Age-Appropriate Advance Care Planning in Children Diagnosed with a Life-Limiting Condition: A Systematic Review
Abstract
1. Introduction
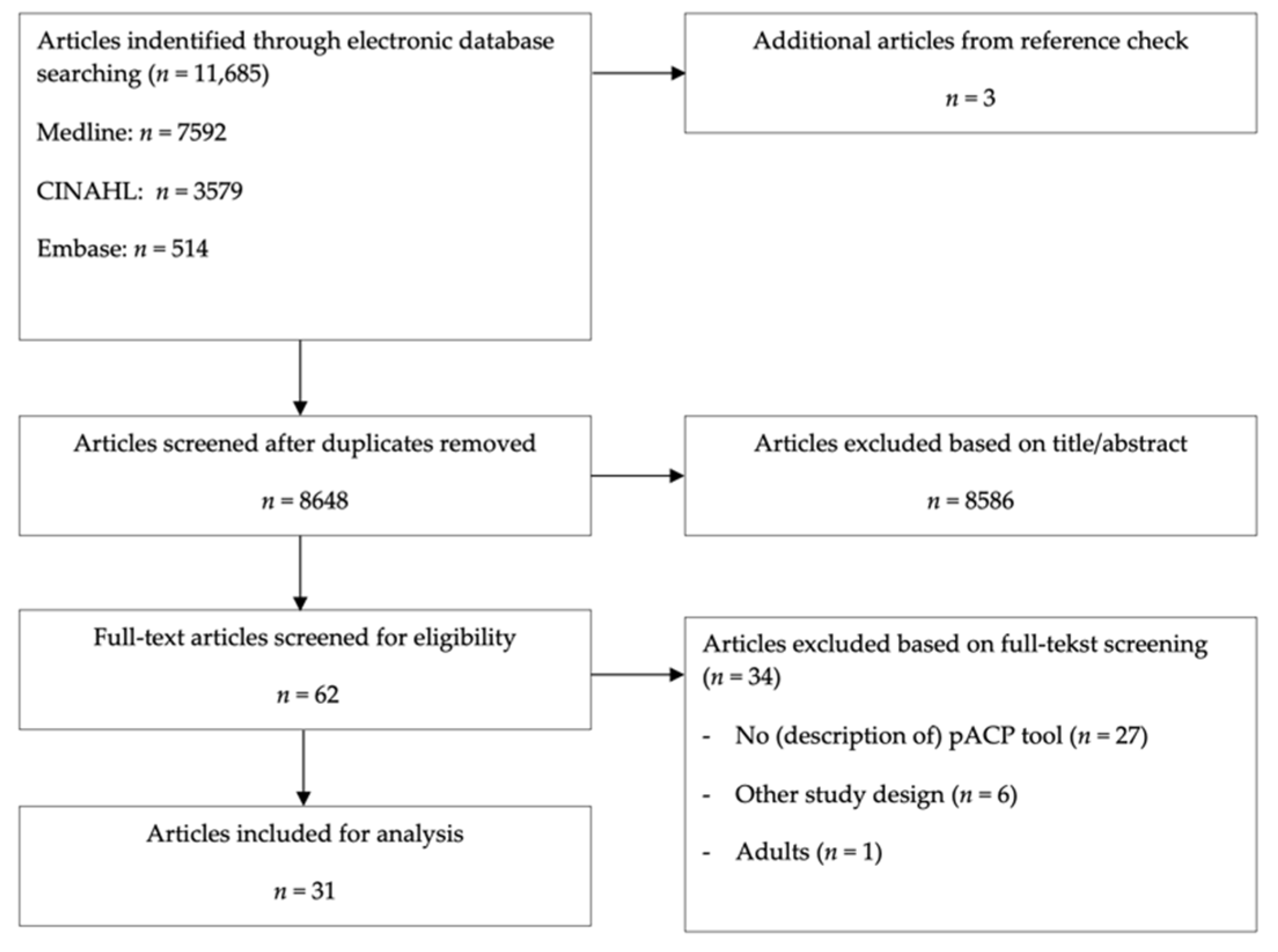
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
3.1. Risk of Bias and Quality of Reporting
3.2. Intervention Characteristics
3.3. Attention to Age Appropriateness
3.4. Factors Influencing Age Appropriateness
4. Discussion
4.1. Defining Age Appropriateness in pACP
4.2. Recommendations for Future Research
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Dallas, 2016 [31] | Lyon, 2009 [32] | Lyon, 2009 [33] | Lyon, 2010 [34] | Lyon, 2013 [35] | Lyon, 2014 [36] | |
|---|---|---|---|---|---|---|
| + | + | + | + | ? | + |
| + | + | + | + | + | + |
| - | - | - | - | - | - |
| + | - | ? | ? | - | ? |
| + | + | ? | + | + | + |
| + | - | + | + | + | + |
| 5 | 3 | 3 | 4 | 3 | 4 |
| Hays, 2006 [37] | Hendricks, 2017 [38] | Jacobs, 2015 [39] | Kazmerski, 2016 [40] | Kline, 2012 (MM) [46] | Lyon, 2019 (MM) [47] | Moody, 2020 [41] | Wiener, 2008 (MM) [49] | Wiener, 2012 (MM) [48] | Friebert, 2020 [42] | Noyes, 2013 (MM) [50] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Selection process of study population | + | + | + | + | + | + | + | + | + | + | - |
| 2. Comparability of compared groups | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 3. Standardized protocol for the use of the ACP tool | - | + | + | + | - | + | + | + | + | + | + |
| 4. Standardized protocol for measuring the outcome | + | + | + | - | + | + | + | + | + | + | + |
| 5. Missing data with regard to inclusion of follow-up or incomplete data | + | + | + | - | - | + | - | - | - | + | - |
| 6. Adjustment for confounders | - | - | NA | NA | + | - | - | NA | NA | + | NA |
| 7. Selective outcome reporting | + | + | + | - | + | + | + | + | + | + | - |
| Total score (out of 7) | 4 | 5 | 5 | 2 | 4 | 5 | 4 | 4 | 4 | 6 | 2 |
| Fahner, 2020 [11] | Feraco, 2018 [43] | Finlay, 2008[45] | Hartley, 2016[44] | Noyes, 2013 (MM) [50] | Kline, 2012 (MM) [46] | Lyon, 2019 (MM) [47] | Wiener, 2012 (MM) [48] | Wiener, 2008 (MM) [49] | |
|---|---|---|---|---|---|---|---|---|---|
| +/- | + | - | +/- | - | +/- | - | - | + |
| - | + | - | - | - | - | - | + | + |
| - | - | - | - | - | - | - | - | - |
| - | + | - | - | - | - | - | - | - |
| - | - | - | - | - | - | +/- | - | + |
| - | - | - | - | - | - | - | - | - |
| - | - | - | - | - | - | - | - | - |
| - | - | - | - | - | - | - | - | - |
| + | + | + | + | + | + | + | - | + |
| - | + | - | + | + | - | - | - | - |
| - | + | - | - | + | - | - | - | - |
| + | + | + | + | + | + | + | + | + |
| - | +/- | - | - | + | +/- | +/- | - | + |
| - | +/- | - | - | +/- | - | - | - | - |
| - | - | - | - | - | - | + | - | - |
| + | + | +/- | - | +/- | + | + | + | + |
| - | + | - | + | +/- | - | + | - | + |
| - | - | - | - | - | - | - | - | + |
| + | + | - | + | + | + | + | - | - |
| - | - | - | - | + | - | - | - | - |
| - | + | - | - | - | - | + | - | + |
| - | - | - | + | - | - | - | - | - |
| - | - | - | - | - | - | - | - | - |
| - | + | - | - | - | - | - | - | - |
| - | - | - | - | - | - | - | - | - |
| - | + | - | + | - | - | - | - | - |
| - | + | - | - | + | - | - | - | - |
| - | - | + | - | - | - | - | - | - |
| + | + | - | + | +/- | +/- | +/- | +/- | - |
| + | + | +/- | + | + | + | - | + | + |
| + | + | - | + | + | - | - | - | - |
| + | - | - | - | - | - | - | - | - |
| Total + | 8.5 | 18 | 4 | 10.5 | 12 | 6 | 8.5 | 4.5 | 11 |
References
- Nap-van der Vlist, M.M.; van der Sprenkel, E.E.B.; Nijhof, L.N.; Grootenhuis, M.A.; van der Ent, C.K.; Swart, J.F.; van Royen-Kerkhof, A.; van Grotel, M.; van de Putte, E.M.; Nijhof, S.L.; et al. Daily Life Participation in Childhood Chronic Disease: A Qualitative Study on the Child’s and Parent’s Perspective. BMJ Paediatr. Open 2021, 5, e001057. [Google Scholar] [CrossRef] [PubMed]
- Feudtner, C.; Rosenberg, A.R.; Boss, R.D.; Wiener, L.; Lyon, M.E.; Hinds, P.S.; Bluebond-Langner, M.; Wolfe, J. Challenges and Priorities for Pediatric Palliative Care Research in the U.S. and Similar Practice Settings: Report From a Pediatric Palliative Care Research Network Workshop. J. Pain Symptom Manag. 2019, 58, 909–917.e3. [Google Scholar] [CrossRef] [PubMed]
- Hinds, P.S.; Menard, J.C.; Jacobs, S.S. The Child’s Voice in Pediatric Palliative and End-of-Life Care. Prog. Palliat. Care 2012, 20, 337–342. [Google Scholar] [CrossRef]
- Hein, I.M.; Troost, P.W.; Broersma, A.; de Vries, M.C.; Daams, J.G.; Lindauer, R.J.L. Why Is It Hard to Make Progress in Assessing Children’s Decision-Making Competence? BMC Med. Ethics 2015, 16, 1. [Google Scholar] [CrossRef]
- Fahner, J.C.; Rietjens, J.A.C.; Heide, A.; Delden, J.J.M.; Kars, M.C. Survey of Paediatricians Caring for Children with Life-limiting Conditions Found That They Were Involved in Advance Care Planning. Acta Paediatr. 2020, 109, 1011–1018. [Google Scholar] [CrossRef]
- Durall, A.; Zurakowski, D.; Wolfe, J. Barriers to Conducting Advance Care Discussions for Children with Life-Threatening Conditions. Pediatrics 2012, 129, e975–e982. [Google Scholar] [CrossRef]
- Sanderson, A.; Zurakowski, D.; Wolfe, J. Clinician Perspectives Regarding the Do-Not-Resuscitate Order. JAMA Pediatrics 2013, 167, 954–958. [Google Scholar] [CrossRef]
- Feenstra, B.; Boland, L.; Lawson, M.L.; Harrison, D.; Kryworuchko, J.; Leblanc, M.; Stacey, D. Interventions to Support Children’s Engagement in Health-Related Decisions: A Systematic Review. BMC Pediatrics 2014, 14, 109. [Google Scholar] [CrossRef]
- van Driessche, A.; de Vleminck, A.; Gilissen, J.; Kars, M.C.; van der Werff ten Bosch, J.; Deliens, L.; Cohen, J.; Beernaert, K. Advance Care Planning for Adolescents with Cancer and Their Parents: Study Protocol of the BOOST PACP Multi-Centre Randomised Controlled Trial and Process Evaluation. BMC Pediatrics 2021, 21, 376. [Google Scholar] [CrossRef]
- Myers, J.; Cosby, R.; Gzik, D.; Harle, I.; Harrold, D.; Incardona, N.; Walton, T. Provider Tools for Advance Care Planning and Goals of Care Discussions: A Systematic Review. Am. J. Hosp. Palliat. Med. 2018, 35, 1123–1132. [Google Scholar] [CrossRef]
