1. Introduction
The global demand for fish and fish products has been increasing in parallel with population growth and economic development. To reduce the fishing pressure on commercially important species, the effective utilization of noncommercial or underutilized species is becoming increasingly important. Surimi production represents a practical strategy for utilizing these resources, as surimi can be further processed into a variety of gelled food products, such as fish balls and crab-flavored kamaboko. These surimi-based products are categorized as hydrocolloid gels. During surimi gel production, fish meat is homogenized and its proteins are solubilized under high ionic strength conditions through the addition of salt, followed by gelation mediated by catalytic cross-linking and thermal aggregation of proteins. In surimi gels, myosin heavy chain (MHC), a myofibrillar protein with a molecular weight of approximately 220 kDa, plays a pivotal role in the formation of the gel network [
1].
Species-specific differences in the thermal behavior of surimi gel properties complicate surimi processing, particularly due to variations in the temperature at which gelation or disintegration occurs [
2,
3]. Transglutaminase (TGase) is known as the enzyme that catalyzes the polymerization reaction between MHC molecules through the formation of ε-(γ-Glu)-Lys isopeptide bonds [
4,
5,
6,
7]. TGase are widely expressed in the muscle tissues of various fish species, but their activity or temperature dependency is highly species-specific [
8]. Conversely, proteases expressed in fish muscle tissues interfere with surimi gelation, and their enzymatic activity and temperature dependency are also highly species-specific [
2,
3,
5,
7]. The disintegration of surimi gel due to MHC degradation by proteases can be explained by the following mechanisms: degradation of MHCs proceeds prior to polymerization, known as
modori, and degradation of polymerized MHCs, referred to as
himodori [
5,
9].
To maximize the cross-linking of MHCs catalyzed by TGase and to minimize their proteolytic degradation, a combination of different heating temperatures—known as two-step heating—is commonly employed in the surimi food industry [
10]. However, the prediction of surimi gel properties under varying two-step heating conditions remains challenging, as no kinetic model has yet been proposed to explain the dynamics of MHC and the associated mechanical properties during surimi gelation and disintegration. A kinetic model that explains the dynamics of MHC, including the competition among TGase-catalyzed polymerization, non-enzymatic polymerization, and proteolytic degradation, is necessary for a comprehensive understanding of the mechanisms underlying surimi gelation and disintegration. First, it is necessary to develop individual kinetic models that describe
modori or
himodori and TGase-catalyzed MHC polymerization, respectively. These models should then be integrated to construct a comprehensive model capable of describing the competitive dynamics among these processes. However, few studies have elucidated the detailed relationship between MHC polymerization/degradation during
himodori and the mechanical properties of surimi gels, and sufficient empirical data for model construction are still lacking. Therefore, the present study aimed to construct a fundamental model describing the competition between non-enzymatic polymerization and proteolytic degradation of MHC. We focused on golden threadfin bream
Nemipterus virgatus, which exhibits low TGase activity and high protease activity at elevated temperatures (>50 °C) [
3,
8,
11], and developed a model of MHC dynamics during surimi disintegration caused by
himodori.
3. Discussion
