A Review of the Development of Biopolymer Hydrogel-Based Scaffold Materials for Drug Delivery and Tissue Engineering Applications
Abstract
1. Introduction
2. Properties and Advantages of Biopolymer Hydrogels
2.1. Biocompatibility of Biopolymer Hydrogels
2.2. Biodegradability of Biopolymer Hydrogels
2.3. Hydrophilicity of Biopolymer Hydrogels
3. Tunable Physical and Chemical Properties of Biopolymer Hydrogels
4. Fabrication Techniques of Biopolymer Hydrogels
4.1. Chemical Crosslinking Method
4.2. Physical Crosslinking Method
4.3. Three-Dimensional (3D) Printing Method
4.4. Electrospinning Method
5. Biopolymer Hydrogel-Based Scaffold Materials for Drug Delivery Applications
5.1. Sustained Release Systems
5.2. Targeted Delivery System
5.3. Thermoresponsive and pH-Responsive Hydrogels
6. Biopolymer Hydrogel-Based Scaffold Materials for Tissue Engineering Applications
6.1. Cartilage and Bone Regeneration
6.2. Wound Healing
6.3. Soft Tissue
7. Challenges and Future Directions
7.1. Challenges
7.2. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater. Sci. Mater. Med. 2019, 30, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D Printing of Hydrogels: Rational Design Strategies and Emerging Biomedical Applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar]
- Liu, X.; Wu, K.; Gao, L.; Wang, L.; Shi, X. Biomaterial strategies for the application of reproductive tissue engineering. Bioact. Mater. 2022, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Champeau, M.; Heinze, D.A.; Viana, T.N.; de Souza, E.R.; Chinellato, A.C.; Titotto, S. 4D Printing of Hydrogels: A Review. Adv. Funct. Mater. 2020, 30, 1910606. [Google Scholar] [CrossRef]
- Boobalasibi, S.; Kabilan, B.; Dinesh, A.; Patil, R.P.; Radhakrishnan, K.; Gnanasekaran, L.; Manikandan, E.; Mohanavel, V.; Ayyar, M.; Iqbal, M.; et al. Review on Magnetic Nanoparticle-Infused Polymer Nanocomposites for Enhanced Photothermal Performance. Semiconductors 2024, 58, 1027. [Google Scholar] [CrossRef]
- El-Bahrawy, N.R.; Elgharbawy, H.; Elmekawy, A.; Salem, M.; Morsy, R. Development of porous hydroxyapatite/PVA/gelatin/alginate hybrid flexible scaffolds with improved mechanical properties for bone tissue engineering. Mater. Chem. Phys. 2024, 319, 129332. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J. Hydrophilic polymers. Polymers 2019, 11, 693. [Google Scholar] [CrossRef]
- Miri, Z.; Haugen, H.J.; Loca, D.; Rossi, F.; Perale, G.; Moghanian, A.; Ma, Q. Review on the strategies to improve the mechanical strength of highly porous bone bioceramic scaffolds. J. Eur. Ceram. Soc. 2024, 44, 23. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.J.; Yang, Y.; Chen, Y.; Zhu, X.; You, X. Biopolymer-based self-healing hydrogels: A short review. Giant 2023, 16, 100188. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, S.; Ke, Y.; Ding, L.; Zeng, X.; Magdassi, S.; Long, Y. 4D Printed Hydrogels: Fabrication, Materials, and Applications. Adv. Mater. Technol. 2020, 5, 2000034. [Google Scholar] [CrossRef]
- Rastogi, P.; Kandasubramanian, B. Review of Alginate-Based Hydrogel Bioprinting for Application in Tissue Engineering. Biofabrication 2019, 11, 042001. [Google Scholar] [CrossRef] [PubMed]
- Puertas-Bartolomé, M.; Mora-Boza, A.; García-Fernández, L. Emerging Biofabrication Techniques: A Review on Natural Polymers for Biomedical Applications. Polymers 2021, 13, 1209. [Google Scholar] [CrossRef]
- Thirumalai, D.; Santhamoorthy, M.; Kim, S.C.; Lim, H.R. Conductive polymer-based hydrogels for wearable electrochemical biosensors. Gels 2024, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, Y.; Ren, A.; Xiang, T.; Zhou, S. Degradable and Recyclable Hydrogels for Sustainable Bioelectronics. ACS Appl. Mater. Interfaces 2024, 16, 32887. [Google Scholar] [CrossRef]
- Mohan, A.; Suresh, R.; Ashwini, M.; Periyasami, G.; Guganathan, L.; Lin, M.C.; Kumarasamy, K.; Kim, S.C.; Santhamoorthy, M. Alginate functionalized magnetic-silica composites for pH-responsive drug delivery and magnetic hyperthermia applications. Mater. Lett. 2024, 362, 136088. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, X.; Ma, X.; Wang, W.; Yan, F.; Zhao, X.; Chu, X.; Xu, W.; Sun, C. A Review of the Properties and Applications of Bioadhesive Hydrogels. Polym. Chem. 2021, 12, 3721. [Google Scholar] [CrossRef]
- Erol, O.; Pantula, A.; Liu, W.; Gracias, D.H. Transformer Hydrogels: A Review. Adv. Mater. Technol. 2019, 4, 1900043. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future-Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef]
