Development of Eco-Friendly Date Palm Biomass-Based Hydrogels for Enhanced Water Retention in Soil
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of DPR and Pretreatment
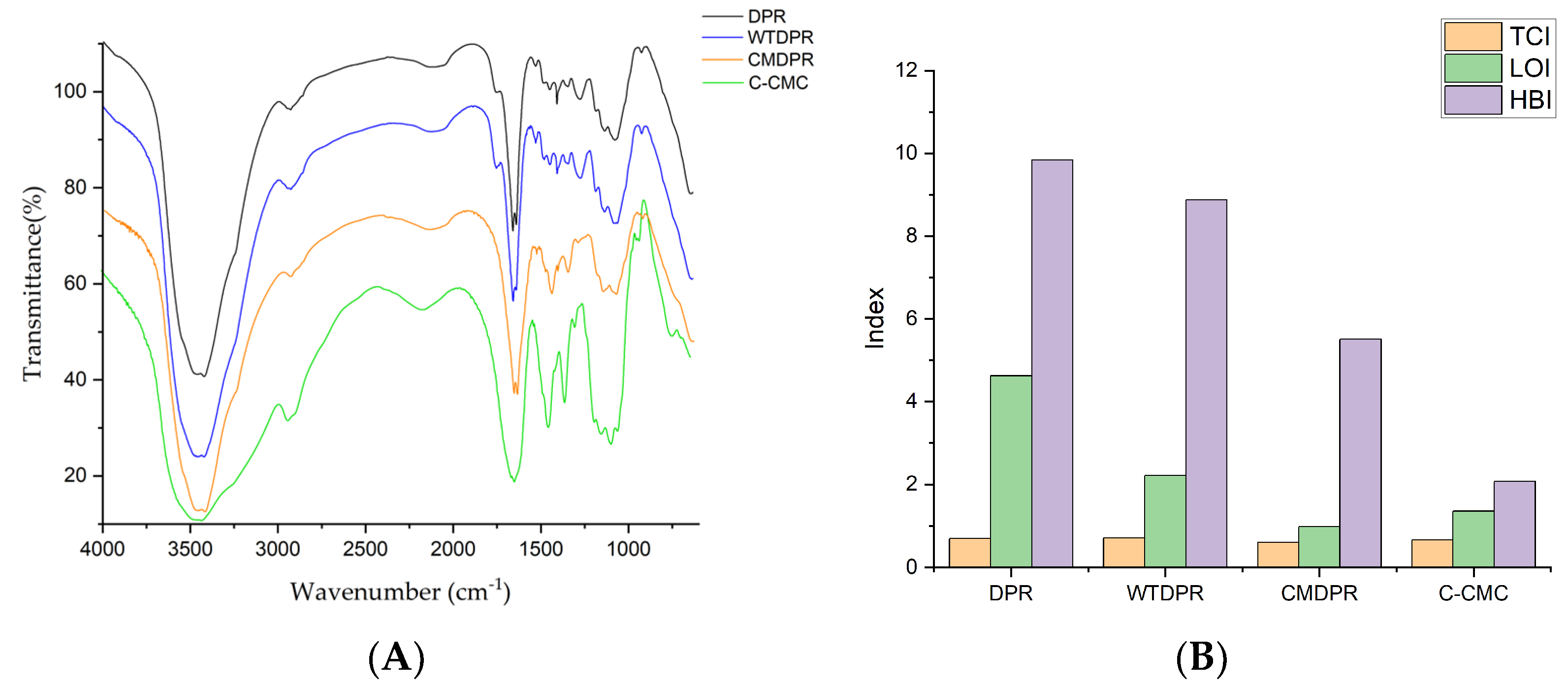
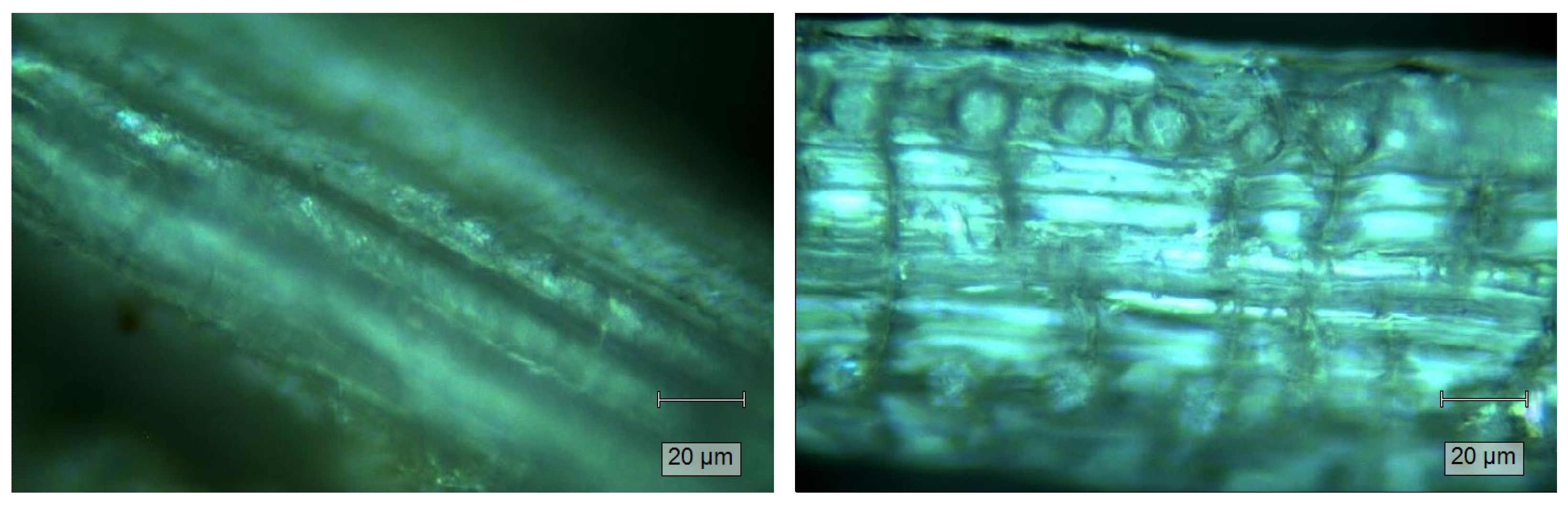
2.2. Characterization of DPR and WTDPR
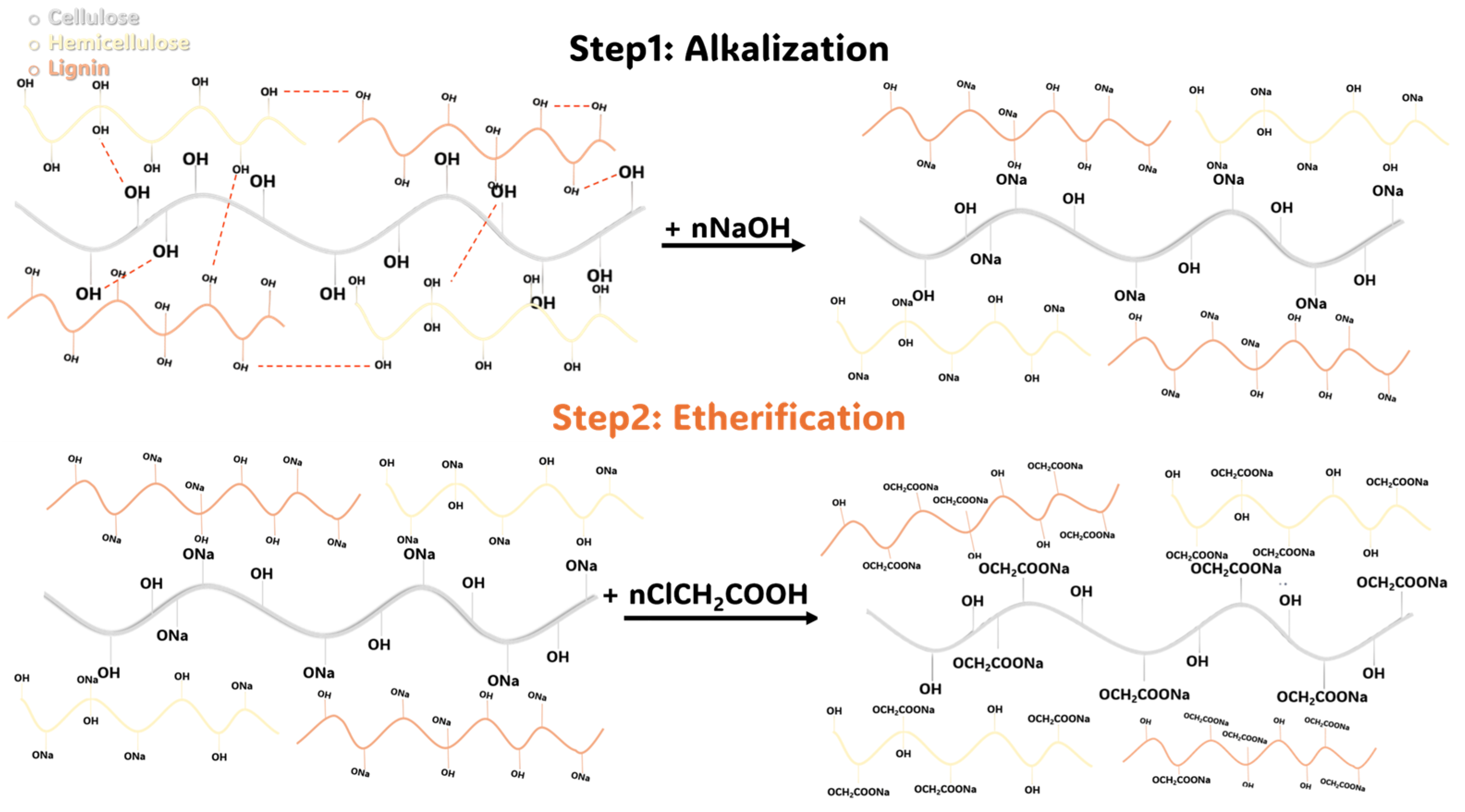
2.3. Preparation of CMDPR, Degree of Substitution, and Yield
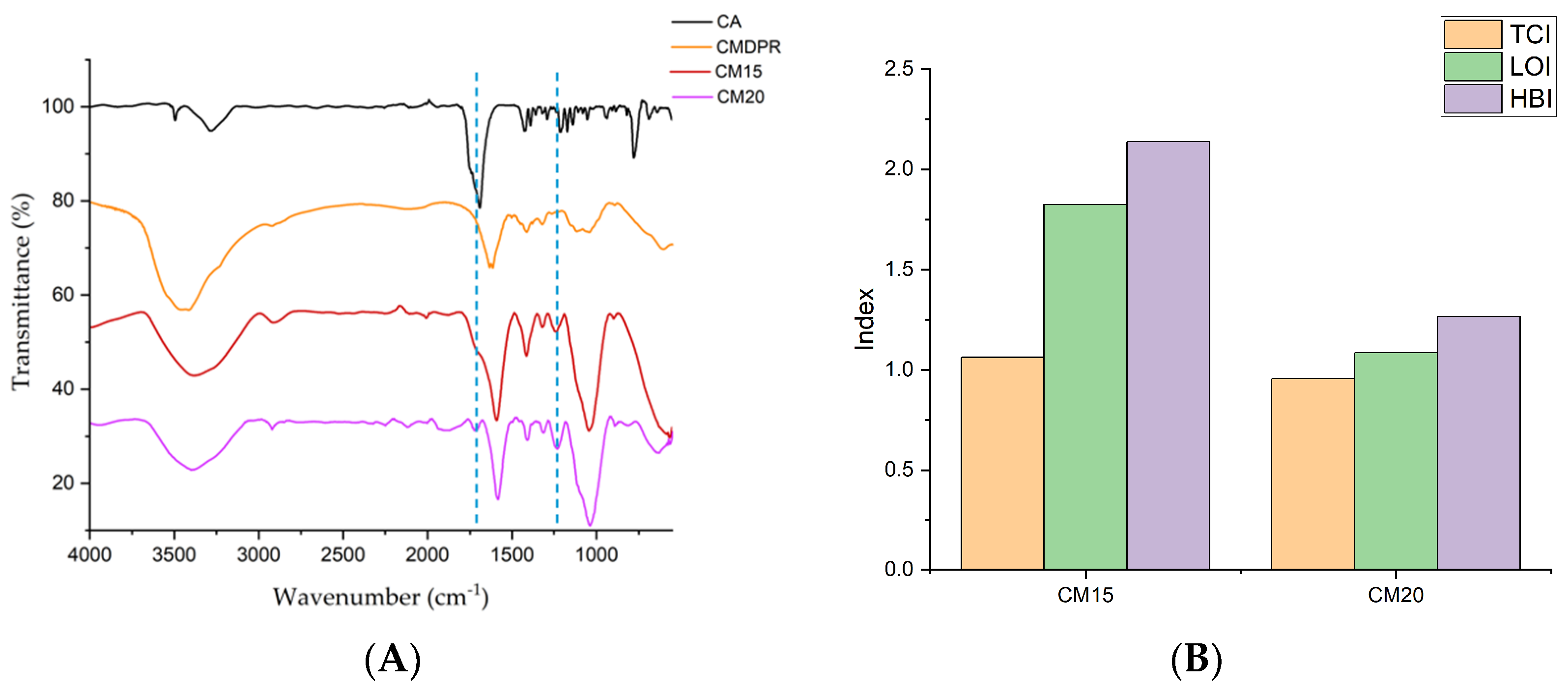
2.4. Characterization of CMDPR and C-CMC
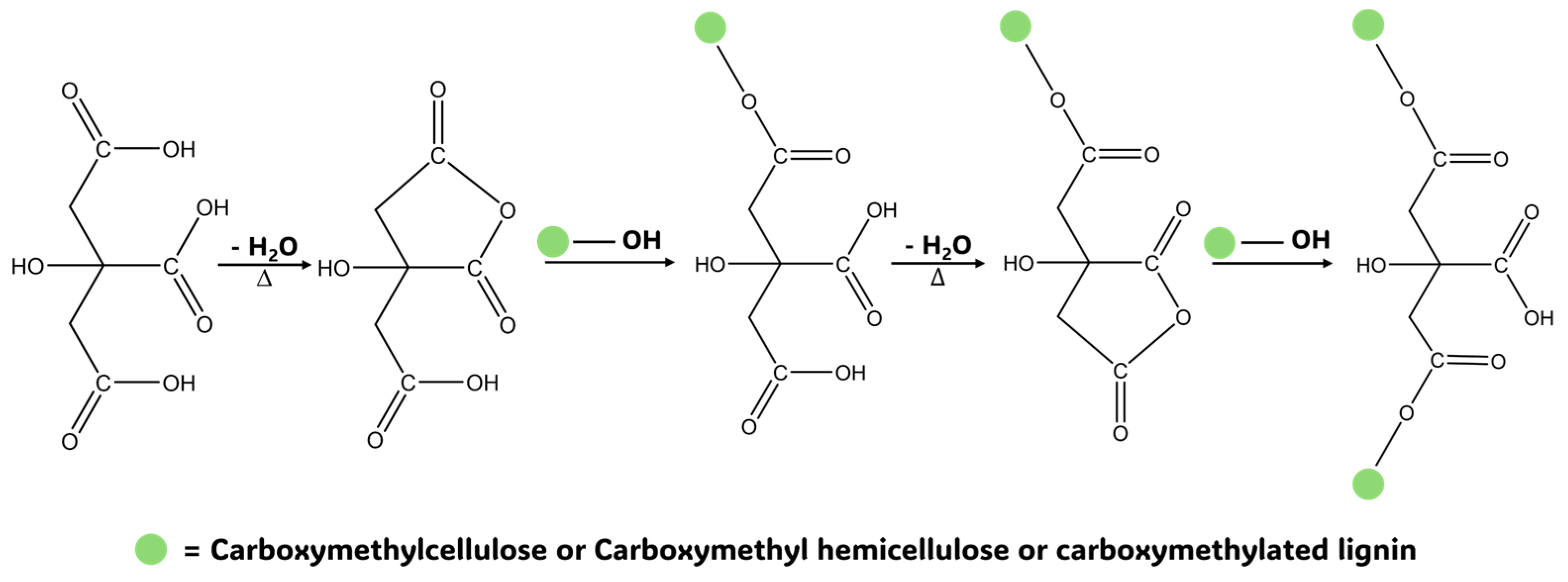
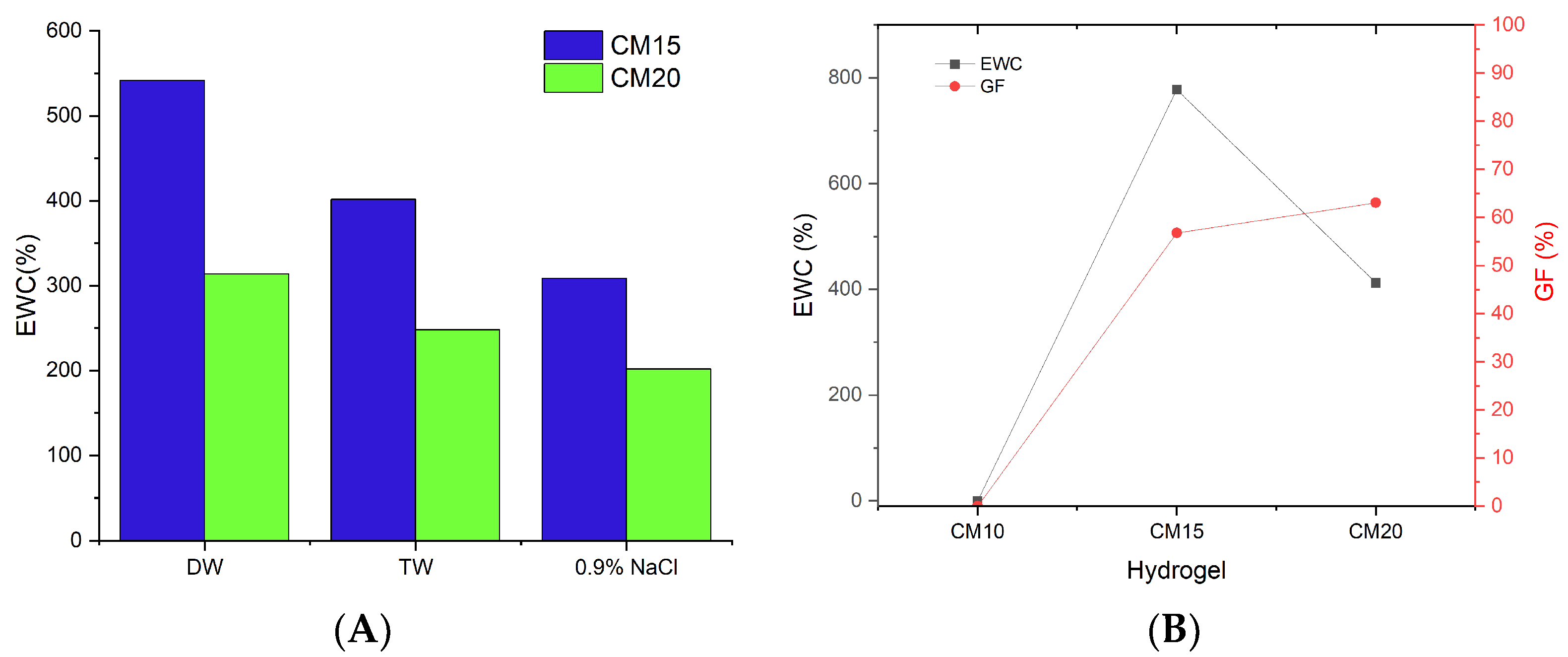
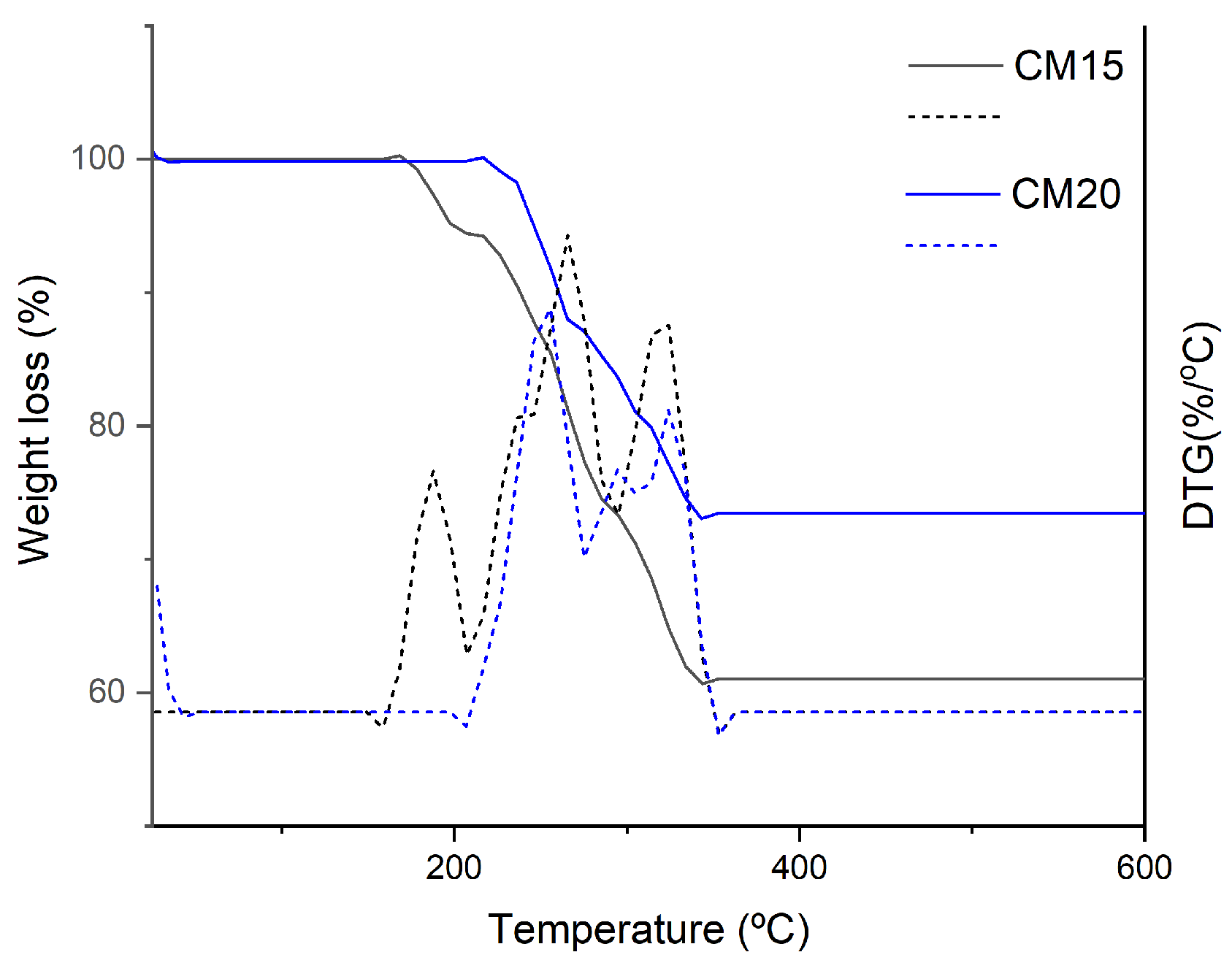
2.5. Hydrogels Fabrication and Characterization
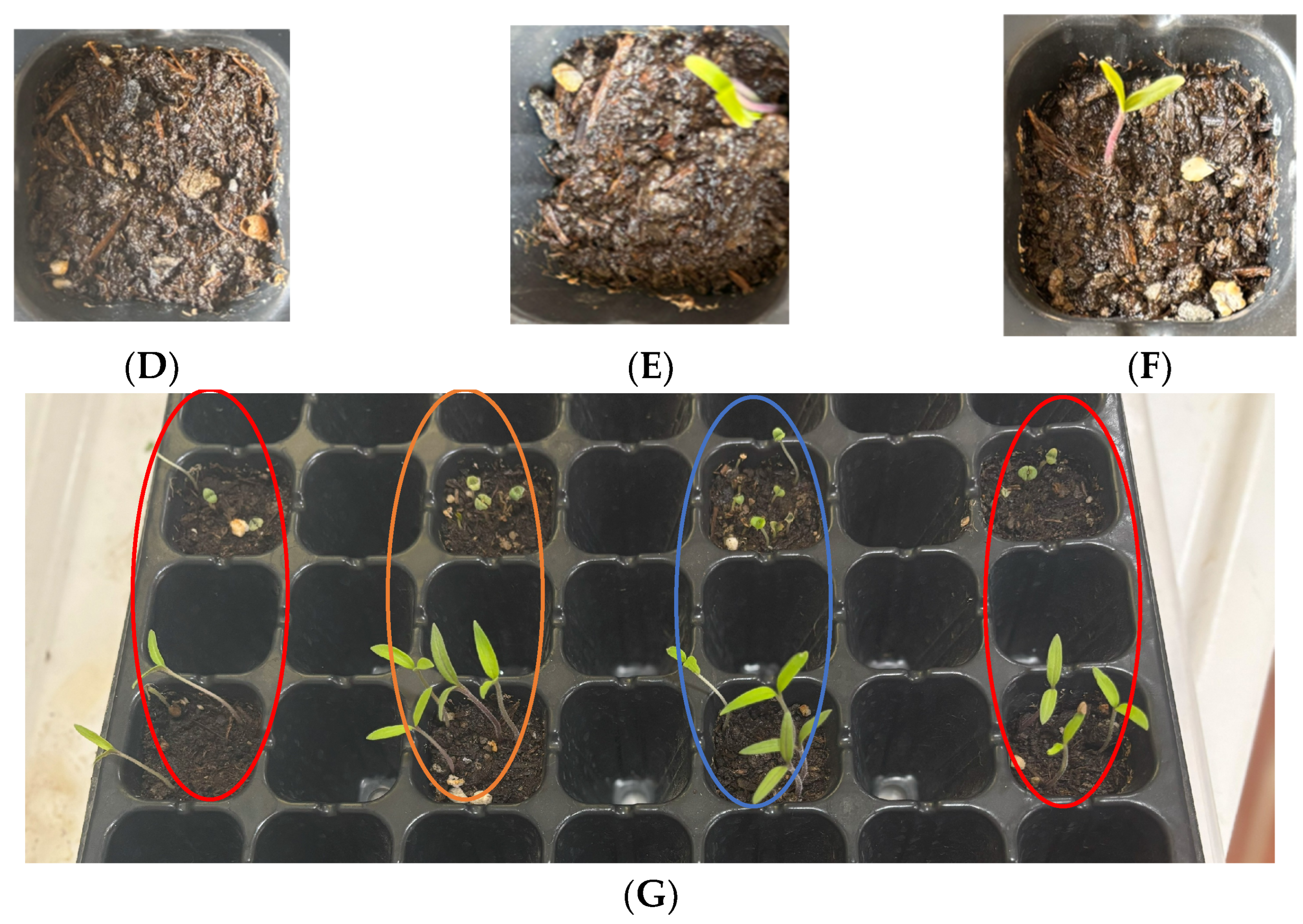
2.6. Hydrogels’ Effect on Germination
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Biomass Compositional Analysis and Pretreatment
4.3. Biomass Carboxymethylation
4.4. CMDPR Degree of Substitution and Yield
- A is milliequivalents of acid consumed per gram of sample.
- B is NaOH solution added amount in mL.
- C is the concentration of the NaOH solution in normality.
- D is the amount of HCl consumed in the titration in mL.
- E is the concentration of the HCl in normality.
- F is acidified CMDPR used (g).
- The value 162 is the gram molecular mass of the anhydrous glucose unit of cellulose.
- The value 584 is the net increase in molecular mass for each carboxymethyl group substituted.
- Wp is the weight of produced CMDPR.
- W0 is the weight of WTDPR.
4.5. Hydrogels Fabrication
4.6. Hydrogel Properties
4.6.1. Gel Fraction
4.6.2. Equilibrium Swelling Capacity
4.7. Effects of Hydrogels on Germination
4.8. Characterization
4.8.1. Fourier Transform Infrared Spectrometry (FTIR)
4.8.2. Microscope Imaging
4.8.3. Dynamic Light Scattering (DLS)
4.8.4. Thermal Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adjuik, T.A.; Nokes, S.E.; Montross, M.D.; Wendroth, O. Lignin-Based Hydrogel for Water Retention in Silt Loam Soil. In Proceedings of the American Society of Agricultural and Biological Engineers Annual International Meeting, ASABE 2021, Virtual, 12–16 July 2021; American Society of Agricultural and Biological Engineers: Saint Joseph, MI, USA, 2021; Volume 2, pp. 657–669. [Google Scholar]
- Elhag, M. Evaluation of Different Soil Salinity Mapping Using Remote Sensing Techniques in Arid Ecosystems, Saudi Arabia. J. Sens. 2016, 2016, 7596175. [Google Scholar] [CrossRef]
- Vedovello, P.; Sanches, L.V.; da Silva Teodoro, G.; Majaron, V.F.; Bortoletto-Santos, R.; Ribeiro, C.; Putti, F.F. An Overview of Polymeric Hydrogel Applications for Sustainable Agriculture. Agriculture 2024, 14, 840. [Google Scholar] [CrossRef]
- Patra, S.K.; Poddar, R.; Brestic, M.; Acharjee, P.U.; Bhattacharya, P.; Sengupta, S.; Pal, P.; Bam, N.; Biswas, B.; Barek, V.; et al. Prospects of Hydrogels in Agriculture for Enhancing Crop and Water Productivity under Water Deficit Condition. Int. J. Polym. Sci. 2022, 2022, 4914836. [Google Scholar] [CrossRef]
- Cannazza, G.; Cataldo, A.; de Benedetto, E.; Demitri, C.; Madaghiele, M.; Sannino, A. Experimental Assessment of the Use of a Novel Superabsorbent Polymer (SAP) for the Optimization of Water Consumption in Agricultural Irrigation Process. Water 2014, 6, 2056–2069. [Google Scholar] [CrossRef]
- Koech, R.; Langat, P. Improving Irrigation Water Use Efficiency: A Review of Advances, Challenges and Opportunities in the Australian Context. Water 2018, 10, 1771. [Google Scholar] [CrossRef]