The present study investigated the competition between the non-enzymatic polymerization and proteolytic degradation of MHC, and their effects on the gelation of golden threadfin bream
N. virgatus surimi.
N. virgatus is one of the important fish species used as a raw material for surimi in Asia. Consequently, insights into the
himodori phenomenon of
N. virgatus surimi have been accumulated over a long period [
3,
12,
13,
14,
15,
16], and research aimed at improving its gel properties continues to be actively conducted [
11,
17,
18,
19,
20,
21]. First, we confirmed that proteases in
N. virgatus surimi are activated by heating at 60 °C, which leads to gel disintegration (
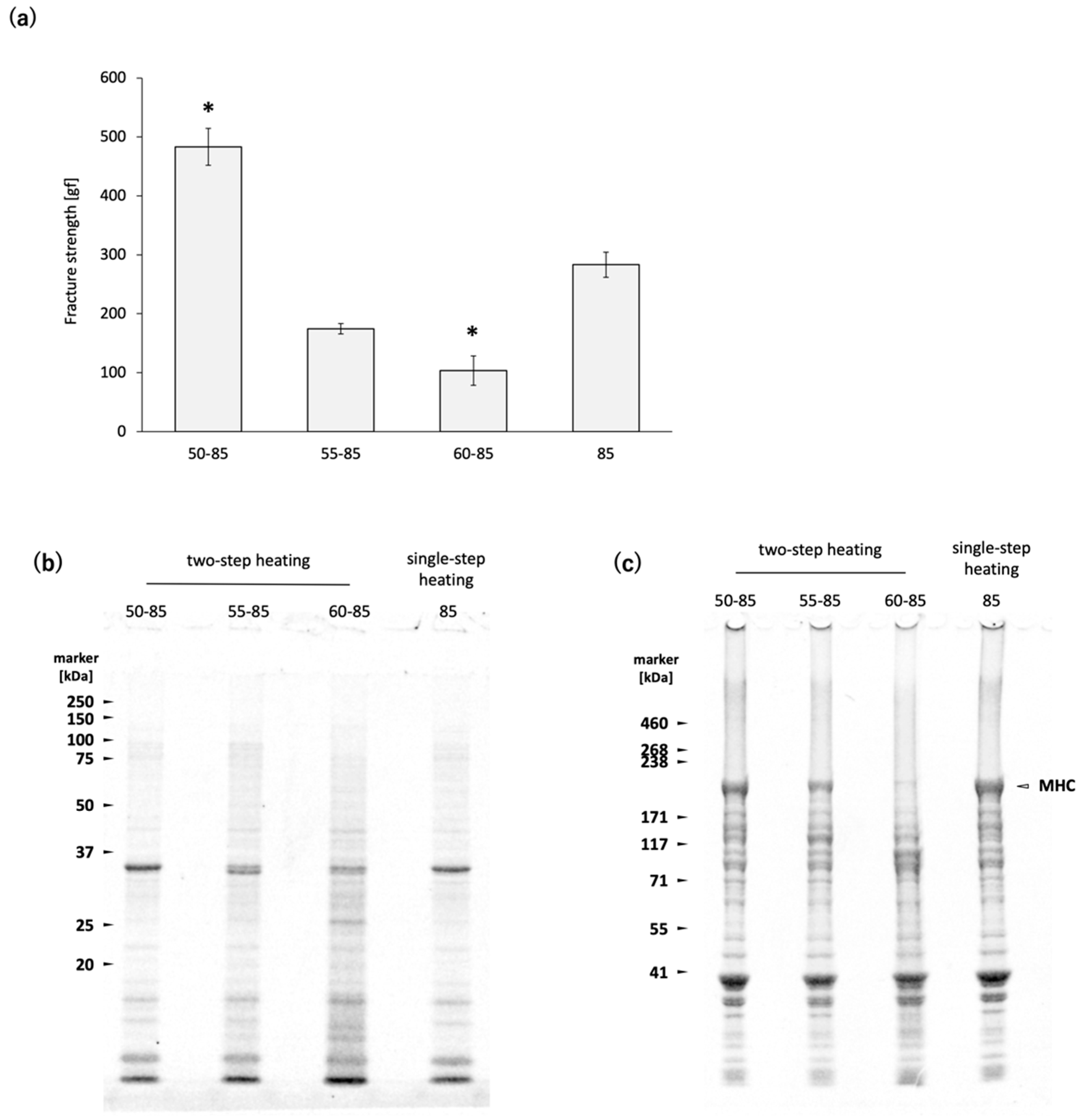
Figure 1a) [
3,
13]. The protease responsible for gel disintegration at 60 °C was purified and identified as a sarcoplasmic serine proteinase with a molecular weight of 77 kDa [
13]. A sarcoplasmic serine proteinase with a molecular weight of 540 kDa, which has an optimum temperature of 50 °C, was also purified from
N. virgatus meat [
14]. Kinoshita et al. (1990) [
3] referred to the protease activated at 60 °C as Sp-60-MIP, and the one activated at 50 °C as Sp-50-MIP. They reported that the
N. virgatus meat used for the purification of Sp-50-MIP exhibited gel disintegration when heated at 50 °C, but not at 60 °C [
14]. In the present study, the
N. virgatus surimi exhibited gel disintegration when heated at 60 °C, but not at 50 °C (
Figure 1a). The results of SDS-PAGE indicate that MHC degradation was clearly promoted at 60 °C, whereas heating at 50 °C did not induce noticeable degradation (
Figure 1c). Seasonal variations and freeze-thaw effects are possible factors contributing to this reversal [
22,
23]; however, further investigation is needed in future studies.
TGase in white croaker
Pennahia argentata, a species commonly used as surimi ingredients in Japan due to its strong gelation properties, has been reported to show higher peak activity around 30 °C and little to no activity at 50 °C [
5,
8]. In contrast, TGase in
N. virgatus meat exhibits relatively lower activity but operates over a broader temperature range, with an optimum around 40 °C [
8]. During two-step heating 50, the slower rate of temperature increase compared to single-step heating 85 results in a longer residence time within the temperature range conductive to TGase activity in the
N. virgatus surimi. Although the
N. virgatus surimi gel prepared by two-step heating 50 exhibited higher fracture strength than that prepared by single-step heating 85 (
Figure 1a), the MHC band intensities remained at comparable levels (
Figure 1c). Since TGase-catalyzed MHC polymerization typically results in a decrease in MHC band intensity, as observed by SDS-PAGE, the observed difference in fracture strength between two-step heating 50 and single-step heating 85 cannot be attributed to TGase activity. These results confirmed the negligible activity of TGase during heating at 50–85 °C in the
N. virgatus surimi used in the present study. The thermal denaturation point of myosin is approximately 40–50 °C in many fish species [
24]. During two-step heating 50, the progression from thermal unfolding to aggregation of MHC occurs more gradually compared to single-step heating 85. It has been reported that purified MHC derived from
Cyprinus carpio forms multimers at 30 °C through association via subfragment-1 [
25]. Based on these findings, it is considered that during heating at 50 °C for 60 min, MHC multimerization progresses in the surimi of
N. virgatus, leading to the formation of MHC clusters, which subsequently bind to each other at 85 °C. This slower polymerization process may contribute to the formation of a finer gel network, thereby enhancing the fracture strength of the surimi gel.
