Psychosocial Considerations for the Child with Rare Disease: A Review with Recommendations and Calls to Action
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. The Child with RD
3.1.1. Intersecting Identities and Experiences
3.1.2. Behavioral Health
3.1.3. Communication
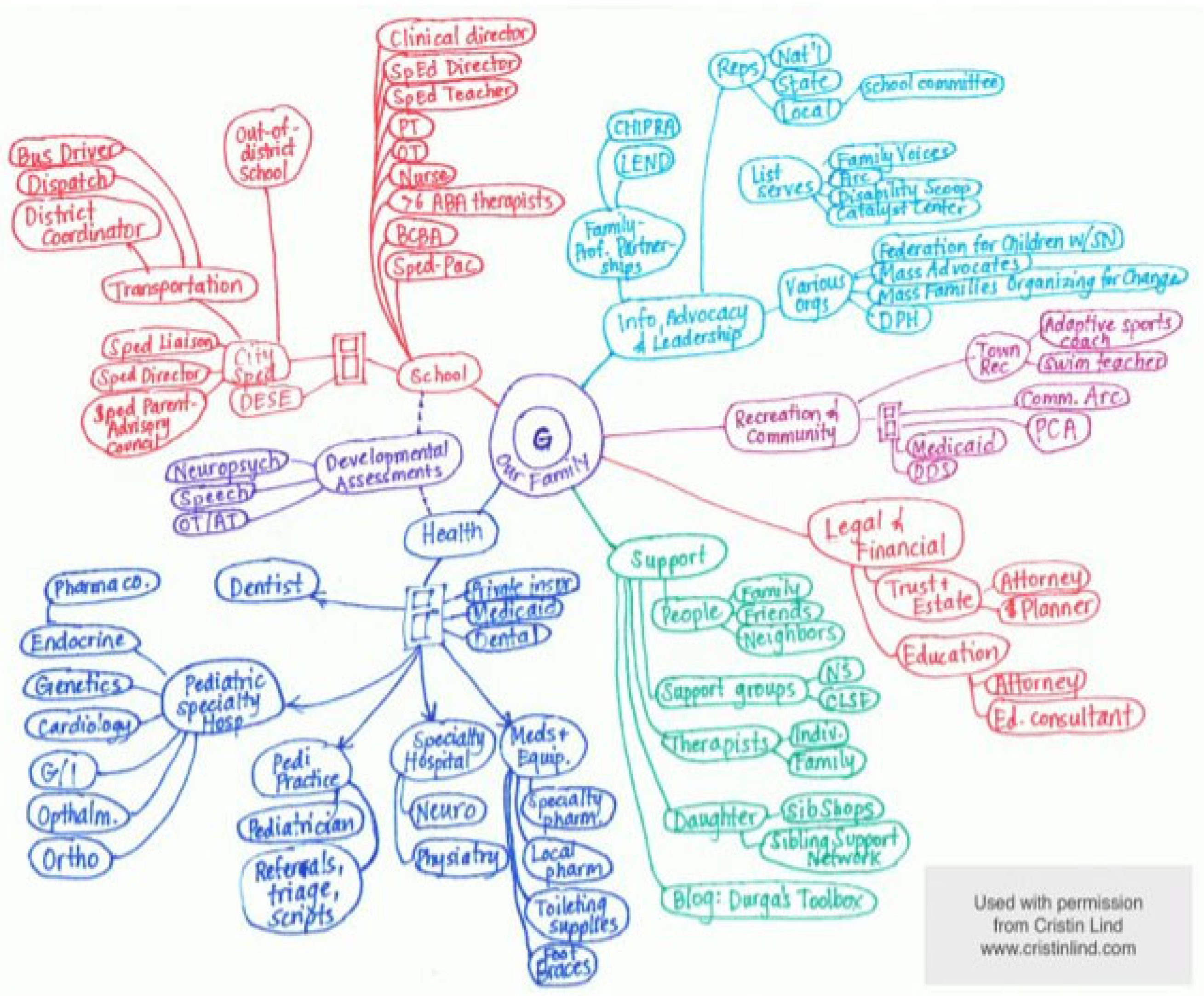
3.2. RD in the Family
3.3. Social Determinants of Health
3.4. Home Care
3.5. The Search for Answers
3.6. Barriers to Wellbeing
3.7. Coordination of Care
3.8. Access to Information
3.9. The Family Is Part of the Care Team
3.9.1. Advisory Councils and Boards
3.9.2. The Siblings
3.10. Access Barriers in the Community
3.10.1. Community Activities and Transportation
3.10.2. Appropriate and Fair Education
3.11. The Healthcare Team
3.12. Transition to Adult Care
4. Recommendations
4.1. Support Pathways
4.1.1. The Child and Family
4.1.2. The Siblings
- Parents, be aware that while attending to the needs of the child with RD, you may be neglecting—or creating unfair expectations for—your other children.
- Siblings can learn to participate in the family and feel pride and love in helping their brother or sister with their health.
- Try to establish some balance between the needs of your child with a chronic health problem or disability and those of your other children.
- Keep in mind that siblings need to have honest information about the condition and to have their questions listened to and answered.
- Spending small amounts of quality time with each child individually as much as possible may help.
