Abstract
In the past, an anteriorly located anus was often misdiagnosed and treated as an anorectal malformation (ARM) with a perineal fistula (PF). The paper aims to define the criteria for a normal anus, an anterior anus (AA) as an anatomic variant, and milder types of ARM such as congenital anal stenosis (CAS) and PF. An extensive literature search was performed by a working group of the ARM-Net Consortium concerning the subject “Normal Anus, AA, and mild ARM”. A consensus on definitions, clinical characteristics, diagnostic management, and treatment modalities was established, and a diagnostic algorithm was proposed. The algorithm enables pediatricians, midwives, gynecologists, and surgeons to make a timely correct diagnosis of any abnormally looking anus and initiate further management if needed. Thus, the routine physical inspection of a newborn should include the inspection of the anus and define its position, relation to the external sphincter, and caliber. A correct diagnosis and use of the presented terminology will avoid misclassifications and allow the initiation of correct management. This will provide a reliable comparison of different therapeutic management and outcomes of these patient cohorts in the future.
1. Introduction
Clinicians can be confronted with an abnormal aspect of the anus in newborns. The questions “What is defining a normal anus?” and “When is an anomaly considered an anorectal malformation (ARM)?” remain controversial in the literature. There are a great variability of terms, and the difference between normal anatomy, an anatomic variant, and a pathologic one is often unclear.
These conditions represent the less complex end of the spectrum of ARM but also mild forms like perineal fistulas (PF) can cause significant sequelae, such as chronic constipation [1,2], unnecessary colostomy [1,2,3], overflow-fecal incontinence with severe psychosocial stress [4,5,6], urinary tract infections [7], obstetric injuries [8], and even acute bowel perforation [1,3,9,10] with lethal outcome in the newborn period [1,9,10,11]. It is known that mild forms of ARM may be diagnosed late or even remain undetected [1,12,13]. This is true especially in countries with lower socio-economic facilities [14,15], but even in renowned European centers, between 8.7% and 46% of newborns with ARM are discharged from the birth unit without the correct diagnosis [3,16,17,18]. The rarity of ARMs creates challenges in timely clinical diagnosis. Enhancing clinicians’ awareness and providing clinical tools to differentiate ARM from non-pathological variants would decrease morbidity and potential mortality [10,16,18,19].
Clear criteria are required to determine whether the appearance is still within the normal spectrum, or if a specific ARM is present [1,2,3,9,10,11,16,18,19,20,21,22].
The aim of this paper is to define the criteria for a normal anus, an anterior anus (AA) as an anatomic variant, a mild ARM such as congenital anal stenosis (CAS), and PF. In addition, the ARM-Net Consortium seeks to clarify these entities and outline the diagnostic work-up based on published evidence and personal experiences to provide a timely management approach and avoid complications and unnecessary surgery.
2. Materials and Methods
The ARM-Net Consortium, founded in 2010 by European pediatric surgeons, epidemiologists, geneticists, psychologists, and representatives of patient organizations to collect and exchange data and knowledge about ARM to improve clinical care and quality of life of ARM patients by promoting research on genetic, epidemiologic and clinical subjects [23], assembled consecutive working groups on the subject “Normal Anus, AA, and mild ARM” to clarify the differentiation among these entities. After performing an extensive literature search, the working group established a consensus on definitions, diagnostic management, and treatment modalities, providing recommendations in a diagnostic algorithm, which was presented at the annual meeting of the ARM-Net Consortium in October 2021. Finally, a consensus was obtained from the whole ARM-Net Consortium for the present paper in its final form.
3. Results
3.1. Terminology and Definitions
The ARM-Net consortium agreed on the following terminology and related definitions:
- Normal anus: Lies in a normal position along the perineum between the fourchette (girls) or scrotum (boys) and the coccyx. It is of normal caliber and circumferentially surrounded by the sphincter muscle complex (Figure 1a).
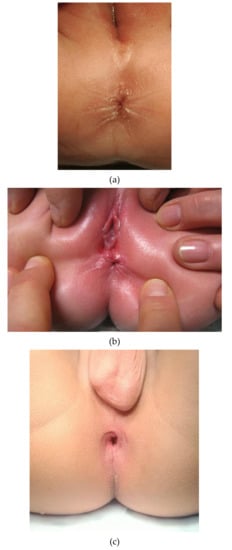 Figure 1. (a) Picture of a normal anus in a female infant. (b) Picture of an anterior anus in a female infant. Note the proximity of the anal opening to the external genitalia. (c). Picture of a non-stenotic perineal fistula. The sphincter muscle complex does not encircle the anal opening along the anterior margin.
Figure 1. (a) Picture of a normal anus in a female infant. (b) Picture of an anterior anus in a female infant. Note the proximity of the anal opening to the external genitalia. (c). Picture of a non-stenotic perineal fistula. The sphincter muscle complex does not encircle the anal opening along the anterior margin. - Anterior anus (AA): Considered a normal anatomic variant and defines an anus that is anteriorly located in the perineum, yet fully surrounded by the sphincter muscle complex [24,25,26,27,28,29], and has a normal caliber [30] (Figure 1b). There is no concomitant ARM (such as rectovaginal H-type fistula, Currarino syndrome, etc.).
- Perineal fistula (PF): Anus is anteriorly located in the perineum and is not completely surrounded by the sphincter muscle complex [29]. It can have a normal or diminished caliber, further defined as non-stenotic or stenotic PF, respectively (Figure 1c).
- Congenital anal stenosis (CAS): Anus lies in a normal position, completely surrounded by the sphincter muscle complex, but is too narrow. It may be partly covered by a median bar or membrane, usually located at the dentate line [13].
3.1.1. Diagnostic Algorithm
To differentiate among these entities, a diagnostic algorithm (Figure 2) is proposed based on the following questions:
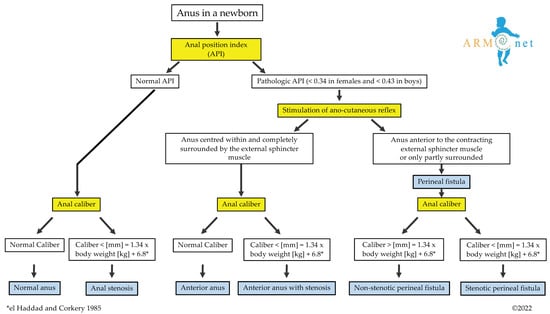
Figure 2.
The algorithm shows the diagnostic pathway that leads to diagnosing a normal anus, anterior anus with or without stenosis, congenital anal stenosis, and perineal fistula (stenotic or non-stenotic). * The anal caliber is measured according to the equation presented by el Haddad and Corkery [21].
How to Determine Whether the Position of the Anus Is Normal?
The normal position of an anus was formerly thought to lie in the midway between the vaginal fourchette and coccyx in girls and the scrotal crease and coccyx in boys [31,32]. Closer observation, using serial measurements, stated that in girls, the anus lies closer to the posterior labial commissure than the midpoint of the perineum [24,26,27,33,34,35,36,37,38,39].
In 1984, the Anal Position Index (API) was proposed by Reisner and colleagues to determine the position of the anal opening in the pelvic floor [38]. API is the ratio of the perineal length divided by the length of the complete posterior pelvic floor, that is, the distance of the fourchette/the scrotal-perineal junction to the center of the anus divided by the distance from the fourchette/scrotal-perineal junction to the tip of the coccyx in girls/boys, respectively (Figure 3).
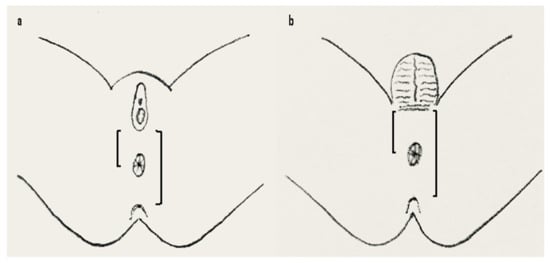
Figure 3.