- Ishraque Ahmad, S.; Ahmad, R.; Shoeb Khan, M.; Kant, R.; Shahid, S.; Gautam, L.; Mustafa Hasan, G.; Hassan, I.M. Chitin and its derivatives: Structural properties and biomedical applications. Int. J. Biomacromol. 2020, 164, 526. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133. [Google Scholar] [CrossRef]
- Sampath, U.G.T.M.; Ching, Y.C.; Chuah, C.H.; Singh, R.; Lin, P.C. Preparation and characterization of nanocellulose reinforced semi-interpenetrating polymer network of chitosan hydrogel. Cellulose 2017, 24, 2215. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.; Patoary, M.K.; Zhang, M.; Mussana, H.; Li, M.; Naeem, M.A.; Mushtaq, M.; Farooq, A.; Liu, L. Cellulose from sources to nanocellulose and an overview of synthesis and properties of nanocellulose/zinc oxide nanocomposite materials. Int. J. Biol. Macromol. 2020, 154, 1050. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, V.K.; Singh, R.; Singh, K.; Dash, K.K.; Malik, S. Unveiling the potential of starch-blended biodegradable polymers for substantializing the eco-friendly innovations. J. Agric. Food Res. 2024, 15, 101065. [Google Scholar] [CrossRef]
- Chavez-Esquivel, G.; Cervantes-Cuevas, H.; Vera-Ramírez, M.A. Effect of dual modification with citric acid combined with ultrasonication on hydrolysis kinetics, morphology and structure of corn starch dispersions. Int. J. Biol. Macromol. 2022, 222, 1688. [Google Scholar] [CrossRef]
- Grosjean, M.; Gangolphe, L.; Nottelet, B. Degradable Self-Healable Networks for Use in Biomedical Applications. Adv. Funct. Mater. 2023, 33, 2205315. [Google Scholar] [CrossRef]
- Adjuik, T.A.; Nokes, S.E.; Montross, M.D. Biodegradability of Bio-Based and Synthetic Hydrogels as Sustainable Soil Amendments: A Review. J. Appl. Polym. Sci. 2023, 140, e53655. [Google Scholar] [CrossRef]
- Hu, B.; Gao, J.; Lu, Y.; Wang, Y. Applications of Degradable Hydrogels in Novel Approaches to Disease Treatment and New Modes of Drug Delivery. Pharmaceutics 2023, 15, 2370. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Suresh, R.; Ramkumar, V.; Guganathan, L.; Thirupathi, K.; Periyasami, G.; Mohan, A.; Kim, S.C. Amidoxime functionalized mesoporous silica nanoparticles for pH-responsive delivery of anticancer drug. Z. Phys. Chem. 2024, 238, 2135. [Google Scholar] [CrossRef]
- Levalley, P.J.; Neelarapu, R.; Sutherland, B.P.; Dasgupta, S.; Kloxin, C.J.; Kloxin, A.M. Photolabile Linkers: Exploiting Labile Bond Chemistry to Control Mode and Rate of Hydrogel Degradation and Protein Release. J. Am. Chem. Soc. 2020, 142, 4671. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Santhamoorthy, M.; Phan, T.T.V.; Kim, S.C. pNIPAm-Based pH and Thermoresponsive Copolymer Hydrogel for Hydrophobic and Hydrophilic Drug Delivery. Gels 2024, 10, 184. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2019, 12, 2653. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, V.; Raorane, C.J.; Christy, H.J.; Anandhi, S.; Santhamoorthy, S.; Kamachiyappan, P.; Ashokkumar, A.; Balamurugan, S.; Kim, S.C. Hydrogen-bonded keto-enol mechanized chalcone material for optical and antibiofilm applications. J. Mol. Struct. 2023, 1292, 136109. [Google Scholar] [CrossRef]
- Pan, X.; Li, Y.; Pang, W.; Xue, Y.; Wang, Z.; Jiang, C.; Shen, C.; Liu, Q.; Liu, L. Preparation, characterisation and comparison of glabridin loaded hydrogel forming microneedles by chemical and physical cross-linking. Int. J. Pharm. 2022, 617, 121612. [Google Scholar] [CrossRef]
- Sana, S.S.; Santhamoorthy, M.; Haldar, R.; Raorane, C.J.; Iravani, S.; Varma, R.S.; Kim, S.C. Recent advances on MXene-based hydrogels for antibacterial and drug delivery applications. Process. Biochem. 2023, 132, 200. [Google Scholar] [CrossRef]
- Yom-Tov, O.; Seliktar, D.; Bianco-Peled, H. PEG-Thiol based hydrogels with controllable properties. Eur. Polym. J. 2016, 74, 1. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Tapaswi, P.T.; Park, S.S.; Mathew, A.; Cho, H.J.; Ha, C.S. Ion-imprinted mesoporous silica hybrids for selective recognition of target metal ions. Micropor. Mesopor. Mater. 2013, 180, 162. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Kim, S.C. Dual pH-and Thermo-Sensitive Poly (N-Isopropylacrylamide-co-Allylamine) Nanogels for Curcumin Delivery: Swelling–Deswelling Behavior and Phase Transition Mechanism. Gels 2023, 9, 536. [Google Scholar] [CrossRef]