- Fahner, J.; Rietjens, J.; van der Heide, A.; Milota, M.; van Delden, J.; Kars, M. Evaluation Showed That Stakeholders Valued the Support Provided by the Implementing Pediatric Advance Care Planning Toolkit. Acta Paediatr. Int. J. Paediatr. 2020, 110, 237–246. [Google Scholar] [CrossRef]
- Fahner, J.C.; Beunders, A.J.M.; van der Heide, A.; Rietjens, J.A.C.; Vanderschuren, M.M.; van Delden, J.J.M.; Kars, M.C. Interventions Guiding Advance Care Planning Conversations: A Systematic Review. J. Am. Med. Dir. Assoc. 2018, 20, 227–248. [Google Scholar] [CrossRef]
- Lotz, J.D.; Jox, R.J.; Borasio, G.D.; Führer, M. Pediatric Advance Care Planning: A Systematic Review. Pediatrics 2013, 131, e873–e880. [Google Scholar] [CrossRef]
- Hughes, B.; O’Brien, M.R.; Flynn, A.; Knighting, K. The Engagement of Young People in Their Own Advance Care Planning Process: A Systematic Narrative Synthesis. Palliat. Med. 2018, 32, 1147–1166. [Google Scholar] [CrossRef]
- Lotz, J.D.; Daxer, M.; Jox, R.J.; Borasio, G.D.; Führer, M. “Hope for the Best, Prepare for the Worst”: A Qualitative Interview Study on Parents’ Needs and Fears in Pediatric Advance Care Planning. Palliat. Med. 2017, 31, 764–771. [Google Scholar] [CrossRef]
- Needle, J.S.; Peden-McAlpine, C.; Liaschenko, J.; Koschmann, K.; Sanders, N.; Smith, A.; Schellinger, S.E.; Lyon, M.E. “Can You Tell Me Why You Made That Choice?”: A Qualitative Study of the Influences on Treatment Decisions in Advance Care Planning among Adolescents and Young Adults Undergoing Bone Marrow Transplant. Palliat. Med. 2019, 34, 281–290. [Google Scholar] [CrossRef]
- Wadsworth, B. Piaget’s Theory of Cognitive Development: An Introduction for Students of Psychology and Education; McKay: New York, NY, USA, 1971. [Google Scholar]
- Coyne, I. Children’s Participation in Consultations and Decision-Making at Health Service Level: A Review of the Literature. Int. J. Nurs. Stud. 2008, 45, 1682–1689. [Google Scholar] [CrossRef]
- Kars, M.C.; Grypdonck, M.H.F.; van Delden, J.J.M. Being a Parent of a Child with Cancer throughout the End-of-Life Course. Oncol. Nurs. Forum 2011, 38, E260–E271. [Google Scholar] [CrossRef]
- Kars, M.C.; Grypdonck, M.H.F.; de Bock, L.C.; van Delden, J.J.M. The Parents’ Ability to Attend to the “Voice of Their Child” with Incurable Cancer during the Palliative Phase. Health Psychol. 2015, 34, 446–452. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Zwakman, M.; Verberne, L.M.; Kars, M.C.; Hooft, L.; van Delden, J.J.M.; Spijker, R. Introducing PALETTE: An Iterative Method for Conducting a Literature Search for a Review in Palliative Care. BMC Palliat. Care 2018, 17, 82. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Rietjens, J.A.C.; Sudore, R.L.; Connolly, M.; van Delden, J.J.; Drickamer, M.A.; Droger, M.; van der Heide, A.; Heyland, D.K.; Houttekier, D.; Janssen, D.J.A.; et al. Definition and Recommendations for Advance Care Planning: An International Consensus Supported by the European Association for Palliative Care. Lancet Oncol. 2017, 18, e543–e551. [Google Scholar] [CrossRef]
- Schulz, R.; Czaia, S.J.; McKay, J.R.; Ory, M.G.; Belle, S.H. Intervention Taxonomy (ITAX): Describing Essential Features of Interventions. Am. J. Health Behav. 2010, 34, 811–821. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P., Green, S., Eds.; Version 5.1.0.; John Wiley and Sons: West Sussex, UK, 2011. [Google Scholar]
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated Criteria for Reporting Qualitative Research (COREQ): A 32-Item Checklist for Interviews and Focus Groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef]
- Dixon-Woods, M.; Sutton, A.; Shaw, R.; Miller, T.; Smith, J.; Young, B.; Bonas, S.; Booth, A.; Jones, D. Appraising Qualitative Research for Inclusion in Systematic Reviews: A Quantitative and Qualitative Comparison of Three Methods. J. Health Serv. Res. Policy 2007, 12, 42–47. [Google Scholar] [CrossRef]
- Popay, J.; Roberts, H.; Sowden, A.; Petticrew, M. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: A Product from the ESRC Methods Programme; Version 1; Lancaster University: Lancaster, UK, 2006. [Google Scholar]
- Dierckx de Casterlé, B.; Gastmans, C.; Bryon, E.; Denier, Y. QUAGOL: A Guide for Qualitative Data Analysis. Int. J. Nurs. Stud. 2012, 49, 360–371. [Google Scholar] [CrossRef]
- Dallas, R.H.; Kimmel, A.; Wilkins, M.L.; Rana, S.; Garcia, A.; Cheng, Y.I.; Wang, J.; Lyon, M.E. Acceptability of Family-Centered Advanced Care Planning for Adolescents with HIV. Pediatrics 2016, 138, e20161854. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.E.; Garvie, P.A.; Briggs, L.; He, J.; McCarter, R.; D’Angelo, L.J. Development, Feasibility, and Acceptability of the Family/Adolescent- Centered (FACE) Advance Care Planning Intervention for Adolescents with HIV. J. Palliat. Med. 2009, 12, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.E.; Garvie, P.A.; McCarter, R.; Briggs, L.; He, J.; D’Angelo, L.J. Who Will Speak for Me? Improving End-of-Life Decision-Making for Adolescents with HIV and Their Families. Pediatrics 2009, 123, e199–e206. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.E.; Garvie, P.A.; Briggs, L.; He, J.; Malow, R.; D’angelo, L.J.; Mccarter, R. Is It Safe? Talking to Teens with HIV/AIDS about Death and Dying: A 3-Month Evaluation of Family Centered Advance Care (FACe) Planning-Anxiety, Depression, Quality of Life. Dove Press J. 2010, 2, 27–37. [Google Scholar] [CrossRef]
- Lyon, M.E.; Jacobs, S.; Briggs, L.; Cheng, Y.I.; Wang, J. Family-Centered Advance Care Planning for Teens with Cancer. JAMA Pediatrics 2013, 167, 460–467. [Google Scholar] [CrossRef]
- Lyon, M.E.; Jacobs, S.; Briggs, L.; Cheng, Y.I.; Wang, J. A Longitudinal, Randomized, Controlled Trial of Advance Care Planning for Teens with Cancer: Anxiety, Depression, Quality of Life, Advance Directives, Spirituality. J. Adolesc. Health 2014, 54, 710–717. [Google Scholar] [CrossRef]