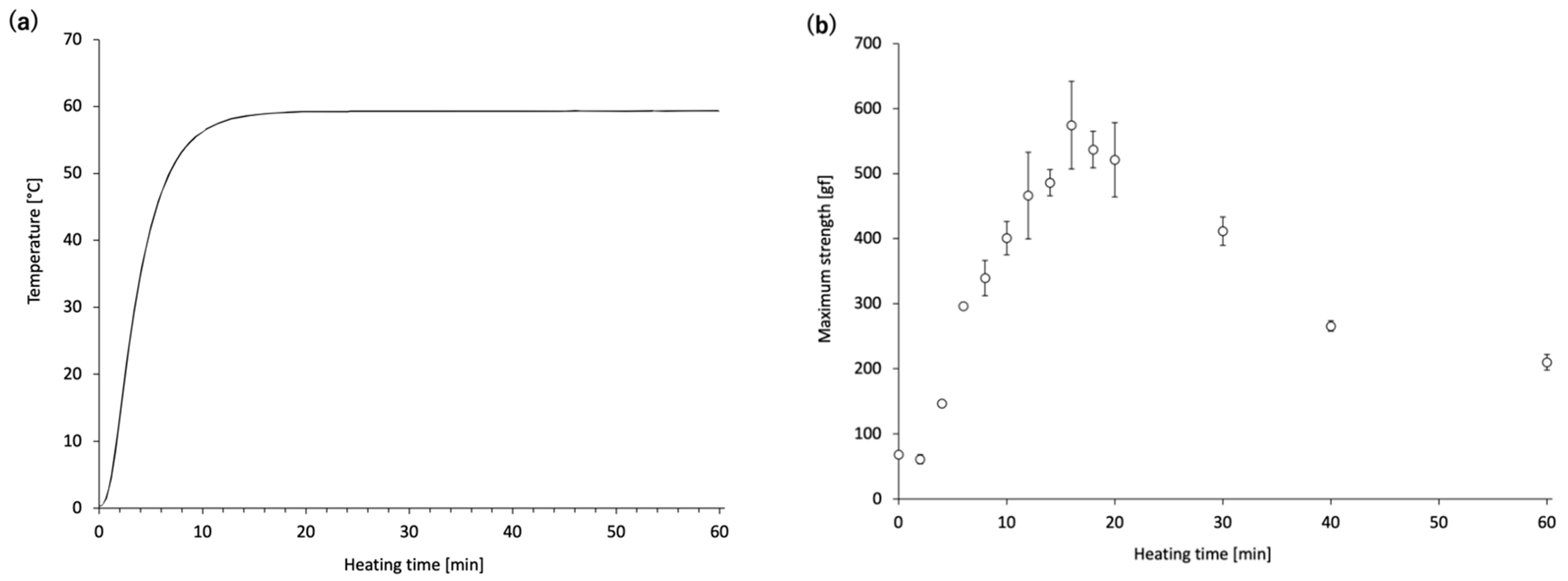
Next, time-course changes in the mechanical properties and MHC monomer levels of the
N. virgatus surimi during heating at 60 °C were investigated. The maximum strength of the
N. virgatus surimi reached its highest value after 16 min of heating (
Figure 2b), which corresponded to the time when the core temperature of the surimi sample reached 60 °C (
Figure 2a). These results suggest that gelation of the surimi progressed until the core temperature reached approximately 60 °C; however, gel disintegration began once the core temperature reached this point.