- Wang, R.; Liu, L.; He, X.; Xia, Z.; Zhao, Z.; Xi, Z.; Yu, J.; Wang, J. Dynamic Crosslinked Injectable Mussel-Inspired Hydrogels with Adhesive, Self-Healing, and Biodegradation Properties. Polymers 2023, 15, 1876. [Google Scholar] [CrossRef]
- Pan, H.; Tong, M.; Wang, X.; Huang, B.; Yu, X.; Zhang, C.; Wang, Y. Fully Biobased High-Strength and High-Toughness Double Cross-Linked Cellulose Hydrogel for Flexible Electrolytes. ACS Sust. Chem. Eng. 2024, 12, 18231–18244. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Hubbe, M.; Pal, L. Flexible and Pressure-Responsive Sensors from Cellulose Fibers Coated with Multiwalled Carbon Nanotubes. ACS Appl. Electron. Mater. 2019, 1, 1179. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Ramkumar, V.; Thirupathi, K.; Gnanasekaran, L.; Karuppannan, V.; Phan, T.T.V.; Kim, S.C. L-Lysine functionalized mesoporous silica hybrid nanoparticles for pH-responsive delivery of curcumin. Pharmaceutics 2023, 15, 1631. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.M.; Liu, R.J.; Yan, Q. Biological Stimuli-Responsive Polymer Systems: Design, Construction and Controlled Self-Assembly. Chin. J. Polym. Sci. 2018, 36, 347. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Santhamoorthy, M. Preparation of dual pH-and temperature-sensitive nanogels for curcumin delivery. Mater. Proc. 2023, 14, 71. [Google Scholar] [CrossRef]
- Wang, C.; Lai, J.; Li, K.; Zhu, S.; Lu, B.; Liu, J.; Tang, Y.; Wei, Y. Cryogenic 3D Printing of Dual- Delivery Scaffolds for Improved Bone Regeneration with Enhanced Vascularization. Bioact. Mater. 2021, 6, 137. [Google Scholar] [CrossRef]
- Barrett-Catton, E.; Ross, M.L.; Asuri, P. Multifunctional Hydrogel Nanocomposites for Biomedical Applications. Polymers 2021, 13, 856. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Vanaraj, R.; Thirupathi, K.; Ulagesan, S.; Nam, T.J.; Phan, T.T.V.; Kim, S.C. L- lysine-modified pNIPAm-co-GMA copolymer hydrogel for pH-and temperature-responsive drug delivery and fluorescence imaging applications. Gels 2023, 9, 363. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Park, J.H.; Bae, J.H.; Kim, S.H.; Ha, C.S. Mesoporous organosilica hybrids with a tunable amphoteric framework for controlled drug delivery. J. Mater. Chem. B. 2014, 2, 6487. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Adamek, P.; Paul, G.R.; Qin, X.H.; Rubert, M.; Muller, R. Optimization of mechanical stiffness and cell density of 3D bioprinted cell-laden scaffolds improves extracellular matrix mineralization and cellular organization for bone tissue engineering. Acta Biomater. 2020, 114, 307. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.S.; Yoo, H.S. Electrospun nanofibrils embedded hydrogel composites for cell cultivation in a biomimetic environment. RSC Adv. 2017, 7, 54246. [Google Scholar] [CrossRef]
- Wei, X.; Luo, Y.; Huang, P. 3D bioprinting of alginate scaffolds with controlled micropores by leaching of recrystallized salts. Polym. Bull. 2019, 76, 6077. [Google Scholar] [CrossRef]
- Thirupathi, K.; Santhamoorthy, M.; Radhakrishnan, S.; Ulagesan, S.; Nam, T.J.; Phan, T.T.V.; Kim, S.C. Thermosensitive polymer-modified mesoporous silica for pH and temperature-responsive drug delivery. Pharmaceutics 2023, 15, 795. [Google Scholar] [CrossRef] [PubMed]
- Tapaswi, P.K.; Moorthy, M.S.; Park, S.S.; Ha, C.S. Fast, selective adsorption of Cu2+ from aqueous mixed metal ions solution using 1, 4, 7-triazacyclononane modified SBA-15 silica adsorbent (SBA-TACN). J. Solid State Chem. 2014, 211, 191. [Google Scholar] [CrossRef]
- Blevins, K.M.; Danilkowicz, R.M.; Fletcher, A.N.; Allen, N.B.; Johnson, L.G.; Adams, S.B. In situ 3D bioprinting of musculoskeletal tissues in orthopedic surgery. J. 3D Print. Med. 2022, 6, 25. [Google Scholar] [CrossRef]
- Witzler, M.; Büchner, D.; Shoushrah, S.; Babczyk, P.; Baranova, J.; Witzleben, S.; Tobiasch, E.; Schulze, M. Polysaccharide-Based Systems for Targeted Stem Cell Differentiation and Bone Regeneration. Biomolecules 2019, 9, 840. [Google Scholar] [CrossRef]
- Basanth, A.; Mayilswamy, N.; Kandasubramanian, B. Bone regeneration by biodegradable polymers. Polym. Plast. Technol. Mater. 2022, 61, 816. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Thirupathi, K.; Krishnan, S.; Guganathan, L.; Dave, S.; Phan, T.T.V.; Kim, S.C. Preparation of magnetic iron oxide incorporated mesoporous silica hybrid composites for ph and temperature-sensitive drug delivery. Magnetochemistry 2023, 9, 81. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Z.-G.; Xiao, Z.-G. Preparation, physicochemical characterization and in vitro release behavior of resveratrol-loaded oxidized gellan gum/resistant starch hydrogel beads. Carbohydr. Polym. 2021, 260, 117794. [Google Scholar] [CrossRef]