- Support groups involving other siblings in a comparable situation can play a pivotal role in siblings’ coping and thriving amidst this challenging situation.
4.2. Support and Collabortion Pathways
4.2.1. Behavioral Health
4.2.2. Partnership
4.3. Patient Family Advisory Councils or Boards
- Use evolving recruitment methods
- Prepare for effective participation
- Ensure diversity within PFACs
- Outline terms for orientation and participation.
5. Calls to Action
5.1. Include the Child Directly
5.2. Leverage Collaborative Communication
- 1.
- Facilitate Transition to Adult Care
- 2.
- Access and Share Essential Information
- 3.
- Sustain Strategically
5.3. Educators
5.4. Investigators
5.5. Policy Advocates and Change-Makers
5.5.1. Improve SDH at Multiple Levels
5.5.2. Improve Home and Community Based Services
5.5.3. Improve Accessibility in Communities
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richter, T.; Nestler-Parr, S.; Babela, R.; Khan, Z.M.; Tesoro, T.; Molsen, E.; Hughes, D. Rare Disease Terminology and Definitions-A Systematic Global Review: Report of the ISPOR Rare Disease Special Interest Group. Value Health 2015, 18, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Caplan, A. The Orphan Drug Act Revisited. JAMA 2019, 321, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Schuller, Y.; Gispen-de Wied, C.; Hollak, C.E.M.; Leufkens, H.G.M.; Stoyanova-Beninska, V. Dose-Finding Studies Among Orphan Drugs Approved in the EU: A Retrospective Analysis. J. Clin. Pharmacol. 2019, 59, 229–244. [Google Scholar] [CrossRef]
- Harada, K.; Toriyabe, K.; Ono, S. Survey of Japanese Orphan Drug Program: Factors Related to Successful Marketing Approval. J. Clin. Phamacol. 2020, 60, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S.A.; Palacios, M.G.; Palacios, R.F. The impact of our fight. Cancer Rep. 2021, 5, 1–5. [Google Scholar] [CrossRef]
- Boycott, K.M.; Vanstone, M.R.; Bulman, D.E.; MacKenzie, A.E. Rare-disease genetics in the era of next-generation sequencing: Discovery to translation. Nat. Rev. Genet. 2013, 14, 681–691. [Google Scholar] [CrossRef]
- Germeni, E.; Vallini, I.; Bianchetti, M.G.; Schulz, P.J. Reconstructing normality following the diagnosis of a childhood chronic disease: Does “rare“ make a difference? Eur. J. Pediatrics 2018, 177, 489–495. [Google Scholar] [CrossRef]
- Munro, M.; Cook, A.M.; Bogart, K.R. An inductive qualitative content analysis of stigma experienced by people with rare diseases. Health Psychol. 2021, 22, 1–16. [Google Scholar] [CrossRef]
- Currie, G.; Szabo, J. Social isolation and exclusion: The parents’ experience of caring for children with rare neurodevelopmental disorders. Int. J. Qual. Stud. Health Well Being 2020, 15, 1725362. [Google Scholar] [CrossRef]
- Morgenstern, L.; Wagner, M.; Denecke, J.; Grolle, B.; Johannsen, J.; Wegscheider, K.; Wiegand-Grefe, S. The Need for Psychosocial Support in Parents of Chronically Ill Children. Prax. Kinderpsychol. Kinderpsychiatr. 2017, 66, 687–701. [Google Scholar] [CrossRef]
- Nobuyuki, Y.; Ishiguro, A.; Sakai, H.; Ohfuji, S.; Fukushima, W.; Hirota, Y. Factor-associated caregiver burden in medically complex patients with special health-care needs. Pediatr. Int. 2014, 56, 742–747. [Google Scholar]
- Bogart, K.R.; Frandrup, E.; Locke, T.; Thompson, H.; Weber, N.; Yates, J.; Zike, N.; Hemmesch, A.R. Rare place where I feel normal”: Perceptions of a social support conference among parents of and people with Moebius syndrome. Res. Dev. Disabil. 2017, 64, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Kuo, D.Z.; Cohen, E.; Agrawal, R.; Berry, J.G.; Casey, P.H. A national profile of caregiver challenges among more medically complex children with special health care needs. Pediatr. Adolesc. Med. 2011, 165, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Belzer, L.T. (Children’s Mercy Kansas City, Kansas City, MO, USA). Ryan, on Miya’s Story. Personal Communication, 24 February 2022. [Google Scholar]
- von der Lippe, C.; Diesen, P.S.; Feragen, K.B. Living with a rare disorder: A systematic review of the qualitative literature. Mol. Genet. Genom. Med. 2017, 5, 758–773. [Google Scholar] [CrossRef] [PubMed]
- Baumbusch, J.; Mayer, S.; Sloan-Yip, I. Alone in a Crowd? Parents of Children with Rare Diseases’ Experiences of Navigating the Healthcare System. J. Genet. Couns. 2019, 28, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.B.; Carboni-Jiménez, A.; Cañedo-Ayala, M.; Turner, K.A.; Chiovitti, M.; Levis, A.W.; Thombs, B.D. Perceived Benefits and Facilitators and Barriers to Providing Psychosocial Interventions for Informal Caregivers of People with Rare Diseases: A Scoping Review. Patient Patient Cent. Outcomes Res. 2020, 13, 471–519. [Google Scholar] [CrossRef]
