Study on Filter Cake Removal Fluid of EZFLOW Weak Gel Drilling Fluid
Abstract
1. Introduction
2. Results and Discussion
2.1. Mechanism of Weak Gel Drilling Fluid
2.1.1. Rheological Properties
2.1.2. Plugging Performance
2.2. Construction of Filter Cake Removal Fluid System
2.2.1. Acid Selection
Gel-Breaking Performance Evaluation
- (1)
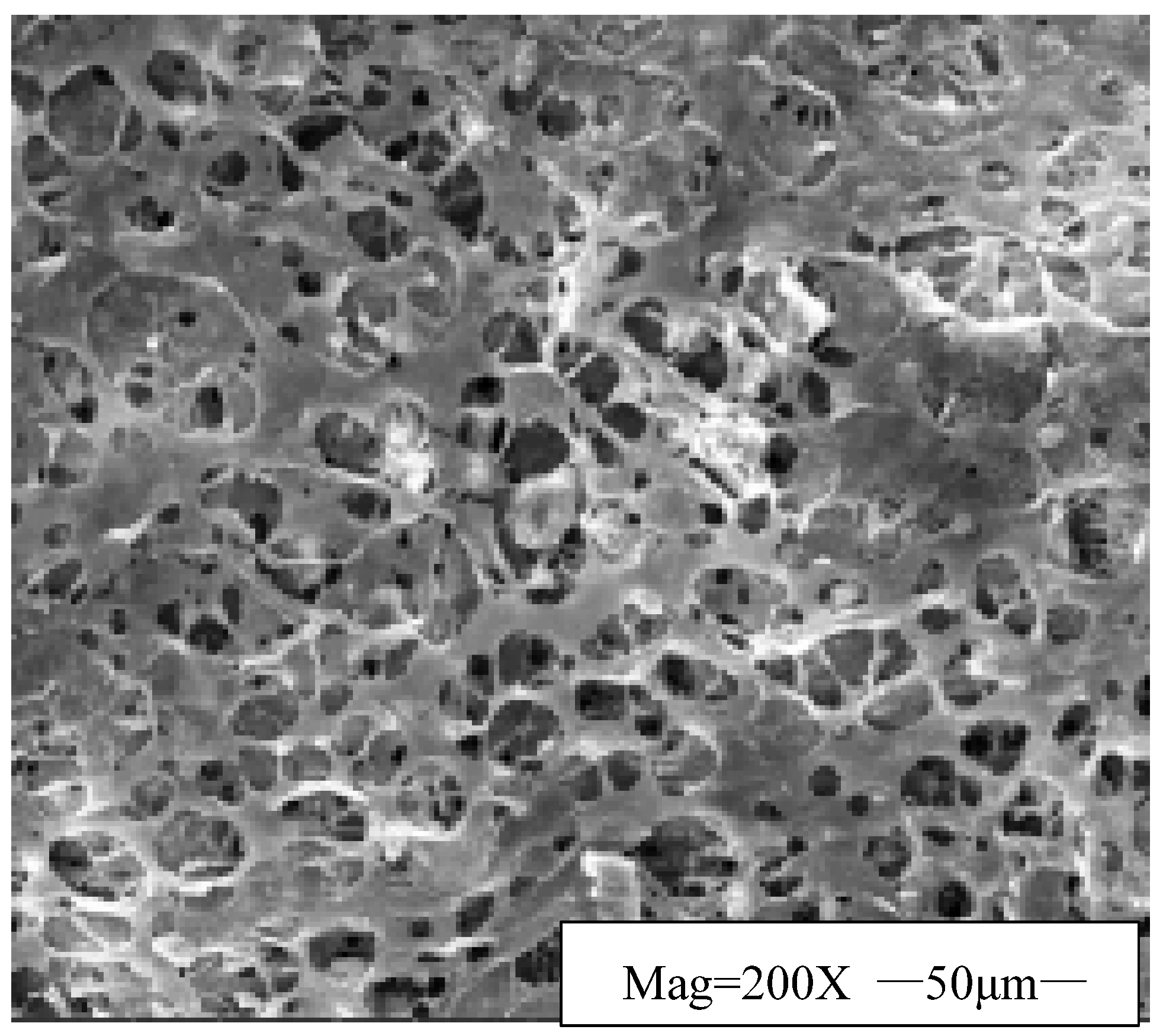
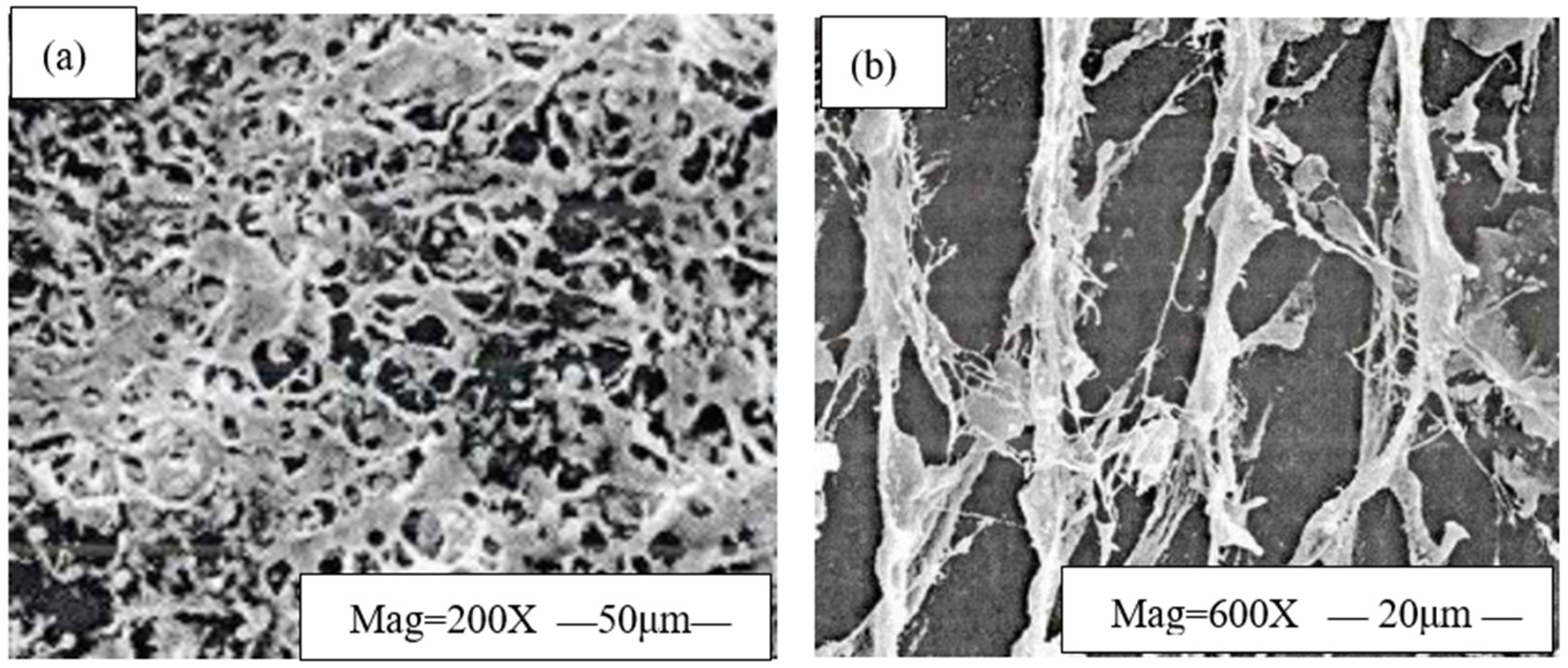
- Effect of HWCP Acid Solution on EZVIS Solution’s Network Structure