API measurement in females (a) and males (b). API is calculated by dividing the distance between the fourchette (a) or scroto-perineal fold (b) to the center of the anus with the distance between fourchette (a) or scroto-perineal fold (b) to the tip of the coccyx marked on a transparent tape or flexible ruler. Anterior displacement is diagnosed when the API is <0.34 in females and <0.43 in boys (overall mean minus 2 standard deviations as presented by Sharma et al. [40]). API = anal position index.
The mean ratio in neonates was 0.44 ± 0.05 and 0.58 ± 0.06 in females and males, respectively, with significantly lower values in females compared to males [38]. Since the introduction of the API, many authors have proven the API is age and ethnicity-independent [24,26,27,28,33,34,35,36,37,39,40].
An AA was defined when the API results in two standard deviations (SD) below the calculated mean [38]. Using API as a diagnostic tool, an AA was diagnosed with an incidence of 24.6% in otherwise healthy boys and 43.4% of girls, respectively, wherefore it was stated as a common anal abnormality [24]. Conversely, Núñez-Ramos and colleagues investigated the API in more than 1000 newborns in two European hospitals. They reported a significantly lower incidence of AA (2.27–2.84% in females and 1.14–2.10% in males) [27], which corresponds to the expected statistical incidence of 2.28%, assuming a normal distribution. Based on a slight variation of mean and SD, different thresholds for diagnosis of AA can be extracted from data presented in the literature (Table 1) [24,27,33,34,35,36,37,38,39,41,42,43,44,45,46].

Table 1.
Results of the API-values and the criteria for anterior displacement according to published studies (adapted from Sharma et al. [40]). Additional API values presented in the literature have been included.
Akbiyik and Kutlu evaluated the external genital proportions in 205 pre-pubertal girls and proposed an equation to estimate the expected perineal length, that is 10.314 mm + (0.230 x kg body weight) [47]. The results of this equation are coherent with studies from adult females, where the mean perineal length was described as 25.6 ± 7.3 mm [48] and 21.3 ± 8.5 mm [49].
Besides an anterior position, a congenital lateral or posterior position of the anal opening has never been reported so far.
How to Determine if the Anal Opening Is Completely Surrounded by the Sphincter Muscle Complex?
Once the anterior position of the anus is confirmed by API, it is necessary to determine the correlation with the external sphincter in order to differentiate AA from a PF. As previously defined, a normal anus, AA, and CAS are completely surrounded by radiate cutaneous wrinkles that, instead, are lacking ventrally in a PF [24,30] (Figure 1c). To diagnose AA or CAS, the anus must lie in the center of the external sphincter. The ano-cutaneous reflex is evoked in lithotomy position with thighs flexed over the abdomen and the examiner checking whether the anus is fully surrounded by the external contracting sphincter [24,26,27,28,30]. This investigation can usually be done without sedation, by simple stimulation of the skin with a cotton swab. In inconclusive cases, electrostimulation in sedation or general anesthesia, avoiding muscle relaxation [50], is recommended to distinguish between these entities [25,29].
How to Determine the Caliber of the Anus in a Newborn?
The calibration of the anus is important to determine the diameter of a PF and rule out CAS. PF has, in most cases, a pathologically small anal caliber. CAS has normal API, complete sphincter encircling, and a diameter smaller than the expected caliber related to the patient’s body weight [21]. CAS is a much rarer form of ARM than PF but presents with similar clinical signs and functional prognosis [51,52].
The examiner’s little finger has been proposed to be the best probe to evaluate the elasticity of an anus in a newborn [21]. However, the little finger of an adult examiner is often too big; therefore, Hegar dilators, whose diameter is expressed in millimeters, should be used [21]. The anal caliber is defined by the Hegar that comfortably fits the anus without resistance and slightly whitens the skin of the anal verge without causing discomfort or pain in an awake neonate. Núñez-Ramos and colleagues reported a size between Hegar 8 and 10 in neonates, with a slight difference between females and males [26,27]. Irrespective of gender, it has been demonstrated that the anal caliber correlates with the body weight in newborns [21,26,27,28]. Thus, an equation has been presented to calculate the expected caliber of a normal anus related to the newborn’s body weight [21].
Equation of the expected anal caliber related to body weight [21]:
Caliber of the anus [mm] = 1.34 × body weight [kg] + 6.8
3.1.2. Further Diagnostic Modalities
Further diagnostic investigations to differentiate AA from PF can be considered if the diagnostic methods described above remain inconclusive. Transperineal ultrasound is a non-invasive diagnostic tool that allows evaluation of the anal position with clear visualization of the sphincter muscle complex [53,54,55]. Anorectal manometry has also been proposed to investigate the high-pressure zone of the lower anal canal and to evaluate ventral muscle deficiency [56,57]. Anal endosonography has been used to depict the anatomical integrity of the anal sphincters in children after surgical correction of ARM [58] but has not been used in newborns yet. External phased magnetic resonance imaging (MRI) may provide good information on the anal sphincters, rectum, and pelvic floor musculature [59,60] and has already been used in infants [61,62]. Although MRI may be performed in a feed and wrap technique when the baby is young, it usually requires general anesthesia in infants older than 3 months [63]. The transanal MRI has also been applied in children to assess the anal sphincters after surgery, but it has not yet been used as a preoperative diagnostic tool [64]. Finally, defecography may also be used to evaluate the anorectal angle (see paragraph below) in patients with chronic constipation [65], though it exposes a very young child to a considerable amount of radiation.
3.2. Clinical Aspects
What are the possible symptoms of AA, PF, and CAS?
3.2.1. Constipation
In literature, various definitions of constipation are used, and patients with AA, PF, or CAS are often pooled together, leading to unclear information on symptoms and outcomes. Indeed, the former literature on AA reported that this entity is commonly associated with constipation, wherefore surgical repair was often performed [31,66,67,68,69,70,71,72]. However, based on the definition of AA given here, a considerable part of these cases would be nowadays termed as “non-stenotic PF”, as their anus is not completely surrounded by the sphincter. In fact, no significant association of AA with constipation has been observed in several recent reports [24,33,36,37,73]. On the other hand, some authors reported a prevalence of chronic constipation as high as 47% in female and 35% in male AA patients aged 3 months to 12 years [26,27,28]. These patients were conservatively managed, even though the authors reported up to 31% of severe constipation in the very first month of life [26,27,28]. Another study on infants diagnosed with AA showed that constipation rose from 10% in the second month of life to 71.4% at 6 months of age [74].
There is a well-defined, conservatively treated cohort of patients from Finland described as “AA”, which, according to our definitions, also included patients with a non-stenotic PF (“mostly surrounded by sphincter“). When their anal caliber was less than Hegar 12, they were described as AA with concomitant mild AS and treated by anal dilation for 6–8 weeks until Hegar 14 was achieved [75,76]. On follow-up, these patients, older than 7 years, presented a rectoanal inhibitory reflex, anal resting, and squeeze pressures comparable to controls [77]. According to a long-term study, constipation affected 36% of patients versus 13% of a control population (p = 0.002); of note, this percentage tended to decline with age [75]. Fecal incontinence is not reported, neither in patients with AA nor in those with non-stenotic PF [75].
3.2.2. Urological and Gynecological Concerns
Because of the greater proximity of the anus to the urethra, a higher rate of urinary tract infections (UTI) in females with AA or non-stenotic PF may be anticipated. Nevertheless, several studies found an equal incidence of lower UTIs compared to controls [73,75]. The gynecologic assessment in post-menarchal girls with AA demonstrated a mean vaginal length of 52 mm (SD: 0.24), comparable with normal reference values for age [78], normal perineal tropism, and normal perineal and vaginal flora [73]. As the study by Duci and colleagues also included patients with non-stenotic PF in their so-called “AA”-population [73], these outcomes favor non-operative management of patients with AA or non-stenotic PF.
