Stimuli-Responsive Hydrogels of Poly(Methacrylic Acid)/Poly(N,N-dimethylacrylamide) Interpenetrating Polymer Networks as Drug Delivery Systems for Promethazine Hydrochloride
Abstract
:1. Introduction
2. Results and Discussion
2.1. Swelling Properties
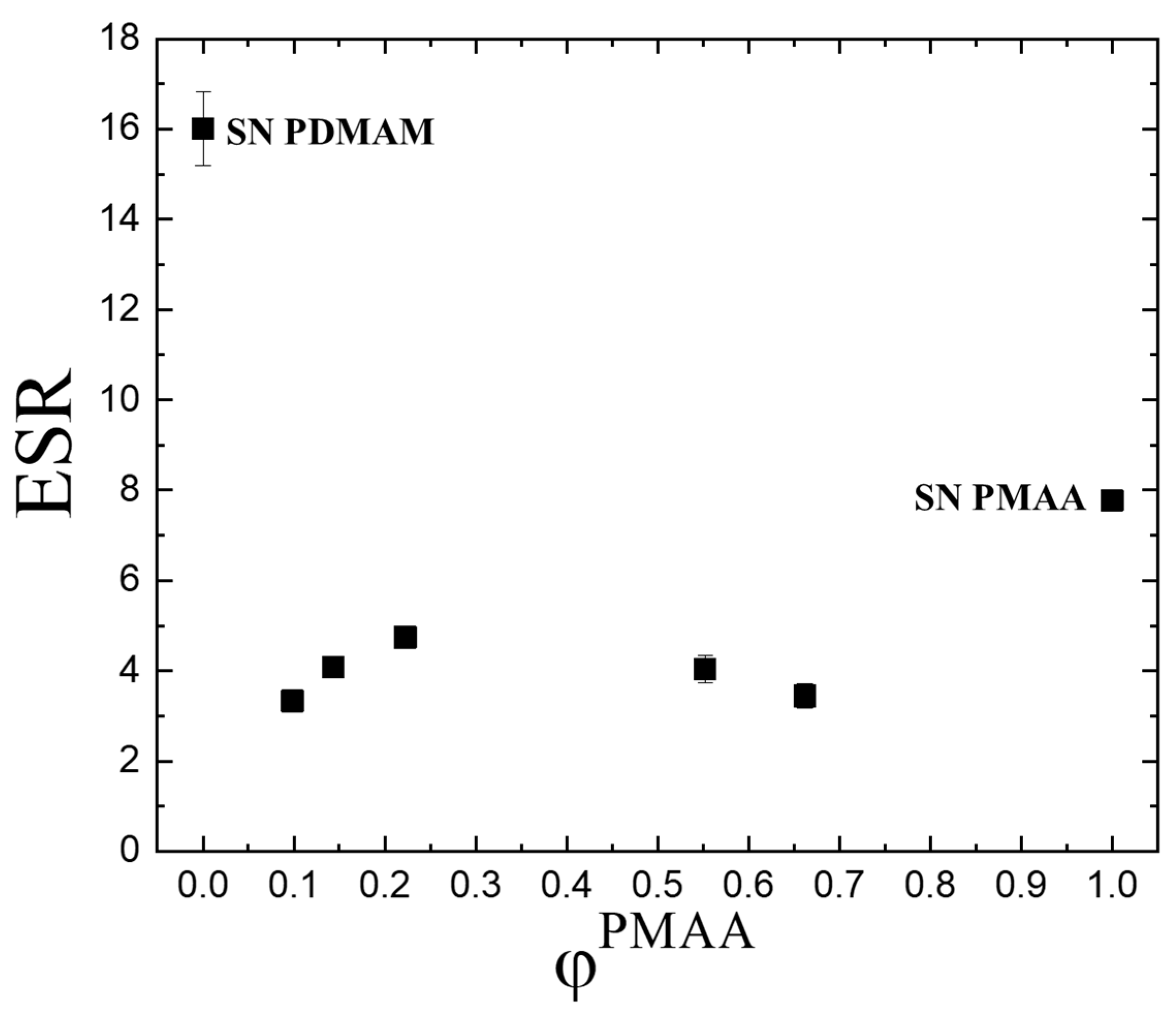
2.1.1. Equilibrium Swelling Ratio (ESR) in Water
- (i)
- Different chemical crosslinking degrees as SN PDMAM is obtained by using 0.1 mol.% crosslinking agent, while SN PMAA is obtained via crosslinking with 4 mol.% of the same crosslinking agent. Thus, a denser network is expected for PMAA which limits the diffusion of the water molecules into it.