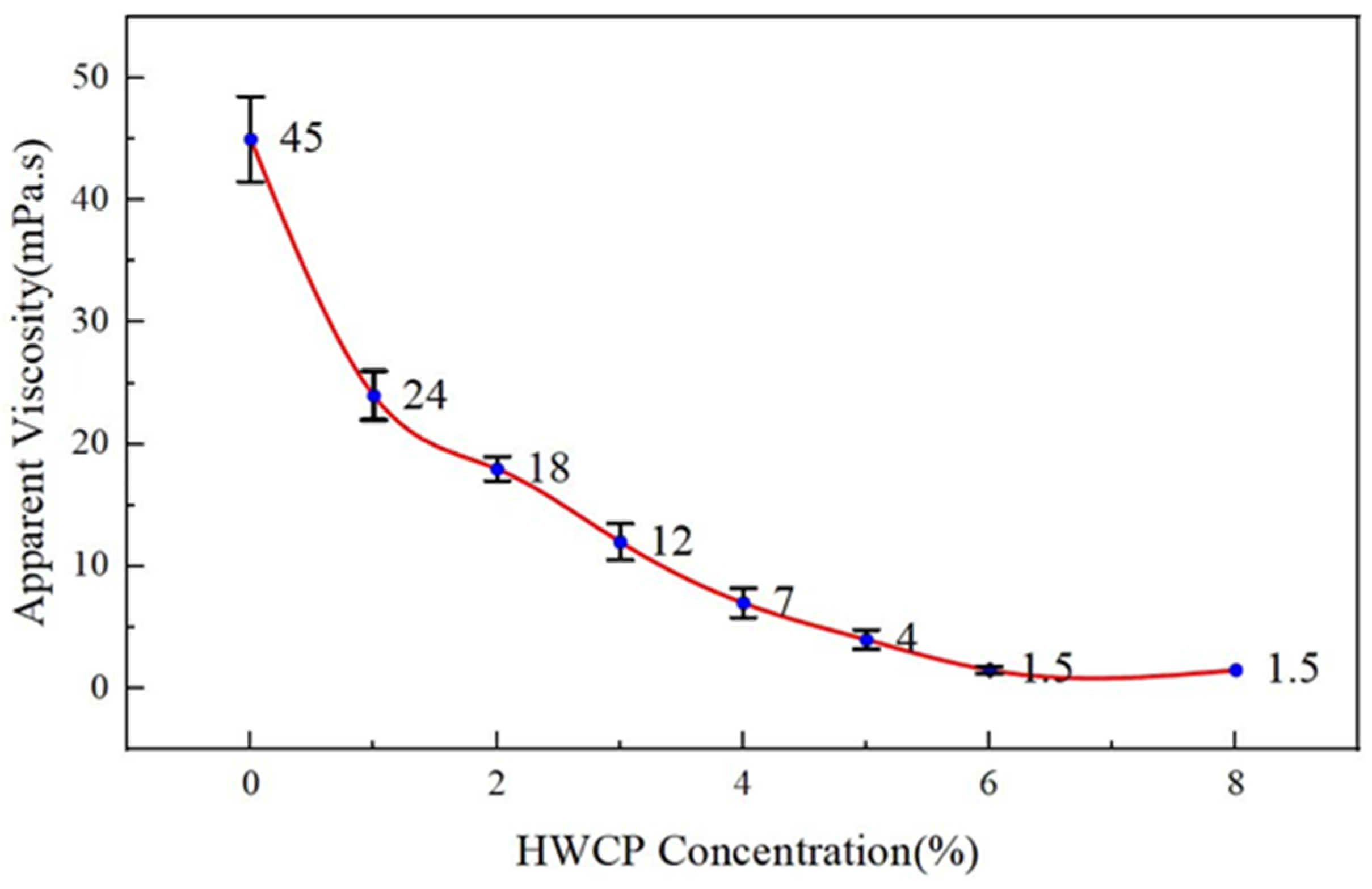
- (2)
- Effect of HWCP Acid Solution Concentration on Apparent Viscosity of EZVIS Solution
Evaluation of Dissolution Capacity
- (1)
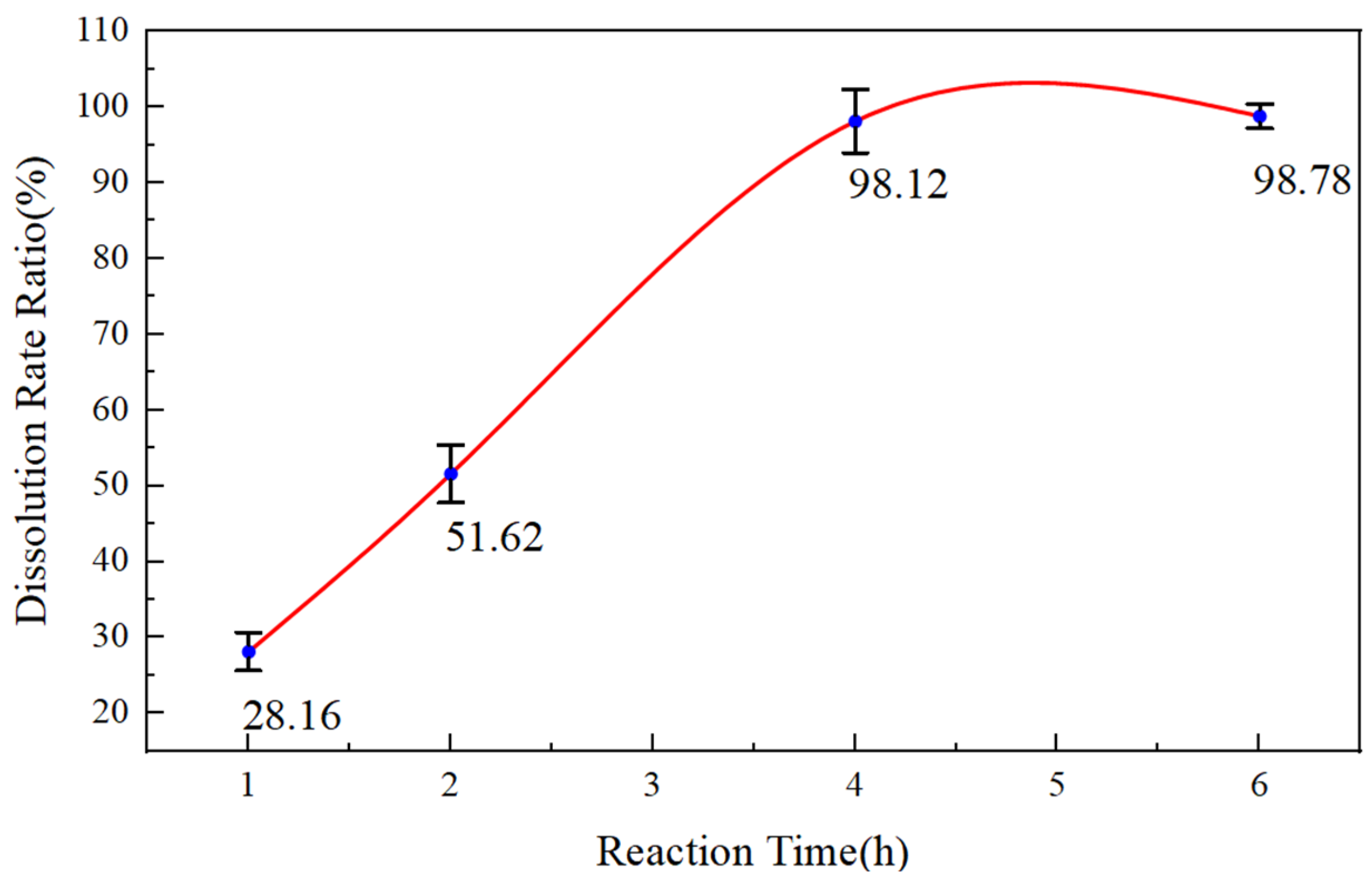
- Dissolution Rate of Temporary Plugging Agent EZCARB for HWCP Acid Solution
- (2)
- Dissolution Rate of Reservoir Cuttings for HWCP Acid Solution
- (3)
- Dissolution Rates of EZCARB and Reservoir Cuttings by Different Concentrations of HWCP Acid Solution