In case of pregnancy in these conservatively treated females, the mode of delivery should be individually discussed with the patients in advance. Although vaginal delivery has been reported in women with a history of ARM, such as rectovestibular or rectoperineal fistula, it has been recommended to evaluate the adequacy of the perineal body case by case [79]. By others, cesarean section (CS) has been suggested as the best delivery mode for all patients with AA/PF to avoid perineal tears and obstetric anal sphincter injury (OASI) [8,75]. Ness has recently reported that women in their first labor are at risk for OASI if the perineal length is less than 30 mm [80]. Another study also stated that women with a low API (<0.42) or a short perineum (<40 mm) were prone to traumatic vaginal delivery in primigravidae, with higher rates of episiotomy and instrumented delivery as well as perineal tears [44]. Eventually, OASI can cause burdensome short- and long-term morbidity, affecting the women’s quality of life due to pain, fecal and/or urinary incontinence, and sexual dysfunction. Therefore, CS in patients with either AA or ARM is most likely highly recommended, although each pregnant woman should be individually counseled and the risk factors and benefits of each mode of delivery outlined.
3.3. Management
Treatment Recommendations for Constipation in Patients with AA, Non-Stenotic PF, or CAS?
In former times, patients with AA were sometimes treated surgically for constipation, but at present, primary conservative management is advocated. Conservative management approaches include stool softeners, laxatives, and transanal bowel management with suppositories or enemas [28]. Based on the long-term outcome of the presented literature, patients with a non-stenotic PF may also be primarily treated by the same conservative management approaches as mentioned for patients with AA [81]. Simple dilations have also been reported as successful in patients with mild stenosis of a PF [13]. Nevertheless, patients with AA or a non-stenotic PF are at risk of developing a rectal cul-de-sac and therefore need to be closely followed to avoid complications later in life.
The mechanism for developing a rectal cul-de-sac was attributed to the incomplete straightening of the rectoanal angle at defecation in case of a ventral malposition of the bowel opening. The descending feces push against the dorsal wall of the anorectal junction, which bulges out, eventually resulting in a dorsal bag or “cul-de-sac”, which might make complete defecation nearly impossible [28,56,67]. Thus, in case of refractory constipation to medical treatment, these patients may eventually benefit from surgical correction [67,70]. Surgical techniques aim to eliminate the cul-de-sac and align the anal canal with the rectum [56,62]. To address that, various techniques such as posterior anoplasty [69,70,71], posterior anoplasty with the complete division of the external sphincter fibers [31], anal transposition [56,57], cutback procedures [66,81,82,83], as well as PSARP [62,84] have been reported in the literature. If surgery is indicated, surgical techniques should aim to preserve the native mechanism of continence, as advocated for any mild form of ARMs [13].
Congenital anal stenosis (CAS) in an orthotopic anus is usually treated by serial anal dilatations, but surgery is required when a median bar, complete membrane, or severe forms are present [13,28]. The rare form of a funnel anus, commonly associated with Currarino syndrome, is characterized by a skin-lined deep anal funnel and the stenotic anal skin-rectum junction, which may also be treated by serial anal dilations [13,85].
In the case of Hegar-dilations, it is paramount that painful dilations are avoided, as they can cause dysfunctional defecation, constipation, and overflow incontinence later in life [86,87].
3.4. Genetical Concerns
Is Genetic Analysis Warranted in Patients with AA, PF, or CAS?
Patients with a mild ARM (or any other congenital disability) are prone to display additional congenital anomalies. Therefore, every ARM patient should undergo a thorough clinical examination and so-called VACTERL-screening (search for vertebral, cardiac, trachea-esophageal, renal, and limb malformations). Apart from the diagnostic work-up, it is also important to investigate the patient’s family history. Heritability has been reported for patients with either a vestibular or perineal fistula [88]. Concerning patients with AA, Duci and colleagues reported familial occurrence in 5/50 patients of their “AA” population [73]. It has also been reported that risk factors for AA are female gender, high maternal age, and later birth order [74].
Patients with “AA” have also been described as suffering from other congenital malformations or syndromes. However, under the present definitions, it may be these patients were not actually born with a non-stenotic PF. Also, the association of “AA” and perineal groove has been reported [13]. Indeed, Figure 4 shows a patient with “AA”, Pierre-Robin syndrome, and perineal groove.
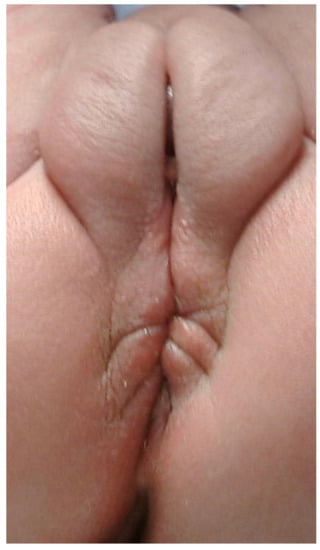
Figure 4.
Picture of a female newborn with AA and a perineal groove. The patient also has Pierre-Robin syndrome.
“AA” was also detected in patients with esophageal atresia, cardiac anomalies, Di-George Syndrome, and Down syndrome [73]. Moreover, a female patient with “AA” was reported to present a disorder of sex differentiation with an accessory phallus, an accessory phallic urethra, and a perineal lipoma [89]. Female patients diagnosed with “ectopic anus”, Hirschsprung disease, and Currarino syndrome at the age of 25 years [90] or affected by X-linked Opitz G/BBB syndrome [91] or Baller-Gerold syndrome [92,93] were also described. An “anterior ectopic anus” was also seen with a rectourethral fistula and a fusiform megalourethra as features of the abdominal muscle deficiency syndrome [94], and in another patient with complete duplication of the bladder, urethra, uterus, and vagina [95]. It was also documented in a female patient with partial trisomy 11q syndrome and deletion 1q44 syndrome [96], in another female with partial monosomy 9p and partial trisomy 18q and stenotic anal opening [97], and in combination with polythelia [98]. The occurrence of propionic acidemia in three siblings with “ectopic anus” in one and a PF in a second sibling from consanguineous parents was suggested for an autosomal recessive genetic inheritance [99]. “AA” was also documented as a feature of an unknown mandibulofacial dysostosis syndrome occurring with duodenal and biliary atresia associated with facial, thyroid, and auditory apparatus abnormalities [100]. Furthermore, “AA” was observed in patients with Juberg-Hayward syndrome [101]. In combination with the cardinal manifestations of aplasia cutis and epibulbar dermoid, “AA” was mentioned with other features such as laryngomalacia, microcephaly, and significant developmental delay to describe an oculo-ectodermal syndrome with possibly recessive inheritance [102].
These examples highlight the importance of investigating the family history and performing a physical examination and VACTERL-screening of patients with mild ARM, but also with mere AA, to not miss possible syndromes or other congenital anomalies. However, because of the noxious side effects of radiation, especially in children, radiologic examinations should be only performed upon abnormal clinical or ultrasound/MRI findings. The concept that AA might display an increased risk for associated malformations or if this current assumption is based on biased data from the past when AA and non-stenotic PF-patients were not differentiated from each other requires further studies.
4. Discussion
The term “ectopic anus” was first used in 1958 to describe an anal opening anterior to the external sphincter [66]. A full twenty years later, Hendren reported on patients with an “anterior anal opening” treated with anoplasty for persistent constipation [69].
According to the present knowledge, an anteriorly located anus (AA) is defined as a normal variant of an anus located more anteriorly along the perineal body with a normal caliber, completely surrounded by the anal sphincter complex [24,25,26,27,28,29]. Yet, the occurrence of AA in patients with a genetically recognized syndrome, as well as the observed rate of stenosis of AA [13,28,75], may suggest that AA should be considered a mild form of ARM. The terms “anterior anus”, “anterior displacement of the anus”, ”anteriorly displaced anus”, “anteposition of the anus”, “anterior ectopic anus”, ”ectopic anus”, “anterior perineal anus”, or “anus perinei ventralis” have been variably used to describe either patients with AA according to the present definition, or patients with a bowel opening not completely surrounded by the sphincter muscle complex, which we propose to term consistently as PF [13,24,25,26,27,28,29,30,31,33,34,35,36,37,38,39,41,44,45,46,53,55,56,57,61,62,66,67,68,69,70,71,72,73,74,75,77,82,83,84,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110]. Because of this unclear terminology, management and outcome parameters could not be reliably compared.