- Ghanem, A.M.; Alamri, Y.A. The Impact of the Green Middle East Initiative on Sustainable Development in the Kingdom of Saudi Arabia. J. Saudi Soc. Agric. Sci. 2023, 22, 35–46. [Google Scholar] [CrossRef]
- Sojka, R.E.; Bjorneberg, D.L.; Entry, J.A.; Lentz, R.D.; Orts, W.J. Polyacrylamide in Agriculture and Environmental Land Management. Adv. Agron. 2007, 92, 75–162. [Google Scholar]
- Chamorro, A.F.; Palencia, M.; Arrieta, Á.A. Development of High-Efficiency Fertilizer by Hydrogels Obtained from Cassava Starch and Citric Acid for Slow Release of Ammonium and Potassium. Gels 2024, 10, 434. [Google Scholar] [CrossRef]
- Tariq, Z.; Iqbal, D.N.; Rizwan, M.; Ahmad, M.; Faheem, M.; Ahmed, M. Significance of Biopolymer-Based Hydrogels and Their Applications in Agriculture: A Review in Perspective of Synthesis and Their Degree of Swelling for Water Holding. RSC Adv. 2023, 13, 24731–24754. [Google Scholar] [CrossRef]
- Zhao, B.; Moore, J.S. Fast PH- and Ionic Strength-Responsive Hydrogels in Microchannels. Langmuir 2001, 17, 4758–4763. [Google Scholar] [CrossRef]
- Adjuik, T.A.; Nokes, S.E.; Montross, M.D. Biodegradability of Bio-Based and Synthetic Hydrogels as Sustainable Soil Amendments: A Review. J. Appl. Polym. Sci. 2023, 140, e53655. [Google Scholar] [CrossRef]
- Adjuik, T.A.; Nokes, S.E.; Montross, M.D.; Wendroth, O. The Impacts of Bio-Based and Synthetic Hydrogels on Soil Hydraulic Properties: A Review. Polymers 2022, 14, 4721. [Google Scholar] [CrossRef]
- Agbna, G.H.D.; Zaidi, S.J. Hydrogel Performance in Boosting Plant Resilience to Water Stress—A Review. Gels 2025, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of Cellulose-Based Superabsorbent Hydrogels as Water Reservoir in Agriculture. Int. J. Polym. Sci. 2013, 2013, 435073. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Kaewpirom, S. Potassium Release Kinetics and Water Retention of Controlled-Release Fertilizers Based on Chitosan Hydrogels. J. Polym. Environ. 2010, 18, 413–421. [Google Scholar] [CrossRef]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef]
- Gschwend, F.J.V.; Brandt-Talbot, A.; Chambon, C.L.; Hallett, J.P. Ultra-Low Cost Ionic Liquids for the Delignification of Biomass. In Ionic Liquids: Current State and Future Directions; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2017; Volume 1250, pp. 209–223. [Google Scholar]
- Shahi, N.; Wang, P.; Adhikari, S.; Min, B.; Rangari, V.K. Biopolymers Fractionation and Synthesis of Nanocellulose/Silica Nanoparticles from Agricultural Byproducts. ACS Sustain. Chem. Eng. 2021, 9, 6284–6295. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Shrestha, S.; Kognou, A.L.M.; Zhang, J.; Qin, W. Different Facets of Lignocellulosic Biomass Including Pectin and Its Perspectives. Waste Biomass Valorization 2021, 12, 4805–4823. [Google Scholar] [CrossRef]
- Gabrielii, I.; Gatenholm, P. Preparation and Properties of Hydrogels Based on Hemicellulose. J. Appl. Polym. Sci. 1998, 69, 1661–1667. [Google Scholar] [CrossRef]
- Palantöken, S.; Bethke, K.; Zivanovic, V.; Kalinka, G.; Kneipp, J.; Rademann, K. Cellulose Hydrogels Physically Crosslinked by Glycine: Synthesis, Characterization, Thermal and Mechanical Properties. J. Appl. Polym. Sci. 2020, 137, 48380. [Google Scholar] [CrossRef]
