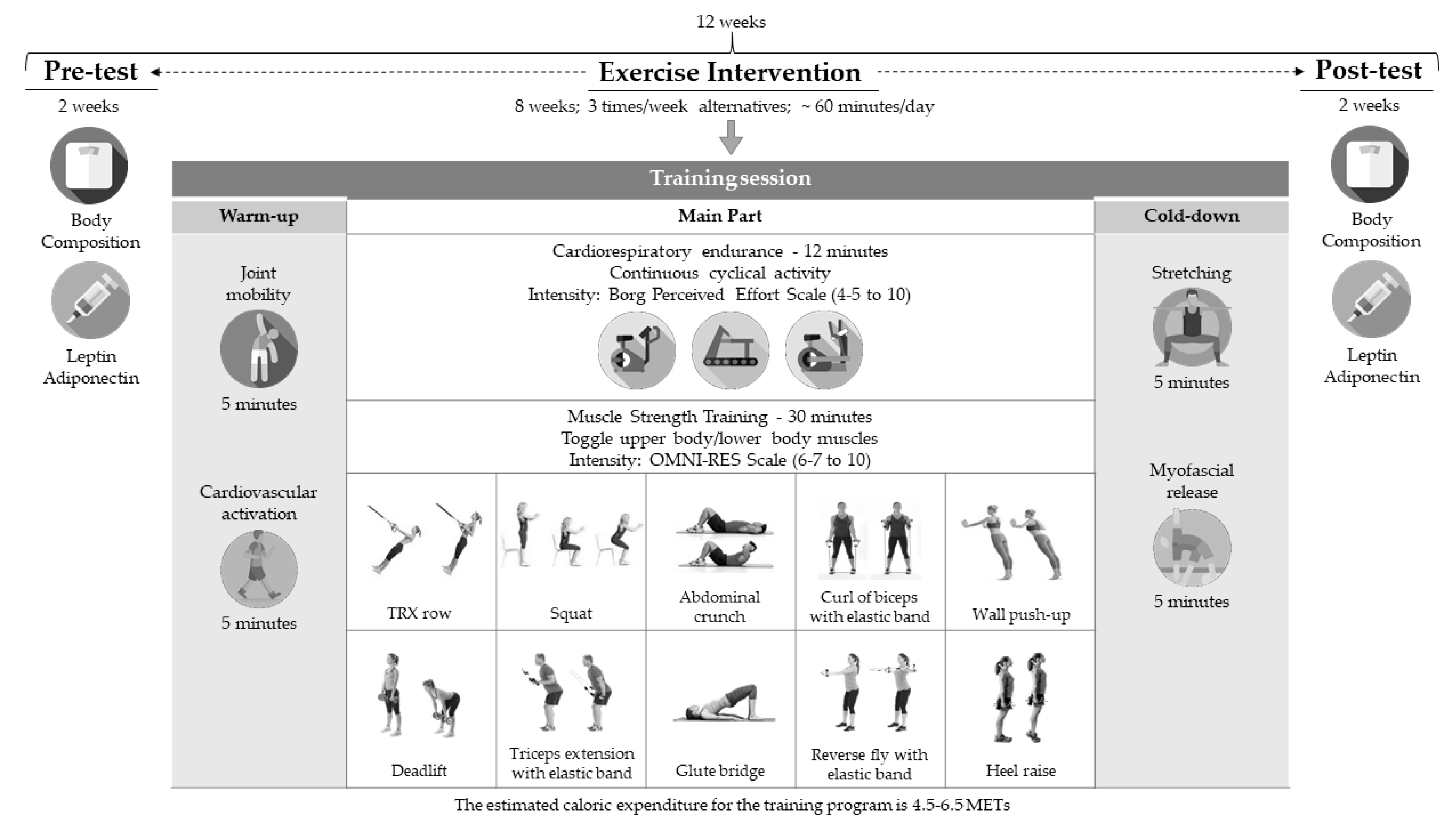
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- W.H.O. World Health Organization. Obesity and Overweight Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 July 2020).