- (ii)
- The pH of the media as PMAA is a pH-sensitive polymer. As distilled water was used as the swelling media, its pH is a little bit above and comparable to the pKa of the pendant carboxylic groups in PMAA which defines the possibility part of them to be in their neutral form while other part are ions. That means that the neutral carboxylic groups could form hydrogen bonds between themselves, thus forming an additional physical network on the top of the existing chemical one. At the same time, for PMMA, it is known that their side -CH3 groups which are directly bound to the macromolecular backbone could form via hydrophobic interactions between themselves small hydrophobic clusters which again act as physical network junctions. Thus, both factors work with a reduced swelling ability of PMAA as compared to PDMAM.
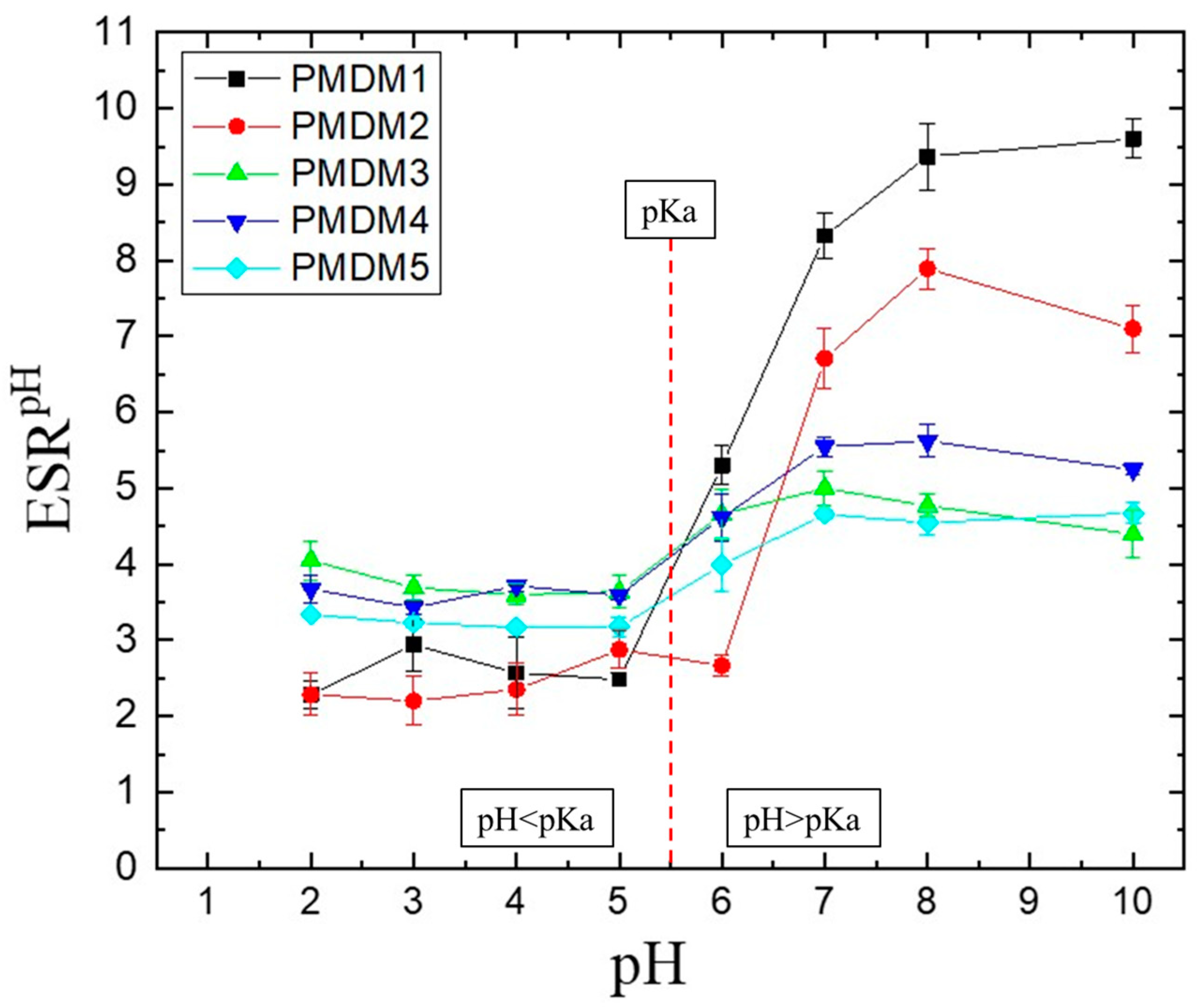