An anterior position of an anus is diagnosed when the API is 2 SD below the calculated mean [38,40]. However, the calculations of mean and SD are based on the concept of a normal distribution, but in physiological conditions, a skewed distribution more often applies. Percentile values would be more helpful, and thus, future studies on patients with AA or API measurements should also report non-parametric values of the API besides mean and SD, to reduce this bias. The reliability of the API measurements performed by different investigators has not been evaluated yet. By comparing the measurements of different investigators in our clinics, we identified this as a possible point of concern. Therefore, it will be the objective of a future ARM-Net study.
We consider AA a normal anatomic variant of the anus with a normal anal canal. Recent long-term outcomes proving normal bowel control, normal frequency of lower urinary tract symptoms, as well as normal perineal tropism and perineal and vaginal colonization, all favor non-operative management of the affected children. In this context, we fully respect the groundbreaking work from the Helsinki group concerning the conservative treatment of patients with mild forms of ARM, even though patients with the anus not completely surrounded by the external sphincter muscle were included in their “AA” group [75]. If uniform definitions are used, it will be possible to reliably compare the series of patients from different centers in the future.
Procedures, such as anal dilations, are not required for AA unless stenosis is encountered, but might be of benefit in the case of CAS [13,75]. Painful dilations must be avoided, as they can cause dysfunctional defecation, constipation, and overflow incontinence later in life [86,87].
According to present knowledge, the “fistula” in ARM represents an ectopic anal canal and should be preserved as far as possible to improve the chance for fecal continence [13,77,81,111].
AA may be a hint to investigate for suspected syndromes. In families with ARM-affected members, AA may signify a possible genetic involvement. It is unknown whether AA, according to the present definition, may be associated with other congenital anomalies. Therefore, a thorough physical examination and VACTERL-screening are suggested in every child with AA as in any other ARM patient. Future studies will reveal whether children with AA may present a greater risk for other congenital anomalies or if the management can be limited to an accurate physical examination.
Environmental factors also play a role in the etiology of AA. Prenatal phthalate metabolite exposure was documented to reduce the anogenital distance in newborns with unknown consequences [112,113]. Exposure to the pesticide dichlorodiphenyltrichloroethane (DDT) metabolite 1,1-dichloro-2,2-bis (p-chlorophenyl) ethylene (DDE) during the first trimester of pregnancy has already been associated with a significant reduction of the API in boys (ß = −0.02; p = 0.02) [45]. However, a more recent study could not confirm an association between maternal DDT and DDE isomers and the anogenital distance between boys and girls at birth but noted a significant association between the maternal serum concentration of o,p’-DDE, the breakdown product of 1,1,1-trichloro-2-(2-chlorophenyl)-2-(4-chlorophenyl) ethane (o,p’-DDT) at delivery with a shorter ano-fourchette distance at 1-year of age [42]. These environmental factors might cause a greater variability of API measurements than expected [114,115].
Children with AA have been reported to experience normal bowel control and a normal stooling pattern. In some cases, however, defecation disorders may develop in certain patients. Indeed, AA was often seen as the cause of chronic constipation in many patients who presented with a large cul-de-sac and were candidates for surgical management. However, cases of AA and PF are not clearly distinguished from each other in most of the papers, leading to pooled therapeutic results. Conservative management such as diet, stool softeners, laxatives, and rectal irrigation should always be the first-line treatment option in a child with AA who develops signs of constipation. As the onset of constipation has been reported in patients with AA already during infancy, we advise closely following children with AA in their first year of life. According to a long-term study by Kyrklund and colleagues, constipation declines with age and can be successfully managed by medical treatment [75]. Both Kyrklund and Duci, who included non-stenotic PF-patients with an anal opening mostly surrounded by sphincter in their AA population, proved that these patients have a similar satisfactory long-term outcome regarding bowel function under conservative management [73,75].
Hence, we suggest this patient group should be conservatively managed, except (i) in case of a stenotic PF (we recommend Hegar ≤ 8 in a term newborn), (ii) in case of a PF surrounded by the sphincter less than 75% of the anal circumference, or (iii) in patients with AA or non-stenotic PF who develop refractory constipation on conservative management.
Until now, no studies compare obstetric complications in mothers with conservatively versus surgically treated PF, outlining the need for individual counseling concerning the mode of delivery. Future studies addressing the mode of delivery are mandatory. They might change the recommendations given here for CS in women with a history of ARM and the conservative treatment of female patients with non-stenotic PF or AA.
5. Conclusions
We seek to clarify the differentiation between anatomic variants and mild forms of ARM (PF, CAS) by providing a clinical decision tool to determine which condition is present in an abnormally looking anus at birth. Part of the presented algorithm is the proposal of a binding terminology for AA and PF. This common language is a prerequisite for further research comparing the results of different treatments, namely conservative and surgical, and for better defining the best way to manage the affected children.
Author Contributions
E.S. conceived the idea. Conceptualization and methodology were performed by E.S. and E.E.A., E.S., I.S., C.E.J.S., E.J.; P.M. performed the literature search and discussed the topic. E.E.A. and E.S. drafted the diagnostic algorithm. E.S. established and updated Table 1. E.E.A. wrote the first draft of the manuscript. P.M. and I.S. provided the pictures of the patients. E.J. and I.A.L.M.v.R. performed validation and formal analysis. All authors contributed to the final version of the manuscript through reviewing and editing. The entire ARM-Net Consortium has read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were not applicable since this review is descriptive and did not use human data. Parental approval was given for publishing pictures of their child according to ethical approval from the local institutions.
Informed Consent Statement
Parental informed consent was obtained to publish pictures. Otherwise, it was not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The members of the working group “AA and PF” of the ARM-Net Consortium would like to thank Carlo Marcelis, clinical geneticist Radboudumc Nijmegen, The Netherlands, for giving his opinion on AA, syndromology, and genetic association. Furthermore, we thank all participating pediatric surgeons for their contribution in reading the paper and disclosing their opinions about the proposed definitions and diagnostic algorithm. This helped us achieve a uniform consensus on the definition of AA, stenotic and non-stenotic PF, and CAS. Members of the ARM-Net consortium not mentioned as coauthors by person: Dalia Aminoff, AIMAR, patient organization, Italy; Piero Bagolan and Barbara Iacobelli, Ospedale Bambino Gesù, Rome, Italy; Ivo de Blaauw and Herjan van der Steeg, Dept. of Surgery-Pediatric Surgery, Radboudumc Nijmegen, The Netherlands; Paul Broens, University Medical Center Groningen, The Netherlands; Hakan Çavuşoğlu, Gazi University, Faculty of Medicine, Ankara, Turkey; Stefan Deluggi and Johanna Ludwiczek, Kepler University Hospital GmbH, Linz, Austria; Emre Divarci, Department of Paediatric Surgery at the Ege UniversityMedical School, İzmir, Turkey; María Fanjul, Hospital Gregorio Marañón, Madrid, Spain; Francesco Fascetti-Leon, Pediatric Surgery Unit, University of Padua, Italy; Araceli García Vázquez, Hospital 12 de Octubre Madrid, Spain; Carlos Giné, Hospital Vall d’Hebron, Barcelona, Spain; Stefano Giuliani, Great Ormond Street Hospital, London, United Kingdom; Ramon Gorter, Amsterdam University Medical Centers, The Netherlands; Jan Goseman and Martin Lacher, University Hospital Leipzig, Germany; Caterina Grano, Sapienza University of Rome, Italy; Sabine Grasshoff-Derr, Buergerhospital and Clementine Childrens Hospital, Frankfurt, Germany; Stefan Holland-Cunz, University Children’s Hospital, Basel, Switzerland; Justin de Jong, Pediatric Surgical Center of Amsterdam, Amsterdam, The Netherlands; Ernesto Leva and Anna Morandi, Dept. of Pediatric Surgery, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milano, Italy; Gabriele Lisi, Santo Spirito Civil Hospital, Pescara, Italy; Igor Makedonsky, Children’s Hospital Dnepropetrovsk, Ukraine; Carlo Marcelis, Dept. Human Genetics, Radboudumc, Nijmegen, The Netherlands; Marc Miserez, Department of Abdominal Surgery at the University Hospitals of the Katholieke Universiteit Leuven, Belgium; Mazeena Mohideen, SoMA Austria, patient organization, Austria; Onur Ozen, Dept. Pediatric Surgery, Gazi University, Faculty of Medicine, Ankara, Turkey; Alessio Pini Prato, Azienda Ospedaliera Nazionale SS A. e B. e Cesare Arrigo, Italy; Carlos Reck-Burneo, Medical University Wien, Austria; Heiko Reutter, University of Bonn, Germany; Stephan Rohleder, Medical University Hospital, Mainz, Germany; Nicole Schwarzer, SoMA e.V., patient organization, Germany; Pernilla Stenström, Lund University, Skane University Hospital, Lund, Sweden; Holger Till, Dept. of Pediatric and Adolescent Surgery, Medical University of Graz, Austria; Chris Verhaak, Amalia Children’s Hospital, Radboudumc Nijmegen, The Netherlands; Alejandra Vilanova-Sánchez, University Hospital La Paz, Madrid, Spain; Patrick Volk, Dept. Surgery, University Hospital Heidelberg, Heidelberg, Germany; Marieke Witvliet, Wilhelmina Children’s Hospital, Utrecht, The Netherlands.