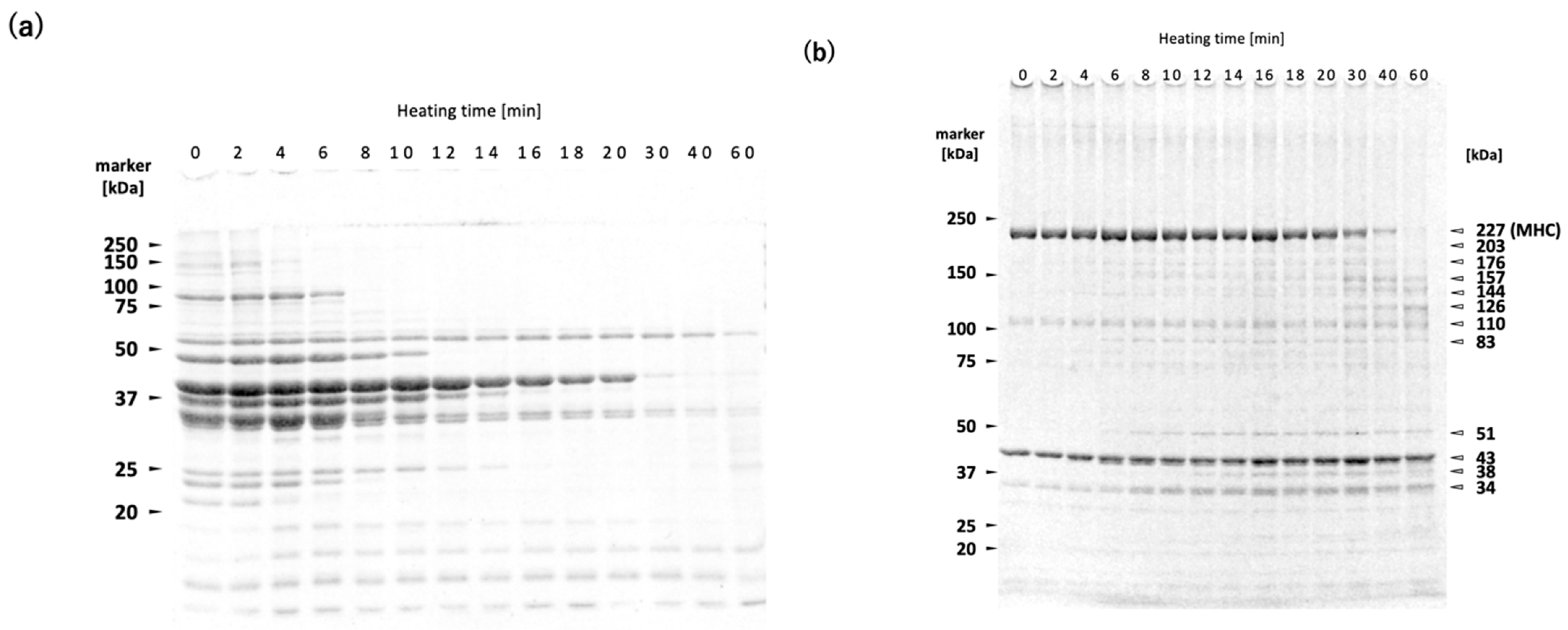
The WSP decreased with increasing heating time (
Figure 3a). The WSP consists primarily of sarcoplasmic proteins, including glycolytic enzymes. It has been shown that WSP of both rainbow trout
Oncorhynchus mykiss meat and deep-sea bonefish
Pterothrissus gissu surimi begin to undergo thermal aggregation above approximately 40 °C [
26,
27]. Although the thermal denaturation point of sarcoplasmic proteins varies depending on fish species and their living environment, it can be estimated to fall within the range of 40–50 °C in
N. virgatus surimi. Therefore, the decrease in WSP with increasing heating time at 60 °C can be attributed to thermal aggregation rather than proteolytic degradation (
Figure 3a). The bands at 34, 38, 51, and 83 kDa observed in the reduced USP are likely sarcoplasmic proteins that became insoluble through thermal aggregation, as indicated by the increase in their band intensities with prolonged heating (
Figure 3b).
A decrease in MHC band intensity in
N. virgatus surimi during heating at 60 °C was observed in both the reduced and non-reduced USP fractions (
Figure 3b,d). The decrease observed in the reduced USP indicated proteolytic degradation of MHC, as it was accompanied by an increase in the staining intensities at 126, 144, and 157 kDa (
Figure 3b,c). On the other hand, the decrease observed in the non-reduced USP indicates both non-enzymatic polymerization and proteolytic degradation of MHC (
Figure 3d,e). The decrease in MHC intensity in the reduced USP began after approximately 20 min of heating (
Figure 3b,c), whereas in the non-reduced USP, the decrease was observed as early as 2 min into heating (
Figure 3d,e). Taken together, these results suggest that thermal polymerization of MHCs via disulfide bonding occurred prior to their proteolytic degradation in the