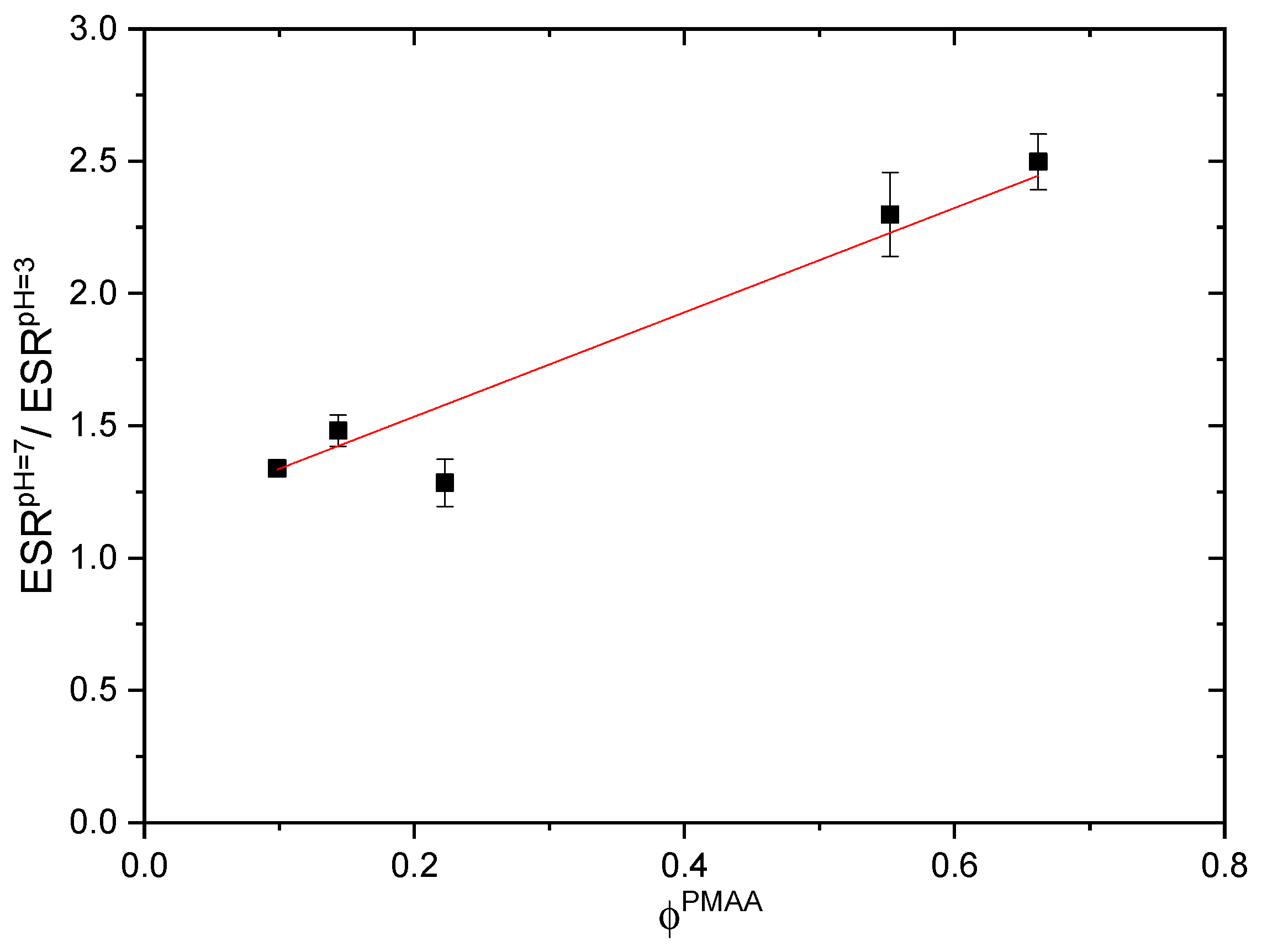
2.1.2. pH-Responsiveness
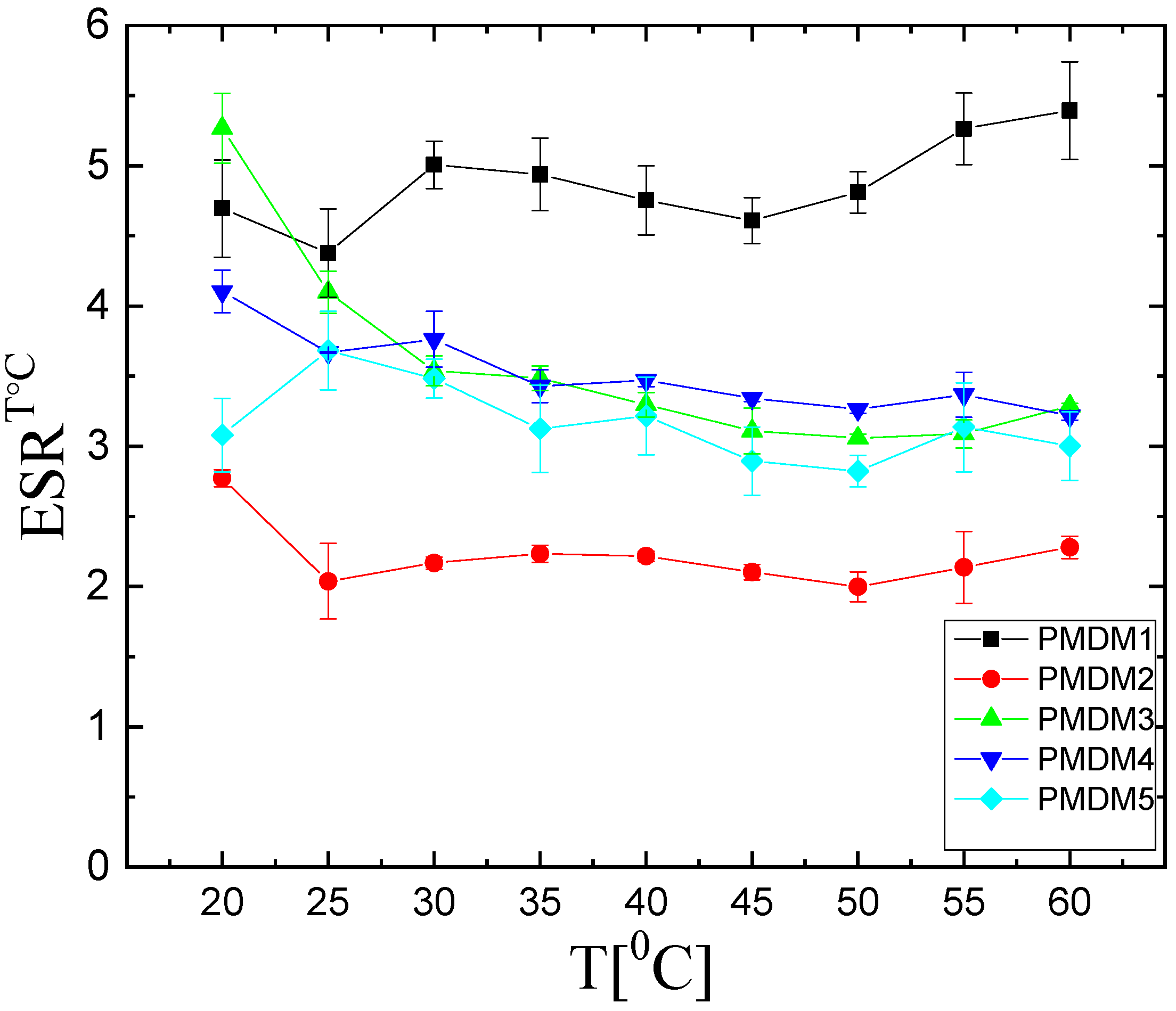
2.1.3. Temperature Responsiveness
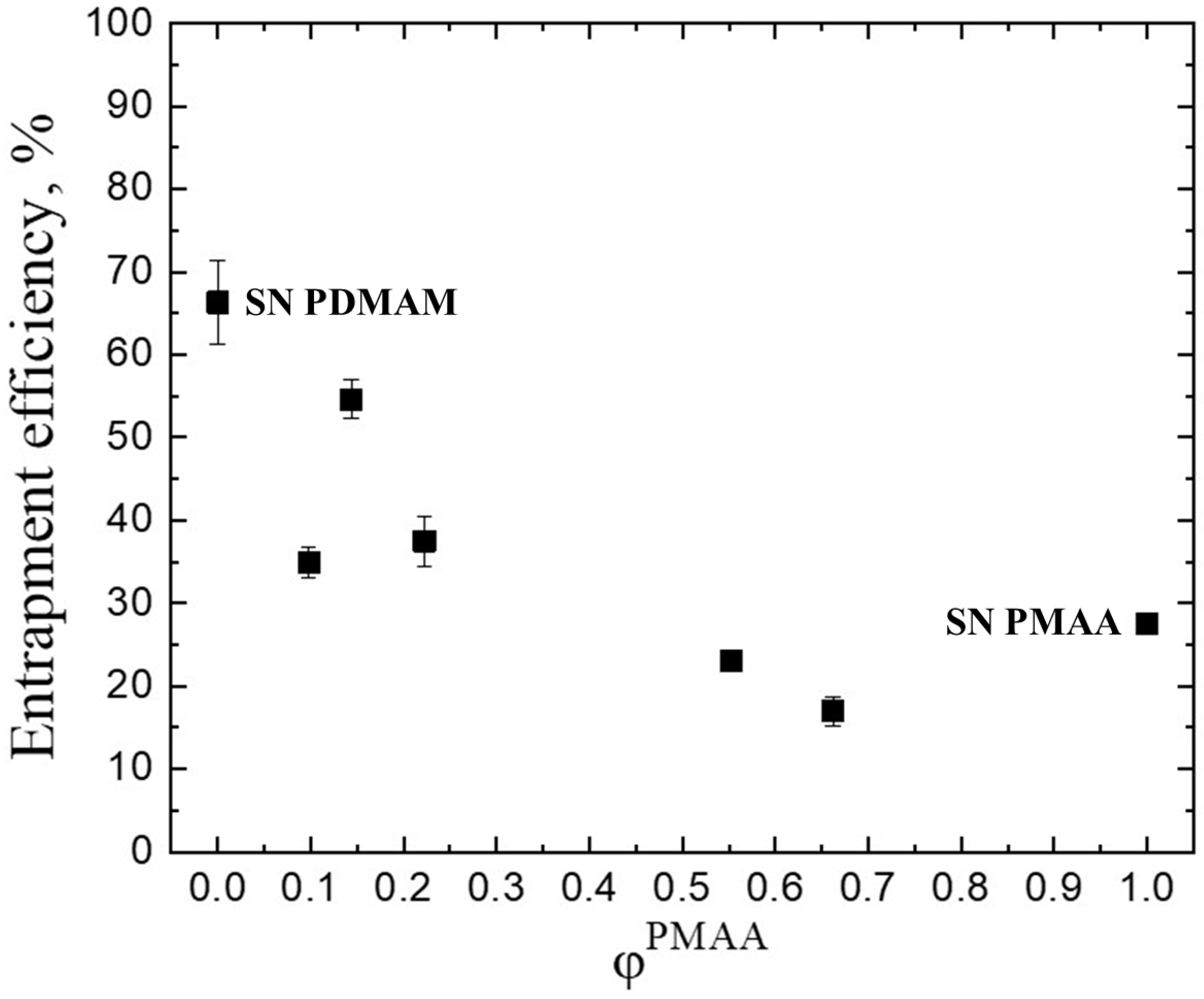
2.2. Drug Loading
2.3. Drug Release
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. IPN’s Synthesis
4.3. Swelling Properties
4.3.1. Equilibrium Swelling Ratio
4.3.2. pH Responsiveness
4.3.3. Temperature Responsiveness
4.4. Drug Loading
4.5. In Vitro Drug Release
4.6. Drug Release Kinetics
- Zero order (ZO)
- First order (FO)
- Higuchi model (HM)
- Korsmeyer–Peppas model
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IPN | Interpenetrating polymer network(s) |
| SN | Single network(s) |
| PMAA | Poly(methacrylic acid) |
| PDMAM | Poly(N,N-dimethylacrylamide) |
| PMH | Promethazine hydrochloride |
| ESR | Equilibrium swelling ratio |
| EE | Entrapment efficiency |
References