- Hays, R.M.; Valentine, J.; Haynes, G.; Russell Geyer, J.; Villareale, N.; Mckinstry, B.; Varni, J.W.; Churchill, S.S. The Seattle Pediatric Palliative Care Project: Effects on Family Satisfaction and Health-Related Quality of Life. J. Palliat. Med. 2006, 9, 716–728. [Google Scholar] [CrossRef]
- Hendricks-Ferguson, V.L.; Pradhan, K.; Shih, C.S.; Gauvain, K.M.; Kane, J.R.; Liu, J.; Haase, J.E. Pilot Evaluation of a Palliative and End-of-Life Communication Intervention for Parents of Children with a Brain Tumor. J. Pediatric Oncol. Nurs. 2017, 34, 203–213. [Google Scholar] [CrossRef]
- Jacobs, S.; Perez, J.; Cheng, Y.I.; Sill, A.; Wang, J.; Lyon, M.E. Adolescent End of Life Preferences and Congruence with Their Parents’ Preferences: Results of a Survey of Adolescents with Cancer. Pediatric Blood Cancer 2015, 62, 710–714. [Google Scholar] [CrossRef]
- Kazmerski, T.M.; Weiner, D.J.; Matisko, J.; Schachner, D.; Lerch, W.; May, C.; Maurer, S.H. Advance Care Planning in Adolescents with Cystic Fibrosis: A Quality Improvement Project. Pediatric Pulmonol. 2016, 51, 1304–1310. [Google Scholar] [CrossRef]
- Moody, K.M.; Hendricks-Ferguson, V.L.; Baker, R.; Perkins, S.; Haase, J.E. A Pilot Study of the Effects of COMPLETE: A Communication Plan Early Through End of Life, on End-of-Life Outcomes in Children with Cancer. J. Pain Symptom Manag. 2020, 60, 417–421. [Google Scholar] [CrossRef]
- Friebert, S.; Grossoehme, D.H.; Baker, J.N.; Needle, J.; Thompkins, J.D.; Cheng, Y.I.; Wang, J.; Lyon, M.E. Congruence Gaps Between Adolescents with Cancer and Their Families Regarding Values, Goals, and Beliefs About End-of-Life Care. JAMA Netw Open 2020, 3, e205424. [Google Scholar] [CrossRef]
- Feraco, A.M.; Brand, S.R.; Gagne, J.; Sullivan, A.; Block, S.D.; Wolfe, J. Development of the “Day 100 Talk”: Addressing Existing Communication Gaps during the Early Cancer Treatment Period in Childhood Cancer. Pediatric Blood Cancer 2018, 65, e269–e272. [Google Scholar] [CrossRef]
- Hartley, G.; Berger, Z.; Maynard, L. The Development and Evaluation of a Holistic Needs Assessment within Children’s Palliative Care. Int. J. Palliat. Nurs. 2016, 22, 236–242. [Google Scholar] [CrossRef]
- Finlay, F.; Lewis, M.; Lenton, S.; Poon, M. Planning for the End of Children’s Lives -the Lifetime Framework. Child: Care Health Dev. 2008, 34, 542–544. [Google Scholar] [CrossRef]
- Kline, C.; Reineke, A.; Auger, J.A.; Willert, J.; Roberts, W.; Schiff, D. Effects of a Unique Pediatric Hematology-Oncology Palliative Care Program on Medical Decision-Making and Communication between Healthcare Providers and Families: Results of a Supportive Care Survey. Prog. Palliat. Care 2012, 20, 13–18. [Google Scholar] [CrossRef]
- Lyon, M.E.; Thompkins, J.D.; Fratantoni, K.; Fraser, J.L.; Schellinger, S.E.; Briggs, L.; Friebert, S.; Aoun, S.; Cheng, Y.I.; Wang, J. Family Caregivers of Children and Adolescents with Rare Diseases: A Novel Palliative Care Intervention. BMJ Supportive Palliat. Care 2019, 1–10. [Google Scholar] [CrossRef]
- Wiener, L.; Zadeh, S.; Battles, H.; Baird, K.; Ballard, E.; Osherow, J.; Pao, M. Allowing Adolescents and Young Adults to Plan Their End-of-Life Care. Pediatrics 2012, 130, 897–905. [Google Scholar] [CrossRef]
- Wiener, L.; Ballard, E.; Brennan, T.; Battles, H.; Martinez, P.; Pao, M. How i Wish to Be Remembered: The Use of an Advance Care Planning Document in Adolescent and Young Adult Populations. J. Palliat. Med. 2008, 11, 1309–1313. [Google Scholar] [CrossRef]
- Noyes, J.; Hastings, R.P.; Lewis, M.; Hain, R.; Bennett, V.; Hobson, L.; Spencer, L.H. Planning Ahead with Children with Life-Limiting Conditions and Their Families: Development, Implementation and Evaluation of ‘My Choices’. BMC Palliat. Care 2013, 12, 5. [Google Scholar] [CrossRef]
- Christenson, K.; Lybrand, S.A.; Hubbard, C.R.; Hubble, R.A.; Ahsens, L.; Black, P. Including the Perspective of the Adolescent in Palliative Care Preferences. J. Pediatric Health Care 2010, 24, 286–291. [Google Scholar] [CrossRef]
- Curtin, K.B.; Watson, A.E.; Wang, J.; Okonkwo, O.C.; Lyon, M.E. Pediatric Advance Care Planning (PACP) for Teens with Cancer and Their Families: Design of a Dyadic, Longitudinal RCCT. Contemp. Clin. Trials 2017, 62, 121–129. [Google Scholar] [CrossRef]
- Dallas, R.H.; Wilkins, M.L.; Wang, J.; Garcia, A.; Lyon, M.E. Longitudinal Pediatric Palliative Care: Quality of Life & Spiritual Struggle (FACE): Design and Methods. Contemp. Clin. Trials 2012, 33, 1033–1043. [Google Scholar] [CrossRef]
- Fraser, J.; Harris, N.; Berringer, A.J.; Prescott, H.; Finlay, F. Advanced Care Planning in Children with Life-Limiting Conditions-The Wishes Document. Arch. Dis. Child. 2010, 95, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, A.; Peacock, M.; Arber, A. Helping Young People Who Have Learning Disabilities and Their Families to Plan End of Life Care: The ADVANCE Toolkit. Learn. Disabil. Pract. 2018, 21, 33. [Google Scholar] [CrossRef]
- Zadeh, S.; Pao, M.; Wiener, L. Opening End-of-Life Discussions: How to Introduce Voicing My CHOiCESTM, an Advance Care Planning Guide for Adolescents and Young Adults. Palliat. Supportive Care 2015, 13, 591–599. [Google Scholar] [CrossRef] [PubMed]
- van Breemen, C.; Johnston, J.; Carwana, M.; Louie, P. Serious Illness Conversations in Pediatrics: A Case Review. Children 2020, 7, 102. [Google Scholar] [CrossRef]
- Baker, J.N.; Hinds, P.S.; Spunt, S.L.; Barfield, R.C.; Allen, C.; Powell, B.C.; Anderson, L.H.; Kane, J.R. Integration of Palliative Care Practices into the Ongoing Care of Children with Cancer: Individualized Care Planning and Coordination. Pediatric Clin. N. Am. 2008, 55, 223–250. [Google Scholar] [CrossRef]
- Snaman, J.M.; Blazin, L.; Holder, R.L.; Wolfe, J.; Baker, J.N. Identifying and Quantifying Adolescent and Young Adult Patient Preferences in Cancer Care: Development of a Conjoint Analysis-Based Decision-Making Tool. J. Adolesc. Young Adult Oncol. 2019, 8, 212–216. [Google Scholar] [CrossRef]