- Brugallé, E.; Antoine, P.; Geerts, L.; Bellengier, L.; Manouvrier-Hanu, S.; Fantini-Hauwel, C. Growing up with a rare genetic disease: An interpretative phenomenological analysis of living with Holt-Oram syndrome. Disabil. Rehabil. 2021, 43, 2304–2311. [Google Scholar] [CrossRef]
- McPherson, M.; Arango, P.; Fox, H.; Lauver, C.; McManus, M.; Newacheck, P.; Perrin, J.; Shonkoff, J.; Strickland, B. A new definition of children with special healthcare needs. Pediatrics 1998, 102, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Kuo, D.; Goudie, A.; Cohen, E.; Houtrow, A.; Agrawal, R.; Carle, A.C.; Wells, N. Inequities In Health Care Needs For Children With Medical Complexity. Health Aff. 2014, 33, 2190–2198. [Google Scholar] [CrossRef]
- Cohen, E.; Kuo, D.Z.; Agrawal, R.; Berry, J.G.; Bhagat, S.K.M.; Simon, T.D.; Srivastava, R. Children with medical complexity: An emerging population for clinical and research initiatives. Pediatrics 2011, 127, 529–538. [Google Scholar] [CrossRef]
- Zurynski, Y.; Deverell, M.; Dalkeith, T.; Johnson, S.; Christodoulou, J.; Leonard, H.; Elliott, E.J. Australian children living with rare diseases: Experiences of diagnosis and perceived consequences of diagnostic delays. Orphanet J. Rare Dis. 2017, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Zurynski, Y.; Altman, L. Families as Partners: Co-design of a localised model of care for children with medical complexity living in rural Australia and evaluation using the Paediatric Integrated Care Survey (PICS). Int. J. Integr. Care 2019, 19, 616. [Google Scholar] [CrossRef]
- Pawliuk, C.; Widger, K.; Dewan, T.; Brander, G.; Brown, H.L.; Hermansen, A.M.; Grégoire, M.C.; Steele, R.; Siden, H.H. Scoping review of symptoms in children with rare, progressive, life-threatening disorders. BMJ Support Palliat Care 2020, 10, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Quaye, A.A.; Coyne, I.; Söderbäck, M.; Hallström, I.K. Children’s active participation in decision-making processes during hospitalisation: An observational study. J. Clin. Nurs. 2019, 28, 4525–4537. [Google Scholar] [CrossRef]
- Abrams, J.A.; Tabaac, A.; Jung, S.; Else-Quest, N.M. Considerations for employing intersectionality in qualitative health research. Soc. Sci. Med. 2020, 258, 113138. [Google Scholar] [CrossRef]
- Fraiman, Y.S.; Wojcik, M.H. The influence of social determinants of health on the genetic diagnostic odyssey: Who remains undiagnosed, why, and to what effect? Pediatr. Res. 2021, 89, 295–300. [Google Scholar] [CrossRef]
- Mattson, G.; Kuo, D.Z.; Yogman, M.; Baum, R.; Gambon, T.B.; Lavin, A.; Esparza, R.M.; Nasir, A.A.; Wissow, L.S.; Apkon, S.; et al. Psychosocial Factors in Children and Youth With Special Health Care Needs and Their Families. Pediatrics 2019, 143, e20183171. [Google Scholar] [CrossRef]
- Bravo, L.; Killela, M.K.; Reyes, B.L.; Santos, K.M.B.; Torres, V.; Huang, C.-C.; Jacob, E. Self-Management, Self-Efficacy, and Health-Related Quality of Life in Children With Chronic Illness and Medical Complexity. J. Pediatr. Health Care Off. Publ. Natl. Assoc. Pediatr. Nurse Assoc. Pract. 2020, 34, 304–314. [Google Scholar] [CrossRef]
- Adams, C.D.; Streisand, R.M.; Zawacki, T.; Joseph, K.E. Living With a Chronic Illness: A Measure of Social Functioning for Children and Adolescents. J. Pediatric Psychol. 2002, 27, 593–605. [Google Scholar] [CrossRef]
- Cole, T.; McKendrick, F.; Titman, P.; Cant, A.J.; Pearce, M.S.; Cale, C.M.; Goldblatt, D.; Gennery, A.R. Health Related Quality of Life and Emotional Health in Children with Chronic Granulomatous Disease: A Comparison of Those Managed Conservatively with Those That Have Undergone Haematopoietic Stem Cell Transplant. J. Clin. Immunol. 2013, 33, 8–13. [Google Scholar] [CrossRef]
- Adama, E.A.; Arabiat, D.; Foster, M.J.; Afrifa-Yamoah, E.; Runions, K.; Vithiatharan, R.; Lin, A. The psychosocial impact of rare diseases among children and adolescents attending mainstream schools in Western Australia. Int. J. Incl. Educ. 2021, 1–14. [Google Scholar] [CrossRef]
- Lum, A.; Wakefield, C.E.; Donnan, B.; Burns, M.A.; Fardell, J.E.; Jaffe, A.; Kasparian, N.A.; Kennedy, S.E.; Leach, S.T.; Lemberg, D.A.; et al. Facilitating engagement with school in students with chronic illness through positive education: A mixed-methods comparison study. Sch. Psychol. 2019, 34, 677–686. [Google Scholar] [CrossRef]
- Conti-Ramsden, G.; Durkin, K. What Factors Influence Language Impairment? Considering Resilience as well as Risk. Folia Phoniatr Logop 2015, 67, 293–299. [Google Scholar] [CrossRef]