- Wu, L.; Huang, S.; Zheng, J.; Qiu, Z.; Lin, X.; Qin, Y. Synthesis and Characterization of Biomass Lignin-Based PVA Super-Absorbent Hydrogel. Int. J. Biol. Macromol. 2019, 140, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.; Qaisar, K.; Nawaz, A.; Akram, F.; Mukhtar, H.; Zohu, X.; Xu, Y.; Mumtaz, M.; Rashid, U.; Ghani, W.; et al. Advances in Valorization of Lignocellulosic Biomass towards Energy Generation. Catalysts 2021, 11, 309. [Google Scholar] [CrossRef]
- Verdía Barbará, P.; Abouelela Rafat, A.; Hallett, J.P.; Brandt-Talbot, A. Purifying Cellulose from Major Waste Streams Using Ionic Liquids and Deep Eutectic Solvents. Curr. Opin. Green Sustain. Chem. 2023, 41, 100783. [Google Scholar] [CrossRef]
- Sosa, F.H.B.; Dias, R.M.; da Costa Lopes, A.M.; Coutinho, J.A.P.; da Costa, M.C. Fast and Efficient Method to Evaluate the Potential of Eutectic Solvents Todissolve Lignocellulosic Components. Sustainability 2020, 12, 3358. [Google Scholar] [CrossRef]
- Shaikh, H.M.; Anis, A.; Poulose, A.M.; Al-Zahrani, S.M.; Madhar, N.A.; Alhamidi, A.; Aldeligan, S.H.; Alsubaie, F.S. Synthesis and Characterization of Cellulose Triacetate Obtained from Date Palm (Phoenix dactylifera L.) Trunk Mesh-Derived Cellulose. Molecules 2022, 27, 1434. [Google Scholar] [CrossRef]
- Nasatto, P.; Pignon, F.; Silveira, J.; Duarte, M.; Noseda, M.; Rinaudo, M. Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef]
- Chakka, V.P.; Zhou, T. Carboxymethylation of Polysaccharides: Synthesis and Bioactivities. Int. J. Biol. Macromol. 2020, 165, 2425–2431. [Google Scholar] [CrossRef]
- Saadaoui, N.; Rouilly, A.; Fares, K.; Rigal, L. Characterization of Date Palm Lignocellulosic By-Products and Self-Bonded Composite Materials Obtained Thereof. Mater. Des. 2013, 50, 302–308. [Google Scholar] [CrossRef]
- Shaikh, H.M.; Anis, A.; Poulose, A.M.; Al-Zahrani, S.M.; Madhar, N.A.; Alhamidi, A.; Alam, M.A. Isolation and Characterization of Alpha and Nanocrystalline Cellulose from Date Palm (Phoenix dactylifera L.) Trunk Mesh. Polymers 2021, 13, 1893. [Google Scholar] [CrossRef]
- Galiwango, E.; Abdel Rahman, N.S.; Al-Marzouqi, A.H.; Abu-Omar, M.M.; Khaleel, A.A. Isolation and Characterization of Cellulose and α-Cellulose from Date Palm Biomass Waste. Heliyon 2019, 5, e02937. [Google Scholar] [CrossRef] [PubMed]
- Ghori, W.; Saba, N.; Jawaid, M.; Asim, M. A Review on Date Palm (Phoenix dactylifera) Fibers and Its Polymer Composites. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 012009. [Google Scholar] [CrossRef]
- Haddadou, I.; Aliouche, D.; Brosse, N.; Amirou, S. Characterization of Cellulose Prepared from Some Algerian Lignocellulosic Materials (Zeen Oak Wood, Aleppo Pine Wood and Date Palm Rachis). Eur. J. Wood Wood Prod. 2015, 73, 419–421. [Google Scholar] [CrossRef]
- Mirmehdi, S.M.; Zeinaly, F.; Dabbagh, F. Date Palm Wood Flour as Filler of Linear Low-Density Polyethylene. Compos. B Eng. 2014, 56, 137–141. [Google Scholar] [CrossRef]
- Amirou, S.; Zerizer, A.; Pizzi, A.; Haddadou, I.; Zhou, X. Particleboards Production from Date Palm Biomass. Eur. J. Wood Wood Prod. 2013, 71, 717–723. [Google Scholar] [CrossRef]
- Suriyatem, R.; Noikang, N.; Kankam, T.; Jantanasakulwong, K.; Leksawasdi, N.; Phimolsiripol, Y.; Insomphun, C.; Seesuriyachan, P.; Chaiyaso, T.; Jantrawut, P.; et al. Physical Properties of Carboxymethyl Cellulose from Palm Bunch and Bagasse Agricultural Wastes: Effect of Delignification with Hydrogen Peroxide. Polymers 2020, 12, 1505. [Google Scholar] [CrossRef]
- Rahman, M.S.; Mondal, M.I.H.; Yeasmin, M.S.; Abu Sayeed, M.; Hossain, M.A.; Ahmed, M.B. Conversion of Lignocellulosic Corn Agro-Waste into Cellulose Derivative and Its Potential Application as Pharmaceutical Excipient. Processes 2020, 8, 711. [Google Scholar] [CrossRef]