- Toce, S.; Collins, M.A. The FOOTPRINTS SM Model of Pediatric Palliative Care. J. Palliat. Med. 2003, 6, 989–1000. [Google Scholar] [CrossRef]
- Freyer, D.R. Care of the Dying Adolescent: Special Considerations. Pediatrics 2004, 113, 381–388. [Google Scholar] [CrossRef]
- Longbottom, S.; Slaughter, V. Sources of Children’s Knowledge about Death and Dying. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170267. [Google Scholar] [CrossRef]
- Fender, J.G.; Crowley, K. How Parent Explanation Changes What Children Learn from Everyday Scientific Thinking. J. Appl. Dev. Psychol. 2007, 28, 189–210. [Google Scholar] [CrossRef]
- Grootens-Wiegers, P.; Hein, I.M.; van den Broek, J.M.; de Vries, M.C. Medical Decision-Making in Children and Adolescents: Developmental and Neuroscientific Aspects. BMC Pediatrics 2017, 17, 120. [Google Scholar] [CrossRef]
- Appelbaum, P.; Grisso, T.; Appelbaum, P.S.; Grisso, T. The MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR); Professional Resource Press: Sarasota, FL, USA, 2001. [Google Scholar]
- Appelbaum, P.S.; Grisso, T. Assessing Patients’ Capacities to Consent to Treatment. N. Engl. J. Med. 1988, 319, 1635–1638. [Google Scholar] [CrossRef]
- Shaffer, D.; Kipp, K. Developmental Psychology; Thomson Wadsworth: Belmont, NSW, Australia, 2007. [Google Scholar]
- Reed, J.; Warner-Rogers, J. Child Neuropsychology; Concept, Theory and Practice; Reed, J., Warner-rogers, J., Eds.; Wiley-Blackwell: Oxford, UK, 2008. [Google Scholar]
- Rueda, M.R.; Fan, J.; McCandliss, B.D.; Halparin, J.D.; Gruber, D.B.; Lercari, L.P.; Posner, M.I. Development of Attentional Networks in Childhood. Neuropsychologia 2004, 42, 1029–1040. [Google Scholar] [CrossRef]
- Waszak, F.; Li, S.-C.; Hommel, B. The Development of Attentional Networks: Cross-Sectional Findings from a Life Span Sample. Dev. Psychol. 2010, 46, 337–349. [Google Scholar] [CrossRef]
- Guillery-Girard, B.; Martins, S.; Deshayes, S.; Hertz-Pannier, L.; Chiron, C.; Jambaqué, I.; Landeau, B.; Clochon, P.; Chételat, G.; Eustache, F. Developmental Trajectories of Associative Memory from Childhood to Adulthood: A Behavioral and Neuroimaging Study. Front. Behav. Neurosci. 2013, 7, 126. [Google Scholar] [CrossRef]
- Thaler, N.S.; Goldstein, G.; Pettegrew, J.W.; Luther, J.F.; Reynolds, C.R.; Allen, D.N. Developmental Aspects of Working and Associative Memory. Arch. Clin. Neuropsychol. 2013, 28, 348–355. [Google Scholar] [CrossRef][Green Version]
- Rhodes, S.M.; Murphy, D.; Hancock, P.J.B. Developmental Changes in the Engagement of Episodic Retrieval Processes and Their Relationship with Working Memory during the Period of Middle Childhood. Br. J. Dev. Psychol. 2011, 29, 865–882. [Google Scholar] [CrossRef]
- Sprondel, V.; Kipp, K.H.; Mecklinger, A. Developmental Changes in Item and Source Memory: Evidence From an ERP Recognition Memory Study with Children, Adolescents, and Adults. Child Dev. 2011, 82, 1638–1953. [Google Scholar] [CrossRef]
- Czernochowski, D.; Mecklinger, A.; Johansson, M. Age-Related Changes in the Control of Episodic Retrieval: An ERP Study of Recognition Memory in Children and Adults. Dev. Sci. 2009, 12, 1026–1040. [Google Scholar] [CrossRef]
- Cotton, C.R.; Range, L. Children’s Death Concepts: Relationship to Cognitive Functioning, Age, Experience with Death, Fear of Death, and Hopelessness. J. Clin. Child Adolesc. Psychol. 1990, 19, 123–127. [Google Scholar] [CrossRef]
- Hunter, S.B.; Smith, D.E. Predictors of Children’s Understandings of Death: Age, Cognitive Ability, Death Experience and Maternal Communicative Competence. OMEGA-J. Death Dying 2008, 57, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, K.S.; Gutiérrez, I.T.; Schein, S.S., IV. COGNITIVE DIMENSIONS OF DEATH IN CONTEXT. Monogr. Soc. Res. Child Dev. 2014, 79, 62–82. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, V. Young Children’s Understanding of Death. Aust. Psychol. 2005, 40, 179–186. [Google Scholar] [CrossRef]
- Slaughter, V.; Lyons, M. Learning about Life and Death in Early Childhood. Cogn. Psychol. 2003, 46, 1–30. [Google Scholar] [CrossRef]
- Waxman, S.; Medin, D. Experience and Cultural Models Matter: Placing Firm Limits on Childhood Anthropocentrism. Hum. Dev. 2007, 50, 23–30. [Google Scholar] [CrossRef]
- Markovits, H. The Development of Abstract Conditional Reasoning; Barrouillet, P., Gauffroy, C., Eds.; Psychology Press: London, UK, 2013. [Google Scholar]
- Pillow, B.H.; Pearson, R.M.; Hecht, M.; Bremer, A. Children’s and Adults’ Judgments of the Certainty of Deductive Inferences, Inductive Inferences, and Guesses. J. Genet. Psychol. 2010, 171, 203–217. [Google Scholar] [CrossRef]
- Grisso, T.; Appelbaum, P.S.; Hill-Fotouhi, C. The MacCAT-T: A Clinical Tool to Assess Patients’ Capacities to Make Treatment Decisions. Psychiatr. Serv. 1997, 48, 1415–1419. [Google Scholar] [CrossRef]
- Frith, U.; Frith, C.D. Development and Neurophysiology of Mentalizing. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003, 358, 459–473. [Google Scholar] [CrossRef]
- Abu-Akel, A. A Neurobiological Mapping of Theory of Mind. Brain Res. Rev. 2003, 43, 29–40. [Google Scholar] [CrossRef]
- Christensen, T. Sick Girl Speaks. In Sick Girl Speaks; Christensen, T., Ed.; iUniverse: New York, NY, USA, 2007. [Google Scholar]
- Blakemore, S.-J.; Mills, K.L. Is Adolescence a Sensitive Period for Sociocultural Processing? Annu. Rev. Psychol. 2014, 65, 187–207. [Google Scholar] [CrossRef]
- Casey, B.J.; Jones, R.M.; Hare, T.A. The Adolescent Brain. Ann. N. Y. Acad. Sci. USA 2008, 1124, 111–126. [Google Scholar] [CrossRef]
- Giesbertz, N.A.A.; Bredenoord, A.L.; van Delden, J.J.M. Clarifying Assent in Pediatric Research. Eur. J. Hum. Genet. 2014, 22, 266–269. [Google Scholar] [CrossRef]
- National Institutes of Health Children’s Assent to Clinical Trial Participation. Online Resource from National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/treatment/clinical-trials/patient-safety/childrens-assent (accessed on 10 April 2022).