2.2.2. Dosage Optimization of Corrosion Inhibitor HWCI
2.3. Performance Evaluation of Filter Cake Removal Fluid System
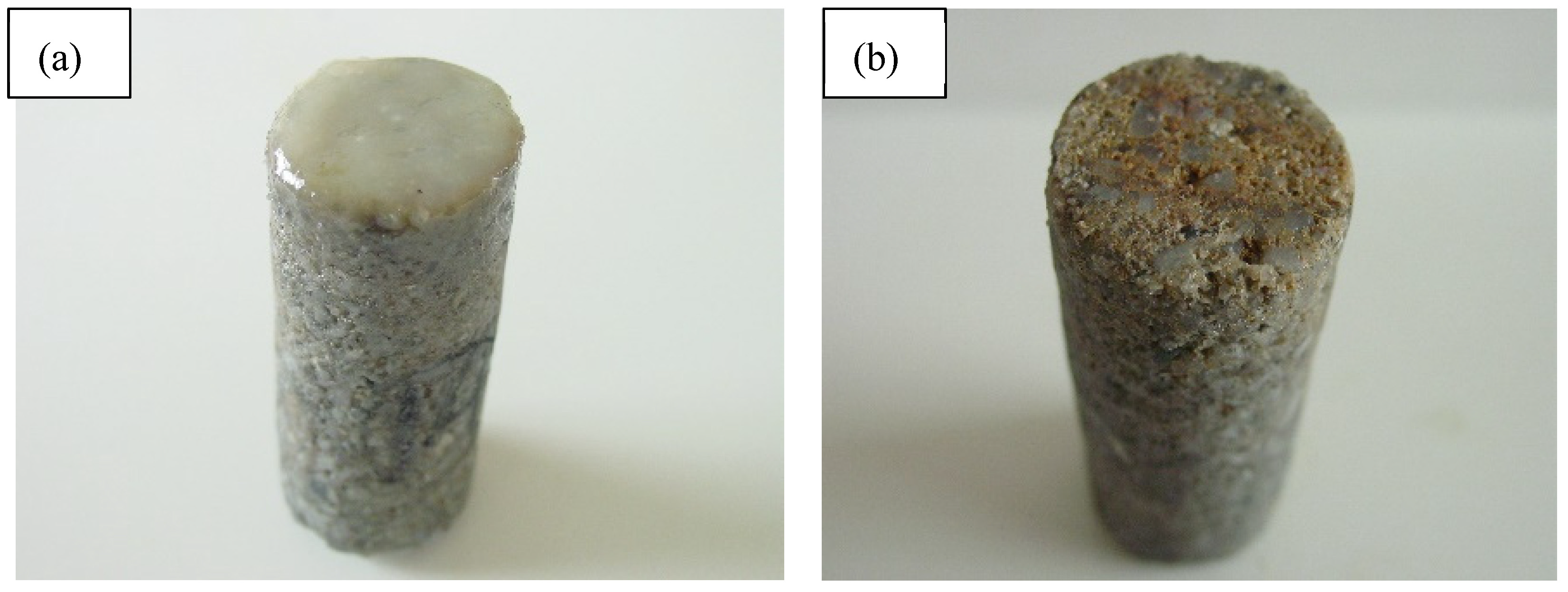
2.3.1. Evaluation of Filter Cake Removal Ability
2.3.2. Reservoir Protection Performance Evaluation
3. Conclusions
- (1)
- The microstructure of EZFLOW weak gel drilling fluid consists of a reversible network and sol particles either embedded within the network or encapsulated at its nodes. This unique spatial network endows the system with exceptional rheological properties, good plugging performance, and lubricity.
- (2)
- Based on the components of EZFLOW weak gel drilling fluid’s filter cake, a filter cake removal fluid formula was established: filtered seawater + 6% composite organic acid HWCP + 3% corrosion inhibitor HWCI. This fluid not only removes the filter cake, degrades the polymer EZVIS, and dissolves the temporary plugging agent EZCARB to protect the reservoir, but also provides a retarded deep-acidizing effect, enhancing reservoir permeability and productivity.
- (3)
- After treatment with the filter cake removal fluid, the filter cake on the core end face of the EZFLOW weak gel drilling fluid-contaminated reservoir core is almost completely removed, with a core permeability recovery rate of over 95%. This indicates the fluid’s excellent filter cake removal and strong reservoir protection capabilities.