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, L.; Diaz-Amaya, S. Composite Biomaterials; Elsevier Ebooks; Elsevier: Amsterdam, The Netherlands, 2022; pp. 149–169. [Google Scholar]
- Simeonov, M.; Kostova, B.; Vassileva, E. Interpenetrating Polymer Networks of Poly (Methacrylic Acid) and Polyacrylamide: Synthesis, Characterization and Potential Application for Sustained Drug Delivery. RSC Adv. 2016, 6, 64239–64246. [Google Scholar] [CrossRef]
- Jenkins, A.D.; Kratochvíl, P.; Stepto, R.F.T.; Suter, U.W. Glossary of Basic Terms in Polymer Science (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2287–2311. [Google Scholar]
- Simeonov, M.; Kostova, B.; Mihaylova, R.; Vassileva, E. Hydrogels of Poly (2-hydroxyethyl methacrylate) and Poly (N,N-dimethylacrylamide) Interpenetrating Polymer Networks as Dermal Delivery Systems for Dexamethasone. Pharmaceutics 2025, 17, 62. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Goswami, S.; Das, S.; Bhattacharjee, S.; Bhaladhare, S. pH-Responsive, Stable, and Biocompatible Functional Nanogels Based on Chitosan (CS)/Poly Methacrylic Acid (PMAA) Polymers: Synthesis and Characterization. Mater. Today Commun. 2023, 36, 106541. [Google Scholar] [CrossRef]
- Simeonov, M.; Monova, A.; Kostova, B.; Vassileva, E. Drug Transport in Stimuli Responsive Acrylic and Methacrylic Interpenetrating Polymer Networks. J. Appl. Polym. Sci. 2017, 134, 45380. [Google Scholar] [CrossRef]
- Mann, J.L.; Grosskopf, A.K.; Smith, A.A.A.; Appel, E.A. Highly Branched Polydimethylacrylamide Copolymers as Functional Biomaterials. Biomacromolecules 2021, 22, 86–94. [Google Scholar] [CrossRef]
- National Cancer Institute Promethazine Hydrochloride. Cancer.gov. Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/promethazine-hydrochloride (accessed on 21 January 2025).