- Feudtner, C.; Kang, T.I.; Hexem, K.R.; Friedrichsdorf, S.J.; Osenga, K.; Siden, H.; Friebert, S.E.; Hays, R.M.; Dussel, V.; Wolfe, J. Pediatric Palliative Care Patients: A Prospective Multicenter Cohort Study. Pediatrics 2011, 127, 1094–1101. [Google Scholar] [CrossRef]
- Shehata, G.A. Childhood Cognitive Impairment. Acta Psychopathol. 2016, 02, 37. [Google Scholar] [CrossRef]
- Ferraz de Arruda-Colli, M.N.; Sansom-Daly, U.; dos Santos, M.A.; Wiener, L. Considerations for the Cross-Cultural Adaptation of an Advance Care Planning Guide for Youth with Cancer. Clin. Pract. Pediatric Psychol. 2018, 6, 341–354. [Google Scholar] [CrossRef]
- Huang, C.-Y. How Culture Influences Children’s Development. Online Resource from the Conversation. Available online: https://theconversation.com/how-culture-influences-childrens-development-99791 (accessed on 8 April 2022).
- Jaakkola, R.O.; Slaughter, V. Children’s Body Knowledge: Understanding ‘Life’ as a Biological Goal. Br. J. Dev. Psychol. 2002, 20, 325–342. [Google Scholar] [CrossRef]
- Piers, R.; Albers, G.; Gilissen, J.; de Lepeleire, J.; Steyaert, J.; van Mechelen, W.; Steeman, E.; Dillen, L.; vanden Berghe, P.; van den Block, L. Advance Care Planning in Dementia: Recommendations for Healthcare Professionals. BMC Palliat. Care 2018, 17, 88. [Google Scholar] [CrossRef]
- Robinson, L.; Tang, E.; Taylor, J.-P. Dementia: Timely Diagnosis and Early Intervention. BMJ 2015, 350, h3029. [Google Scholar] [CrossRef]
- Hegde, S.; Ellajosyula, R. Capacity Issues and Decision-Making in Dementia. Ann. Indian Acad. Neurol. 2016, 19, 34–39. [Google Scholar] [CrossRef]

| (critical illness[MeSH Terms] OR critical illness*[tiab] OR "critically ill"[tiab] OR life limiting condition*[tiab] OR life-limiting disease*[tiab] OR life threatening illness*[tiab] OR life limiting illness*[tiab] OR life threatening condition*[tiab] OR serious illness*[tiab] OR palliative care[MeSH] OR terminal care[MeSH] OR "palliative care"[tiab] OR "palliative medicine"[tiab] OR "palliative nursing"[tiab] OR "palliative period"[tiab] OR "palliative phase"[tiab] OR "palliative therapy"[tiab] OR palliative treatment*[tiab] OR "palliative supportive care"[tiab] OR "terminal care"[tiab] OR "terminal medicine"[tiab] OR "terminal period"[tiab] OR "terminal phase"[tiab] OR EOL[tiab] OR end of life*[tiab]) |
| And |
| ("advance care planning"[MeSH] OR "advance directives"[MeSH] OR "decision making"[MeSH] OR "living wills"[MeSH] OR "patient participation"[MeSH] OR advance care plan*[tiab] OR ACP[tiab] OR pACP[tiab] OR advance decision*[tiab] OR advance directive*[tiab] OR advance medical directive*[tiab] OR advance healthcare planning*[tiab] OR advance medical planning*[tiab] OR advance statement*[tiab] OR "do not hospitalize"[tiab] OR "do not hospitalise"[tiab] OR "do not resuscitate"[tiab] OR "do not attempt cardiopulmonary resuscitation"[tiab] OR "DNR order"[tiab] OR DNACPR[tiab] OR "planning ahead"[tiab] OR "refusal of treatment"[tiab] OR treatment limitation*[tiab] OR conversation guide*[tiab] OR guide*[tiab] OR program*[tiab] OR procedure*[tiab] OR practice*[tiab] OR treatment limiting*[tiab] OR shared decision*[tiab] OR "patient participation"[tiab] OR "patient involvement"[tiab] OR "child centered care"[tiab] OR "person centered care"[tiab] OR "patient centered care"[tiab]) |
| And |
| (Infan*[tiab] OR toddler*[tiab] OR minor[tiab] OR minors*[tiab] OR boy[tiab] OR boys[tiab] OR boyfriend[tiab] OR boyfriends[tiab] OR boyhood[tiab] OR girl[tiab] OR girls[tiab] OR girlfriend[tiab] OR girlfriends[tiab] OR kid[tiab] OR kids[tiab] OR child[tiab] OR children*[tiab] OR schoolchild*[tiab] OR school child*[tiab] OR adolescen*[tiab] OR juvenil*[tiab] OR youth*[tiab] OR teen*[tiab] OR underage*[tiab] OR pubescen*[tiab] OR puberty[tiab] OR pediatrics[MESH] OR pediatric[tiab] OR pediatrics[tiab] OR paediatric[tiab] OR paediatrics[tiab] OR school[tiab] OR school*[tiab] OR prematur*[tiab] OR preterm*[tiab] OR youth[tiab] OR youths[tiab] OR teen[tiab] OR teens[tiab] OR teenager[tiab] OR youngster*[tiab] OR child[MeSH] OR neonat*[tiab] OR baby[tiab] OR babies[tiab] OR toddler*[tiab] OR newborn*[tiab] OR postneonat*[tiab] OR postnat*[tiab] OR perinat*[tiab] OR preschool*[tiab] OR suckling*[tiab] OR picu[tiab] OR nicu[tiab] OR neo-nat*[tiab] OR neonat*[tiab] OR premature*[tiab] OR postmature*[tiab] OR pre-mature*[tiab] OR post-mature*[tiab] OR preterm*[tiab] OR pre-term*[tiab] OR playgroup*[tiab] OR play-group*[tiab] OR playschool*[tiab] OR prepube*[tiab] OR preadolescen*[tiab] OR junior high*[tiab] OR highschool*[tiab] OR senior high[tiab] OR young people*[tiab]) |
| Author, Year, Country * | Aim | Population (Age in Years), n | Outcome Parameters | Risk of Bias Total Score (6) |
|---|---|---|---|---|
| Dallas, 2016, USA [31] | FACE (FAmily/Adolescent-CEntered Advance Care Planning) vs. Healthy Living Control Condition | Adolescents with HIV (14–21) and their family decision maker, dyads n = 97 (I: 48, C: 49) | FACE:
| 5 |
| Lyon, 2009, USA [32] | FACE vs. Healthy Living Control Condition | Adolescents with HIV/AIDS (14–21) and surrogate, dyads n = 38 (I: 20, C: 18) | FACE:
| 3 |
| Lyon, 2009, USA [33] | FACE vs. Healthy Living Control Condition | Adolescents with HIV/AIDS (14–21) and surrogate, dyads n = 38 (I: 18, C: 17) | FACE:
| 3 |
| Lyon, 2010, USA [34] | FACE vs. Healthy Living Control Condition | Adolescents with HIV (14–21) and legal guardian, dyads n = 38 (I: 18, C: 17) | FACE:
| 4 |
| Lyon, 2013, USA [35] | FACE vs. Treatment as Usual | Adolescent with cancer (14–21) and their Surrogate, dyads n = 30 (I: 17, C: 13) | FACE:
| 3 |
| Lyon, 2014, USA [36] | FACE vs. Treatment as Usual | Adolescent with cancer (14–21) and their surrogate, dyads n = 30 (I: 17, C: 13) | FACE-TC (Family/Adolescent-Centered Advance Care Planning for Teens with Cancer):
| 4 |
| Author, Year, Country * | Aim (A), Design (D) | Population (Age in Years), n | Outcomes | Risk of Bias Total Score (6) |
|---|---|---|---|---|
| Friebert, 2020, USA [42] | A: To assess adolescents’ EOL needs and family congruence D: Survey study from intervention arm FACE-TC (FAmily/Adolescent-CEntered Advance Care Planning for Teens with Cancer) (session 1) RCT | Adolescents with cancer (14–21) and their legal or chosen guardian, dyads n = 80 | FACE-TC
| 6 |
| Hays, 2006, USA [37] | A: To assess the effects of DMT (Decision-Making Tool) on family satisfaction and QOL non-experimental pre-test and post-test D: Nonexperimental pre-test, post-test comparison study | Children and adolescents with potentially life-limiting illness (0–22) and their parents, dyads n = 41 | DMT:
| 4 |
| Hendricks, 2017, USA [38] | A: To evaluate COMPLETE (Communication Plan: Early through End of Life intervention) on the parent and provider levels and to describe the given parental responses. D: Prospective, longitudinal, single-group pilot study | Parents of children (0–18) with a brain tumor and a poor prognosis, mostly mothers; parents n = 13 and children n = 11 | COMPLETE:
