Evaluation of SARS-CoV-2 Serological Testing in Patients with Multiple Myeloma and Other Hematologic Malignancies on Monoclonal Antibody Therapies
Abstract
1. Introduction
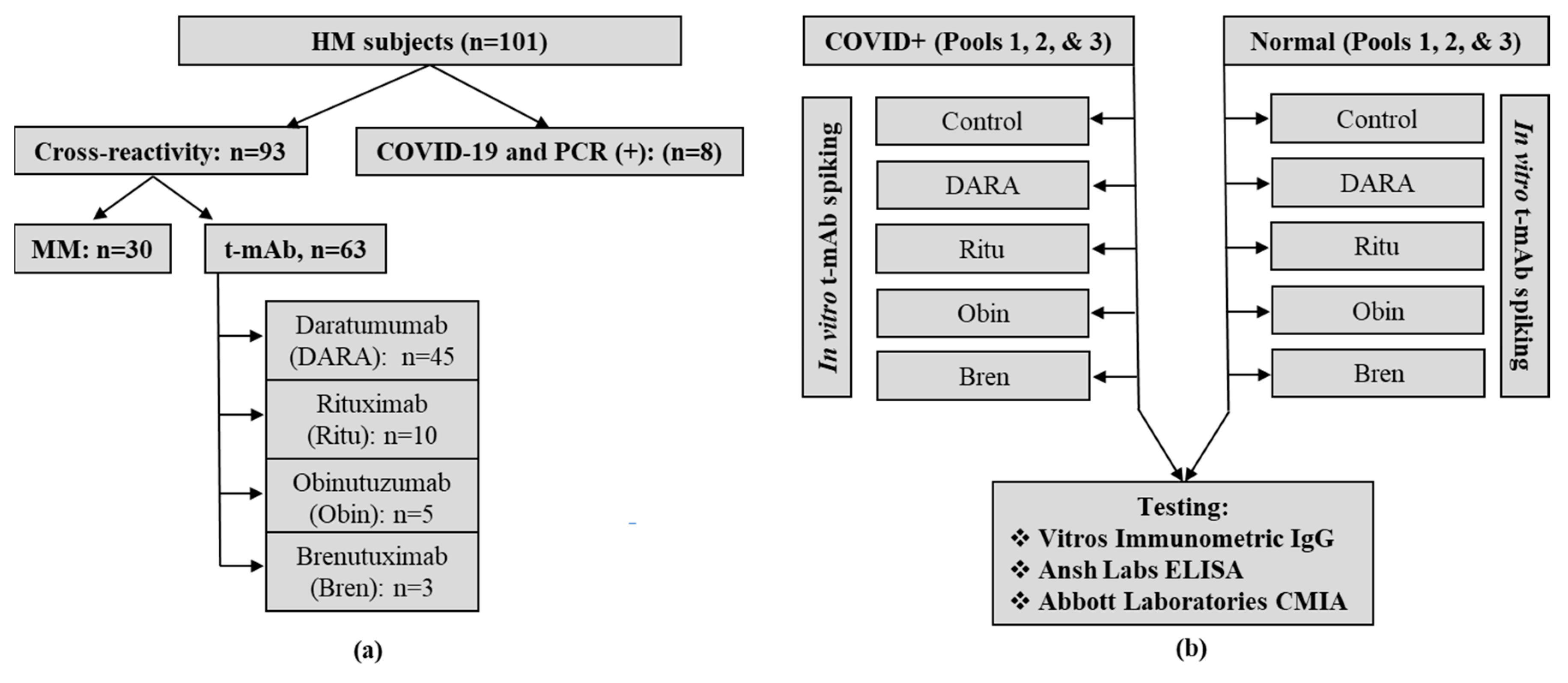
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, L.Y.W.; Cazier, J.-B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, A.N.; Chackathayil, J.; Cheng, V.W.; Curley, H.M.; Fittall, M.W.; et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [PubMed]
- He, W.; Chen, L.; Yuan, G.; Fang, Y.; Chen, W.; Wu, D.; Liang, B.; Lu, X.; Ma, Y.; Li, L.; et al. COVID-19 in persons with haematological cancers. Leukemia 2020, 34, 1637–1645. [Google Scholar] [PubMed]
- Aries, J.A.; Davies, J.K.; Auer, R.L.; Hallam, S.L.; Montoto, S.; Smith, M.; Sevillano, B.; Foggo, V.; Wrench, B.; Zegocki, K.; et al. Clinical outcome of coronavirus disease 2019 in haemato-oncology patients. Br. J. Haematol. 2020, 190, e64–e67. [Google Scholar] [PubMed]
- Lee, S.J.; Borrello, I. Role of the immune response in disease progression and therapy in multiple myeloma. Cancer Treat. Res. 2016, 169, 207–225. [Google Scholar] [PubMed]
- Kumar, S.K.; Anderson, K.C. Immune therapies in multiple myeloma. Clin. Cancer Res. 2016, 22, 5453–5460. [Google Scholar] [PubMed]
- Blimark, C.; Holmberg, E.; Mellqvist, U.-H.; Landgren, O.; Björkholm, M.; Hultcrantz, M.; Kjellander, C.; Turesson, I.; Kristinsson, S.Y. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica 2015, 100, 107–113. [Google Scholar] [PubMed]
- Wang, B.; Van Oekelen, O.; Mouhieddine, T.H.; Del Valle, D.M.; Richter, J.; Cho, H.J.; Richard, S.; Chari, A.; Gnjatic, S.; Merad, M.; et al. A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward. J. Hematol. Oncol. 2020, 13, 94. [Google Scholar] [PubMed]
- Cook, G.; Ashcroft, A.J.; Pratt, G.; Popat, R.; Ramasamy, K.; Kaiser, M.; Jenner, M.; Henshaw, S.; Hall, R.; Sive, J.; et al. Real-world assessment of the clinical impact of symptomatic infection with severe acute respiratory syndrome coronavirus (COVID-19 disease) in patients with multiple myeloma receiving systemic anti-cancer therapy. Br. J. Haematol. 2020, 190, e83–e86. [Google Scholar]
- Long, Q.; Liu, B.-Z.; Deng, H.-J.; Wu, G.-C.; Deng, K.; Chen, Y.-K.; Liao, P.; Qiu, J.-F.; Lin, Y.; Cai, X.-F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar]
