Recent Advances in the Development of Protein- and Peptide-Based Subunit Vaccines against Tuberculosis
Abstract
1. Introduction
2. BCG: A Cornerstone in TB Handling
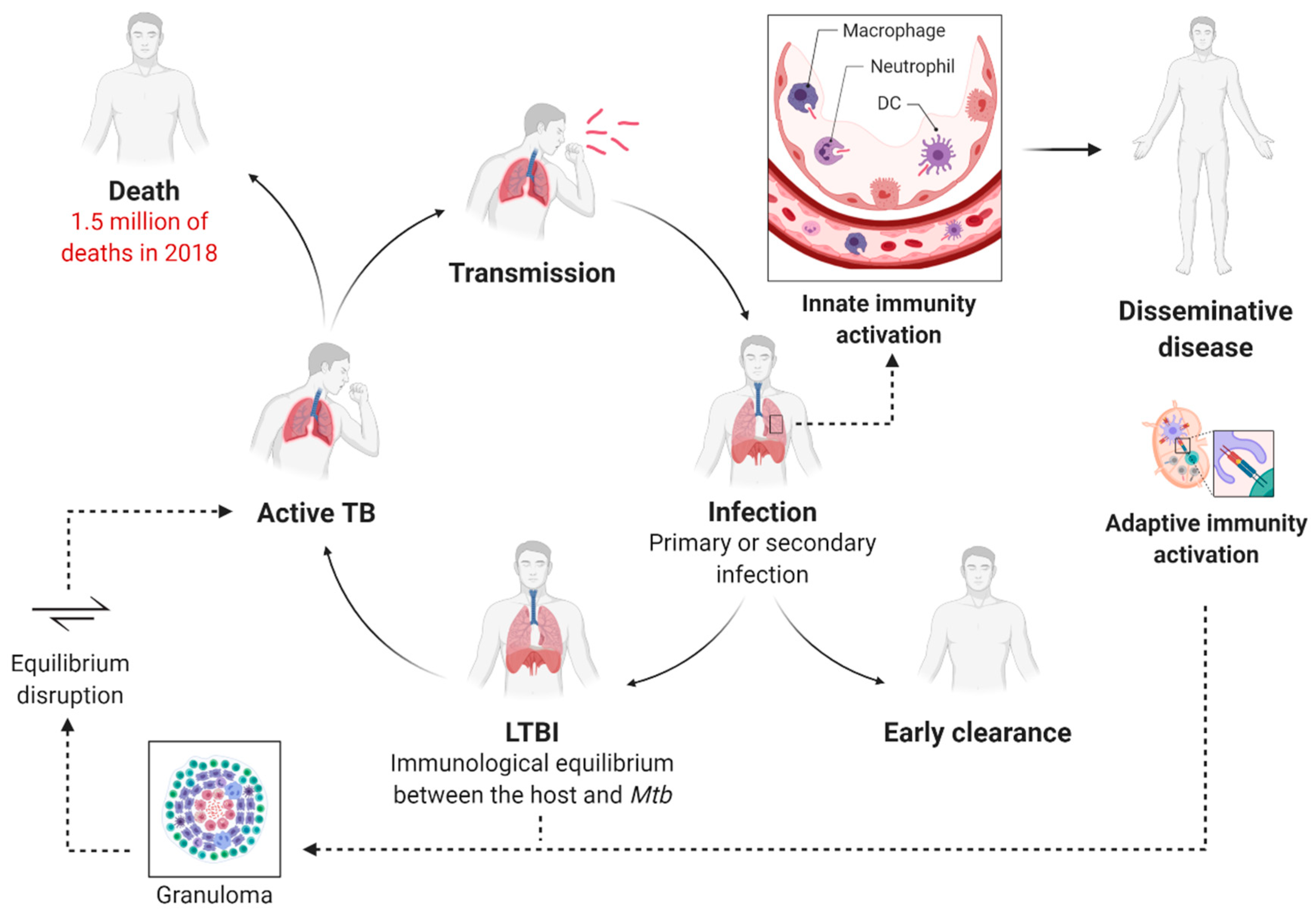
3. Tuberculosis Immunopathology
4. Strategic Goals for New TB Vaccine Development
4.1. Target Population
4.2. Tuberculosis Vaccine Pipeline
4.3. Subunit Vaccines against Tuberculosis
4.3.1. Protein Antigens and Immunological Response
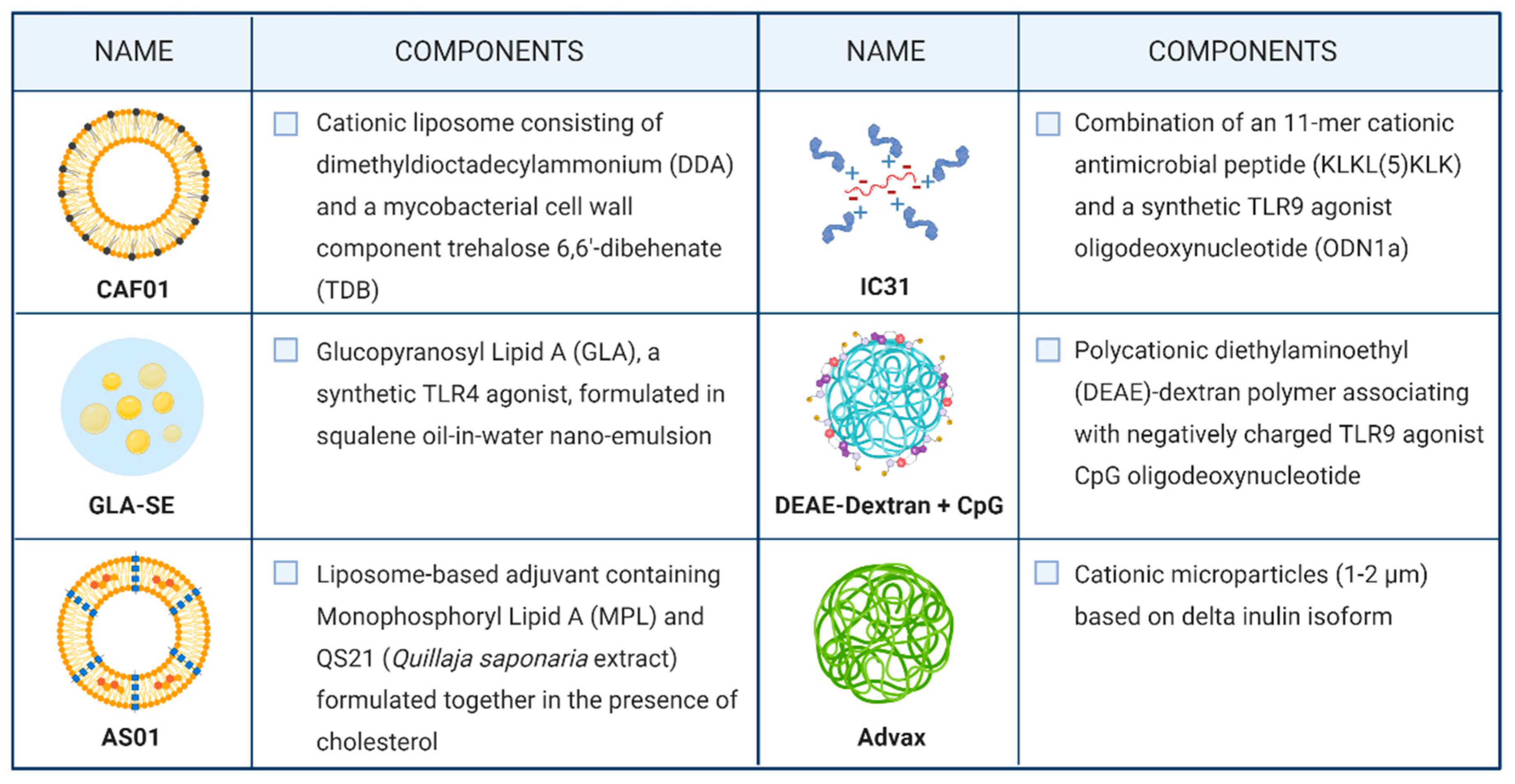
4.3.2. Adjuvants and Formulation Strategies for Subunit Vaccines
4.3.3. Peptide-Based Subunit Vaccines
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2020; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Manyazewal, T.; Woldeamanuel, Y.; Blumberg, H.M.; Fekadu, A.; Marconi, V.C. The fight to end tuberculosis must not be forgotten in the COVID-19 outbreak. Nat. Med. 2020, 26, 811–812. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Gumbo, T.; Gandhi, N.R.; Murray, M.; Theron, G.; Udwadia, Z.; Migliori, G.B.; Warren, R. Global control of tuberculosis: From extensively drug-resistant to untreatable tuberculosis. Lancet Respir. Med. 2014, 2, 321–338. [Google Scholar] [CrossRef]