- Moreira, P.H.S.S.; de Oliveira Freitas, J.C.; Braga, R.M.; Araújo, R.M.; de Souza, M.A.F. Production of Carboxymethyl Lignin from Sugar Cane Bagasse: A Cement Retarder Additive for Oilwell Application. Ind. Crops Prod. 2018, 116, 144–149. [Google Scholar] [CrossRef]
- Alsulami, R.A.; El-Sayed, S.A.; Eltaher, M.A.; Mohammad, A.; Almitani, K.H.; Mostafa, M.E. Thermal Decomposition Characterization and Kinetic Parameters Estimation for Date Palm Wastes and Their Blends Using TGA. Fuel 2023, 334, 126600. [Google Scholar] [CrossRef]
- Younis, M.; Farag, H.A.; Alhamdan, A.; Aboelasaad, G.; Zein El-Abedein, A.I.; Kamel, R.M. Utilization of Palm Residues for Biochar Production Using Continuous Flow Pyrolysis Unit. Food Chem. X 2023, 20, 100903. [Google Scholar] [CrossRef]
- Sun; Tomkinson, J.; Zhu, W.; Wang, S.Q. Delignification of Maize Stems by Peroxymonosulfuric Acid, Peroxyformic Acid, Peracetic Acid, and Hydrogen Peroxide. 1. Physicochemical and Structural Characterization of the Solubilized Lignins. J. Agric. Food Chem. 2000, 48, 1253–1262. [Google Scholar] [CrossRef]
- Saleem, H.; Saud, A.; Zaidi, S.J. Sustainable Preparation of Graphene Quantum Dots from Leaves of Date Palm Tree. ACS Omega 2023, 8, 28098–28108. [Google Scholar] [CrossRef] [PubMed]
- Sizirici, B.; Fseha, Y.H.; Yildiz, I.; Delclos, T.; Khaleel, A. The Effect of Pyrolysis Temperature and Feedstock on Date Palm Waste Derived Biochar to Remove Single and Multi-Metals in Aqueous Solutions. Sustain. Environ. Res. 2021, 31, 9. [Google Scholar] [CrossRef]
- Zrelli, A.; Elfalleh, W.; Ghorbal, A.; Chaouachi, B. Valorization of Date Palm Wastes by Lignin Extraction to Be Used for the Improvement of Polymeric Membrane Characteristics. Period. Polytech. Chem. Eng. 2022, 66, 70–81. [Google Scholar] [CrossRef]
- Boumediri, H.; Bezazi, A.; Del Pino, G.G.; Haddad, A.; Scarpa, F.; Dufresne, A. Extraction and Characterization of Vascular Bundle and Fiber Strand from Date Palm Rachis as Potential Bio-Reinforcement in Composite. Carbohydr. Polym. 2019, 222, 114997. [Google Scholar] [CrossRef]
- Bezazi, A.; Belaadi, A.; Bourchak, M.; Scarpa, F.; Boba, K. Novel Extraction Techniques, Chemical and Mechanical Characterisation of Agave americana L. Natural Fibres. Compos. B Eng. 2014, 66, 194–203. [Google Scholar] [CrossRef]
- Kasyapi, N.; Chaudhary, V.; Bhowmick, A.K. Bionanowhiskers from Jute: Preparation and Characterization. Carbohydr. Polym. 2013, 92, 1116–1123. [Google Scholar] [CrossRef]
- Bezazi, A.; Boumediri, H.; Garcia del Pino, G.; Bezzazi, B.; Scarpa, F.; Reis, P.N.B.; Dufresne, A. Alkali Treatment Effect on Physicochemical and Tensile Properties of Date Palm Rachis Fibers. J. Nat. Fibers 2020, 19, 3770–3787. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Effect of Hot Water Extraction and Liquid Hot Water Pretreatment on the Fungal Degradation of Biomass Feedstocks. Bioresour. Technol. 2011, 102, 9788–9793. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of Certain Infrared Bands to Cellulose Crystallinity and Crystal Lattice Type. Part II. A New Infrared Ratio for Estimation of Crystallinity in Celluloses I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of Certain Infrared Bands to Cellulose Crystallinity and Crystal Latticed Type. Part I. Spectra of Lattice Types I, II, III and of Amorphous Cellulose. J. Appl. Polym. Sci. 1964, 8, 1311–1324. [Google Scholar] [CrossRef]