4. Materials and Methods
4.1. Materials
4.2. Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiang, X.; Yang, H.L.; Liu, X.L. Research and Application of EZFLOW Solid-Free Weak Gel Drilling Fluid in Horizontal Wells in Shallow Gas Fields in the Western South China Sea. Pet. Drill. Tech. 2018, 46, 38–43. [Google Scholar]
- Wan, X. Application of new drilling fluid on open hole completion in unconsolidated sandstone horizontal well. Petrochem. Ind. Appl. 2018, 37, 51–54. [Google Scholar]
- Xiang, C.G. Development and Working Mechanism Analysis of a New Weak Gel Water Based Drilling Fluid. Drill. Fluid Complet. Fluid 2015, 32, 11–14. [Google Scholar]
- Li, X.D.; Xiong, Z.Q.; Fu, F. Research and application of environment-friendly weak-gel drilling fluid for wall and core protection. Geol. Explor. 2024, 60, 581–591. [Google Scholar]
- Bai, X.D.; Zheng, X.X.; Wang, H. Synthesis and Characterization of A Weak Gel Gelling Agent AM/AMPS/OA8 in Water-based Drilling Fluid. Chin. J. Synth. Chem. 2017, 25, 886–891. [Google Scholar]
- Tao, M.; Luo, G.; Luo, X. Development and performance study of polymer weak gel for drilling fluids. Mod. Chem. Ind. 2018, 38, 132–136. [Google Scholar]
- Wang, G.C.; Liu, W.C.; Chen, X.M. Technology of compound salt weak gel drilling fluid for long horizontal wells in deep shale oil reservoirs. Oil Drill. Prod. Technol. 2023, 45, 675–681. [Google Scholar]
- Sun, P.H.; Han, M.; Cao, H.; Liu, W.S.; Zhang, S.H.; Zhu, J.Y. Development and Performance Evaluation of Solid-Free Drilling Fluid for CBM Reservoir Drilling in Central Hunan. Energies 2020, 13, 4857. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, J.S.; Huang, X.B.; Lv, K.H.; Wang, J.T.; Geng, Y.; Fan, J.H.; He, J.; Dai, J.J. Preparation of polymer-coated SiO2 aerogel for weakening wellbore instability caused by heat transfer between the drilling fluid and well wall. Geoenergy Sci. Eng. 2025, 247, 213611. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, F.W.; Tang, Y.H.; Wang, Y.Y.; He, X.Y.; Wang, T.F.; Wu, J. The Simulation of Ester Lubricants and Their Application in Weak Gel Drilling Fluids. Gels 2024, 10, 178. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Chen, S.N.; Zhou, F.S.; Wei, Z.J. Gel Stability of Calcium Bentonite Suspension in Brine and Its Application in Water-Based Drilling Fluids. Gels 2022, 8, 643. [Google Scholar] [CrossRef] [PubMed]
- Erge, O.; Gu, Q.F.; Chen, D.M.; Van, O.E. MPD choke control considering the thixotropic gelation behavior of drilling fluids. J. Pet. Sci. Eng. 2022, 208, 109793. [Google Scholar] [CrossRef]
- Zou, Y.; Zhu, X.; Wu, X. Experimental Study on the influence of Positive Gel Drilling Fluid on Shale Wellbore Stability. Chem. Technol. Fuels Oils 2021, 57, 188–195. [Google Scholar] [CrossRef]
- Luis, H.; Quitian, A.; Raquel, S.S.; Yamid, J.; Garcia, B.; Eduardo, M.G.; Vladimir, B.B.; Oriana, P.C.; Admilson, T.F. Stress Overshoot Analysis in Flow Start-Up Tests: Aging Time Fitting of the Different Gel-Based Drilling Fluids. Gels 2025, 11, 127. [Google Scholar] [CrossRef]
- Géssica, P.; Andrade, E.V.; Galdino, J.F.; Franco, A.T.; Alves, E.; Waldmann, A. Influence of pressure on the gel strength and on the solid-like behavior for an inverted emulsion drilling fluid. J. Pet. Sci. Eng. 2022, 219, 111114. [Google Scholar]