- Snowling, M.J.; Bishop, D.V.M.; Stothard, S.E.; Chipchase, B.; Kaplan, C. Psychosocial outcomes at 15 years of children with a preschool history of speech-language impairment. J. Child Psychol. Psychiatry 2006, 47, 759–765. [Google Scholar] [CrossRef]
- Yew, S.G.K.; O’Kearney, R. Emotional and behavioural outcomes later in childhood and adolescence for children with specific language impairments: Meta-analyses of controlled prospective studies. J. Child Psychol. Psychiatry 2013, 54, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.A.; Patton, E.; Freebairn, L.; Tag, J.; Iyengar, S.K.; Stein, C.M.; Taylor, H.G. Psychosocial co-morbidities in adolescents and adults with histories of communication disorders. J. Commun. Disord. 2016, 61, 60–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoover, C.G.; Coller, R.J.; Houtrow, A.; Harris, D.; Agrawal, R.; Turchi, R. Understanding Caregiving and Caregivers: Supporting CYSHCN at Home. Acad. Pediatr. 2022, 22, S14–S21. [Google Scholar] [CrossRef] [PubMed]
- Medway, M.; Tong, A.; Craig, J.C.; Kim, S.; Mackie, F.; McTaggart, S.; Walker, A.; Wong, G. Parental perspectives on the financial impact of caring for a child with CKD. Am. J. Kidney Dis. 2015, 65, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Shriver, J. Cost of Caregiving on Parents of Children With Medical Complexity and Life-Limiting Conditions. Pediatrics 2021, 148, e2021050222. [Google Scholar] [CrossRef] [PubMed]
- Sarathy, B.; Morris, H.; Tumin, D.; Buckman, C. The Impact of Medical Financial Hardship on Children’s Health. Clin. Pediatr. 2020, 59, 1252–1257. [Google Scholar] [CrossRef]
- Okumura, M.J.; Van Cleave, J.; Gnanasekaran, S.; Houtrow, A. Understanding Factors Associated With Work Loss for Families Caring for CSHCN. Pediatrics 2009, 124, S392–S398. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.C.; Agrawal, R.K.; Davis, M.M. Home Health Care For Children With Medical Complexity: Workforce Gaps, Policy, and Future Directions. Health Aff. Millwood 2019, 38, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.C.; Chorniy, A.; Kwon, S.; Kan, K.; Heard-Garris, N.; Davis, M.M. Children With Special Health Care Needs and Forgone Family Employment. Pediatrics 2021, 148, e2020035378. [Google Scholar] [CrossRef] [PubMed]
- Schall, T.E.; Foster, C.C.; Feudtner, C. Safe Work-Hour Standards for Parents of Children With Medical Complexity. JAMA Pediatr. 2020, 174, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, K.S. Food insufficiency and children with special healthcare needs. Public Health 2019, 167, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Sonik, R.A.; Coleman-Jensen, A.; Parish, S.L. Household food insufficiency, health status and emergency healthcare utilisation among children with and without special healthcare needs. Public Health Nutr. 2020, 23, 3204–3210. [Google Scholar] [CrossRef]
- Fuller, A.E.; Brown, N.M.; Grado, L.; Oyeku, S.O.; Gross, R.S. Material Hardships and Health Care Utilization Among Low-Income Children with Special Health Care Needs. Acad. Pediatr. 2019, 19, 733–739. [Google Scholar] [CrossRef]
- Fuller, A.E.; Garg, A.; Brown, N.M.; Tripodis, Y.; Oyeku, S.O.; Gross, R.S. Relationships BETWEEN Material Hardship, Resilience, and Health Care Use. Pediatrics 2020, 145, e20191975. [Google Scholar] [CrossRef]
- Hounsell, K.G.; Moore, C.; Zahavi, A.; Arje, D.; Weiser, N.; Esser, K.; Netten, K.; Soscia, J.; Cohen, E.; Orkin, J. The Experience of Housing Needs Among Families Caring for Children With Medical Complexity. Pediatrics 2021, 148, e2020018937. [Google Scholar] [CrossRef]
- Berry, J.G. What Children with Medical Complexity, Their Families, and Healthcare Providers Deserve from an Ideal Healthcare System; Lucile Packard Foundation for Children’s Health: Palo Alto, CA, USA, 2015. [Google Scholar]
- Bona, K.; London, W.B.; Guo, D.; Frank, D.A.; Wolfe, J. Trajectory of Material Hardship and Income Poverty in Families of Children Undergoing Chemotherapy: A Prospective Cohort Study. Pediatric Blood Cancer 2016, 63, 105–111. [Google Scholar] [CrossRef]
- AAP Section on Home Health Care. Guidelines for Pediatric Home Health Care; American Academy of Pediatrics: Elk Grove Village, IL, USA, 2008. [Google Scholar] [CrossRef]