Conflicts of Interest
The authors, all members of the ARM-Net Consortium, declare no conflict of interest.
References
- Eltayeb, A.A. Delayed presentation of anorectal malformations: The possible associated morbidity and mortality. Pediatr. Surg. Int. 2010, 26, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Gow, K.W.; Penner, J.G.; Blair, G.K.; Murphy, J.J.; Webber, E.M. Presentation of low anorectal malformations beyond the neonatal period. Pediatrics 2000, 105, E68. [Google Scholar] [CrossRef] [PubMed]
- Turowski, C.; Dingemann, J.; Gillick, J. Delayed diagnosis of imperforate anus: An unacceptable morbidity. Pediatr. Surg. Int. 2010, 26, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Demirogullari, B.; Ozen, I.O.; Karabulut, R.; Turkyilmaz, Z.; Sonmez, K.; Kale, N.; Basaklar, A.C. Colonic motility and functional assessment of the patients with anorectal malformations according to Krickenbeck consensus. J. Pediatr. Surg. 2008, 43, 1839–1843. [Google Scholar] [CrossRef]
- Grano, C.; Fernandes, M.; Bucci, S.; Aminoff, D.; Lucidi, F.; Violani, C. Self-efficacy beliefs, faecal incontinence and health-related quality of life in patients born with anorectal malformations. Colorectal Dis. 2018, 20, 711–718. [Google Scholar] [CrossRef]
- Rajindrajith, S.; Devanarayana, N.M.; Crispus Perera, B.J.; Benninga, M.A. Childhood constipation as an emerging public health problem. World J. Gastroenterol. 2016, 22, 6864–6875. [Google Scholar] [CrossRef]
- Stenström, P.; Sandelin, H.; Emblem, R.; Björnland, K. Lower urinary tract symptoms in children with anorectal malformations with rectoperineal fistulas. J. Pediatr. Surg. 2016, 51, 1234–1240. [Google Scholar] [CrossRef]
- Stenström, P.; Hambraeus, M.; Arnbjörnsson, E.; Örnö, A.K. Pelvic floor in females with anorectal malformations-findings on perineal ultrasonography and aspects of delivery mode. J. Pediatr. Surg. 2015, 50, 622–629. [Google Scholar] [CrossRef]
- Lindley, R.M.; Shawis, R.N.; Roberts, J.P. Delays in the diagnosis of anorectal malformations are common and significantly increase serious early complications. Acta Paediatr. 2006, 95, 364–368. [Google Scholar] [CrossRef]
- Wilson, B.E.; Etheridge, C.E.; Soundappan, S.V.; Holland, A.J. Delayed diagnosis of anorectal malformations: Are current guidelines sufficient? J. Paediatr. Child Health. 2010, 46, 268–272. [Google Scholar] [CrossRef]
- Haider, N.; Fisher, R. Mortality and morbidity associated with late diagnosis of anorectal malformations in children. Surgeon 2007, 5, 327–330. [Google Scholar] [CrossRef]
- De Blaauw, I.; Midrio, P.; Breech, L.; Bischoff, A.; Dickie, B.; Versteegh, H.P.; Peña, A.; Levitt, M.A. Treatment of adults with unrecognized or inadequately repaired anorectal malformations: 17 cases of rectovestibular and rectoperineal fistulas. J. Pediatr. Adolesc. Gynecol. 2013, 26, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Pakarinen, M.P.; Rintala, R.J. Management and outcome of low anorectal malformations. Pediatr. Surg. Int. 2010, 26, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Lawal, T.A. Overview of Anorectal Malformations in Africa. Front. Surg. 2019, 5, 7. [Google Scholar] [CrossRef]
- Vd Merwe, E.; Cox, S.; Numanoglu, A. Anorectal malformations, associated congenital anomalies and their investigation in a South African setting. Pediatr. Surg. Int. 2017, 33, 875–882. [Google Scholar] [CrossRef]
- Aldeiri, B.; Davidson, J.R.; Eaton, S.; Coletta, R.; Cardoso Almeida, A.; Long, A.M.; Knight, M.; Cross, K.M.; Chouikh, T.; Iacobelli, B.D.; et al. Variations in the Detection of Anorectal Anomalies at Birth among European Cities. Eur. J. Pediatr. Surg. 2020, 30, 287–292. [Google Scholar] [CrossRef]
- Jonker, J.E.; Trzpis, M.; Broens, P.M.A. Underdiagnosis of Mild Congenital Anorectal Malformations. J. Pediatr. 2017, 186, 101–104. [Google Scholar] [CrossRef]
- Tareen, F.; Coyle, D.; Aworanti, O.M.; Gillick, J. Delayed diagnosis of anorectal malformation—A persistent problem. Ir. Med. J. 2013, 106, 238–240. [Google Scholar]
- Wilson, B.E.; Holland, A.J. Comment on Turowski et al.: Delayed diagnosis of imperforate anus: An unacceptable morbidity. Pediatr. Surg. Int. 2011, 27, 443–444. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Committee on Fetus and Newborn. Hospital stay for healthy term newborns. Pediatrics 2010, 125, 405–409. [Google Scholar] [CrossRef]
- El Haddad, M.; Corkery, J.J. The anus in the newborn. Pediatrics 1985, 76, 927–928. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.D.; Ludwig, K.A. Neonatal colon perforation due to anorectal malformations: Can it be avoided? World J. Gastroenterol. 2013, 19, 3915–3917. [Google Scholar] [CrossRef] [PubMed]
- De Blaauw, I.; Wijers, C.H.; Schmiedeke, E.; Holland-Cunz, S.; Gamba, P.; Marcelis, C.L.; Reutter, H.; Aminoff, D.; Schipper, M.; Schwarzer, N.; et al. First results of a European multi-center registry of patients with anorectal malformations. J. Pediatr. Surg. 2013, 48, 2530–2535. [Google Scholar] [CrossRef] [PubMed]
- Herek, O.; Polat, A. Incidence of anterior displacement of the anus and its relationship to constipation in children. Surg. Today 2004, 34, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Iwai, J.; Takahashi, H.; Maie, M.; Ohnuma, N.; Etoh, T.; Tanabe, M.; Aoyagi, H.; Shinbo, K. Diagnosis of anterior perineal anus (APA)—Significance of electromyographic evaluation of the external anal sphincter location. Nihon Heikatsukin Gakkai Zasshi 1988, 24, 193–203. (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- Núñez-Ramos, R.; González-Velasco, M.; Núñez Núñez, R.; Enriquez Zarabozo, E.; Vargas Muñoz, I.; Blesa Sánchez, E. Valoración de la posición del ano en recién nacidos y en niños con estreñimiento crónico. Incidencia del ano anterior ectópico Evaluation of the anal position in newborns and children with chronic constipation. Incidence of anterior ectopic anus. Cir. Pediatr. 2011, 24, 84–89. [Google Scholar] [PubMed]
- Núñez-Ramos, R.; Fabbro, M.A.; González-Velasco, M.; Núñez Núñez, R.; Romanato, B.; Vecchiato, L.; D’Agostino, S.; Blesa Sánchez, E. Determination of the anal position in newborns and in children with chronic constipation: Comparative study in two European healthcare centres. Pediatr. Surg. Int. 2011, 27, 1111–1115. [Google Scholar] [CrossRef]