- Osmałek, T.Z.; Froelich, A.; Soból, M.; Milanowski, B.; Skotnicki, M.; Kunstman, P.; Szybowicz, M. Gellan gum macrobeads loaded with naproxen: The impact of various naturally derived polymers on pH-dependent behavior. J. Biomater. Appl. 2018, 33, 140. [Google Scholar] [CrossRef]
- Liu, L.; Yao, W.; Xie, X.; Gao, J.; Lu, X. pH-sensitive dual drug loaded janus nanoparticles by oral delivery for multimodal analgesia. J. Nanobiotechnology 2021, 19, 235. [Google Scholar] [CrossRef]
- Fraser, D.; Nguyen, T.; Kotelsky, A.; Lee, W.; Buckley, M.; Benoit, D.S.W. Hydrogel Swelling- Mediated Strain Induces Cell Alignment at Dentin Interfaces. ACS Biomater. Sci. Eng. 2022, 8, 3568. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, K.; Phan, T.T.V.; Santhamoorthy, M.; Ramkumar, V.; Kim, S.C. pH and thermoresponsive PNIPAm-co-polyacrylamide hydrogel for dual stimuli-responsive controlled drug delivery. Polymers 2022, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Ren, T.; Chen, G.; Zhou, Z.; Li, Z.; Wu, W.; Huang, L. High-performance porous copolymer hydrogel for oceanic electricity generation. Chem. Eng. J. 2023, 456, 140983. [Google Scholar] [CrossRef]
- Ulagesan, S.; Santhamoorthy, M.; Phan, T.T.V.; Alagumalai, K.; Thirupathi, K.; Kim, S.C.; Nam, T.J.; Choi, Y.H. Mesoporous silica (SBA-15) with enriched amidoxime functionalities for pH- controlled anticancer drug delivery. Inorg. Chem. Commun. 2022, 146, 110132. [Google Scholar] [CrossRef]
- Li, H.; Li, B.; Lv, D.; Li, W.; Lu, Y.; Luo, G. Biomater releasing drug responsively to promote wound healing via regulation of pathological microenvironment. Adv. Drug Deliv. Rev. 2023, 196, 114778. [Google Scholar] [CrossRef]
- Shi, X.; Cheng, Y.; Wang, J.; Chen, H.; Wang, X.; Li, X.; Tan, W.; Tan, Z. 3D printed intelligent scaffold prevents recurrence and distal metastasis of breast cancer. Theranostics 2020, 10, 10652. [Google Scholar] [CrossRef]
- Wu, W.; Luo, L.; Wang, Y.; Wu, Q.; Dai, H.-B.; Li, J.-S.; Durkan, C.; Wang, N.; Wang, G.-X. Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics 2018, 8, 3038. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Phan, T.T.V.; Ramkumar, V.; Raorane, C.J.; Thirupathi, K.; Kim, S.C. Thermo- sensitive poly (N-isopropylacrylamide-co-polyacrylamide) hydrogel for pH-responsive therapeutic delivery. Polymers 2022, 14, 4128. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, X.; Huang, R.; Chen, H.; Shi, X.; Wang, J.; Tan, W.; Tan, Z. E-jet 3D printed drug delivery implants to inhibit growth and metastasis of orthotopic breast cancer. Biomaterials 2020, 230, 119618. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Ma, P.X.; Guo, B.; Du, Y. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 2019, 536, 224. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117. [Google Scholar] [CrossRef]
- Dang, H.P.; Shafiee, A.; Lahr, C.A.; Dargaville, T.R.; Tran, P.A. Local Doxorubicin Delivery via 3D- Printed Porous Scaffolds Reduces Systemic Cytotoxicity and Breast Cancer Recurrence in Mice. Adv. Ther. 2020, 3, 2000056. [Google Scholar] [CrossRef]
- Wang, D.; Xia, Y.; Zhang, D.; Sun, X.; Chen, X.; Oliver, S.; Shi, S.; Lei, L. Hydrogen-bonding reinforced injectable hydrogels: Application as a thermo-triggered drug controlled-release system. ACS Appl. Polym. Mater. 2020, 2, 1587. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Kunasekaran, U.; Thirupathi, K.; Thirumalai, D.; Kim, S.C. Fluorophore- modified hybrid mesoporous silica nanosensor for selective sensing of mercury ions from aqueous solution and biological cells. Mater. Lett. 2022, 313, 131786. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Park, S.S.; Mathew, A.; Lee, S.H.; Lee, W.K.; Ha, C.S. Amidoxime functionalized SBA-15 for selective adsorption of Li+ ions. Sci. Adv. Mater. 2014, 6, 1611. [Google Scholar] [CrossRef]
- Chen, J.; Peng, Q.; Peng, X.; Han, L.; Wang, X.; Wang, J.; Zeng, H. Recent advances in mechano- responsive hydrogels for biomedical applications. ACS Appl. Polym. Mater. 2020, 2, 1092. [Google Scholar] [CrossRef]