N. virgatus surimi during heating at 60 °C.
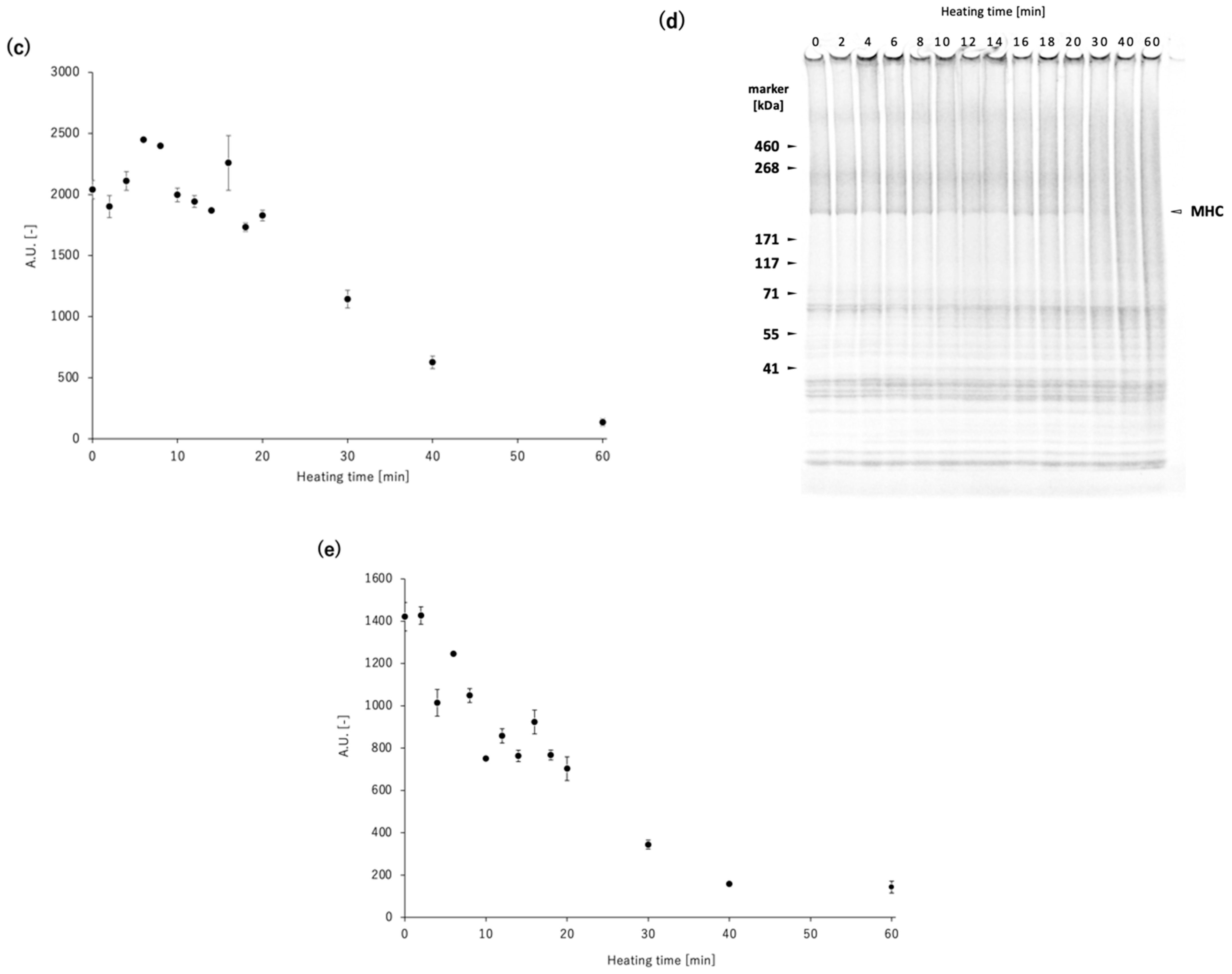
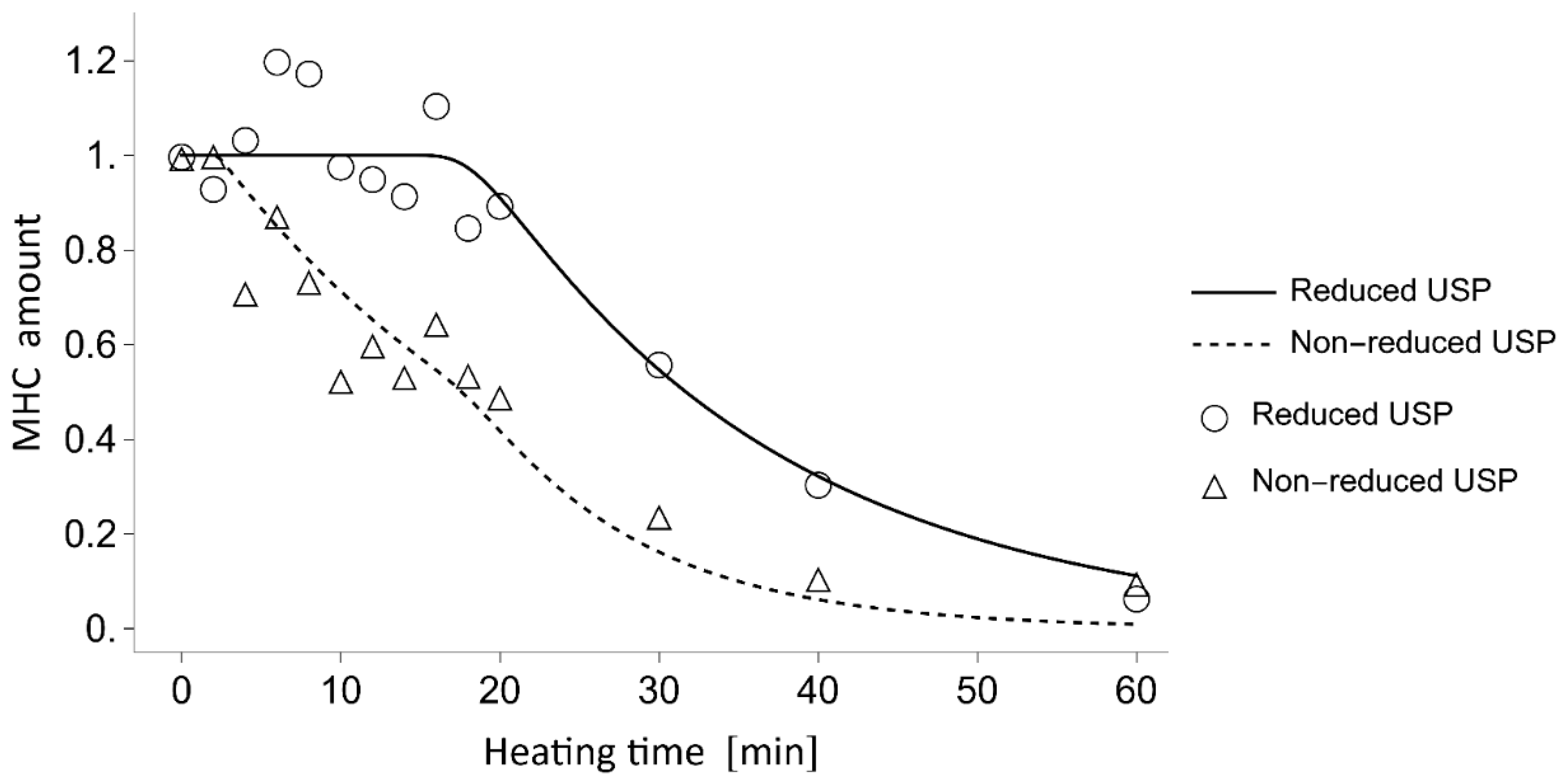
To elucidate the competition between non-enzymatic polymerization and proteolytic degradation of MHC in the
N. virgatus surimi during heating at 60 °C, the experimentally measured values were compared with those calculated using a mathematical model (
Figure 4). The model incorporates only non-enzymatic polymerization and proteolytic degradation of MHC, as TGase activity was confirmed to be negligible during heating at 50–60 °C (
Figure 1c), as described above. The decrease in MHC levels in the reduced USP during heating at 60 °C, which exhibited a delay of approximately 16 min, was accurately traced by the estimated values (
Figure 4). Thus, this delay can be attributed to the temperature-dependent activation of proteases. In both the measured and calculated data, the decrease in MHC levels in the non-reduced USP began as early as 2 min into heating (
Figure 4). This result suggests that the 2 min delay before the decrease in MHC level in the non-reduced USP, due to disulfide bonding, can be explained by the time required to reach the myosin denaturation point. A comparison between the MHC dynamics estimated by mathematical modeling and the measured time-course changes in maximum strength indicates that gelation progresses through the non-enzymatic polymerization of MHC prior to the gel disintegration caused by the proteolytic degradation of MHC in
N. virgatus surimi. Considering the difference in onset time between non-enzymatic polymerization (~2 min) and proteolytic degradation (~16 min), not only unpolymerized but also polymerized MHC, as described in Equation (1), was significantly degraded, which contributed markedly to the reduction in the mechanical properties of the
N. virgatus surimi.
It has been suggested that proteolytic degradation of the pre-formed gel network (
himodori) underlies the mechanism of gel disintegration in
N. virgatus surimi [
3,
5,