- Núñez-Ramos, R.; Fabbro, M.A.; González-Velasco, M.; Vargas Muñoz, I.; Núñez Núñez, R. The anal position index and the anal caliber in the newborn: Anterior ectopic anus and constipation. In Constipation in Children: Diagnosis and Treatment; Núñez, R., Fabbro, M.A., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 151–165. [Google Scholar]
- Peña, A. Comments on anterior ectopic anus. Pediatr. Surg. Int. 2004, 20, 902. [Google Scholar] [CrossRef]
- Herek, O. Anterior ectopic anus: An accurate definitive term for choice of treatment? Pediatr. Surg. Int. 2001, 17, 501. [Google Scholar] [CrossRef]
- Leape, L.L.; Ramenofsky, M.L. Anterior ectopic anus: A common cause of constipation in children. J. Pediatr. Surg. 1978, 13, 627–630. [Google Scholar] [CrossRef]
- Skandalakis, J.E.; Kingsnorth, A.N.; Colborn, G.L.; Weidman, T.A. Large intestine and anorectum. In Skandalakis’s Surgical Anatomy: The Embryology and Anatomy Basis of Modern Surgery; Skandalakis, J.E., Colborn, G.L., Weidman, T.A., Eds.; Paschalidis Medical Publications: Athens, Greece, 2004; pp. 899–914. [Google Scholar]
- Bar-Maor, J.A.; Eitan, A. Determination of the normal position of the anus (with reference to idiopathic constipation). J. Pediatr. Gastroenterol. Nutr. 1987, 6, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.T.; Lee, H.C.; Wang, W.N.; Yeung, C.Y.; Jiang, C.B. Determination of the normal position of the anus in Taiwanese infants. Pediatr. Neonatol. 2009, 50, 158–161. [Google Scholar] [CrossRef]
- Davari, H.A.; Hosseinpour, M. The anal position index: A simple method to define the normal position of the anus in neonate. Acta Paediatr. 2006, 95, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Genç, A.; Taneli, C.; Tansuğ, N.; Kasirga, E.; Yilmaz, D.; Küçükoğlu, T.; Onağ, A. Evaluation of the location of the anus by a modified technique in the neonate. J. Pediatr. Surg. 2002, 37, 80–82. [Google Scholar] [CrossRef]
- Mohta, A.; Goel, M.R. Determination of anal position index. Indian Pediatr. 2004, 41, 91–92. [Google Scholar]
- Reisner, S.H.; Sivan, Y.; Nitzan, M.; Merlob, P. Determination of anterior displacement of the anus in newborn infants and children. Pediatrics 1984, 73, 216–217. [Google Scholar] [CrossRef]
- Rerksuppaphol, S.; Rerksuppaphol, L. Normal anal position index in Thai newborns. J. Med. Assoc. Thai. 2008, 91, 1839–1844. [Google Scholar]
- Sharma, S.; Perumal, V.; Sharma, K.; Gupta, D.K. Varied parameters and utility of the anal position index: A systematic review and meta-analysis. Pediatr. Surg. Int. 2021, 37, 469–477. [Google Scholar] [CrossRef]
- Alemrajabi, M.; Moradi, M.; Jahangiri, F.; Marzbali, F. Anal position index; can it predict pelvic organ disorders in adults? J. Coloproctol. 2019, 39, 237–241. [Google Scholar] [CrossRef]
- Bornman, M.S.; Chevrier, J.; Rauch, S.; Crause, M.; Obida, M.; Sathyanarayana, S.; Barr, D.B.; Eskenazi, B. Dichlorodiphenyltrichloroethane exposure and anogenital distance in the Venda Health Examination of Mothers, Babies and their Environment (VHEMBE) birth cohort study, South Africa. Andrology 2016, 4, 608–615. [Google Scholar] [CrossRef]
- Patel, J.N.; Kumar, A.; Yadav, P.S.; Chadha, R.; Datta, V.; Roy Choudhury, S. The position of the anal dimple in newborns and infants with anorectal malformations and its correlation with the normal anal position. J. Pediatr. Surg. 2018, 53, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Rizk, D.E.; Thomas, L. Relationship between the length of the perineum and position of the anus and vaginal delivery in primigravidae. Int. Urogynecol. J. Pelvic. Floor Dysfunct. 2000, 11, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sanchez, L.; Zepeda, M.; Cebrián, M.E.; Belkind-Gerson, J.; Garcia-Hernandez, R.M.; Belkind-Valdovinos, U.; López-Carrillo, L. Dichlorodiphenyldichloroethylene exposure during the first trimester of pregnancy alters the anal position in male infants. Ann. N.Y. Acad. Sci. 2008, 1140, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tufekci, S.; Yesildag, E. Determination of the Normal Anal Location in Neonates: A Prospective Cross-Sectional Study. Med. Bull Haseki 2021, 59, 330–334. [Google Scholar] [CrossRef]
- Akbiyik, F.; Kutlu, A.O. External genital proportions in pre-pubertal girls: A morphometric reference for female genitoplasty. J. Urol. 2010, 184, 1476–1481. [Google Scholar] [CrossRef]
- Ellibeş Kaya, A.; Doğan, O.; Yassa, M.; Başbuğ, A.; Özcan, C.; Çalışkan, E. Do external female genital measurements affect genital perception and sexual function and orgasm? Turk. J. Obstet. Gynecol. 2020, 17, 175–181. [Google Scholar] [CrossRef]
- Kreklau, A.; Vâz, I.; Oehme, F.; Strub, F.; Brechbühl, R.; Christmann, C.; Günthert, A. Measurements of a ‘normal vulva’ in women aged 15–84: A cross-sectional prospective single-centre study. BJOG 2018, 125, 1656–1661. [Google Scholar] [CrossRef]
- Mixa, V.; Skába, R.; Kraus, J.; Cvachovec, K. Influence of anesthesia on the results of intraoperative diagnostic electromyostimulation in patients with anorectal malformation. J. Pediatr. Surg. 2011, 46, 2135–2139. [Google Scholar] [CrossRef]
- Chatterjee, S.K. Rare/Regional Variants. In Anorectal Malformations in Children. Embriology, Diagnosis, Surgical Treatment, Follow-Up; Holschneider, A.M., Hutson, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 252–262. [Google Scholar]
- Holschneider, A.; Hutson, J.; Peña, A.; Beket, E.; Chatterjee, S.; Coran, A.; Davies, M.; Georgeson, K.; Grosfeld, J.; Gupta, D.; et al. Preliminary report on the International Conference for the Development of Standards for the Treatment of Anorectal Malformations. J. Pediatr. Surg. 2005, 40, 1521–1526. [Google Scholar] [CrossRef]
- Bruzeau, A.H.; Moriau, D.; Bahans, C.; Mounayer, C.; Spampinato, G.; Guigonis, V.; Ballouhey, Q.; Fourcade, L. Perineal ultrasound in infants with anteriorly displaced anus: A new decision-making tool for the surgeon? Eur. J. Radiol. 2021, 142, 109854. [Google Scholar] [CrossRef]
- Casson Masselin, M.; Moriau, D.; Bahans, C.; Spampinato, G.; Guigonis, V.; Ballouhey, Q.; Fourcade, L. Transperineal ultrasound to assess anal positioning in female neonates. Med. Ultrason. 2021, 23, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Haber, H.P.; Warmann, S.W.; Fuchs, J. Transperineal sonography of the anal sphincter complex in neonates and infants: Differentiation of anteriorly displaced anus from low-type imperforate anus with perineal fistula. Ultraschall Med. 2008, 29, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Kerremans, R.P.; Pennickx, F.M.; Beckers, J.P. Functional evaluation of ectopic anus and its surgical consequences. Am. J. Dis. Child. 1974, 128, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Schuster, T.; Joppich, I.; Schneider, K.; Jobst, G. A computerised vector manometry study of the so-called ectopic anus. Pediatr. Surg. Int. 2000, 16, 8–14. [Google Scholar] [CrossRef]