- Nada, A.M.A.; Hassan, M.L. Thermal Behavior of Cellulose and Some Cellulose Derivatives. Polym. Degrad. Stab. 2000, 67, 111–115. [Google Scholar] [CrossRef]
- Hrčka, R.; Kučerová, V.; Hönig, V. Dry-Matter Loss and Changes in the Chemical Composition of Spruce Wood after Long-Term Storing in the Form of Roundwood. Polymers 2022, 14, 3400. [Google Scholar] [CrossRef]
- Singh, J.K.; Chaurasia, B.; Dubey, A.; Noguera, A.M.F.; Gupta, A.; Kothari, R.; Upadhyaya, C.P.; Kumar, A.; Hashem, A.; Alqarawi, A.A.; et al. Biological Characterization and Instrumental Analytical Comparison of Two Biorefining Pretreatments for Water Hyacinth (Eicchornia crassipes) Biomass Hydrolysis. Sustainability 2021, 13, 245. [Google Scholar] [CrossRef]
- Krutul, D.; Szadkowski, J.; Výbohová, E.; Kučerová, V.; Čabalová, I.; Antczak, A.; Szadkowska, D.; Drożdżek, M.; Zawadzki, J. Effect of Steam Explosion Pretreatment on Chosen Saccharides Yield and Cellulose Structure from Fast-Growing Poplar (Populus Deltoides × Maximowiczii) Wood. Wood Sci. Technol. 2024, 58, 441–458. [Google Scholar] [CrossRef]
- Dos Santos Dias, D.; Otoni, C.G.; da Silva, R.R.; Meneguin, A.B.; Mattoso, L.H.C.; da Barud, H.S.; Ribeiro, C.A. Large Scale Manufacturing of Puree-Only Edible Films from Onion Bulb (Allium cepa L.): Probing Production and Structure–Processing–Property Correlations. Ind. Crops Prod. 2020, 145, 111847. [Google Scholar] [CrossRef]
- Rafidison, B.H.; Ramasawmy, H.; Chummun, J.; Florens, F.B.V. Using Infrared Spectrum Analyses to Predict Tensile Strength of Fibres in a Group of Closely Related Plant Species: Case of Mascarenes Pandanus spp. SN Appl. Sci. 2020, 2, 1922. [Google Scholar] [CrossRef]
- Kučerová, V.; Výbohová, E.; Hönig, V.; Čabalová, I. Chemical Changes within Solids during Liquid Hot Water Pretreatment of Wood. BioResources 2020, 15, 38. [Google Scholar] [CrossRef]
- Hrčka, R.; Kučerová, V.; Hýrošová, T.; Hönig, V. Cell Wall Saturation Limit and Selected Properties of Thermally Modified Oak Wood and Cellulose. Forests 2020, 11, 640. [Google Scholar] [CrossRef]
- Kačík, F.; Šmíra, P.; Kačíková, D.; Vel’ková, V.; Nasswettrová, A.; Vacek, V. Chemical Alterations of Pine Wood Saccharides during Heat Sterilisation. Carbohydr. Polym. 2015, 117, 681–686. [Google Scholar] [CrossRef]
- Isroi; Ishola, M.; Millati, R.; Syamsiah, S.; Cahyanto, M.; Niklasson, C.; Taherzadeh, M. Structural Changes of Oil Palm Empty Fruit Bunch (OPEFB) after Fungal and Phosphoric Acid Pretreatment. Molecules 2012, 17, 14995–15012. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Berglund, L.A. Recycling without Fiber Degradation—Strong Paper Structures for 3D Forming Based on Nanostructurally Tailored Wood Holocellulose Fibers. ACS Sustain. Chem. Eng. 2020, 8, 1146–1154. [Google Scholar] [CrossRef]
- Huang, C.; Cheng, J.; Zhan, Y.; Liu, X.; Wang, J.; Wang, Y.; Yoo, C.G.; Fang, G.; Meng, X.; Ragauskas, A.J.; et al. Utilization of Guaiacol-Based Deep Eutectic Solvent for Achieving a Sustainable Biorefinery. Bioresour. Technol. 2022, 362, 127771. [Google Scholar] [CrossRef]
- Wahlström, N.; Edlund, U.; Pavia, H.; Toth, G.; Jaworski, A.; Pell, A.J.; Choong, F.X.; Shirani, H.; Nilsson, K.P.R.; Richter-Dahlfors, A. Cellulose from the Green Macroalgae Ulva lactuca: Isolation, Characterization, Optotracing, and Production of Cellulose Nanofibrils. Cellulose 2020, 27, 3707–3725. [Google Scholar] [CrossRef]