- King, R.I.; Florkowski, C.M. How paraproteins can affect laboratory assays: spurious results and biological effects. Pathology 2010, 42, 397–401. [Google Scholar] [PubMed]
- Willrich, M.A.V.; Ladwig, P.M.; Andreguetto, B.D.; Barnidge, D.R.; Murray, D.L.; Katzmann, A.J.; Snyder, M.R. Monoclonal antibody therapeutics as potential interferences on protein electrophoresis and immunofixation. Clin. Chem. Lab. Med. 2016, 54, 1085–1093. [Google Scholar]
- McDonald, S.; Courtney, D.M.; Clark, A.E.; Muthukumar, A.; Lee, F.; Balani, J.; Mahimainathan, L.; Bararia, A.; Oliver, D.; Sarode, R.; et al. Diagnostic performance of a rapid point-of-care test for SARS-CoV-2 in an urban emergency department setting. Acad. Emerg. Med. 2020, 27, 764–766. [Google Scholar]
| (a) | |||||||||||||
| Specimen | Vitros Immunodiagnostics | Ansh Labs | Abbott Laboratories | ||||||||||
| Result | Interpretation | Result | Interpretation | Result | Interpretation | ||||||||
| MM (n = 30) mean ± SD | 0.030 ± 0.009 | NR | 2.107 ± 0.842 | NR | 0.030 ± 0.081 | Neg | |||||||
| HM + t-mAb (n = 63) mean ± SD | DARA (n = 45) | 0.026 ± 0.007 | NR | 2.393 ± 1.130 | NR | 0.028 ± 0.084 | Neg | ||||||
| Ritu (n = 10) | 0.040 ± 0.012 | NR | 2.360 ± 1.313 | NR | 0.067 ± 0.156 | Neg | |||||||
| Obin (n = 5) | 0.042 ± 0.019 | NR | 2.640 ± 1.165 | NR | 0.080 ± 0.140 | Neg | |||||||
| Bren (n = 3) | 0.050 ± 0.010 | NR | 1.700 ± 0.721 | NR | 0.027 ± 0.015 | Neg | |||||||
| HM + CoV (n = 8) Index or Unit (*) | Patient #1 | 10.2 | R | 10.2 | INT | 1.80 | Pos | ||||||
| Patient #2 | 211 | R | 211 | R | 5.65 | Pos | |||||||
| Patient #3 | 840 | R | 118.98 | R | 7.12 | Pos | |||||||
| Patient #4 | 770 | R | 95.55 | R | 7.32 | Pos | |||||||
| Patient #5 | 810 | R | 130.72 | R | 8.11 | Pos | |||||||
| Patient #6 | 940 | R | 132.42 | R | 7.62 | Pos | |||||||
| Patient #7 | 590 | R | 82.97 | R | 7.58 | Pos | |||||||
| Patient #8 | 620 | R | 34.71 | R | 7.05 | Pos | |||||||
| (b) | |||||||||||||
| Assay Type | Pooled Normal Samples—Index/Unit (Non-Reactive/Negative) | Pooled COVID-19 Samples—Index/Unit (Reactive/Positive) | |||||||||||
| Control | DARA | Ritu | Obin | Bren | Control | DARA | Ritu | Obin | Bren | ||||