- Suárez-Carmona, W.; Sánchez-Oliver, A.J.; González-Jurado, J.A. Pathophysiology of obesity: Current view. Rev. Chil. Nutr. 2017, 44, 226–233. [Google Scholar]
- Sánchez-Oliver, A.J.; Martín-García, C.; Gálvez-Ruiz, P.; González-Jurado, J.A. Mortality and Economic Expenses of Cardiovascular Diseases Caused by Physical Inactivity in Spain. J. Phys. Educ. Sport 2018, 18, 1420. [Google Scholar]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galán-Lopez, P.; Sánchez-Oliver, A.J.; Pihu, M.; Gísladóttír, T.; Domínguez, R.; Ries, F. Association between Adherence to the Mediterranean Diet and Physical Fitness with Body Composition Parameters in 1717 European Adolescents: The AdolesHealth Study. Nutrients 2020, 12, 77. [Google Scholar]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Young, D.R.; Hivert, M.-F.; Alhassan, S.; Camhi, S.M.; Ferguson, J.F.; Katzmarzyk, P.T.; Lewis, C.E.; Owen, N.; Perry, C.K.; Siddique, J.; et al. Sedentary Behavior and Cardiovascular Morbidity and Mortality: A Science Advisory From the American Heart Association. Circulation 2016, 134, e262–e279. [Google Scholar] [CrossRef]
- Cai, D.; Khor, S. “Hypothalamic Microinflammation” Paradigm in Aging and Metabolic Diseases. Cell Metab. 2019, 30, 19–35. [Google Scholar] [CrossRef]
- León-Pedroza, J.I.; González-Tapia, L.A.; del Olmo-Gil, E.; Castellanos-Rodríguez, D.; Escobedo, G.; González-Chávez, A. Low-grade systemic inflammation and the development of metabolic diseases: From the molecular evidence to the clinical practice. Cirugía Cir. 2015, 83, 543–551. [Google Scholar]
- Izaola, O.; de Luis, D.; Sajoux, I.; Domingo, J.C.; Vidal, M. Inflammation and obesity (Lipoinflammation). Nutr. Hosp. 2015, 31, 2352–2358. [Google Scholar] [CrossRef] [Green Version]
- Klöting, N.; Blüher, M.J.R.i.E.; Disorders, M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 277–287. [Google Scholar]
- Francisco, V.; Pino, J.; Campos-Cabaleiro, V.; Ruiz-Fernández, C.; Mera, A.; González-Gay, M.A.; Gómez, R.; Gualillo, O. Obesity, fat mass and immune system: Role for leptin. Front. Physiol. 2018, 9, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obata, Y.; Yamada, Y.; Takahi, Y.; Baden, M.Y.; Saisho, K.; Tamba, S.; Yamamoto, K.; Umeda, M.; Furubayashi, A.; Matsuzawa, Y. Relationship between serum adiponectin levels and age in healthy subjects and patients with type 2 diabetes. Clin. Endocrinol. 2013, 79, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.-H.; Hsu, L.-A.; Wu, S.; Teng, M.-S.; Sun, Y.-C.; Ko, Y.-L. Leptin-to-adiponectin ratio is related to low grade inflammation and insulin resistance independent of obesity in non-diabetic Taiwanese: A cross-sectional cohort study. Acta Cardiol. Sin. 2014, 30, 204. [Google Scholar] [PubMed]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gómez-Ambrosi, J. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutrients 2019, 11, 454. [Google Scholar] [CrossRef] [Green Version]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Gómez-Ambrosi, J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018, 7, 57–62. [Google Scholar] [CrossRef]
- Soltani, N.; Marandi, S.M.; Kazemi, M.; Esmaeil, N. The Exercise Training Modulatory Effects on the Obesity-Induced Immunometabolic Dysfunctions. Diabetes Metab. Syndr. Obes. 2020, 13, 785–810. [Google Scholar] [CrossRef] [Green Version]