13]. The present study quantitatively elucidated these details using a mathematical model. Elucidating the molecular mechanisms underlying surimi gelation and disintegration enables the prediction of gel properties under varying heating temperatures and with the addition of TGase or protease inhibitors. Therefore, our approach may facilitate the utilization of noncommercial fish species for surimi processing by enabling better control over surimi gel properties. However, it is also necessary to construct a mathematical model that can quantitatively describe the relationship between MHC polymerization and degradation, and the mechanical properties of surimi gels.
5. Materials and Methods
5.1. Material
Fresh specimens of N. virgatus were purchased at a local market in Tokyo, Japan. The fish were transported to the laboratory on ice. Filleting was performed in a cold room, and the deboned, skinned flesh was stored at −35 °C until further use.
5.2. Surimi Preparation
N. virgatus surimi was prepared according to the method described by Kominami et al. (2025) [
28] with slight modifications. Briefly, frozen flesh was ground with 3% (
w/
w) NaCl after thawing overnight at 4 °C. The salt-ground surimi was then packed and sealed into plastic jars of either 20 mL (φ42 × 26 mm) or 5.5 mL (φ29 × 16 mm) capacity. All operations were conducted at 4°C.
The surimi-filled 20 mL jars were subjected to a temperature-controlled water bath heating under the following conditions: 50, 55, and 60 °C for 60 min, followed by heating at 85 °C for 20 min (referred to as two-step heating 50–85, 55–85, and 60–85), or a single-step heating at 85 °C for 20 min (referred to as single-step heating 85). Immediately after heating, the jars were rapidly cooled in ice water. Following overnight storage in ice water, the N. virgatus surimi gels were subjected to fracture strength measurements.
The 5.5 mL surimi-filled jars were heated in a 60 °C water bath for varying durations (2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 30, 40, and 60 min), then rapidly cooled and stored overnight in ice water. The temperature near the center of the surimi was monitored during 60 °C heating using a coated thermocouple wire (Type T, outer diameter: 0.9 mm, wire diameter: 0.254 mm), and data were recorded using a multichannel recorder (MCR-4TC, T&D Corporation, Nagano, Japan). Some of the 5.5 mL surimi-filled jars were stored in ice water without heating and used as unheated controls. Both unheated and heated N. virgatus surimi samples were subjected to constant-speed compression testing.