| 5 |
| Jacobs, 2015, USA [39] | A: To examine EOL family congruence D: Survey study from intervention arm RCT provider post-hoc survey | Adolescents with cancer (14–21) and their legal or chosen guardian, dyads n = 17 and clinicians n = 30 | FACE-TC:
| 5 |
| Kazmerski, 2016, USA [40] | A: To assess patient and provider attitudes and preferences towards VMC (Voicing My Choices) D: Pre–post-test training survey quality improvement study | Patients with advanced CF (≤22); patients n = 12, providers (pre-training) n = 6, and providers (post-training) n = 7 | Patient and provider (pre- and post-training):
| 2 |
| Moody, 2020, USA [41] | A: To assess effects of COMPLETE on EOL outcomes D: Two-phase, single-arm, two-center prospective pre–post-intervention pilot study | Phase I: Parents of children with newly diagnosed cancer (1–<18 months), parents n = 21 and children n = 18 Phase II: Parents of children with any prognosis, parents n = 20 and children n = 17 | COMPLETE:
| 4 |
| Author, Year, Country * | Aim (A), Design (D) | Population (Age in Years), n | Outcome Parameters | Risk of Bias Total Score (6) | Quality of Reporting Total Score (32) | |
|---|---|---|---|---|---|---|
| Quantitative | Qualitative | |||||
| Kline, 2012, USA [46] | A: To assess family satisfaction and preferences with their palliative care program and its DMT tool (Decision-Making Tool) D: Supportive care survey and open-ended questions interview study | Guardians of high-risk hemato-oncology pediatric patients (mean of 9.7), n = 20 (quantitative outcomes) and n = 6 (qualitative outcomes) |
| Open-ended questions on the palliative care program and DMT; questions NS | 4 | 6 |
| Lyon, 2019, USA [47] | A: To assess the feasibility and acceptability of FACE-Rare (FAmily-CEntered pediatric Advance Care Planning-Rare) D: Pre–post-test questionnaire study | Pediatric patients with rare diseases (≥1–≤21) and their legal guardians or family caregivers (all mothers), dyads n = 6 | FACE-Rare
| Questions NS | 5 | 8.5 |
| Noyes, 2013, UK [50] | A: To evaluate ‘My Choices’ and enhance future care planning D: Pre–post-test questionnaire (quantitative) and semi-structured interview (qualitative) study | Children and young people (0–≥16) with complex health and palliative care needs, as well as their parents and health-care providers, children n = 11 parents n = 12, bereaved parents n = 3, professionals n = 13 (qualitative outcomes), professionals (pre-study) n = 27, and professionals (post-study) n = 20 (quantitative outcomes) | Professionals evaluating My Choices on preferred:
| Views of parents, children, and professionals on the My Choices booklets; questions/themes NS | 2 | 12 |
| Wiener, 2008, USA [49] | A: To assess the acceptability of Five Wishes, helpfulness, and defining important EOL concerns D: Descriptive study data and closed- and open-response interviews | Adolescents and young adults with HIV-1 or metastatic/recurrent cancer (16–28), n = 20 | Five Wishes:
| Adjustments to the Five Wishes document | 4 | 11 |
| Wiener, 2012, USA [48] | A: To assess and compare the usefulness, helpfulness, and stressfulness of the MTMWMV (My Thoughts, My Wishes, My Voice) with the Five Wishes D: Descriptive study data and closed- and open-response interviews | AYAs with metastatic or recurrent cancer or HIV infection (16–28), n = 52 | Evaluating both tools regarding:
| Adjustments to the MTMWMV document | 4 | 4.5 |
| Author, Year, Country * | Aim (A), Design (D) | Population (Age in Years), n | Outcomes | Quality of Reporting Total Score |
|---|---|---|---|---|
| Fahner, 2020, the Netherlands [11] | A: To evaluate the acceptability of content of IMPACT (Implementing Pediatric Advance Care Planning Toolkit) D: Qualitative pilot study | Children with life-limiting diseases (0–<18), children n = 27, parents n = 41, physicians n = 11, and nurses n = 7 |
| 8.5 |
| Feraco, 2018, USA [43] | A: To address and ameliorate existing communication gaps in cancer care and to incorporate resulting knowledge in the development of the D100 (the Day 100 talk) D: Qualitative semi-structured interview study | Children, adolescents, and young adults undergoing cancer treatment for from 1 to <7 months (≥13), as well as their parents and oncology providers, adolescents n = 5, parents n = 6, and providers n = 11 | Perceived communication gaps in cancer care | 18 |
| Finlay, 2008, UK [45] | A: To enhance family engagement in EOL planning through incorporating the results in their 3 × 3 framework D: Documentary analysis study | Children with non-malignant life-limiting illnesses (2–16 months), n = 8 | Content of EOL plans | 4 |
| Hartley, 2016, UK [44] | A: To evaluate the assessment of family needs and concerns by the HNA tool (Holistic Needs Assessment) D: Qualitative analysis study and qualitative pilot study | Care managers employed by Anglia’s Children’s Hospices, n = 7 |
| 10.5 |
| Author, Year, Country * | Aim (A), Design (D) | Population (Age in Years), n | Outcomes | Quality Appraisal |
|---|---|---|---|---|
| Baker, 2008, USA [58] | A: To assess clinical gaps in pediatric cancer care and to enhance this by integrating these aspects in the tool D: Narrative review study | Children with cancer (NS) and their parents, n = NA | The development of the Individualized Care Coordination Plan | NA |
| Christenson, 2010, USA [51] | A: To present communication gaps in palliative care of adolescents and to improve this by using the CCCT (Comfort Care Communication Tool) D: Case report study | Woman with CF (18), n = 1 | One case study | NA |
| Curtin, 2017, USA [52] | A: To assess FACE-TC (FAmily-CEntered pediatric Advance Care Planning-Rare) efficacy on family congruence, quality of life and early ACP document completion D: Study protocol of a dyadic, longitudinal RCT | AYAs (14–20) with cancer and their family decision maker), dyads n = 130 | Design of dyadic, longitudinal RCT | NA |
| Dallas, 2012, USA [53] | A: To assess long-term FACE (FAmily/Adolescent-CEntered Advance Care Planning) efficacy on EOL care and tries to enhance physical, psychological, spiritual well-being D: Study protocol of a dyadic, longitudinal RCT | Adolescents with HIV (14–21) and their family decision makers (>21), n = 130 | Design of dyadic, longitudinal RCT | NA |
| Fraser, 2010, UK [54] | A: To present the importance of sensitive pediatric EOL planning and to describe the history and format of the Wishes document D: Narrative review study | NA (NS) | The importance of EOL planning The development of the Wishes document | NA |
| Gallagher, 2018, UK [55] | A: To highlight the importance of knowledge and skills required to engage with children with learning disabilities in their EOL planning D: Narrative review study | NA (NS) | The importance of and challenges in EOL planning ADVANCE toolkit content | NA |