- Boss, R.D.; Raisanen, J.C.; Detwiler, K.; Fratantoni, K.; Huff, S.M.; Neubauer, K.; Donohue, P.K. Lived Experience of Pediatric Home Health Care Among Families of Children With Medical Complexity. Clin. Pediatr. 2020, 59, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.C.; Kwon, S.; Whitlow, L.; Cullen, J.P.; Agrawal, R.K.; Goodman, D.; Davis, M.M. Connecting Hospital to Home: Characteristics of and Rehospitalization Rates in Hospitalized Children With Private-Duty Nursing. Hosp. Pediatr. 2019, 9, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Weaver, M.S.; Wichman, B.; Bace, S.; Schroeder, D.; Vail, C.; Wichman, C.; Macfadyen, A. Measuring the Impact of the Home Health Nursing Shortage on Family Caregivers of Children Receiving Palliative Care. J. Hosp. Palliat. Nurs. 2018, 20, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Deuitch, N.T.; Beckman, E.; Halley, M.C.; Young, J.L.; Reuter, C.M.; Kohler, J.; Bernstein, J.A.; Wheeler, M.T.; Ormond, K.E.; Tabor, H.K.; et al. “Doctors can read about it, they can know about it, but they’ve never lived with it“: How parents use social media throughout the diagnostic odyssey. J. Genet. Couns. 2021, 30, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Hayakawa, I.; Abe, Y. Diagnostic odyssey of acute disseminated encephalomyelitis in children. Sci. Rep. 2021, 11, 21954. [Google Scholar] [CrossRef]
- Nevin, S.M.; Wakefield, C.E.; Barlow-Stewart, K.; McGill, B.C.; Bye, A.; Palmer, E.E.; Dale, R.C.; Gill, D.; Children’s Hospital At Westmead Neurology Group; Cogenes Group. Psychosocial impact of genetic testing on parents of children with developmental and epileptic encephalopathy. Dev. Med. Child Neurol. 2021, 64, 95–101. [Google Scholar] [CrossRef]
- Tumiene, B. Unmet psychosocial needs of parents of children with rare, complex, and severe genetic diseases. Dev. Med. Child Neurol. 2021, 64, 13. [Google Scholar] [CrossRef]
- Mann, K.; Alvey, J.; Marty, C.; Murphy, N. Health-Related Quality of Life and Family Functioning of Parents of Children with Medical Complexity. Curr. Phys. Med. Rehabil. Rep. 2019, 7, 23–29. [Google Scholar] [CrossRef]
- Anderson, M.; Elliott, E.J.; Zurynski, Y.A. Australian families living with rare disease: Experiences of diagnosis, health services use and needs for psychosocial support. Orphanet J. Rare Dis. 2013, 8, 22. [Google Scholar] [CrossRef]
- Bayer, N.D.; Wang, H.; Yu, J.A.; Kuo, D.Z.; Halterman, J.S.; Li, Y. A National Mental Health Profile of Parents of Children With Medical Complexity. Pediatrics 2021, 148, e2020023358. [Google Scholar] [CrossRef]
- Commodari, E. Children staying in hospital: A research on psychological stress of caregivers. Ital. J. Pediatr. 2010, 36, 40. [Google Scholar] [CrossRef] [PubMed]
- Minor, H.G.; Carlson, L.E.; Mackenzie, M.J.; Zernicke, K.; Jones, L. Evaluation of a Mindfulness-Based Stress Reduction (MBSR) Program for Caregivers of Children with Chronic Conditions. Soc. Work. Health Care 2006, 43, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Brannan, A.M.; Heflinger, C.A. Caregiver, child, family, and service system contributors to caregiver strain in two child mental health service systems. J. Behav. Health Serv. Res. 2006, 33, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, S.C.; Given, B.; Petlick, N.H.; Bemis, A. Supporting family caregivers in providing care. In Patient Safety and Quality: An Evidence-Based Handbook for Nurses; AHRQ Publication, Agency for Healthcare Research and Quality; U.S. Department of Health and Human Services: Rockville, MD, USA, 2008; Volume 1, pp. 341–404. [Google Scholar]
- Cardinali, P.; Migliorini, L.; Rania, N. The Caregiving Experiences of Fathers and Mothers of Children With Rare Diseases in Italy: Challenges and Social Support Perceptions. Front. Psychol. 2019, 10, 1780. [Google Scholar] [CrossRef]
- Cohen, J.S.; Biesecker, B.B. Quality of life in rare genetic conditions: A systematic review of the literature. Am. J. Med. Genet. Part A 2010, 152, 1136–1156. [Google Scholar] [CrossRef]
- Sapp, J.C.; Dong, D.; Stark, C.; Ivey, L.E.; Hooker, G.; Biesecker, L.G.; Biesecker, B.B. Parental attitudes, values, and beliefs toward the return of results from exome sequencing in children. Clin. Genet. 2014, 85, 120–126. [Google Scholar] [CrossRef]
- Dellve, L.; Samuelsson, L.; Tallborn, A.; Fasth, A.; Hallberg, L.R.-M. Stress and well-being among parents of children with rare diseases: A prospective intervention study. J. Adv. Nurs. 2006, 53, 392–402. [Google Scholar] [CrossRef]
- Mooney-Doyle, K.; Lindley, L.C. Family and Child Characteristics Associated With Caregiver Challenges for Medically Complex Children. Fam. Community Health 2020, 43, 74–81. [Google Scholar] [CrossRef]
- Coquillette, M.; Cox, J.E.; Cheek, S.; Webster, R.A. Social Work Services Utilization by Children with Medical Complexity. Matern. Child Health J. 2015, 19, 2707–2713. [Google Scholar] [CrossRef]