- Goh, J.; Goh, K.P.; Abbasi, A. Exercise and Adipose Tissue Macrophages: New Frontiers in Obesity Research? Front. Endocrinol. 2016, 14, 65. [Google Scholar] [CrossRef]
- Bruun, J.M.; Helge, J.W.; Richelsen, B.; Stallknecht, B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E961–E967. [Google Scholar] [CrossRef]
- Silveira, L.S.; Antunes, B.D.M.M.; Minari, A.; Dos Santos, R.V.T.; Neto, J.C.R.; Lira, F.S. Macrophage Polarization: Implications on Metabolic Diseases and the Role of Exercise. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 115–132. [Google Scholar] [CrossRef] [Green Version]
- Dieli-Conwright, C.M.; Parmentier, J.-H.; Sami, N.; Lee, K.; Spicer, D.; Mack, W.J.; Sattler, F.; Mittelman, S.D. Adipose tissue inflammation in breast cancer survivors: Effects of a 16-week combined aerobic and resistance exercise training intervention. Breast Cancer Res. Treat. 2018, 168, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Sun, Y.; Woods, J.A. Exercise and the Regulation of Inflammatory Responses. Prog. Mol. Biol. Transl. Sci. 2015, 135, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.A.; Vieira, V.J.; Keylock, K.T. Exercise, inflammation, and innate immunity. Immunol. Allergy Clin. N. Am. 2009, 29, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Lehnig, A.C.; Stanford, K.I. Exercise-induced adaptations to white and brown adipose tissue. J. Exp. Biol. 2018, 221, jeb161570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kader, S.M.A.; Al-Jiffri, O.H.; Al-Shreef, F.M. Aerobic exercises alleviate symptoms of fatigue related to inflammatory cytokines in obese patients with type 2 diabetes. Afr. Health Sci. 2015, 15, 1142–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, N.; Ruan, Y.; Gao, X.; Sun, J. Systematic Review and Meta-Analysis of Randomized, Controlled Trials on the Effect of Exercise on Serum Leptin and Adiponectin in Overweight and Obese Individuals. Horm. Metab. Res. 2017, 49, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Annibalini, G.; Lucertini, F.; Agostini, D.; Vallorani, L.; Gioacchini, A.; Barbieri, E.; Guescini, M.; Casadei, L.; Passalia, A.; Del Sal, M. Concurrent aerobic and resistance training has anti-inflammatory effects and increases both plasma and leukocyte levels of IGF-1 in late middle-aged type 2 diabetic patients. Oxidative Med. Cell. Longev. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Venojärvi, M.; Wasenius, N.; Manderoos, S.; Heinonen, O.J.; Hernelahti, M.; Lindholm, H.; Surakka, J.; Lindström, J.; Aunola, S.; Atalay, M.; et al. Nordic walking decreased circulating chemerin and leptin concentrations in middle-aged men with impaired glucose regulation. Ann. Med. 2013, 45, 162–170. [Google Scholar] [CrossRef]
- Winzer, B.M.; Paratz, J.D.; Whitehead, J.P.; Whiteman, D.C.; Reeves, M.M. The feasibility of an exercise intervention in males at risk of oesophageal adenocarcinoma: A randomized controlled trial. PLoS ONE 2015, 10, e0117922. [Google Scholar] [CrossRef] [Green Version]
- Ligibel, J.A.; Giobbie-Hurder, A.; Olenczuk, D.; Campbell, N.; Salinardi, T.; Winer, E.P.; Mantzoros, C.S. Impact of a mixed strength and endurance exercise intervention on levels of adiponectin, high molecular weight adiponectin and leptin in breast cancer survivors. Cancer Causes Control. 2009, 20, 1523–1528. [Google Scholar] [CrossRef]