- Shirisha, S.; Sahoo, S.K.; Veena, T.; Rao, Y.M. Design and Evaluation of Different Transdermal Therapeutic Systems of Promethazine Hydrochloride. Ind. J. Pharm. Educ. 2024, 58, s139–s148. [Google Scholar] [CrossRef]
- Boraste, S.V.; Patil, S.B. Formulation Development and Evaluation of Nasal in Situ Gel of Promethazine Hydrochloride. Drug Dev. Ind. Pharm. 2024, 50, 11–22. [Google Scholar] [CrossRef]
- McDonough, J.A.; Persyn, J.T.; Nino, J.A.; Dixon, H.; Boland, E.J.; Wang, Z.; Putcha, L. Microcapsule-Gel Formulation of Promethazine HCl for Controlled Nasal Delivery: A Motion Sickness Medication. J. Microencapsul. 2007, 24, 109–116. [Google Scholar] [CrossRef]
- Alyami, H.S.; Ibrahim, M.A.; Alyami, M.H.; Dahmash, E.Z.; Almeanazel, O.T.; Algahtani, T.S.; Alanazi, F.; Alshora, D.H. Formulation of Sublingual Promethazine Hydrochloride Tablets for Rapid Relief of Motion Sickness. Saudi Pharm. J. 2021, 29, 478–486. [Google Scholar] [CrossRef]
- Shah, J.N.; Shah, K.N.; Mehta, T.A. Hydroxy Propyl β-Cyclodextrin Complexation of Promethazine Hydrochloride for the Formulation of Fast Dissolving Sublingual Film: In Vitro and in Vivo Evaluation. J. Pharm. Investig. 2015, 45, 91–99. [Google Scholar] [CrossRef]
- Vismari, L.; Pires, M.L.N.; Benedito-Silva, A.A.; Calil, H.M. Bioavailability of Immediate and Controlled Release Formulations of Lithium Carbonate. Rev. Bras. Psiquiatr. 2002, 24, 74–79. [Google Scholar] [CrossRef]
- Zhang, J.; Peppas, N.A. Molecular Interactions in Poly (Methacrylic Acid)/Poly (N-isopropyl Acrylamide) Interpenetrating Polymer Networks. J. Appl. Polym. Sci. 2001, 82, 1077–1082. [Google Scholar] [CrossRef]
- Maroni, A.; Moutaharrik, S.; Zema, L.; Gazzaniga, A. Enteric Coatings for Colonic Drug Delivery: State of the Art. Expert Opin. Drug Deliv. 2017, 14, 1027–1029. [Google Scholar] [CrossRef] [PubMed]
- Gvozdeva, Y.; Staynova, R. pH-Dependent Drug Delivery Systems for Ulcerative Colitis Treatment. Pharmaceutics 2025, 17, 226. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution Properties of Poly (N-Isopropylacrylamide). J. Macromol. Sci. Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Mueller, K.F. Thermotropic Aqueous Gels and Solutions of N,N-Dimethylacrylamide-Acrylate Copolymers. Polymer 1992, 33, 3470–3476. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6014, Promethazine Hydrochloride. Nih.gov. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Promethazine-hydrochloride (accessed on 31 January 2025).