- Gao, J.; Karp, J.M.; Langer, R.; Joshi, N. The future of drug delivery. Chem. Mater. 2023, 35, 359. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, P.; Zhou, Y.; Zhou, X.; Ma, X.; Tan, T.; Cao, H. Hydrogel-encapsulated medium chain lipid-modified zeolite imidazole framework-90 as a promising platform for oral delivery of proteins. J. Control Release 2024, 367, 93. [Google Scholar] [CrossRef]
- Periyasamy, T.; Asrafali, S.P.; Haldhar, R.; Madhappan, S.; Vanaraj, R.; Raorane, C.J.; Kim, S.C. Modified cotton sponge with bio-based polybenzoxazine for plasticizer absorption and oil–water separation. ACS Appl. Mater. 2022, 4, 950. [Google Scholar] [CrossRef]
- Meng, D.; Lei, H.; Zheng, X.; Han, Y.; Sun, R.; Zhao, D.; Liu, R. A temperature-sensitive phase- change hydrogel of tamoxifen achieves the long-acting antitumor activation on breast cancer cells. Onco Targets Ther. 2019, 12, 3919. [Google Scholar] [CrossRef] [PubMed]
- Santhamoorthy, M.; Thirupathi, K.; Periyasamy, T.; Thirumalai, D.; Ramkumar, V.; Kim, S.C. Ethidium bromide-bridged mesoporous silica hybrid nanocarriers for fluorescence cell imaging and drug delivery applications. New J. Chem. 2021, 45, 20641. [Google Scholar] [CrossRef]
- Zhang, N.; Zheng, S.; Pan, Z.; Liu, Z. Phase Transition Effects on Mechanical Properties of NIPA Hydrogel. Polymers 2018, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gao, Z.; Liu, Y.; Huang, Z.; Chai, C.; Hao, J. Multiple Cross-Linking-Dominated Metal−Ligand Coordinated Hydrogels with Tunable Strength and Thermosensitivity. ACS Appl. Polym. Mater. 2019, 1, 2370. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Park, S.S.; Selvaraj, M.; Ha, C.S. Cyclic ligand functionalized mesoporous silica (SBA-15) for selective adsorption of Co2+ ion from artificial seawater. J. Nanosci. Nanotechnol. 2014, 14, 8891. [Google Scholar] [CrossRef]
- Manivasagan, P.; Hoang, G.; Moorthy, M.S.; Mondal, S.; Doan, V.H.M.; Kim, H.; Phan, T.T.V.; Nguyen, T.P.; Oh, J. Chitosan/fucoidan multilayer coating of gold nanorods as highly efficient near- infrared photothermal agents for cancer therapy. Carbohyd. Polym. 2019, 211, 360. [Google Scholar] [CrossRef]
- Park, S.S.; Moorthy, M.S.; Song, H.J.; Ha, C.S. Functionalized mesoporous silicas with crown ether moieties for selective adsorption of lithium ions in artificial sea water. J. Nanosci. Nanotechnol. 2014, 14, 8845. [Google Scholar] [CrossRef]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers 2021, 13, 2086. [Google Scholar] [CrossRef]
- Mondal, S.; Hoang, G.; Manivasagan, P.; Moorthy, M.S.; Kim, H.H.; Phan, T.T.V.; Oh, J. Comparative characterization of biogenic and chemical synthesized hydroxyapatite biomaterials for potential biomedical application. Mater. Chem. Phys. 2019, 228, 344. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.; Zhao, B.; Pinnaratip, R.; et al. Catechol-Functionalized Hydrogels: Biomimetic Design, Adhesion Mechanism, and Biomedical Applications. Chem. Soc. Rev. 2020, 49, 433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, F.; Chen, X.; Wang, X.-W.; Zhang, W.-B.; Peng, J.; Li, J.; Zhai, M. Stretchable, Conductive, and Self-Healing Hydrogel with Super Metal Adhesion. Chem. Mater. 2018, 30, 4289. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Hoang, G.; Manivasagan, P.; Mondal, S.; Phan, T.T.V.; Kim, H.; Oh, J. Chitosan oligosaccharide coated mesoporous silica nanoparticles for pH-stimuli responsive drug delivery applications. J. Porous. Mater. 2019, 26, 217. [Google Scholar] [CrossRef]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering Hydrogel Adhesion for Biomedical Applications via Chemical Design of the Junction. ACS Biomater. Sci. Eng. 2021, 7, 4048. [Google Scholar] [CrossRef]
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications. Gels 2021, 7, 77. [Google Scholar] [CrossRef]
- Cook, M.T.; Haddow, P.; Kirton, S.B.; McAuley, W.J. Polymers Exhibiting Lower Critical Solution Temperatures as a Route to Thermoreversible Gelators for Healthcare. Adv. Funct. Mater. 2021, 31, 2008123. [Google Scholar] [CrossRef]
- Hosseini, V.; Maroufi, N.F.; Saghati, S.; Asadi, N.; Darabi, M.; Ahmad, S.N.S.; Hosseini, H.; Reza, R. Current progress in hepatic tissue regeneration by tissue engineering. J. Transl. Med. BioMed. Cent. 2019, 17, 1. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Hoang, G.; Nguyen, T.P.; Kim, H.H.; Mondal, S.; Manivasagan, P.; Moorthy, M.S.; Lee, K.D.; Junghwan, O. Chitosan as a stabilizer and size-control agent for synthesis of porous flower-shaped palladium nanoparticles and their applications on photo-based therapies. Carbohyd. Polym. 2019, 205, 340. [Google Scholar] [CrossRef]