- Dorneles, G.P.; Haddad, D.O.; Fagundes, V.O.; Vargas, B.K.; Kloecker, A.; Romão, P.R.; Peres, A. High intensity interval exercise decreases IL-8 and enhances the immunomodulatory cytokine interleukin-10 in lean and overweight–obese individuals. Cytokine 2016, 77, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borg, E.; Kaijser, L. A comparison between three rating scales for perceived exertion and two different work tests. Scand. J. Med. Sci. Sports 2006, 16, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Hopkins, W.; Marshall, S.; Batterham, A.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, A.; Dutheil, F.; Drapeau, V.; Metz, L.; Lesour, B.; Chapier, R.; Pereira, B.; Verney, J.; Baker, J.S.; Vinet, A.; et al. Long-term effects of high-intensity resistance and endurance exercise on plasma leptin and ghrelin in overweight individuals: The RESOLVE Study. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2019, 44, 1172–1179. [Google Scholar] [CrossRef]
- de Souza, D.C.; Matos, V.A.F.; dos Santos, V.O.A.; Medeiros, I.F.; Marinho, C.S.R.; Nascimento, P.R.P.; Dorneles, G.P.; Peres, A.; Müller, C.H.; Krause, M.; et al. Effects of high-intensity interval and moderate-intensity continuous exercise on inflammatory, leptin, IgA, and lipid peroxidation responses in obese males. Front. Physiol. 2018, 9, 567. [Google Scholar] [CrossRef] [Green Version]
- Rostás, I.; Pótó, L.; Mátrai, P.; Hegyi, P.; Tenk, J.; Garami, A.; Illés, A.; Solymár, M.; Pétervári, E.; Szűcs, Á.; et al. In middle-aged and old obese patients, training intervention reduces leptin level: A meta-analysis. PLoS ONE 2017, 12, e0182801. [Google Scholar] [CrossRef]
- Salvadori, A.; Fanari, P.; Brunani, A.; Marzullo, P.; Codecasa, F.; Tovaglieri, I.; Cornacchia, M.; Palmulli, P.; Longhini, E. Leptin level lowers in proportion to the amount of aerobic work after four weeks of training in obesity. Horm. Metab. Res. 2015, 47, 225–231. [Google Scholar] [CrossRef]
- Bagheri, R.; Rashidlamir, A.; Ashtary-Larky, D.; Wong, A.; Grubbs, B.; Motevalli, M.S.; Baker, J.S.; Laher, I.; Zouhal, H. Effects of green tea extract supplementation and endurance training on irisin, pro-inflammatory cytokines, and adiponectin concentrations in overweight middle-aged men. Eur. J. Appl. Physiol. 2020, 120, 915–923. [Google Scholar] [CrossRef]
- Kim, D.Y.; Seo, B.D.; Kim, D.J. Effect of walking exercise on changes in cardiorespiratory fitness, metabolic syndrome markers, and high-molecular-weight adiponectin in obese middle-aged women. J. Phys. Ther. Sci. 2014, 26, 1723–1727. [Google Scholar] [CrossRef] [Green Version]
- Saunders, T.J.; Palombella, A.; McGuire, K.A.; Janiszewski, P.M.; Després, J.P.; Ross, R. Acute exercise increases adiponectin levels in abdominally obese men. J. Nutr. Metab. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, K.R.; Blaszczak, A.; Haus, J.M.; Patrick-Melin, A.; Fealy, C.E.; Solomon, T.P.J.; Kalinski, M.I.; Kirwan, J.P. A 7-d exercise program increases high-molecular weight adiponectin in obese adults. Med. Sci. Sports Exerc. 2012, 44, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, J.; Izquierdo, M.; Martínez-Labari, C.; Ortega, F.; Grijalba, A.; Forga, L.; Idoate, F.; García-Unciti, M.; Fernández-Real, J.M.; Gorostiaga, E.M. Resistance training improves cardiovascular risk factors in obese women despite a significative decrease in serum adiponectin levels. Obesity 2010, 18, 535–541. [Google Scholar] [CrossRef] [PubMed]