- Seki, Y.; Yurdakoc, K. Synthesis of pH Dependent Chitosan-EPI Hydrogel Films and Their Application Forin Vitro Release of Promethazine Hydrochloride. J. Appl. Polym. Sci. 2008, 109, 683–690. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–86. [Google Scholar]
- Miyaguchi, T.; Akimoto, T. Intrinsic Randomness of Transport Coefficient in Subdiffusion with Static Disorder. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2011, 83 Pt 1, 031926. [Google Scholar] [CrossRef]
- Mayo Clinic. Promethazine (Oral Route). Available online: https://www.mayoclinic.org/drugs-supplements/promethazine-oral-route/description/drg-20070609 (accessed on 2 March 2025).
- Pourjavadi, A.; Mahdavinia, G.R. Superabsorbency, pH-Sensitivity and Swelling Kinetics of Partially Hydrolyzed Chitosan-g poly (Acrylamide) Hydrogels. Turk. J. Chem. 2006, 30, 595–608. Available online: https://journals.tubitak.gov.tr/chem/vol30/iss5/7 (accessed on 10 January 2025).
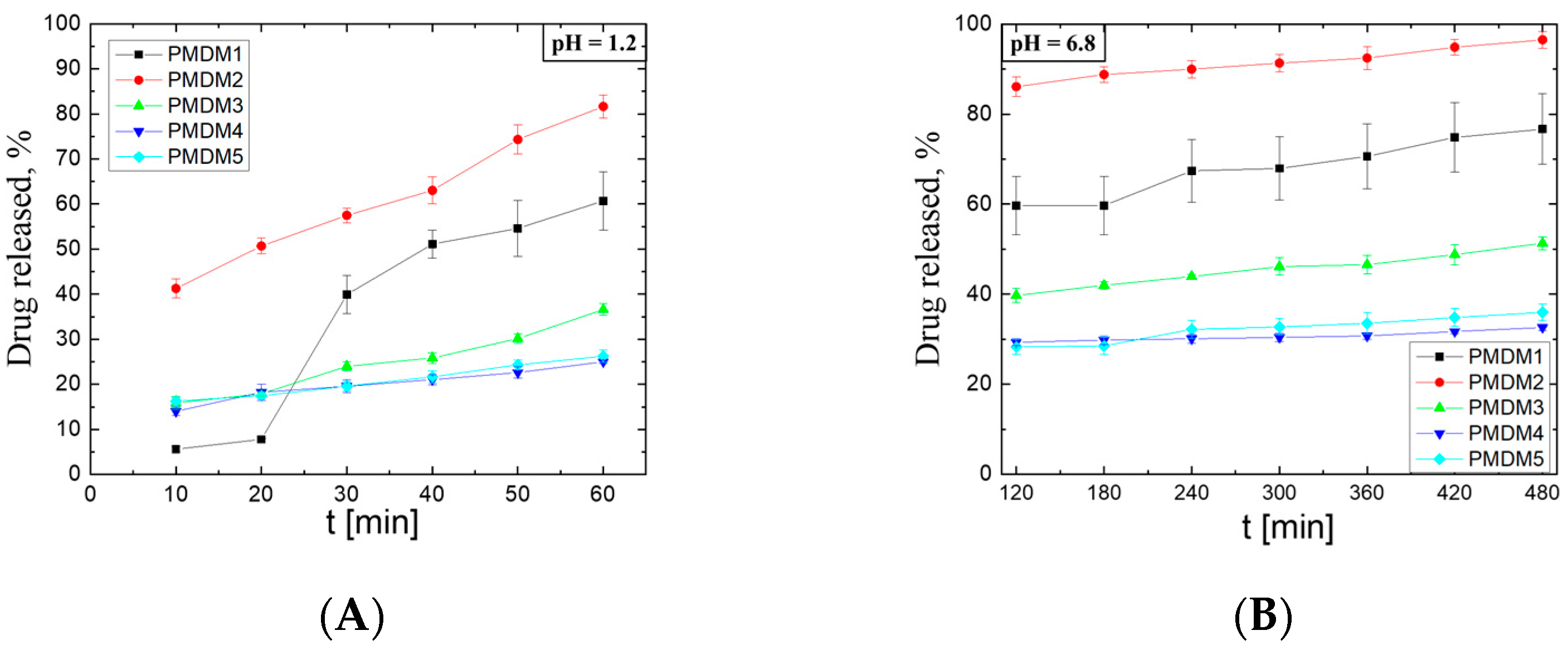

| pH = 1.2 | PMDM1 | PMDM2 | PMDM3 | PMDM4 | PMDM5 | |
|---|---|---|---|---|---|---|
| Zero order | K0 | 0.568 | 0.552 | 0.454 | 0.328 | 0.205 |
| R02 | 0.932 | 0.992 | 0.975 | 0.962 | 0.991 | |
| First order | K1 | 3.3 × 10−1 | 1 × 10−2 | 2.4 × 10−3 | 1.1 × 10−3 | 1.1 × 10−3 |
| R12 | 0.932 | 0.956 | 0.965 | 0.964 | 0.989 | |
| Higuchi | KH | 4.378 | 6.010 | 4.902 | 3.639 | 2.216 |
| RH2 | 0.950 | 0.973 | 0.939 | 0.979 | 0.956 | |
| Korsmeyer–Peppas | KKP | 0.0567 | 0.1679 | 0.0501 | 0.0698 | 0.0824 |
| n | 0.579 | 0.372 | 0.461 | 0.305 | 0.269 | |
| RKP2 | 0.950 | 0.969 | 0.935 | 0.984 | 0.920 | |
| D1.2 (m2/s) | 2.02 × 10−06 | 6.24 × 10−10 | 2.35 × 10−10 | 5.4 × 10−12 | 1.69 × 10−12 | |
| pH = 6.8 | PMDM1 | PMDM2 | PMDM3 | PMDM4 | PMDM5 | |
|---|---|---|---|---|---|---|
| Zero order | K0 | 0.0259 | 0.0184 | 0.0340 | 0.0117 | 0.0227 |
| R02 | 0.934 | 0.977 | 0.985 | 0.942 | 0.919 | |
| First order | K1 | 8.1 × 10−4 | 1.6 × 10−3 | 2 × 10−4 | 4 × 10−5 | 1 × 10−4 |