- Egorikhina, M.N.; Bronnikova, I.I.; Rubtsova, Y.P.; Charykova, I.N.; Bugrova, M.L.; Linkova, D.D.; Aleynik, D.Y. Aspects of In Vitro Biodegradation of Hybrid Fibrin–Collagen Scaffolds. Polymers 2021, 13, 3470. [Google Scholar] [CrossRef]
- Wang, F.; Cai, X.; Shen, Y.; Meng, L. Cell–Scaffold Interactions in Tissue Engineering for Oral and Craniofacial Reconstruction. Bioact. Mater. 2023, 23, 16. [Google Scholar] [CrossRef]
- Manivasagan, P.; Jun, S.W.; Truong, N.T.P.; Hoang, G.; Mondal, S.; Moorthy, M.S.; Kim, H.; Phan, T.T.V.; Doan, V.H.M.; Kim, C.S.; et al. A multifunctional near-infrared laser-triggered drug delivery system using folic acid conjugated chitosan oligosaccharide encapsulated gold nanorods for targeted chemo-photothermal therapy. J. Mater. Chem. B 2019, 7, 3811. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Seo, H.; Manivasagan, P.; Moorthy, M.S.; Park, S.; Oh, J. In vitro photodynamic effect of phycocyanin against breast cancer cells. Molecules 2016, 21, 1470. [Google Scholar] [CrossRef]
- Haugen, H.J.; Basu, P.; Sukul, M.; Mano, J.F.; Reseland, J.E. Injectable Biomaterials for Dental Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3442. [Google Scholar] [CrossRef]
- Shi, J.; Yu, L.; Ding, J. PEG-Based Thermosensitive and Biodegradable Hydrogels. Acta Biomater. 2021, 128, 42. [Google Scholar] [CrossRef] [PubMed]
- de Melo, B.A.G.; Jodat, Y.A.; Cruz, E.M.; Benincasa, J.C.; Shin, S.R.; Porcionatto, M.A. Strategies to Use Fibrinogen as Bioink for 3D Bioprinting Fibrin-Based Soft and Hard Tissues. Acta Biomater. 2020, 117, 60. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, M.; Li, P.; Gan, D.; Yan, L.; Xu, J.; Wang, K.; Fang, L.; Chan, C.W.; Zhang, H.; et al. Mussel-inspired tissue-adhesive hydrogel based on the polydopamine-chondroitin sulfate complex for growth-factor-free cartilage regeneration. ACS Appl. Mater. Interfaces 2018, 10, 28015. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Li, Y.; Ding, X.; Li, D.; Shen, C.; Xu, F.J. Dual-Crosslinked Amorphous Polysaccharide Hydrogels Based on Chitosan/Alginate for Wound Healing Applications. Macromol. Rapid Commun. 2018, 39, 1800069. [Google Scholar] [CrossRef]
- Saunders, L.; Ma, P.X. Self-Healing Supramolecular Hydrogels for Tissue Engineering Applications. Macromol. Biosci. 2019, 19, e1800313. [Google Scholar] [CrossRef]
- Teng, L.; Chen, Y.; Jia, Y.-G.; Ren, L. Supramolecular and dynamic covalent hydrogel scaffolds: From gelation chemistry to enhanced cell retention and cartilage regeneration. J. Mater. Chem. B 2019, 7, 6705. [Google Scholar] [CrossRef]
- Mondal, S.; Hoang, G.; Manivasagan, P.; Moorthy, M.S.; Nguyen, T.P.; Phan, T.T.V.; Kim, H.H.; Kim, M.H.; Nam, S.Y.; Oh, J. Nano-hydroxyapatite bioactive glass composite scaffold with enhanced mechanical and biological performance for tissue engineering application. Ceram. Int. 2018, 44, 15735. [Google Scholar] [CrossRef]
- Xu, Y.; Rothe, R.; Voigt, D.; Sayed, A.; Huang, C.; Hauser, S.; Lee, P.-W.; Cui, M.; Sáenz, J.P.; Boccaccini, A.R.; et al. A self-assembled dynamic extracellular matrix-like hydrogel system with multi-scale structures for cell bioengineering applications. Acta Biomater. 2023, 162, 211. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Gosecka, M.; Bodaghi, M.; Crespy, D.; Youssef, G.; Dodda, J.M.; Wong, S.H.D.; Imran, A.B.; Gosecki, M.; Jobdeedamrong, A.; et al. Engineering multifunctional dynamic hydrogel for biomedical and tissue regenerative applications. Chem. Eng. J. 2024, 487, 150403. [Google Scholar] [CrossRef]
- Guo, B.; Liang, Y.; Dong, R. Physical dynamic double-network hydrogels as dressings to facilitate tissue repair. Nat. Protoc. 2023, 18, 3322. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Li, Y.; Niu, X.; Zhao, B.; Wang, Y.; Bao, C.; Xie, Z.; Lin, Q.; Zhu, L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 2017, 9, 4430. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhou, J.; Liu, B.; Liang, C.; Pan, X.; Zhang, Y.; Zhang, Y.; Wang, Y.; Shao, L.; Wang, J.; et al. Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Mater. Sci. Eng. C. 2019, 99, 322. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, B.; Yin, P.; Zhao, L.-S.; Wang, Y.; Fu, Z.; Dang, R.; Xu, J.; Zhang, J.; Wen, N. Integration of Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomes with Hydroxyapatite-Embedded Hyaluronic Acid-Alginate Hydrogel for Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 1590. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081. [Google Scholar] [CrossRef]