- de Piano-Ganen, A.; Masquio, D.C.L.; Dâmaso, A.R.; Oyama, L.M.; Estadella, D.; Chamas, A.; do Nascimento, C.M.P.O. Serum myristic fatty acid negatively correlates with anti-inflammatory adiponectin/leptin ratio in obese adolescents: Effects of long-Term therapy. Mundo Saude 2017, 40, 537–554. [Google Scholar] [CrossRef]
- Musil, F.; Blaha, V.; Ticha, A.; Hyspler, R.; Haluzik, M.; Lesna, J.; Smahelova, A.; Sobotka, L. Effects of body weight reduction on plasma leptin and adiponectin/leptin ratio in obese patients with type 1 diabetes mellitus. Physiol. Res. 2015, 64, 221–228. [Google Scholar] [CrossRef]
- Mirza, S.; Qu, H.Q.; Li, Q.; Martinez, P.J.; Rentfro, A.R.; McCormick, J.B.; Fisher-Hoch, S.P. Adiponectin/leptin ratio and metabolic syndrome in a mexican american population. Clin. Investig. Med. 2011, 34, E290–E297. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Nam, J.S.; Yeo, D.W.; Kim, K.R.; Suh, S.H.; Ahn, C.W. The effects of aerobic exercise training on serum osteocalcin, adipocytokines and insulin resistance on obese young males. Clin. Endocrinol. 2015, 82, 686–694. [Google Scholar] [CrossRef]
- Abbenhardt, C.; McTiernan, A.; Alfano, C.M.; Wener, M.H.; Campbell, K.L.; Duggan, C.; Foster-Schubert, K.E.; Kong, A.; Toriola, A.T.; Potter, J.D.; et al. Effects of individual and combined dietary weight loss and exercise interventions in postmenopausal women on adiponectin and leptin levels. J. Intern. Med. 2013, 274, 163–175. [Google Scholar] [CrossRef]
- Beavers, K.M.; Ambrosius, W.T.; Nicklas, B.J.; Rejeski, W.J. Independent and combined effects of physical activity and weight loss on inflammatory biomarkers in overweight and obese older adults. J. Am. Geriatr. Soc. 2013, 61, 1089–1094. [Google Scholar] [CrossRef] [Green Version]
- Auerbach, P.; Nordby, P.; Bendtsen, L.Q.; Mehlsen, J.L.; Basnet, S.K.; Vestergaard, H.; Ploug, T.; Stallknecht, B. Differential effects of endurance training and weight loss on plasma adiponectin multimers and adipose tissue macrophages in younger, moderately overweight men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R490–R498. [Google Scholar] [CrossRef]
- Akbarpour, M. The effect of aerobic training on serum adiponectin and leptin levels and inflammatory markers of coronary heart disease in obese men. Biol. Sport 2013, 30, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ackel-D’Elia, C.; Carnier, J.; Bueno, C.R.J.; Campos, R.M.; Sanches, P.L.; Clemente, A.P.; Tufik, S.; de Mello, M.T.; Dâmaso, A.R. Effects of different physical exercises on leptin concentration in obese adolescents. Int. J. Sports Med. 2014, 35, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.C.; Carson, L.; Neal, W.; Baylis, C.; Donley, D.; Yeater, R. Effects of an exercise intervention using Dance Dance Revolution on endothelial function and other risk factors in overweight children. Int. J. Pediatr. Obes. 2009, 4, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Becic, T.; Studenik, C.; Hoffmann, G. Exercise Increases Adiponectin and Reduces Leptin Levels in Prediabetic and Diabetic Individuals: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Med. Sci. 2018, 6, 97. [Google Scholar] [CrossRef] [Green Version]
- Saeidi, A.; Haghighi, M.M.; Kolahdouzi, S.; Daraei, A.; Ben Abderrahmane, A.; Essop, M.F.; Laher, I.; Hackney, A.C.; Zouhal, H. The effects of physical activity on adipokines in individuals with overweight/obesity across the lifespan: A narrative review. Obes Rev. 2020. [Google Scholar] [CrossRef]