| Vitros Immuno-diagnostics | 1 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 135 | 154 | 196 | 137 | 158 | ||
| 2 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 11.5 | 10.9 | 11.0 | 10.9 | 12.1 | |||
| 3 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 460 | 442 | 448 | 448 | 437 | |||
| Ansh Labs | 1 | 1.6 | 1.3 | 1.1 | 2.1 | 3.1 | 98.80 | 96.79 | 85.04 | 100.16 | 81.20 | ||
| 2 | 2.5 | 3.1 | 2.5 | 1.3 | 2.1 | 15.84 | 14.00 | 13.64 | 16.66 | 13.43 | |||
| 3 | 2.4 | 3.1 | 1.5 | 3.6 | 4.1 | 95.16 | 101.68 | 97.32 | 105.14 | 94.67 | |||
| Abbott Laboratories | 1 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 6.89 | 6.89 | 7.15 | 6.89 | 7.03 | ||
| 2 | 0.08 | 0.08 | 0.07 | 0.08 | 0.08 | 1.86 | 1.90 | 1.79 | 1.83 | 1.84 | |||
| 3 | 0.07 | 0.07 | 0.07 | 0.07 | 0.08 | 6.35 | 6.24 | 6.45 | 6.41 | 6.33 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahimainathan, L.; Narasimhan, M.; Corchado, R.; Patel, H.; Kansagra, A.; Devaraj, S.; Geethakumari, P.R.; Muthukumar, A. Evaluation of SARS-CoV-2 Serological Testing in Patients with Multiple Myeloma and Other Hematologic Malignancies on Monoclonal Antibody Therapies. Diagnostics 2020, 10, 992. https://doi.org/10.3390/diagnostics10120992
Mahimainathan L, Narasimhan M, Corchado R, Patel H, Kansagra A, Devaraj S, Geethakumari PR, Muthukumar A. Evaluation of SARS-CoV-2 Serological Testing in Patients with Multiple Myeloma and Other Hematologic Malignancies on Monoclonal Antibody Therapies. Diagnostics. 2020; 10(12):992. https://doi.org/10.3390/diagnostics10120992
Chicago/Turabian StyleMahimainathan, Lenin, Madhusudhanan Narasimhan, Rolando Corchado, Hetalkumari Patel, Ankit Kansagra, Sridevi Devaraj, Praveen Ramakrishnan Geethakumari, and Alagarraju Muthukumar. 2020. "Evaluation of SARS-CoV-2 Serological Testing in Patients with Multiple Myeloma and Other Hematologic Malignancies on Monoclonal Antibody Therapies" Diagnostics 10, no. 12: 992. https://doi.org/10.3390/diagnostics10120992
APA StyleMahimainathan, L., Narasimhan, M., Corchado, R., Patel, H., Kansagra, A., Devaraj, S., Geethakumari, P. R., & Muthukumar, A. (2020). Evaluation of SARS-CoV-2 Serological Testing in Patients with Multiple Myeloma and Other Hematologic Malignancies on Monoclonal Antibody Therapies. Diagnostics, 10(12), 992. https://doi.org/10.3390/diagnostics10120992