- Doyle, M. Peer Support and Mentorship in a US Rare Disease Community: Findings from the Cystinosis in Emerging Adulthood Study. Patient 2015, 8, 65–73. [Google Scholar] [CrossRef]
- Feudtner, C.; Nye, R.T.; Boyden, J.Y.; Schwartz, K.E.; Korn, E.R.; Dewitt, A.G.; Waldman, A.T.; Schwartz, L.A.; Shen, Y.A.; Manocchia, M.; et al. Association Between Children With Life-Threatening Conditions and Their Parents’ and Siblings’ Mental and Physical Health. JAMA Netw. Open 2021, 4, e2137250. [Google Scholar] [CrossRef] [PubMed]
- Committee on Hospital Care. American Academy of Pediatrics. Family-centered care and the pediatrician’s role. Pediatrics 2003, 112 3 Pt 1, 691–696. [Google Scholar]
- Lee, J.H.; Long, D.; Srinivasan, V. Translating scientific evidence into the art of caring for critically ill children across the globe. Transl. Pediatr. 2021, 10, 2643–2645. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, E.M.; Valenzuela-Araujo, D.; Zickafoose, J.S.; Kieffer, E.; DeCamp, L.R. The “Battle” of Managing Language Barriers in Health Care. Clin. Pediatr. 2016, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Santana, S.; Brach, C.; Harris, L.; Ochiai, E.; Blakey, C.; Bevington, F.; Kleinman, D.; Pronk, N. Updating Health Literacy for Healthy People 2030: Defining Its Importance for a New Decade in Public Health. J. Public Health Manag. Pract. 2021, 27, S258–S264. [Google Scholar] [CrossRef]
- Institute of Medicine Committee on Health Literacy. Health Literacy: A Prescription to End Confusion; Nielsen-Bohlman, L., Panzer, A.M., Kindig, D.A., Eds.; National Academy of Sciences: Washington, DC, USA, 2004. [Google Scholar] [CrossRef]
- Yeh, J.; Ostini, R. The impact of health literacy environment on patient stress: A systematic review. BMC Public Health 2020, 20, 749. [Google Scholar] [CrossRef]
- Deavin, A.; Greasley, P.; Dixon, C. Children’s Perspectives on Living With a Sibling With a Chronic Illness. Pediatrics 2018, 142. [Google Scholar] [CrossRef]
- Healthy Children.Org. Siblings of Children with Chronic Illnesses or Disabilities. Available online: https://www.healthychildren.org/English/health-issues/conditions/chronic/Pages/Siblings-of-Children-with-Chronic-Ilnesses.aspx (accessed on 6 March 2022).
- Gan, L.L.; Lum, A.; Wakefield, C.E.; Nandakumar, B.; Fardell, J.E. School Experiences of Siblings of Children with Chronic Illness: A Systematic Literature Review. J. Pediatric Nurs. 2017, 33, 23–32. [Google Scholar] [CrossRef]
- Bezyak, J.L.; Sabella, S.A.; Gattis, R.H. Public Transportation: An Investigation of Barriers for People With Disabilities. J. Disabil. Policy Stud. 2017, 28, 52–60. [Google Scholar] [CrossRef]
- Bezyak, J.L.; Sabella, S.; Hammel, J.; McDonald, K.; Jones, R.A.; Barton, D. Community participation and public transportation barriers experienced by people with disabilities. Disabil. Rehabil. 2020, 42, 3275–3283. [Google Scholar] [CrossRef]
- Moore, A.; Lynch, H. Accessibility and usability of playground environments for children under 12: A scoping review. Scand. J. Occup. Ther. 2015, 22, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.J.; Schelling, A. Postsecondary inclusion for individuals with an intellectual disability and its effects on employment. J. Intellect. Disabil. 2015, 19, 130–148. [Google Scholar] [CrossRef]
- Hewitt-Taylor, J. Children who have complex health needs: Parents’ experiences of their child’s education. Child Care Health Dev. 2009, 35, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.L.; Packman, W.; Packman, S. Psychosocial aspects of patients with Niemann-Pick disease, type B. Am. J. Med. Genet. Part A 2009, 149A, 2430–2436. [Google Scholar] [CrossRef] [PubMed]
- Pittet, I.; Berchtold, A.; Akré, C.; Michaud, P.-A.; Surís, J.-C. Are adolescents with chronic conditions particularly at risk for bullying? Arch. Dis. Child. 2010, 95, 711–716. [Google Scholar] [CrossRef]
- Paz-Lourido, B.; Negre, F.; de la Iglesia, B.; Verger, S. Influence of schooling on the health-related quality of life of children with rare diseases. Health Qual Life Outcomes 2020, 18, 109. [Google Scholar] [CrossRef] [PubMed]
- Verger, S.; Negre, F.; Rosselló, M.R.; Paz-Lourido, B. Inclusion and equity in educational services for children with rare diseases: Challenges and opportunities. Child. Youth Serv. Rev. 2020, 119, 105518. [Google Scholar] [CrossRef]
- Kliegman, R.M.; Ruggeri, B.E.; Smith, M.M. The Team-Based Approach to Undiagnosed and Rare Diseases. Pediatric Clin. North Am. 2017, 64, 17–26. [Google Scholar] [CrossRef]
- Project ECHO. Available online: https://hsc.unm.edu/echo/what-we-do/about-the-echo-model.html (accessed on 11 March 2022).