5.3. Fracture Strength Measurements
The fracture strength of the
N. virgatus surimi gels was determined using a rheometer, according to the method described by Kominami et al. (2020) [
29]. After measurement, the central portion of the sample was excised, flash-frozen in liquid nitrogen, and stored at −80 °C.
5.4. Compression Test
A rheometer (FUDOH RHEOMETER, NRM-2010-CW, Rheotech, Tokyo, Japan) was used to perform compression tests. Since non-gelled surimi lacks shape-retaining properties, it must be evaluated while contained in a plastic jar. Therefore, the evaluation method for gelatin described in the Japanese Industrial Standard K6503 was modified accordingly. In this study, to assess gelation at the center of the jar, a plunger with a diameter (φ5 mm) significantly smaller than that of the jar (φ25 mm) was employed. The 5.5 mL plastic jar with its lid removed was secured onto the sample stage. A cylindrical plunger (φ5 mm) was pressed into the surimi-filled plastic jar at a speed of 1 mm/sec to a depth of 10 mm, and the resulting stress transition of the surimi was recorded using a data logger (NR-HA08, KEYENCE, Osaka, Japan). The maximum stress during compression was defined as the maximum compressive stress (gf) for each sample. After measurement, the central portion of the sample was excised, flash-frozen in liquid nitrogen, and stored at −80 °C.
5.5. Protein Fractionation and SDS-PAGE
Proteins in the
N. virgatus surimi gel were fractionated into a water-soluble protein (WSP) fraction and a urea-solubilized protein fraction (USP) according to the method described by Kominami et al. (2025) [
28] with slight modifications. The frozen surimi sample was ground to a fine powder within the precooled steel cylinders (TH-SPT, Taitec corporation, Saitama, Japan) of an automatic cryogenic crusher (Taitec Freeze Crusher µT-48, Taitec corporation). The frozen powdered sample was suspended in an ice-cold low ionic strength buffer (20 mM KCl, 100 mM Tris-HCl, pH 7.4) at a concentration of 100 mg/mL, followed by centrifugation at 4000 g for 20 min at 4 °C. The supernatant was collected as the WSP fraction. The remaining pellet was washed twice with the low-ionic strength buffer. Subsequently, 40 mg of the resultant pellet was resuspended in 1 mL of either urea-SDS reducing buffer (0.03% bromophenol blue, 3% SDS, 8 M urea, 2 M thiourea, 75 mM dithiothreitol, 50 mM Tris-HCl, pH 6.8) or urea-SDS non-reducing buffer (0.03% bromophenol blue, 3% SDS, 8 M urea, 2 M thiourea, 50 mM Tris-HCl, pH 6.8). The samples were then solubilized overnight at room temperature by gentle inversion using a benchtop tube rotator. Subsequently, they were centrifuged at 12,000 g for 15 min at 20 °C, and the resulting supernatants were collected as the USP fraction.
The WSP fraction was mixed with 4× Laemmli’s sample buffer (Bio-Rad, Hercules, CA, USA) at a 3:1 ratio. The reduced USP fraction was diluted 10-fold with the urea−SDS reducing buffer. Both samples were heated at 95 °C for 3 min prior to SDS-PAGE. The WSP sample was separated using a 12% poly acrylamide gel, while both reduced and non-reduced USP samples were separated using a gradient 2−15% gel (Multi gel II mini 2/15, Cosmo Bio Co., Ltd., Tokyo, Japan). CBB staining and quantitative analysis were performed as described in a previous study [
30]. Briefly, images of the SDS-PAGE bands were acquired using an infrared imaging system (Odyssey Fc Imaging System, LI-COR, Lincoln, NE, USA), configured for an excitation wavelength of 680 nm and an emission wavelength of 700 nm. Densitometric analysis of the acquired images was conducted using ImageJ version 1.51t (US National Institutes of Health, MA, USA).
5.6. Data Analysis
Dunnett’s test was conducted using the package “DescTools” in R v3.4.37 to compare the fracture strength of the surimi gels versus those prepared by single-step heating [
30,
31].