| Male | Female | All | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| Age (yr) | 46.42 ± 5 | 38–54 | 46.36 ± 4.53 | 40–53 | 46.38 ± 4.66 | 38–54 |
| Height (m) | 1.75 ± 0.07 | 1.6–1.9 | 1.61 ± 0.08 | 1.44–1.73 | 1.67 ± 0.10 | 1.44–1.9 |
| Weight (kg) | 109.9 ± 16.35 | 87–140 | 94.67 ± 21.95 | 70.7–147 | 101.73 ± 20.7 | 70.7–147 |
| BMI | 35.98 ± 3.98 | 30.1–42.2 | 36.12 ± 5.87 | 30.05–49.69 | 36.05 ± 4.99 | 30–49.7 |
| BFM (kg) | 43.86 ± 11.08 | 24–58.4 | 45.11 ± 14.70 | 30.8–79.3 | 44.53 ± 12.92 | 24–79.3 |
| BFMP (%) | 39.53 ± 5.94 | 28–48.8 | 47.59 ± 4.63 | 39–53.9 | 43.87 ± 6.59 | 28–53.9 |
| Pre-Test | Post-Test | Change | p§ Value | E.S ¥ | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | CI (95%) | Mean ± SD | CI (95%) | Mean ± SD | CI (95%) | |||
| Adiponecti (ug/mL) | 16.94 ± 4.89 | (14.9–18.6) | 17.3 ± 5.1 | (15.2–19.3) | 0.32 ± 3.04 | (−0.91–1.55) | 0.20 | 0.06 |
| Leptin (ng/mL) | 44.09 ± 24.3 | (34.3–53.9) | 41.6 ± 30.5 | (29.3–53.9) | −2.5 ± 20.1 | (−10.6–5.59) | 0.47 | −0.09 |
| A/L ratio * | 0.5 ± 0.32 | (0.37–0.63) | 0.82 ± 0.79 | (0.5–1.14) | 0.32 ± 0.57 | (0.09–0.55) | 0.009 * | 0.53 |
| Pre-Test | Post-Test | Change | p§ Value | E.S ¥ CI (95%) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | CI (95%) | Mean ± SD | CI (95%) | Mean ± SD | Mean ± SD | |||
| Adiponectin (ug/mL) | 18.24 ± 3.8 | (16.04–20.43) | 18.17 ± 1.16 | (15.67–20.67) | −0.06 ± 3.63 | (−2.16–2.03) | 0.55 | −0.02 |
| Leptin (ng/mL) | 46.5 ± 23.8 | (32.7–60.2) | 46.1 ± 37.6 | (2.44–6.78) | −0.4 ± 21.52 | (−12.8–12.0) | 0.82 | −0.01 |
| A/L ratio * | 0.51 ± 0.28 | (0.35–0.67) | 0.85 ± 0.8 | (0.39–1.31) | 0.34 ± 0.62 | (−0.01–0.7) | 0.05* | 0.55 |
| Pre-Test | Post-Test | Change | p Value § | E.S ¥ | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | CI (95%) | Mean ± SD | CI (95%) | Mean ± SD | CI (95%) | |||
| Adiponectin (ug/mL) | 15.43 ± 5.71 | (11.8–19.1) | 16.2 ± 5.71 | (12.57–19.83) | 0.76 ± 2.25 | (−0.67–2.2) | 0.26 | 0.12 |
| Leptin (ng/mL) | 41.32 ± 25.6 | (25.1–57.6) | 36.36 ± 19.86 | (23.7–48.9) | −4.96 ± 18.82 | (−16.9–6.9) | 0.38 | −0.22 |
| A/L ratio * | 0.49 ± 0.37 | (0.25–0.73) | 0.78 ± 0.81 | (0.27–1.29) | 0.29 ± 0.53 | (−0.05–0.63) | 0.08 | 0.46 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Jurado, J.A.; Suárez-Carmona, W.; López, S.; Sánchez-Oliver, A.J. Changes in Lipoinflammation Markers in People with Obesity after a Concurrent Training Program: A Comparison between Men and Women. Int. J. Environ. Res. Public Health 2020, 17, 6168. https://doi.org/10.3390/ijerph17176168
González-Jurado JA, Suárez-Carmona W, López S, Sánchez-Oliver AJ. Changes in Lipoinflammation Markers in People with Obesity after a Concurrent Training Program: A Comparison between Men and Women. International Journal of Environmental Research and Public Health. 2020; 17(17):6168. https://doi.org/10.3390/ijerph17176168
Chicago/Turabian StyleGonzález-Jurado, José Antonio, Walter Suárez-Carmona, Sergio López, and Antonio Jesús Sánchez-Oliver. 2020. "Changes in Lipoinflammation Markers in People with Obesity after a Concurrent Training Program: A Comparison between Men and Women" International Journal of Environmental Research and Public Health 17, no. 17: 6168. https://doi.org/10.3390/ijerph17176168