- Chen, J.G.; Huang, T.Y.; Liu, R.Q.; Wang, C.Y.; Jiang, H.Y.; Sun, H.Y. Congenital microtia patients: The genetically engineered exosomes released from porous gelatin methacryloyl hydrogel for downstream small RNA profiling, functional modulation of microtia chondrocytes and tissue- engineered ear cartilage regeneration. J. Nanobiotechnology 2022, 20, 164. [Google Scholar] [CrossRef]
- Zhou, B.X.; Jiang, X.L.; Zhou, X.X.; Tan, W.Y.; Luo, H.; Lei, S.R.; Yang, Y. GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: Therapeutic strategies and recent advances. Biomater. Res. 2023, 27, 86. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Bharathiraja, S.; Manivasagan, P.; Oh, Y.; Phan, T.T.V.; Mondal, S.; Kim, H.; Lee, K.D.; Oh, J. Synthesis of Fe3O4 modified mesoporous silica hybrid for pH-responsive drug delivery and magnetic hyperthermia applications. J. Porous Mater. 2018, 25, 1251. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold fabrication technologies and structure/function poperties in bne tssue enineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Xiao, S.N.; Zhao, T.F.; Wang, J.K.; Wang, C.G.; Du, J.N.; Ying, L.W.; Lin, J.T.; Zhang, C.H.; Hu, W.L.; Wang, L.N.; et al. Gelatin methacrylate (GelMA)-Based hydrogels for cell transplantation: An effective strategy for tissue engineering. Stem Cell Rev. Rep. 2019, 15, 664. [Google Scholar] [CrossRef] [PubMed]
- Butenko, S.; Nagalla, R.R.; Guerrero-Juarez, C.F.; Palomba, F.; David, L.-M.; Nguyen, R.Q.; Gay, D.; Almet, A.A.; Digman, M.A.; Nie, Q.; et al. Hydrogel crosslinking modulates macrophages, fibroblasts, and their communication, during wound healing. Nat. Commun. 2024, 15, 6820. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Mondal, S.; Bharathiraja, S.; Manivasagan, P.; Moorthy, M.S.; Oh, J. Optimized Zn-doped hydroxyapatite/doxorubicin bioceramics system for efficient drug delivery and tissue engineering application. Ceram. Int. 2018, 44, 6062. [Google Scholar] [CrossRef]
- Dong, L.L.; Bu, Z.H.; Xiong, Y.Z.; Zhang, H.; Fang, J.H.; Hu, H.X.; Liu, Z.T.; Li, X. Facile extrusion 3D printing of gelatine methacrylate/Laponite nanocomposite hydrogel with high concentration nanoclay for bone tissue regeneration. Int. J. Biol. Macromol. 2021, 188, 72. [Google Scholar] [CrossRef]
- Cidonio, G.; Alcala-Orozco, C.R.; Lim, K.S.; Glinka, M.; Mutreja, I.; Kim, Y.H.; Dawson, J.I.; Woodfield, T.B.F.; Oreffo, R.O.C. Osteogenic and angiogenic tissue formation in high fidelity nanocomposite Laponite-gelatin bioinks. Biofabrication 2019, 11, 035027. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Mondal, S.; Nguyen, T.P.; Kim, H.; Phan, T.T.V.; Lee, K.D.; Oh, J. Biocompatible chitosan oligosaccharide modified gold nanorods as highly effective photothermal agents for ablation of breast cancer cells. Polymers 2018, 10, 232. [Google Scholar] [CrossRef]
- Alves, A.; Miguel, S.P.; Araujo, A.R.; de Jesús Valle, M.J.; Sánchez Navarro, A.; Correia, I.J.; Ribeiro, M.P.; Coutinho, P. Xanthan Gum-Konjac Glucomannan Blend Hydrogel for Wound Healing. Polymers 2020, 12, 99. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Tao, K.; Feng, Q.; Li, F.; Mu, X.; Du, C.; Zhao, R.; Wang, D.; Zhou, X.; et al. Blood-responsive mussel-inspired hydrogels for hemostasis, antibacterial action, and wound healing. Int. J. Biomacromol. 2024, 278, 135038. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, S.; Bui, N.Q.; Manivasagan, P.; Moorthy, M.S.; Mondal, S.; Seo, H.; Phuoc, N.T.; Phan, T.T.V.; Kim, H.; Lee, K.D.; et al. Multimodal tumor-homing chitosan oligosaccharide-coated biocompatible palladium nanoparticles for photo-based imaging and therapy. Sci. Rep. 2018, 8, 500. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Benito-Garzón, L.; Fung, S.; Kohn, J.; Vázquez-Lasa, B.; San Román, J. Bioadhesive functional hydrogels: Controlled release of catechol species with antioxidant and antiinflammatory behavior. Mater. Sci. Eng. C 2019, 105, 110040. [Google Scholar] [CrossRef]
- Cai, F.; Wang, P.; Yuan, M.; Chen, W.; Liu, Y. Hypoxic microenvironment promotes diabetic wound healing by polarizing macrophages to the M2 phenotype in vivo. J. Mol. Histol. 2024, 55, s10735. [Google Scholar] [CrossRef] [PubMed]