| Snaman, 2019, USA [59] | A: To identify high-priority factors in cancer treatment decisions and incorporating this in a new tool D: Descriptive study of tool development | AYAs with newly diagnosed high-risk cancers (NS), their parents, and HCPs, dyads n = 5 and HCP n = 2 | Development of MyPref | NA |
| Toce, 2003, USA [60] | A: To develop a tool that improves the pediatric quality at the EOL D: Descriptive study of tool development | Children with life-threatening conditions (6–>12 months), children n = 83 and continuity providers n = 105 | Development of Footprints | NA |
| Van Breemen, 2020, Canada [57] | A: To describe the steps in the SICG-peds (Serious illness conversations in pediatrics) using one case as an exampleD: Case report study | Child diagnosed with osteosarcoma (11), n = 1 | Content of the SICG-Peds | NA |
| Zadeh, 2015, USA [56] | A: To provide guidelines in the use of Voicing My Choices for health-care providers D: Ethical guide for health-care providers for Voicing My Choices | AYAs living with cancer or pediatric HIV (NS), n = NA | Guidelines in the use of Voicing My Choices | NA |
| Intervention (Country) | Intervention Characteristics | Publications Included | |||
|---|---|---|---|---|---|
| Materials (Ma), Mode (Mo) and Setting (Se) | Aim | Interventionist | Target Population | ||
| 1. Comfort Care Communication Tool (USA) | Ma: Four-quadrant design document Mo: Face-to-face longitudinal conversations Se: NS | To enhance adolescents’ disclosure and person-centered care based on families’ goals | Pediatric Advanced Comfort Care Team Nurse | Adolescents with life-threatening or life-limiting health care conditions | Christenson, 2010 [51] |
| 2. Family-Centered pediatric Advance Care Planning (USA) | Ma: Family-centered ACP survey (session 1), Respecting Choices interview (session 2), and Five Wishes document (session 3) Mo: Three-session face-to-face conversation Se: Outpatient clinic | To facilitate EOL discussions for adolescents and their families | Certified facilitator | Adolescents with cancer, HIV or AIDS and their surrogates | Curtin, 2017 [52] Dallas, 2012 [53] Dallas, 2016 [31] Friebert, 2020 [42] Jacobs, 2015 [39] Lyon, 2009 [32] Lyon, 2009 [33] Lyon, 2010 [34] Lyon, 2013 [35] Lyon, 2014 [36] |
| 3. Family-Centered pediatric Advance Care Planning Rare (USA) | Ma: Conversation card, documentation tool Mo: Four-session interviews, face-to-face or via telemedicine conversation Se: NS | To identify and meet caregiver-centered palliative care needs | Certified clinician | Family caregivers of children and adolescents with rare diseases | Lyon, 2019 [47] |
| 4. Implementing Advance Care Planning Toolkit (NL) | Ma: Information leaflets, preparation cards (child and parent), and conversation guides Mo: Face-to-face conversations, on-off conversation, or multiple conversations Se: Home, inpatient, or outpatient clinic | To prepare children, clinicians and parents for future care, to guide documentation, and to elicit the voice of the child and stimulate a patient-centered approach | Clinician involved in the patient’s care | Children with life-limiting conditions and their families | Fahner, 2020 [11] |
| 5. DAY 100 Talk (UK) | Ma: Family preparatory and summary worksheet and a conversation guide Mo: Fill in up-front and face-to-face longitudinal conversations Se: Outpatient clinic | To enhance families’ disclosure and interdisciplinary guidance | Trained pediatric oncologist and psychosocial clinician | Children, adolescents, and young adults with cancer and their families | Feraco, 2018 [43] |
| 6. 3 × 3 Lifetime Framework (UK) | Ma: 3 × 3 Framework Document Mo: Face-to-face longitudinal conversations Se: NS | To enhance family engagement in EOL planning | Clinicians | Children with non-malignant, life-limiting illnesses and their families | Finlay, 2008 [45] |
| 7. The Wishes Document (UK) | Ma: Hand-held document Mo: Face-to-face longitudinal conversations Se: NS | To enhance family engagement in EOL planning | Clinician involved in the patient’s care | Children, young people with life-limiting conditions and their families | Fraser, 2010 [54] |
| 8. The ADVANCE toolkit (UK) | Ma: Ethical guide Mo: Face-to-face longitudinal conversations Se: Private place | To enhance provider guidance, families’ disclosure, and families’ engagement in EOL planning | Clinician involved in the patient’s care | Young persons with learning disabilities (who are approaching the end of life) and their families | Gallagher, 2018 [55] |
| 9. Holistic Needs Assessment (UK) | Ma: Comprehensive assessment of needs Mo: Face-to-face conversation Se: NS | To enhance person-centered care based on family needs | Senior member of staff | Children in palliative care settings and their family | Hartley, 2016 [44] |
| 10. Decision-making Communication Tool (USA) | Ma: Four domains of decision making Mo: Face-to-face longitudinal conversations Se: Outpatient clinic | To enhance patient–provider communication, decision making, and quality of life, as well as to identify goals of care | Supportive care team clinicians | Pediatric palliative care: infants, children, and adolescents with potentially life-limiting illnesses (oncology) and their families | Kline, 2012 [46] Hays, 2006 [37] |
| 11.Communication Plan: Early through End of Life (USA) | Ma: Conversation guide and visual aids Mo: Three face-to-face conversation sessions, longitudinal revision Se: During clinic appointments | To reduce parental distress | Trained oncology providers | Parents of children with cancer | Hendricks, 2017 [38] Moody, 2020 [41] |
| 12. Voicing my choices (USA) | Ma: Guide adapted from the Five Wishes, completion of the document guide Mo: Longitudinal revision Se: NS | To enhance communication between the patient and caregiver in EOL preferences and care | Clinicians | Adolescents and young people living with a serious illness | Wiener, 2012 [48] Kazmerski, 2016 [40] Zadeh, 2015 [56] |
| 13. My Choices/Choices for My Child Booklets (UK) | Ma: Booklets for children and parents, possibility Mo: To fill in/initiate thinking or face-to-face conversations Se: Home or outpatient clinic | To enhance family engagement in future planning and the disclosure of family preferences | NA | Children with life-limiting conditions from diagnosis onwards and their parents | Noyes, 2013 [50] |
| 14. The Serious Illness Conversation Guide-Peds (SICG-Peds) (Canada) | Ma: Conversation guide Mo: Longitudinal face-to-face or by phone conversations Se: Home or clinic | To enhance understanding of illness and care preferences | Trained pediatrician | Children with serious illness and their parents | Van Breemen, 2020 [57] |
| 15. Five Wishes® (USA) | Ma: Legal document consisting of five wishes Mo: Fill in document Se: NS | To enhance communication in EOL care | Clinicians | Adolescents and young adults living with serious illnesses | Wiener, 2008 [49] |