- Berglund, J.; Mikkelsen, D.; Flanagan, B.M.; Dhital, S.; Gaunitz, S.; Henriksson, G.; Lindström, M.E.; Yakubov, G.E.; Gidley, M.J.; Vilaplana, F. Wood Hemicelluloses Exert Distinct Biomechanical Contributions to Cellulose Fibrillar Networks. Nat. Commun. 2020, 11, 4692. [Google Scholar] [CrossRef] [PubMed]
- Khiari, R.; Mhenni, M.F.; Belgacem, M.N.; Mauret, E. Valorisation of Vegetal Wastes as a Source of Cellulose and Cellulose Derivatives. J. Polym. Environ. 2011, 19, 80–89. [Google Scholar] [CrossRef]
- Jin, Z.; Yu, Y.; Shao, S.; Ye, J.; Lin, L.; Iiyama, K. Lignin as a Cross-Linker of Acrylic Acid-Grafted Carboxymethyl Lignocellulose. J. Wood Sci. 2010, 56, 470–476. [Google Scholar] [CrossRef]
- Rani, D.; Ahuja, M. Carboxymethylation of Lepidium Sativum Polyuronide, Its Characterization and Evaluation as a Nanometric Carrier. Int. J. Biol. Macromol. 2017, 99, 233–240. [Google Scholar] [CrossRef]
- Sankhla, S.; Sardar, H.H.; Neogi, S. Greener Extraction of Highly Crystalline and Thermally Stable Cellulose Micro-Fibers from Sugarcane Bagasse for Cellulose Nano-Fibrils Preparation. Carbohydr. Polym. 2021, 251, 117030. [Google Scholar] [CrossRef]
- Yan, Z.; Li, J.; Chang, S.; Cui, T.; Jiang, Y.; Yu, M.; Zhang, L.; Zhao, G.; Qi, P.; Li, S. Lignin Relocation Contributed to the Alkaline Pretreatment Efficiency of Sweet Sorghum Bagasse. Fuel 2015, 158, 152–158. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and Chemical Modifications of Lignin: Towards Lignin-Based Nanomaterials for Biomedical Applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Lu, Y.; He, Q.; Fan, G.; Cheng, Q.; Song, G. Extraction and Modification of Hemicellulose from Lignocellulosic Biomass: A Review. Green Process. Synth. 2021, 10, 779–804. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Jantrawut, P.; Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; et al. Carboxymethyl Bacterial Cellulose from Nata de Coco: Effects of NaOH. Polymers 2021, 13, 348. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; de Bukzem, A.L.; Ascheri, D.P.R.; Signini, R.; de Aquino, G.L.B. Microwave-Assisted Carboxymethylation of Cellulose Extracted from Brewer’s Spent Grain. Carbohydr. Polym. 2015, 131, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Moussa, I.; Khiari, R.; Moussa, A.; Belgacem, M.N.; Mhenni, M.F. Preparation and Characterization of Carboxymethyl Cellulose with a High Degree of Substitution from Agricultural Wastes. Fibers Polym. 2019, 20, 933–943. [Google Scholar] [CrossRef]
- Yeasmin, M.S.; Mondal, M.I.H. Synthesis of Highly Substituted Carboxymethyl Cellulose Depending on Cellulose Particle Size. Int. J. Biol. Macromol. 2015, 80, 725–731. [Google Scholar] [CrossRef]
- Churam, T.; Usubharatana, P.; Phungrassami, H. Sustainable Production of Carboxymethyl Cellulose: A Biopolymer Alternative from Sugarcane (Saccharum officinarum L.) Leaves. Sustainability 2024, 16, 2352. [Google Scholar] [CrossRef]
- Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; Jantrawut, P.; Sommano, S.R.; et al. Synthesis, Characterization, and Application of Carboxymethyl Cellulose from Asparagus Stalk End. Polymers 2021, 13, 81. [Google Scholar] [CrossRef]
- Lin, B.-J.; Chen, W.-H. Sugarcane Bagasse Pyrolysis in a Carbon Dioxide Atmosphere with Conventional and Microwave-Assisted Heating. Front. Energy Res. 2015, 3, 4. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; Trivedi, N.; Reddy, C.R.K. Synthesis and Characterization of Seaweed Cellulose Derived Carboxymethyl Cellulose. Carbohydr. Polym. 2017, 157, 1604–1610. [Google Scholar] [CrossRef]