- Gourishetti, K.; Keni, R.; Nayak, P.G.; Jitta, S.R.; Bhaskaran, N.A.; Kumar, L.; Kumar, N.; Krishnadas, N.; Shenoy, R.R. Sesamol-Loaded PLGA Nanosuspension for Accelerating Wound Healing in Diabetic Foot Ulcer in Rats. Int. J. Nanomed. 2020, 15, 9265. [Google Scholar] [CrossRef]
- Markiewicz-Gospodarek, A.; Koziol, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice. Int. J. Environ. Res. Public Health 2022, 19, 1338. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, C.; Wang, B.; Yin, J.; Yan, S. Progress in Hydrogels for Skin Wound Repair. Macromol. Biosci. 2022, 22, e2100475. [Google Scholar] [CrossRef]
- Phelan, H.A.; James, H.H.; Hickerson, W.L.; Cockerell, C.J.; Shupp, J.W.; Carter, J.E. Use of 816 Consecutive Burn Wound Biopsies to Inform a Histologic Algorithm for Burn Depth Categorization. J. Burn Care Res. 2021, 42, 1162. [Google Scholar] [CrossRef]
- Salehi, M.; Farzamfar, S.; Bozorgzadeh, S.; Bastami, F. Fabrication of Poly(L-Lactic Acid)/Chitosan Scaffolds by Solid-Liquid Phase Separation Method for Nerve Tissue Engineering: An In Vitro Study on Human Neuroblasts. J. Craniofac. Surg. 2019, 30, 784. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wu, P.; An, G.; Fang, Q.; Zheng, J.; Wang, Y.B. Research advances on the techniques for diagnosing burn wound depth. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi 2022, 38, 481. [Google Scholar]
- Kamolz, L.-P.; Hecker, A. Molecular Mechanisms Related to Burns, Burn Wound Healing and Scarring. Int. J. Mol. Sci. 2023, 24, 8785. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lv, H.; Du, Y.; Zhu, W.; Yang, W.; Wang, X.; Wang, J.; Chen, W. Biologically Modified Implantation as Therapeutic Bioabsorbable Materials for Bone Defect Repair. Regen. Ther. 2022, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Saad, B. Qasim In-vitro and in-vivo degradation studies of freeze gelated porous chitosan composite scaffolds for tissue engineering applications. Polym. Degrad. Stab. 2017, 136, 31. [Google Scholar]
- Zhang, F.; King, M.W. Biodegradable Polymers as the Pivotal Player in the Design of Tissue Engineering Scaffolds. Adv. Healthc. Mater. 2020, 9, 1901358. [Google Scholar] [CrossRef]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef]
- Sahranavard, M.; Zamanian, A.; Ghorbani, F.; Shahrezaee, M.H. A critical review on three dimensional-printed chitosan hydrogels for development of tissue engineering. Bioprinting 2020, 17, e00063. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164. [Google Scholar] [CrossRef]
- Long, L.; Hu, C.; Liu, W.; Wu, C.; Lu, L.; Yang, L.; Wang, Y. Injectable multifunctional hyaluronic acid/methylcellulose hydrogels for chronic wounds repairing. Carbohydr. Polym. 2022, 289, 119456. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Peng, X.; Zhang, L. Chitosan-based drug delivery systems: Current strategic design and potential application in human hard tissue repair. Eur. Polym. J. 2022, 166, 110979. [Google Scholar] [CrossRef]
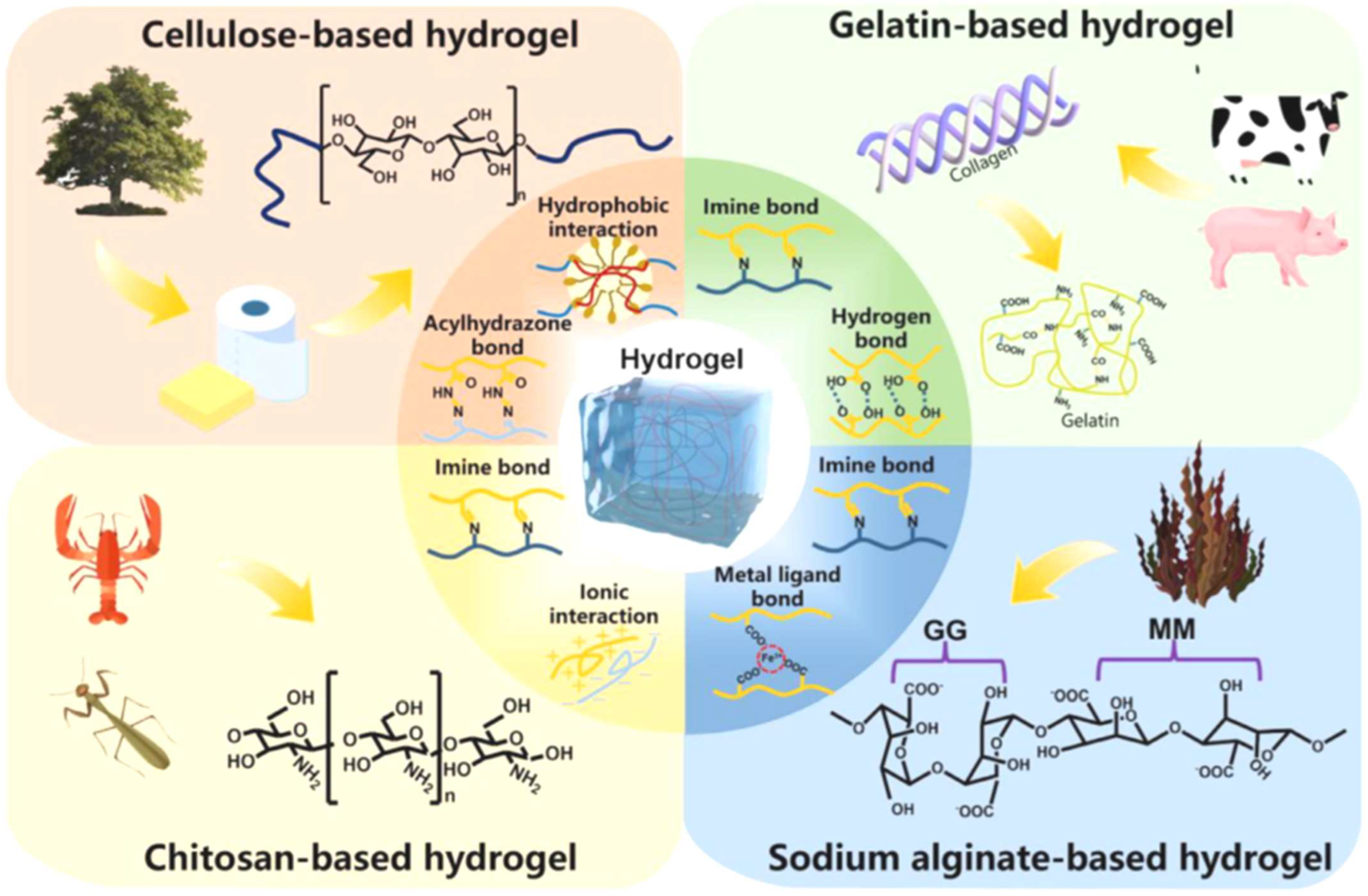









| Biopolymer | Sources | Chemical Structure | Refs. |
|---|---|---|---|
| Chitin | Corals, lamp shells, sponges, squid, and cuttlefish |  | [18,19] |
| Chitosan | Fungi, algae, mollusks, crustaceans, and insects |  | [20,21] |
| Alginate | Seaweed |  | [22] |
| Cellulose | Seaweed, rice husk, and sugarcane bagasse, wood, bamboo, sugarbeet, banana rachis, potato tubers, cotton, hemp, coconut, grass, wheat, rice, and barley |  | [23,24] |
| Starch | Potatoes, maize, cassava, rice, banana, wheat | 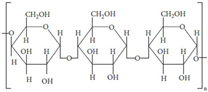 | [25,26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santhamoorthy, M.; Kim, S.-C. A Review of the Development of Biopolymer Hydrogel-Based Scaffold Materials for Drug Delivery and Tissue Engineering Applications. Gels 2025, 11, 178. https://doi.org/10.3390/gels11030178
Santhamoorthy M, Kim S-C. A Review of the Development of Biopolymer Hydrogel-Based Scaffold Materials for Drug Delivery and Tissue Engineering Applications. Gels. 2025; 11(3):178. https://doi.org/10.3390/gels11030178
Chicago/Turabian StyleSanthamoorthy, Madhappan, and Seong-Cheol Kim. 2025. "A Review of the Development of Biopolymer Hydrogel-Based Scaffold Materials for Drug Delivery and Tissue Engineering Applications" Gels 11, no. 3: 178. https://doi.org/10.3390/gels11030178
APA StyleSanthamoorthy, M., & Kim, S.-C. (2025). A Review of the Development of Biopolymer Hydrogel-Based Scaffold Materials for Drug Delivery and Tissue Engineering Applications. Gels, 11(3), 178. https://doi.org/10.3390/gels11030178