- Harder, V.S.; Krulewitz, J.; Jones, C.; Wasserman, R.C.; Shaw, J.S. Effects of Patient-centered Medical Home Transformation on Child Patient Experience. J. Am. Board Fam. Med. JABFM 2016, 29, 60–68. [Google Scholar] [CrossRef][Green Version]
- Kieber-Emmons, A.M.; Miller, W.L. The Patient-Centered Medical Home (PCMH) Framing Typology for Understanding the Structure, Function, and Outcomes of PCMHs. J. Am. Board Fam. Med. 2017, 30, 472–479. [Google Scholar] [CrossRef][Green Version]
- Van Cleave, J.; Okumura, M.J.; Swigonski, N.; O’Connor, K.G.; Mann, M.; Lail, J.L. Medical Homes for Children with Special Health Care Needs: Primary Care or Subspeciality Service? Acad. Pediatr. 2016, 16, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.; Coker, T.R. Social Complexity as a Special Health Care Need in the Medical Home Model. Pediatrics 2018, 142, 1–3. [Google Scholar] [CrossRef]
- Singh, S.P.; Anderson, B.; Liabo, K.; Ganeshamoorthy, T. Supporting young people in their transition to adults’ services: Summary of NICE guidance. BMJ 2016, 353, i2225. [Google Scholar] [CrossRef] [PubMed]
- Romito, B.; Jewell, J.; Jackson, M. Child Life Services. Pediatrics 2021, 147, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, S.; Lee, S.; Mollica, M.; Wiener, L.; Mendley, S.; Adams, L.; Blachman-Demner, D. Navigating Pediatric to Adult Healthcare Transition: A National Institutes of Health Workshop. J. Pediatr. 2022, 244, 234–240. [Google Scholar] [CrossRef]
- Witt, S.; Kristensen, K.; Wiegand-Grefe, S.; Boettcher, J.; Bloemeke, J.; Wingartz, C.; Bullinger, M.; Quitmann, J. Rare pediatric diseases and pathways to psychosocial care: A qualitative interview study with professional experts working with affected families in Germany. Orphanet J. Rare Dis. 2021, 16, 497. [Google Scholar] [CrossRef]
- NORD. RareDiseases.org. Available online: https://rarediseases.org/for-patients-and-families/connect-others/find-patient-organization/ (accessed on 9 March 2022).
- Resources for Parent to Parent Support. Available online: https://www.parentcenterhub.org/parent-to-parent-support/ (accessed on 3 March 2022).
- Parent to Parent New Zealand. Available online: https://parent2parent.org.nz/ (accessed on 3 March 2022).
- Parent to Parent USA. Available online: https://www.p2pusa.org/parents/ (accessed on 3 March 2022).
- Hope Kids. Available online: https://www.hopekids.org/about-us/ (accessed on 3 March 2022).
- Meyer, D. Sibling Support Project: SibShop. Available online: https://siblingsupport.org/ (accessed on 3 March 2022).
- Burkhart, K.; Asogwa, K.; Muzaffar, N.; Gabriel, M. Pediatric Integrated Care Models: A Systematic Review. Clin. Pediatr. 2020, 59, 148–153. [Google Scholar] [CrossRef]
- Bradshaw, S.; Bem, D.; Shaw, K.; Taylor, B.; Chiswell, C.; Salama, M.; Bassett, E.; Kaur, G.; Cummins, C. Improving health, wellbeing and parenting skills in parents of children with special health care needs and medical complexity—A scoping review. BMC Pediatr. 2019, 19, 301. [Google Scholar] [CrossRef]
- Everhart, J.L.; Haskell, H.; Khan, A. Patient- and Family-Centered Care: Leveraging Best Practices to Improve the Care of Hospitalized Children. Pediatr. Clin. N. Am. 2019, 66, 775–789. [Google Scholar] [CrossRef]
- Montalbano, A.; Chadwick, S.; Miller, D.; Taff, K.; De Miranda, E.D.; Pina, K.; Bradley-Ewing, A. Demographic Characteristics Among Members of Patient Family Advisory Councils at a Pediatric Health System. J. Patient Exp. 2021, 8, 23743735211049680. [Google Scholar] [CrossRef]
- Richard, J.; Azar, R.; Doucet, S.; Luke, A. Pediatric Patient and Family Advisory Councils: A Guide to Their Development and Ongoing Implementation. J. Patient Exp. 2020, 7, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Boles, J.; Baddley, D. Enhancing Effective Communication Among Non-Verbal Patients. Pediatr. Nurs. 2018, 44, 144–146. [Google Scholar]
- Yu, J.A.; Henderson, C.; Cook, S.; Ray, K. Family Caregivers of Children With Medical Complexity: Health-Related Quality of Life and Experiences of Care Coordination. Acad. Pediatr. 2020, 20, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Nicholas, D.; Mahant, S.; Weiser, N.; Kanani, R.; Boydell, K.; Cohen, E. Care maps for children with medical complexity. Dev. Med. Child Neurol. 2017, 59, 1299–1306. [Google Scholar] [CrossRef]
- DHHS. Care Coordination. Available online: https://www.ahrq.gov/ncepcr/care/coordination.html (accessed on 11 March 2022).