- Sathawong, S.; Sridach, W.; Techato, K. Lignin: Isolation and Preparing the Lignin Based Hydrogel. J. Environ. Chem. Eng. 2018, 6, 5879–5888. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel Superabsorbent Cellulose-based Hydrogels Crosslinked with Citric Acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Durpekova, S.; Di Martino, A.; Dusankova, M.; Drohsler, P.; Sedlarik, V. Biopolymer Hydrogel Based on Acid Whey and Cellulose Derivatives for Enhancement Water Retention Capacity of Soil and Slow Release of Fertilizers. Polymers 2021, 13, 3274. [Google Scholar] [CrossRef] [PubMed]
- Mignon, A.; De Belie, N.; Dubruel, P.; Van Vlierberghe, S. Superabsorbent Polymers: A Review on the Characteristics and Applications of Synthetic, Polysaccharide-Based, Semi-Synthetic and ‘Smart’ Derivatives. Eur. Polym. J. 2019, 117, 165–178. [Google Scholar] [CrossRef]
- Pintro, J.; Inoue, T.T. A Comparative Study of Determined and Calculated Values of Ionic Strength of Different Nutrient Solutions Consisting of an Ionic Pair. J. Plant. Nutr. 1999, 22, 1223–1231. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, I.C.; Carvalho, S.M.; Mansur, H.S. Bioengineered Water-Responsive Carboxymethyl Cellulose/Poly(Vinyl Alcohol) Hydrogel Hybrids for Wound Dressing and Skin Tissue Engineering Applications. Gels 2023, 9, 166. [Google Scholar] [CrossRef]
- Dai, H.; Huang, H. Enhanced Swelling and Responsive Properties of Pineapple Peel Carboxymethyl Cellulose-g-Poly(Acrylic Acid-Co-Acrylamide) Superabsorbent Hydrogel by the Introduction of Carclazyte. J. Agric. Food. Chem. 2017, 65, 565–574. [Google Scholar] [CrossRef]
- Saputra, A.H.; Hapsari, M.; Pitaloka, A.B.; Wulan, P.P.D.K. Synthesis and Characterization of Hydrogel from Cellulose Derivatives of Water-Hyacinth (Eichhornia crassipes) through Chemical Cross-Linking Method by Using Citric Acid. J. Eng. Sci. Technol. 2015, 10, 75–86. [Google Scholar]
- de Lima, G.F.; de Souza, A.G.; dos Rosa, D.S. Nanocellulose as Reinforcement in Carboxymethylcellulose Superabsorbent Nanocomposite Hydrogels. Macromol. Symp. 2020, 394, 2000126. [Google Scholar] [CrossRef]
- Priya, G.; Narendrakumar, U.; Manjubala, I. Thermal Behavior of Carboxymethyl Cellulose in the Presence of Polycarboxylic Acid Crosslinkers. J. Therm. Anal. Calorim. 2019, 138, 89–95. [Google Scholar] [CrossRef]
- Ghilan, A.; Nita, L.E.; Pamfil, D.; Simionescu, N.; Tudorachi, N.; Rusu, D.; Rusu, A.G.; Bercea, M.; Rosca, I.; Ciolacu, D.E.; et al. One-Step Preparation of Carboxymethyl Cellulose—Phytic Acid Hydrogels with Potential for Biomedical Applications. Gels 2022, 8, 647. [Google Scholar] [CrossRef] [PubMed]
- Pratinthong, K.; Punyodom, W.; Jantrawut, P.; Jantanasakulwong, K.; Tongdeesoontorn, W.; Sriyai, M.; Panyathip, R.; Thanakkasaranee, S.; Worajittiphon, P.; Tanadchangsaeng, N.; et al. Modification of a Carboxymethyl Cellulose/Poly(Vinyl Alcohol) Hydrogel Film with Citric Acid and Glutaraldehyde Crosslink Agents to Enhance the Anti-Inflammatory Effectiveness of Triamcinolone Acetonide in Wound Healing. Polymers 2024, 16, 1798. [Google Scholar] [CrossRef] [PubMed]
- Barbooti, M.M.; Al-Sammerrai, D.A. Thermal Decomposition of Citric Acid. Thermochim. Acta 1986, 98, 119–126. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; Technical Report NREL/TP-510-42622; National Renewable Energy Laboratory: Golden, CO, USA, 2005.