- Keshtgar, A.S.; Athanasakos, E.; Clayden, G.S.; Ward, H.C. Evaluation of outcome of anorectal anomaly in childhood: The role of anorectal manometry and endosonography. Pediatr. Surg. Int. 2008, 24, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Bates, N. Magnetic resonance imaging anatomy of the anal canal. Australas. Radiol. 2004, 48, 443–449. [Google Scholar] [CrossRef]
- Schäfer, A.; Enck, P.; Fürst, G.; Kahn, T.; Frieling, T.; Lübke, H.J. Anatomy of the anal sphincters. Comparison of anal endosonography to magnetic resonance imaging. Dis. Colon. Rectum. 1994, 37, 777–781. [Google Scholar] [CrossRef]
- AbouZeid, A.A.; Mohammad, S.A.; Khairy, K.T. MRI anatomy of anteriorly displaced anus: What obstructs defecation? Pediatr. Radiol. 2014, 44, 831–838. [Google Scholar] [CrossRef]
- Thambidorai, C.R.; Raghu, R.; Zulfiqar, A. Magnetic resonance imaging in anterior ectopic anus. Pediatr. Surg. Int. 2008, 24, 161–165. [Google Scholar] [CrossRef]
- Antonov, N.K.; Ruzal-Shapiro, C.B.; Morel, K.D.; Millar, W.S.; Kashyap, S.; Lauren, C.T.; Garzon, M.C. Feed and Wrap MRI Technique in Infants. Clin. Pediatr. 2017, 56, 1095–1103. [Google Scholar] [CrossRef]
- De Souza, N.M.; Ward, H.C.; Williams, A.D.; Battin, M.; Harris, D.N.; McIver, D.K. Transanal MR imaging after repair of anorectal anomalies in children: Appearances in pull-through versus posterior sagittal reconstructions. AJR Am. J. Roentgenol. 1999, 173, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Piloni, V.; Fioravanti, P.; Spazzafumo, L.; Rossi, B. Measurement of the anorectal angle by defecography for the diagnosis of fecal incontinence. Int. J. Colorectal Dis. 1999, 14, 131–135. [Google Scholar] [CrossRef]
- Bill, A.H., Jr.; Johnson, R.J.; Foster, R.A. Anteriorly placed rectal opening in the perineum ectopic anus; a report of 30 cases. Ann. Surg. 1958, 147, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Ascione, G.; Tamburrini, O.; Settimi, A. Antéposition de l’anus. Cause de constipation chez l’enfant [Anteposition of the anus. A cause of constipation in children]. Chir. Pediatr. 1985, 26, 279–281. [Google Scholar] [PubMed]
- Fukunaga, K.; Kimura, K.; Lawrence, J.P.; Soper, R.T.; Phearman, L.A.; Loening-Baucke, V. Anteriorly located anus: Is constipation caused by abnormal location of the anus? J. Pediatr. Surg. 1996, 31, 245–246. [Google Scholar] [CrossRef]
- Hendren, W.H. Constipation caused by anterior location of the anus and its surgical correction. J. Pediatr. Surg. 1978, 13, 505–512. [Google Scholar] [CrossRef]
- Ishitani, M.B.; Rodgers, B.M. Anteriorly displaced anus: An under-recognized cause of chronic constipation. Pediatr. Surg. Int. 1991, 6, 217–220. [Google Scholar] [CrossRef]
- Tuggle, D.W.; Perkins, T.A.; Tunell, W.P.; Smith, E.I. Operative treatment of anterior ectopic anus: The efficacy and influence of age on results. J. Pediatr. Surg. 1990, 25, 996–997; discussion 997–998. [Google Scholar] [CrossRef]
- Upadhyaya, P. Mid-anal sphincteric malformation, cause of constipation in anterior perineal anus. J. Pediatr. Surg. 1984, 19, 183–186. [Google Scholar] [CrossRef]
- Duci, M.; Fascetti-Leon, F.; Bogana, G.; Gamba, P.; Midrio, P. Conservative management of anterior located anus: A medium-long term follow up. J. Pediatr. Surg. 2021, 56, 2277–2280. [Google Scholar] [CrossRef]
- Rerksuppaphol, S.; Rerksuppaphol, L. Anterior displacement of anus: A common association with constipation in infancy. Asian Biomed. 2010, 4, 595–601. [Google Scholar] [CrossRef]
- Kyrklund, K.; Pakarinen, M.P.; Taskinen, S.; Rintala, R.J. Bowel function and lower urinary tract symptoms in females with anterior anus treated conservatively: Controlled outcomes into adulthood. J. Pediatr. Surg. 2015, 50, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Rintala, R.J. Congenital Anorectal Malformations. In Pediatric Surgery, 2nd ed.; Burge, D.M., Griffiths, M.D., Steinbrecher, H.A., Wheeler, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 155–166. [Google Scholar]
- Kyrklund, K.; Pakarinen, M.P.; Rintala, R.J. Manometric findings in relation to functional outcomes in different types of anorectal malformations. J. Pediatr. Surg. 2017, 52, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Krantz, K.E.; Atkinson, J.P. Pediatric and adolescent gynecology. I. Fundamental considerations. Gross anatomy. Ann. N.Y. Acad. Sci. 1967, 142, 551–575. [Google Scholar] [CrossRef]
- Breech, L. Gynecologic concerns in patients with anorectal malformations. Semin. Pediatr. Surg. 2010, 19, 139–145. [Google Scholar] [CrossRef]
- Ness, W. Obstetric anal sphincter injury: Causes, effects and management. Nurs. Times 2017, 113, 28–32. [Google Scholar]
- Halleran, D.R.; Coyle, D.; Kulaylat, A.N.; Ahmad, H.; Langer, J.C.; Gasior, A.C.; Diefenbach, K.A.; Wood, R.J.; Levitt, M.A. The cutback revisited—The posterior rectal advancement anoplasty for certain anorectal malformations with rectoperineal fistula. J. Pediatr. Surg. 2021, S0022-3468, 845–849. [Google Scholar] [CrossRef]
- Abeyaratne, M. Posterior transposition of anterior ectopic anus. J. Pediatr. Surg. 1991, 26, 725–727. [Google Scholar] [CrossRef]
- Shah, A.J.; Bhattacharjee, N.; Patel, D.N.; Ganatra, J.R. Anal shift: Preliminary results. J. Pediatr. Surg. 2003, 38, 196–198. [Google Scholar] [CrossRef]
- Rawat, J.; Singh, S.; Pant, N. Anorectal Malformations in Adolescent Females: A Retrospective Study. J. Indian Assoc. Pediatr. Surg. 2018, 23, 57–60. [Google Scholar] [CrossRef]