- Magzoub, M.I.; Shamlooh, M.; Salehi, S.; Hussein, I.; Nasser, M.S. Gelation Kinetics of PAM/PEI Based Drilling Mud for Lost Circulation Applications. J. Pet. Sci. Eng. 2021, 200, 108383. [Google Scholar] [CrossRef]
- Fadl, M.A.; Abdou, M.I.; Moustafa, H.Y.; Ahmed, A.E.; Anter, E.; Ahmed, H.E.A. New Comparative Evaluation for the Rheological and Filtration Properties of Water-Based Drilling Fluids Utilizing Sodium Salt of Linear and Cross-Linked Acrylate Polymer Hydrogels. Arab. J. Sci. Eng. 2021, 46, 6989–7017. [Google Scholar] [CrossRef]
- Li, J.; Sun, J.S.; Lv, K.H.; Ji, Y.X.; Ji, J.T.; Liu, J.P. Nano-Modified Polymer Gels as Temperature- and Salt-Resistant Fluid-Loss Additive for Water-Based Drilling Fluids. Gels 2022, 9, 547. [Google Scholar] [CrossRef]
- Zheng, X.; Duan, Z.; Zhuang, Y.; Zhang, S.F.; Cui, X.Y.; Qin, D.H. Application of Solvent-Assisted Dual-Network Hydrogel in Water-Based Drilling Fluid for Lost Circulation Treatment in Fractured Formation. ACS Omega 2024, 9, 1166–1173. [Google Scholar] [CrossRef]
- Sidharth, G.; Shailesh, K.; Amit, K.; Vinay, K.R.; Chandan, G. Development of functional polymer-based clay-free HPHT drilling fluid:Effect of molecular weight and its distribution on drilling fluid performance. Geoenergy Sci. Eng. 2025, 246, 213616. [Google Scholar]
- Li, Z.J.; Huo, L.X.; Zhi, J.Z.; Zhang, S.X.; Wu, X.K. Growth kinetics of Bacillus pasteurii in Xanthan gum solid-free drilling fluid at different temperatures. Geoenergy Sci. Eng. 2023, 223, 211482. [Google Scholar] [CrossRef]
- Li, Z.J.; Zhao, G.; Xiang, C.G. Synthesis and Properties of a Gel Agent with a High Salt Resistance for Use in Weak-Gel-Type Water-Based Drilling Fluid. Arab. J. Sci. Eng. 2022, 47, 12045–12055. [Google Scholar] [CrossRef]
- Shanmugam, S.; Ross, G.; Mbuncha, C.Y.; Santra, A. High performance-branched microgels as universal viscosifiers for water-based drilling fluids. MRS Commun. 2021, 11, 755–761. [Google Scholar] [CrossRef]
- Li, Z.; Xiang, C. Experimental investigation of a new weak-gel-type clay-free and water-based drilling fluid with high-temperature and high-salt resistance for determining its optimized formulation. Energy Sources Part A Recovery Util. Environ. Eff. 2024, 46, 7928–7945. [Google Scholar] [CrossRef]
- Huang, W.L.; Lou, Y.S.; Ma, X.Y.; Xu, H.M.; Wang, Q. Performance Evaluation and Application of the Non-Clay Weak Gel Drilling Fluid for Daniudi Gas Field. Adv. Mater. Res. 2013, 2606, 2861–2864. [Google Scholar] [CrossRef]
- Luo, J.S.; Wang, X.S.; Xu, S.C. Development and application of the non-clay weak gel drilling fluid. Drill. Fluid Complet. Fluid 2002, 19, 10–12. [Google Scholar]
- Wang, J.; Zhang, R.; Nie, M.S. The Study and Application of theWeak gel Drill-in Fluid. Drill. Fluid Complet. Fluid 2008, 25, 6–7. [Google Scholar]
- Shi, P.; Wang, S.B.; Wang, Z.; Liu, Y.J.; Li, X.L.; Li, Y. LNMR analysis of the retention of different guar gum structure in sandstone: Based on a new characterization method. Geoenergy Sci. Eng. 2024, 243, 213113. [Google Scholar] [CrossRef]