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; Technical Report NREL/TP-510-42619; National Renewable Energy Laboratory: Golden, CO, USA, 2005.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012.
- TAPPI, Alpha-, Beta- and Gamma-Cellulose in Pulp T 203 cm-99; Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 1999.
- Azmi, I.S.; Azizan, A.; Mohd Salleh, R. Pretreatment of Oil Palm Frond (OPF) with Ionic Liquid. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012071. [Google Scholar] [CrossRef]





| Biomass Type | Cellulose | Hemicellulose | Lignin | Ash | Extractives | Hot Water Soluble | Ref. |
|---|---|---|---|---|---|---|---|
| Date palm rachis from Jeddah City in Saudi Arabia | 29.82 | 21.69 | 13.74 | 5.89 | 9.5 | 10.84 | This work |
| Date palm rachis from Marrakesh City in Morocco | 39.8 | 31.4 | 14.0 | 9.2 | NA * | 16.8 | [31] |
| Date palm rachis from southern Algeria | 35.87 | NA * | 16.94 | NA * | 8.66 | NA * | [37] |
| Date palm rachis from Biskra City in Algeria | 47.31 | 25.72 | 15.67 | 5.47 | 5.80 | NA * | [35] |
| Date palm rachis from Khoozestan City in Iran | 38.26 | 28.17 | 22.53 | 5.96 | 5.08 | NA * | [36] |
| Peak Assignment | Peak Wavenumber (cm−1) | Ref. | |||||
|---|---|---|---|---|---|---|---|
| −OH stretching | DPR | WTDPR | CMDPR | C-CMC | CM15 | CM20 | |
| −CH stretching | 3400 | 3400 | 3400 | 3400 | 3384 | 3402 | [41] |
| C=O ester or −COOH | 2919 | 2919 | 2919 | 2919 | 2913 | 2923 | [41] |
| C=O conjugated | 1731 | 1731 | - | - | 1726 | 1726 | [42,43] |
| C=C/C=O aromatic or carboxylic acid | 1637 | 1637 | 1637 | - | - | - | [43] |
| C=C aromatic | 1618 | 1618 | 1618 | 1618 | 1587 | 1586 | [44,45] |
| −CH aromatic | 1507 | 1507 | 1507 | - | - | - | [46,47] |
| −CH2 aliphatic/−CH aromatic | 1458 | 1458 | 1458 | - | - | - | [46,47] |
| −CH deformation | 1425 | 1425 | 1422 | 1420 | 1414 | 1414 | [47] |
| −CH rocking | 1374 | 1374 | 1374 | - | - | - | [47] |
| C−O aromatic | 1321 | 1321 | 1330 | 1330 | 1318 | 1316 | [47] |
| C−O−C stretching | 1250 | 1250 | 1270 | - | 1233 | 1235 | [48] |
| C−C/C−O | 1158 | 1158 | 1158 | 1158 | - | - | [46,47] |
| C−O stretching | 1108 | 1108 | 1120 | 1120 | - | - | [49] |
| C−O−C β-glycosidic | 1049 | 1049 | 1049 | 1059 | 1042 | 1042 | [46,47] |
| −OH stretching | 897 | 897 | 897 | 901 | 898 | 898 | [50] |
| Starting Material | Conditions | DS | Ref. |
|---|---|---|---|
| Date palm rachis | 30% NaOH, 1 g substrate/1.2 g MCA, 55 °C, 3.5 h, Isopropanol | 1.14 | This work |
| Date palm rachis | 40% NaOH, 1 g substrate/1.749 g MCA, 80 °C, 8 h in n-Butanol | 1.17 | [68] |
| Posidonia oceanica | 40% NaOH, 1 g substrate/1.749 g MCA, 80 °C, 8 h in n-Butanol | 1 | [68] |
| Cunninghamia lanceolata | 30% NaOH, 1 g substrate/1.4 g MCA, 80 °C, 2 h, Isopropanol | 1.36 | [69] |
| Lepidium Polyuronide | 45% NaOH, 1 g substrate/15 g MCA, 70 °C, 2 h | 1.75 | [70] |
| Sample | Stage | Range | Decomposition Assignment | Residue (%) |
|---|---|---|---|---|
| CMDPR | 1st | 50–180 °C | Volatile compounds and moisture | 45.0% |
| 2nd | 230–324 °C | Carboxyl and hydroxyl groups | ||
| 3rd | 324–373 °C | Cellulose chain | ||
| 4th | 382–421 °C | Methyl-aryl ether bonds of lignin | ||
| 5th | 421–460 °C | Lignin chain | ||
| 6th | 590–600 °C | Oxidation of residual carbonaceous | ||
| C-CMC | 1st | 50–180 °C | Volatile compounds and moisture | 23.8% |
| 2nd | 246–314 °C | Carboxyl and hydroxyl groups | ||
| 3rd | 360–400 °C | Cellulose chain | ||
| 4th | 587.5–600 °C | Oxidation of residual carbonaceous | ||
| CM15 | 1st | 158.8–207.3 °C | Non-crosslinked CA | 67.0% |
| 2nd | 207.3–343.7 °C | Crosslinked CA and CMDPR chain | ||
| CM20 | 1st | 207–343.3 °C | Crosslinked CA and CMDPR chain | 74.1% |
| pH | EC | N | P | K |
|---|---|---|---|---|
| 6–7 | 1.78 µS/cm | 2.08% | 0.015% | 0.22% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsubaie, F.S.; Srdar, M.; Fayraa, O.; Alsulami, F.M.; Omran, F.; Alamry, K.A. Development of Eco-Friendly Date Palm Biomass-Based Hydrogels for Enhanced Water Retention in Soil. Gels 2025, 11, 349. https://doi.org/10.3390/gels11050349
Alsubaie FS, Srdar M, Fayraa O, Alsulami FM, Omran F, Alamry KA. Development of Eco-Friendly Date Palm Biomass-Based Hydrogels for Enhanced Water Retention in Soil. Gels. 2025; 11(5):349. https://doi.org/10.3390/gels11050349
Chicago/Turabian StyleAlsubaie, Faisal S., Mouyed Srdar, Osama Fayraa, Faris M. Alsulami, Feras Omran, and Khalid A. Alamry. 2025. "Development of Eco-Friendly Date Palm Biomass-Based Hydrogels for Enhanced Water Retention in Soil" Gels 11, no. 5: 349. https://doi.org/10.3390/gels11050349
APA StyleAlsubaie, F. S., Srdar, M., Fayraa, O., Alsulami, F. M., Omran, F., & Alamry, K. A. (2025). Development of Eco-Friendly Date Palm Biomass-Based Hydrogels for Enhanced Water Retention in Soil. Gels, 11(5), 349. https://doi.org/10.3390/gels11050349