| R12 | 0.937 | 0.968 | 0.983 | 0.944 | 0.923 | |
| Higuchi | KH | 0.828 | 0.618 | 1.125 | 0.453 | 0.725 |
| RH2 | 0.948 | 0.978 | 0.981 | 0.887 | 0.950 | |
| Korsmeyer–Peppas | KKP | 0.225 | 0.591 | 0.169 | 0.208 | 0.183 |
| n | 0.195 | 0.078 | 0.175 | 0.069 | 0.115 | |
| RKP2 | 0.926 | 0.961 | 0.971 | 0.839 | 0.937 | |
| D6.8 (m2/s) | 1.22 × 10−12 | 7.08 × 10−17 | 4.41 × 10−14 | 7.73 × 10−25 | 7.05 × 10−18 | |
| Sample | SN PMAA | PMDM1 | PMDM2 | PMDM3 | PMDM4 | PMDM5 | SN PDMAM |
|---|---|---|---|---|---|---|---|
| CDMAM [mol L−1] | 0 | 0.5 | 1 | 2 | 3 | 4 | 1 |
| φPMAA | 1 | 0.66 | 0.55 | 0.22 | 0.14 | 0.09 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simeonov, M.; Yildirim, I.; Tzachev, C.T.; Vassileva, E. Stimuli-Responsive Hydrogels of Poly(Methacrylic Acid)/Poly(N,N-dimethylacrylamide) Interpenetrating Polymer Networks as Drug Delivery Systems for Promethazine Hydrochloride. Gels 2025, 11, 240. https://doi.org/10.3390/gels11040240
Simeonov M, Yildirim I, Tzachev CT, Vassileva E. Stimuli-Responsive Hydrogels of Poly(Methacrylic Acid)/Poly(N,N-dimethylacrylamide) Interpenetrating Polymer Networks as Drug Delivery Systems for Promethazine Hydrochloride. Gels. 2025; 11(4):240. https://doi.org/10.3390/gels11040240
Chicago/Turabian StyleSimeonov, Marin, Ioanna Yildirim, Christo T. Tzachev, and Elena Vassileva. 2025. "Stimuli-Responsive Hydrogels of Poly(Methacrylic Acid)/Poly(N,N-dimethylacrylamide) Interpenetrating Polymer Networks as Drug Delivery Systems for Promethazine Hydrochloride" Gels 11, no. 4: 240. https://doi.org/10.3390/gels11040240
APA StyleSimeonov, M., Yildirim, I., Tzachev, C. T., & Vassileva, E. (2025). Stimuli-Responsive Hydrogels of Poly(Methacrylic Acid)/Poly(N,N-dimethylacrylamide) Interpenetrating Polymer Networks as Drug Delivery Systems for Promethazine Hydrochloride. Gels, 11(4), 240. https://doi.org/10.3390/gels11040240