- Wu, D.G.; Wang, L.K.; Zhao, C.Y.; Zhang, Z.J.; Yin, J.Y.; Anwaier, M.; Ren, H.D.; Yang, D.; Yin, S.L.; Cai, Z.L.; et al. Experimental study of reservoir damage of water-based fracturing fluids prepared by different polymers. Pet. Sci. 2024, 21, 3298–3306. [Google Scholar] [CrossRef]
- Huang, Y.P.; Yao, X.L.; Dai, C.L.; Wu, Y.N.; Lin, L.; Yuan, B.A. Supramolecular Reinforced Gel Fracturing Fluid with Low Permeability Damage Applied in Deep Reservoir Hydraulic Fracturing. Gels 2023, 10, 2. [Google Scholar] [CrossRef]
- Neamat, J.; Ali Jagar, A. Field and Experimental Investigations on the Effect of Reservoir Drill-In Fluids on Penetration Rate and Drilling Cost in Horizontal Wells. Gels 2023, 9, 510. [Google Scholar] [CrossRef] [PubMed]
- Dehkohneh, H.Z.; Shahbazi, K.; Abdehvand, B.Z.; Nazemi, R. Experimental investigation of the performance of nano-particles on hybrid gel in lost circulation control. Arab. J. Geosci. 2024, 17, 323. [Google Scholar] [CrossRef]
- Li, X.J.; Zhang, Q.J.; Liu, P.; Li, T.; Liu, G.F.; Liu, Z.K.; Zhao, H.F. Investigation on the microscopic damage mechanism of fracturing fluids to low-permeability sandstone oil reservoir by nuclear magnetic resonance. J. Pet. Sci. Eng. 2022, 209, 109821. [Google Scholar] [CrossRef]
- Taghdimi, R.; Kaffashi, B.; Rasaei, M.R.; Dabiri, M.S.; Hemmati, S.A. Formulating a novel drilling mud using bio-polymers, nanoparticles, and SDS and investigating its rheological behavior, interfacial tension, and formation damage. Sci. Rep. 2023, 13, 12080. [Google Scholar] [CrossRef]
- Wei, H.S.; Zhang, J.B.; Zhang, W.G. UltraFLO Drill-in Fluid. Drill. Fluid Complet. Fluid 2015, 32, 37–39. [Google Scholar]
- Wang, J.S.; Zhou, F.F.; Wang, Q.H. Experimental study on gel breaking of weak gel solid-free drilling fluid. Petrochem. Ind. Appl. 2019, 38, 34–36. [Google Scholar]



| HWCP Concentration (%) | EZCARB Dissolution Rate (%) | Dissolution Rate of Reservoir Cuttings (%) |
|---|---|---|
| 1 | 70.02 | 2.32 |
| 3 | 84.86 | 4.52 |
| 5 | 96.86 | 5.02 |
| 6 | 98.12 | 5.16 |
| 8 | 99.28 | 5.48 |
| Solution | HWCI Concentration (%) | Corrosion Rate (g/m²·h) | Inhibition Efficiency (%) |
|---|---|---|---|
| Filtered seawater + 6% HWCP | 0 | 1.204 | -- |
| 1.0 | 0.371 | 69.19 | |
| 2.0 | 0.103 | 91.45 | |
| 3.0 | 0.052 | 95.68 | |
| 4.0 | 0.049 | 95.93 | |
| 5.0 | 0.045 | 96.26 |
| Contamination Media | K1 (mD) | K2 (mD) | K3 (mD) | K2/K1 (%) | K3/K1 (%) |
|---|---|---|---|---|---|
| EZFLOW weak gel drilling fluid | 6.763 | 5.137 | -- | 75.96 | --- |
| EZFLOW weak gel drilling fluid + filter cake removal fluid | 8.471 | 6.526 | 8.189 | 77.04 | 96.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Hu, Y.; Weng, X. Study on Filter Cake Removal Fluid of EZFLOW Weak Gel Drilling Fluid. Gels 2025, 11, 347. https://doi.org/10.3390/gels11050347
Hu H, Hu Y, Weng X. Study on Filter Cake Removal Fluid of EZFLOW Weak Gel Drilling Fluid. Gels. 2025; 11(5):347. https://doi.org/10.3390/gels11050347
Chicago/Turabian StyleHu, Haohan, Youlin Hu, and Xuejing Weng. 2025. "Study on Filter Cake Removal Fluid of EZFLOW Weak Gel Drilling Fluid" Gels 11, no. 5: 347. https://doi.org/10.3390/gels11050347
APA StyleHu, H., Hu, Y., & Weng, X. (2025). Study on Filter Cake Removal Fluid of EZFLOW Weak Gel Drilling Fluid. Gels, 11(5), 347. https://doi.org/10.3390/gels11050347