- Suomalainen, A.; Wester, T.; Koivusalo, A.; Rintala, R.J.; Pakarinen, M.P. Congenital funnel anus in children: Associated anomalies, surgical management and outcome. Pediatr. Surg. Int. 2007, 23, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Jenetzky, E.; Reckin, S.; Schmiedeke, E.; Schmidt, D.; Schwarzer, N.; Grasshoff-Derr, S.; Zwink, N.; Bartels, E.; Rissmann, A.; Leonhardt, J.; et al. Practice of dilatation after surgical correction in anorectal malformations. Pediatr. Surg. Int. 2012, 28, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Schmiedeke, E.; Busch, M.; Stamatopoulos, E.; Lorenz, C. Multidisciplinary behavioural treatment of fecal incontinence and constipation after correction of anorectal malformation. World J. Pediatr. 2008, 4, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Falcone, R.A., Jr.; Levitt, M.A.; Peña, A.; Bates, M. Increased heritability of certain types of anorectal malformations. J. Pediatr. Surg. 2007, 42, 124–127; discussion 127–128. [Google Scholar] [CrossRef] [PubMed]
- Mahalik, S.K.; Mahajan, J.K.; Sodhi, K.S.; Garge, S.; Vaiphei, K.; Rao, K.L. Rare association in a female DSD case of phallus, accessory phallic urethra, perineal lipoma and anterior ectopic anus. J. Pediatr. Urol. 2013, 9, e39–e42. [Google Scholar] [CrossRef][Green Version]
- Amornfa, J.; Taecholarn, C.; Khaoroptham, S. Currarino syndrome: Report of two cases and review of the literature. J. Med. Assoc. Thai. 2005, 88, 1697–1702. [Google Scholar]
- Meroni, G. X-Linked Opitz G/BBB Syndrome. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2004; pp. 1993–2021. [Google Scholar]
- Anoop, P.; Sasidharan, C.K. Baller-Gerold syndrome. Indian J. Pediatr. 2002, 69, 1097–1098. [Google Scholar] [CrossRef]
- Savarirayan, R.; Tomlinson, P.; Thompson, E. Baller-Gerold syndrome associated with congenital portal venous malformation. J. Med. Genet. 1998, 35, 767–769. [Google Scholar] [CrossRef]
- Wakhlu, A.K.; Wakhlu, A.; Tandon, R.K.; Kureel, S.N. Congenital megalourethra. J. Pediatr. Surg. 1996, 31, 441–443. [Google Scholar] [CrossRef]
- Goh, D.W.; Davey, R.B.; Dewan, P.A. Bladder, urethral, and vaginal duplication. J. Pediatr. Surg. 1995, 30, 125–126. [Google Scholar] [CrossRef]
- Lall, M.; Thakur, S.; Puri, R.; Verma, I.; Mukerji, M.; Jha, P. A 54 Mb 11qter duplication and 0.9 Mb 1q44 deletion in a child with laryngomalacia and agenesis of corpus callosum. Mol. Cytogenet. 2011, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Lin, H.M.; Leung, C.; Lin, S.P.; Su, Y.N.; Su, J.W.; Chen, Y.T.; Wang, W. Partial monosomy 9p (9p22.2-->pter) and partial trisomy 18q (18q21.32-->qter) in a female infant with anorectal malformations. Genet. Couns. 2012, 23, 201–206. [Google Scholar] [PubMed]
- Colombo, M.; Maestri, L.; Lambiase, R.; Magni, L.A. La politelia come spia di altre malformazioni congenite: Un esempio clinico [Polythelia as a sign of other congenital malformations: A clinical example]. Pediatr. Med. Chir. 1994, 16, 399–400. [Google Scholar]
- Branski, D.; Gale, R.; Gross-Kieselstein, E.; Abrahamov, A. Propionic acidemia and anorectal anomalies in three siblings. Am. J. Dis. Child. 1977, 131, 1379–1381. [Google Scholar] [CrossRef] [PubMed]
- Maegawa, G.H.; Chitayat, D.; Blaser, S.; Whyte, H.; Thomas, M.; Kim, P.; Kim, J.; Taylor, G.; McNamara, P.J. Duodenal and biliary atresia associated with facial, thyroid and auditory apparatus abnormalities: A new mandibulofacial dysostosis syndrome? Clin. Dysmorphol. 2006, 15, 191–196. [Google Scholar] [CrossRef]
- Verloes, A.; Le Merrer, M.; Davin, J.C.; Wittamer, P.; Abrassart, C.; Bricteux, G.; Briard, M.L. The orocraniodigital syndrome of Juberg and Hayward. J. Med. Genet. 1992, 29, 262–265. [Google Scholar] [CrossRef]
- James, P.A.; McGaughran, J. A severe case of oculo-ectodermal syndrome? Clin. Dysmorphol. 2002, 11, 179–182. [Google Scholar] [CrossRef]
- Chamaria, K.; Shetty, R. Ectopic anus with barrel gun perineum rare type of anorectal anomaly. J Radiol Case Rep. 2013, 7, 31–36. [Google Scholar] [CrossRef]
- Ottolenghi, A.; Sulpasso, M.; Bianchi, S.; Bettili, G.; Salloum, A.; Liber, H. Ectopic anus in childhood. Eur. J. Pediatr. Surg. 1994, 4, 145–150. [Google Scholar] [CrossRef]
- Pandey, A.; Pandey, P.; Singh, S.P.; Agarwal, S.; Gupta, V.; Verma, R. Histology with immunohistochemistry of the fistula region in female anorectal malformation: Can it be used for neo-anus reconstruction? J. Paediatr. Child Health. 2018, 54, 177–182. [Google Scholar] [CrossRef]
- Petrino, R.A.; Golladay, E.S.; Mollitt, D.L.; Butler, H. Surgically correctable fecal incontinence. South Med. J. 1985, 78, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Lappas, J.C.; Popp, B. Association of anterior ectopic anus and partial absence of levator musculature in a woman with impaired defecation. Report of a case. Dis. Colon. Rectum. 1990, 33, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Tesař, M.; Vávra, P.; Richter, V.; Ihnát, P. Anus perinei ventralis in adulthood—Case report. Rozhl. Chir. 2020, 99, 552–555. [Google Scholar]
- Upadhyaya, V.D.; Bharti, L.K.; Mishra, A.; Yousuf, M.; Mishra, P.; Kumar, B. Constipation after surgery for anorectal malformations: Unrecognised problem until it is a problem. Afr. J. Paediatr. Surg. 2021, 18, 67–71. [Google Scholar] [CrossRef]
- Yeung, C.K.; Lund, L. Laparoscopic cecostomy for anterior ectopic anus with constipation: A new and technical proposal. Eur. J. Pediatr. Surg. 2000, 10, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Ruttenstock, E.M.; Zani, A.; Huber-Zeyringer, A.; Höllwarth, M.E. Pre- and postoperative rectal manometric assessment of patients with anorectal malformations: Should we preserve the fistula? Dis. Colon. Rectum. 2013, 56, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.H.; Sathyanarayana, S.; Barrett, E.S.; Janssen, S.; Liu, F.; Nguyen, R.H.; Redmon, J.B.; TIDES Study Team. First trimester phthalate exposure and anogenital distance in newborns. Hum. Reprod. 2015, 30, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Zarean, M.; Keikha, M.; Feizi, A.; Kazemitabaee, M.; Kelishadi, R. The role of exposure to phthalates in variations of anogenital distance: A systematic review and meta-analysis. Environ. Pollut. 2019, 247, 172–179. [Google Scholar] [CrossRef]
- Romano-Riquer, S.P.; Hernández-Avila, M.; Gladen, B.C.; Cupul-Uicab, L.A.; Longnecker, M.P. Reliability and determinants of anogenital distance and penis dimensions in male newborns from Chiapas, Mexico. Paediatr. Perinat. Epidemiol. 2007, 21, 219–228. [Google Scholar] [CrossRef]
- Salazar-Martinez, E.; Romano-Riquer, P.; Yanez-Marquez, E.; Longnecker, M.P.; Hernandez-Avila, M. Anogenital distance in human male and female newborns: A descriptive, cross-sectional study. Environ. Health. 2004, 3, 8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).