5.7. Mathematical Modelling
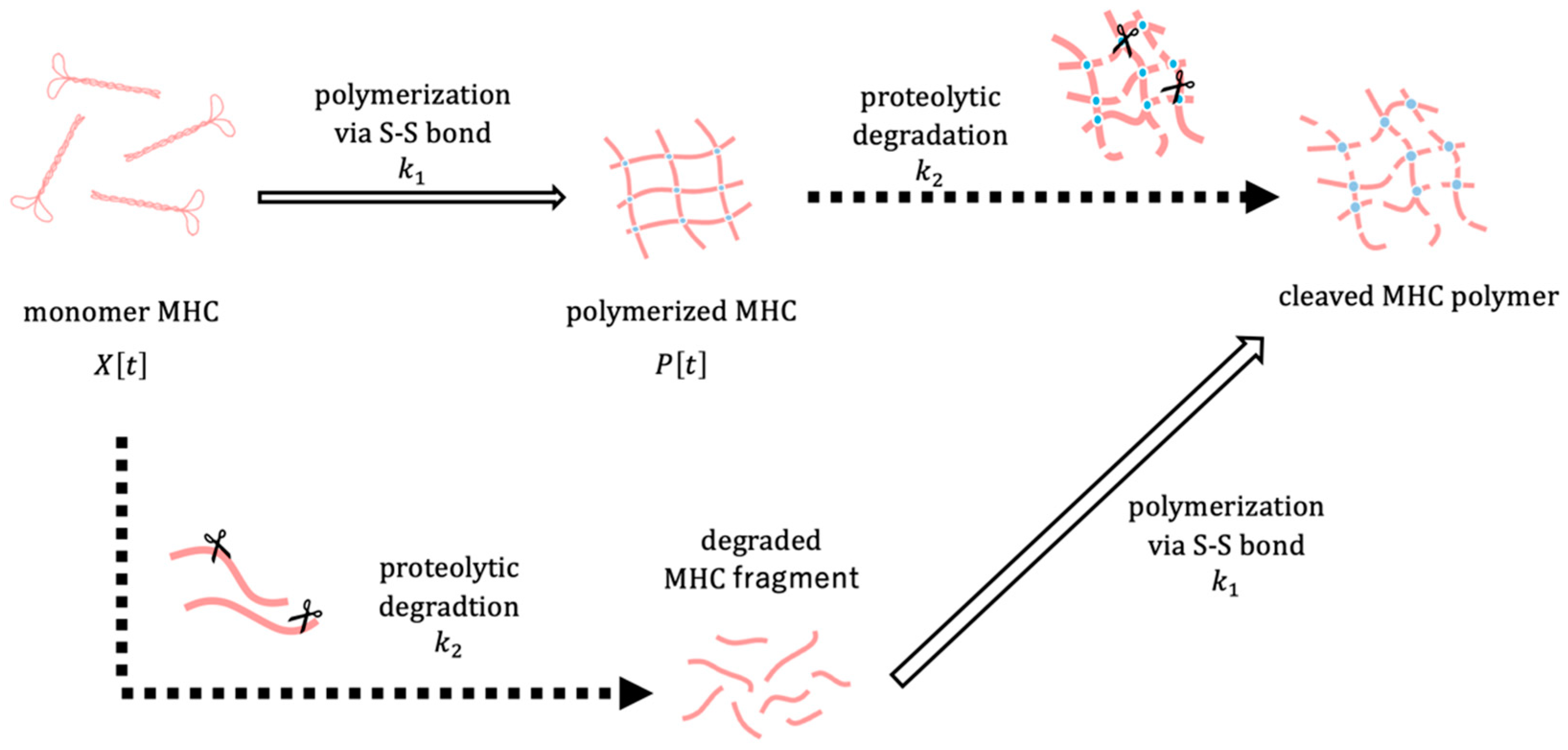
The polymerization and degradation of MHC were described by a system of ordinary differential equations. Let
denote the amount of MHC monomer,
the amount of MHC polymer, and
the core temperature of the surimi sample at time
. To focus on relative changes,
and
represent normalized amounts of MHC monomer and polymer, respectively. The conceptual framework of the model is depicted in
Figure 5. The amount of MHC in the reduced USP corresponds to the sum of
and
P[
t], whereas in the non-reduced USP, it corresponds to
alone. We assume that the polymerization rate of MHC depends on the surimi sample temperature
through a sigmoid function, which reflects the thermal denaturation behaviour of MHC observed in experiments. Considering that MHC degradation is maximized at an optimal temperature, the degradation rate is assumed to be modeled as a Gaussian function dependent on the surimi sample temperature
. The dynamics of MHC polymerization and degradation are described by the following system of differential equations:
with the initial values
,
, and
. The model contains the following parameters:
,
,
,
,
,
,
, and
. The parameter
represents the maximum polymerization rate of MHC. The parameters
and
are the inflection point and the steepness of the sigmoid function, respectively. The parameter
denotes the maximum rate of temperature-dependent degradation. The parameter
is the optimal temperature for MHC degradation and
is the width of a Gaussian function.
is defined as the highest temperature that the surimi sample can reach, i.e., the temperature of the water bath. The parameter
is the rate at which the heating temperature is increased to approach the target temperature
.
Based on measurements of the thermal denaturation of MHC, the parameters
and
were estimated as 29.61 and 22.34, respectively, using the Simple Fit app (v4.33) in OriginPro 2024 SR1. The parameters
and
were set to
and
respectively, from experimental data for the heating temperature. The parameter
was assigned a value of
, since the optimal temperature for MHC degradation is assumed to be
°C, as supported by our findings (
Figure 1) and a previous study [
13]. The parameters
,
, and
were determined by grid search to minimize the residual sum of squares between the numerical results and the experimental data. The values of the optimal parameters were found to be
,
, and
. Estimation of the parameters
, numerical solutions to the differential equations, and the data visualization for comparison with the experimental data were obtained using Wolfram Mathematica 13.1.