| 16.Individualized care planning and coordination (USA) | Ma: Advance care planning documentation tool Mo: Longitudinal revision on timely basis Se: NS | To facilitate integration of palliative care into ongoing care | Clinicians | Children with cancer and their parents | Baker, 2008 [58] |
| 17. MyPref (USA) | Ma: Preference report up-front cancer therapy Mo: Fill in document, longitudinal revision Se: NS | To clarify AYAs’ preferences and to enhance engagement in medical decision making | Oncology providers or other clinicians | AYA patients with relapsed/progressive cancer | Snaman, 2019 [59] |
| 18. FOOTPRINTS (USA) | Ma: Conversation guide, using a discharge order sheet Mo: Longitudinal face-to-face conversations Se: During the interdisciplinary “care conference” | To provide quality of care for the patient, their families, and providers through anticipating their needs on a continual basis | Hospital-based “continuity” pediatrician | Children with life-limiting illnesses and their families | Toce, 2003 [60] |
| Article | Description Concept | Implementation in the Tool Described | Evaluation on Age Appropriateness Stated by Patient/Provider/Family | Recommendations | |||
|---|---|---|---|---|---|---|---|
| Statement of Concept Applied | Elements of Tool | Patient | Provider | Family | |||
| Baker, 2008 [58] | Implicit | No | NS | NS | NS | NS | Yes |
| Christenson, 2010 [51] | Implicit | Yes | Questions adjusted for age and maturity | NS | NS | NS | Yes |
| Curtin, 2017 [52] | Implicit | Yes | NS | NS | NS | NS | No |
| Dallas, 2012 [53] | Implicit | Yes | NS | NS | NS | NS | No |
| Dallas, 2016 [31] | No Description | No | NS | NS | NS | NS | No |
| Fahner, 2020 [11] | Explicit | Yes | Booklets and conversation guides, with language adapted to the children | NS | NS | NS | Yes |
| Feraco, 2018 [43] | No Description | No | NS | NS | NS | NS | No |
| Finlay, 2008 [45] | No Description | No | NS | NS | NS | NS | No |
| Fraser, 2010 [54] | Implicit | No | NS | NS | NS | NS | Yes |
| Friebert, 2020 [42] | Implicit | No | NS | NS | NS | NS | No |
| Gallagher,2018 [55] | Implicit | No | NS | NS | NS | NS | Yes |
| Hartley, 2016 [44] | Implicit | No | NS | NS | NS | NS | No |
| Hays, 2006 [37] | Implicit | No | NS | NS | NS | NS | Yes |
| Hendricks,2017 [38] | No Description | No | NS | NS | NS | NS | No |
| Jacobs, 2015 [39] | No description | No | NS | NS | NS | NS | No |
| Kazmerski, 2016 [40] | Implicit | No | NS | 90% considered VMC (Voicing My Choices) to be age-appropriate; 66% considered ACP to be appropriate to introduce before the age of 18 or at any age | 58% considered VMC to be appropriate for patient population/age group; 50% found the ideal patient age for ACP discussion was >18 years | NS | No |
| Kline, 2012 [46] | No Description | No | NS | NS | NS | NS | No |
| Lyon, 2009 [32] | Explicit | Yes | NS | NS | NS | NS | Yes |
| Lyon, 2009 [33] | Explicit | Yes | NS | NS | NS | NS | No |
| Lyon, 2010 [34] | No Description | No | NS | NS | NS | NS | No |
| Lyon, 2013 [35] | Implicit | No | NS | NS | NS | NS | No |
| Lyon, 2014 [36] | Implicit | Yes | NS | NS | NS | NS | No |
| Lyon, 2019 [47] | No Description | No | NS | NS | NS | NS | No |
| Moody, 2020 [41] | No Description | No | NS | NS | NS | NS | No |
| Noyes, 2013 [50] | Implicit | Yes | Booklets content and images adapted for age | NS | NS | NS | Yes |
| Snaman, 2019 [59] | Explicit | Yes | NS | NS | NS | NS | Yes |
| Van Breemen, 2020 [57] | Implicit | Yes | Family-centered language | NS | NS | NS | No |
| Wiener, 2008 [49] | Implicit | Yes | Age-appropriate images | 90% declared that all statements on EOL care were appropriate and helpful for someone their age | NS | NS | Yes |
| Wiener, 2012 [48] | Implicit | Yes | Wording and questions adjusted for development and a glossary added | No significant tool differences in the degree of help or stress in age groups or differences in document content; AYAs disagreed on whether medical care wishes in the Five Wishes versus MTMWMV (My Thoughts, My Wishes, My Voice) was more appropriate for someone of their age | NS | NS | Yes |
| Zadeh, 2015 [56] | Explicit | Yes | Wording and questions adjusted for development and a glossary added | NS | NS | NS | Yes |
| Toce, 2003 [60] | Implicit | Yes | NS | NS | NS | NS | No |
| Willingness to Participate | Ability to Participate | Developing Social Identity | Legal Responsibilities | |||
|---|---|---|---|---|---|---|
| Decision-Making Capacity | A Child’s Understanding of Their Own Medical Process | Cognitive Impairment | ||||
| Baker, 2008 [58] | x | x | ||||
| Christenson, 2010 [51] | x | x | x | x | ||
| Curtin, 2017 [52] | x | |||||
| Dallas, 2012 [53] | x | x | x | x | x | |
| Dallas, 2016 [31] | x | x | x | |||
| Fahner, 2020 [11] | x | x | ||||
| Feraco, 2018 [43] | x | x | ||||
| Finlay, 2008 [45] | ||||||
| Fraser, 2010 [54] | x | |||||
| Friebert, 2020 [42] | x | x | x | x | ||
| Gallagher, 2018 [55] | x | x | x | |||
| Hartley, 2016 [44] | x | |||||
| Hay, 2006 [37] | ||||||
| Hendricks, 2017 [38] | x | |||||
| Jacobs, 2015 [39] | x | x | x | |||
| Kazmerski, 2016 [40] | x | |||||
| Kline, 2012 [46] | ||||||
| Lyon, 2009 [32] | x | x | x | x | ||
| Lyon, 2009 [33] | x | x | x | x | x | |
| Lyon, 2010 [34] | x | x | x | |||
| Lyon, 2013 [35] | x | x | x | x | ||
| Lyon, 2014 [36] | x | x | x | x | ||
| Lyon, 2019 [47] | ||||||
| Moody, 2020 [41] | ||||||
| Noyes, 2013 [50] | ||||||
| Snaman, 2019 [59] | x | x | ||||
| Toce, 2003 [60] | x | |||||
| van Breemen, 2020 [57] | x | |||||
| Wiener, 2008 [49] | x | x | x | |||
| Wiener, 2012 [48] | x | x | x | x | x | |
| Zadeh, 2015 [56] | x | x | x | x | x | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunetta, J.; Fahner, J.; Legemaat, M.; van den Bergh, E.; Krommenhoek, K.; Prinsze, K.; Kars, M.; Michiels, E. Age-Appropriate Advance Care Planning in Children Diagnosed with a Life-Limiting Condition: A Systematic Review. Children 2022, 9, 830. https://doi.org/10.3390/children9060830
Brunetta J, Fahner J, Legemaat M, van den Bergh E, Krommenhoek K, Prinsze K, Kars M, Michiels E. Age-Appropriate Advance Care Planning in Children Diagnosed with a Life-Limiting Condition: A Systematic Review. Children. 2022; 9(6):830. https://doi.org/10.3390/children9060830
Chicago/Turabian StyleBrunetta, Julie, Jurrianne Fahner, Monique Legemaat, Esther van den Bergh, Koen Krommenhoek, Kyra Prinsze, Marijke Kars, and Erna Michiels. 2022. "Age-Appropriate Advance Care Planning in Children Diagnosed with a Life-Limiting Condition: A Systematic Review" Children 9, no. 6: 830. https://doi.org/10.3390/children9060830
APA StyleBrunetta, J., Fahner, J., Legemaat, M., van den Bergh, E., Krommenhoek, K., Prinsze, K., Kars, M., & Michiels, E. (2022). Age-Appropriate Advance Care Planning in Children Diagnosed with a Life-Limiting Condition: A Systematic Review. Children, 9(6), 830. https://doi.org/10.3390/children9060830