- White, N. Reducing Primary Care Provider Burnout With Pharmacist-Delivered Comprehensive Medication Management. Am. J. Lifestyle Med. 2021, 15, 133–135. [Google Scholar] [CrossRef]
- Stepien, K.M.; Kieć-Wilk, B.; Lampe, C.; Tangeraas, T.; Cefalo, G.; Belmatoug, N.; Francisco, R.; del Toro, M.; Wagner, L.; Lauridsen, A.-G.; et al. Challenges in Transition From Childhood to Adulthood Care in Rare Metabolic Diseases: Results From the First Multi-Center European Survey. Front. Med. 2021, 8, 652358. [Google Scholar] [CrossRef]
- Morrison, A.K.; Glick, A.; Yin, H.S. Health Literacy: Implications for Child Health. Pediatr. Rev. 2019, 40, 263–277. [Google Scholar] [CrossRef]
- Badaczewski, A.; Bauman, L.J.; Blank, A.E.; Dreyer, B.; Abrams, M.A.; Stein, R.E.K.; Roter, D.L.; Hossain, J.; Byck, H.; Sharif, I. Relationship between Teach-back and patient-centered communication in primary care pediatric encounters. Patient Educ. Couns. 2017, 100, 1345–1352. [Google Scholar] [CrossRef]
- Carter, J.; Dwyer, N.; Roselund, J.; Cote, K.; Chase, P.; Martorana, K.; Gustafson, K.; Bergling, E.; Kamalia, R.; DeGrazia, M. Systematic Changes to Help Parents of Medically Complex Infants Manage Medical Expenses. Adv. Neonatal Care 2017, 17, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Nambudiri, V.E. Electronic consultations and clinician burnout: An antidote to our emotional pandemic? J. Am. Med. Inform. Assoc. 2021, 28, 1038–1041. [Google Scholar] [CrossRef]
- Janosy, N.R.; Anderson, C.T.M. Toward physician well-being and the mitigation of burnout. Curr. Opin. Anaesthesiol. 2021, 34, 176–179. [Google Scholar] [CrossRef]
- McKee, K.E.; Tull, A.; Del Carmen, M.G.; Edgman-Levitan, S. Correlation of Provider Burnout With Patient Experience. J. Patient Exp. 2020, 7, 931–936. [Google Scholar] [CrossRef]
- WHO. Social Determinants of Health; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Hood, C.M.; Gennuso, K.P.; Swain, G.R.; Catlin, B.B. County Health Rankings: Relationships Between Determinant Factors and Health Outcomes. Am. J. Prev. Med. 2016, 50, 129–135. [Google Scholar] [CrossRef]
- Berry, J.G.; Harris, D.; Coller, R.J.; Chung, P.J.; Rodean, J.; Macy, M.; Linares, D.E. The Interwoven Nature of Medical and Social Complexity in US Children. JAMA Pediatr. 2020, 174, 891–893. [Google Scholar] [CrossRef]
- Pankewicz, A.; Davis, R.K.; Kim, J.; Antonelli, R.; Rosenberg, H.; Berhane, Z.; Turchi, R.M. Children With Special Needs: Social Determinants of Health and Care Coordination. Clin. Pediatr. (Phila) 2020, 59, 1161–1168. [Google Scholar] [CrossRef]
- Gay, J.C.; Thurm, C.W.; Hall, M.; Fassino, M.J.; Fowler, L.; Palusci, J.V.; Berry, J.G. Home Health Nursing Care and Hospital Use for Medically Complex Children. Pediatrics 2016, 138. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belzer, L.T.; Wright, S.M.; Goodwin, E.J.; Singh, A.; Carter, B.S. Psychosocial Considerations for the Child with Rare Disease: A Review with Recommendations and Calls to Action. Children 2022, 9, 933. https://doi.org/10.3390/children9070933
Belzer LT, Wright SM, Goodwin EJ, Singh A, Carter BS. Psychosocial Considerations for the Child with Rare Disease: A Review with Recommendations and Calls to Action. Children. 2022; 9(7):933. https://doi.org/10.3390/children9070933
Chicago/Turabian StyleBelzer, Leslee T., S. Margaret Wright, Emily J. Goodwin, A. Singh, and Brian S. Carter. 2022. "Psychosocial Considerations for the Child with Rare Disease: A Review with Recommendations and Calls to Action" Children 9, no. 7: 933. https://doi.org/10.3390/children9070933
APA StyleBelzer, L. T., Wright, S. M., Goodwin, E. J., Singh, A., & Carter, B. S. (2022). Psychosocial Considerations for the Child with Rare Disease: A Review with Recommendations and Calls to Action. Children, 9(7), 933. https://doi.org/10.3390/children9070933