- Oh, P.; Pascopella, L.; Barry, P.M.; Flood, J.M. A systematic synthesis of direct costs to treat and manage tuberculosis disease applied to California. BMC Res. Notes 2017, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Diel, R.; Vandeputte, J.; De Vries, G.; Stillo, J.; Wanlin, M.; Nienhaus, A. Costs of tuberculosis disease in the European Union: A systematic analysis and cost calculation. Eur. Respir. J. 2014, 43, 554–565. [Google Scholar] [CrossRef]
- Angelidou, A.; Diray-Arce, J.; Conti, M.G.; Smolen, K.K.; van Haren, S.D.; Dowling, D.J.; Husson, R.N.; Levy, O. BCG as a Case Study for Precision Vaccine Development: Lessons from Vaccine Heterogeneity, Trained Immunity, and Immune Ontogeny. Front. Microbiol. 2020, 11, 332. [Google Scholar] [CrossRef]
- Li, J.; Zhao, A.; Tang, J.; Wang, G.; Shi, Y.; Zhan, L.; Qin, C. Tuberculosis vaccine development: From classic to clinical candidates. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1405–1425. [Google Scholar] [CrossRef]
- Schrager, L.K.; Vekemens, J.; Drager, N.; Lewinsohn, D.M.; Olesen, O.F. The status of tuberculosis vaccine development. Lancet Infect. Dis. 2020, 20, e28–e37. [Google Scholar] [CrossRef]
- Hatherill, M.; White, R.G.; Hawn, T.R. Clinical Development of New TB Vaccines: Recent Advances and Next Steps. Front. Microbiol. 2020, 10, 3154. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E. Vaccination against Tuberculosis: Revamping BCG by Molecular Genetics Guided by Immunology. Front. Immunol. 2020, 11, 316. [Google Scholar] [CrossRef]
- Brazier, B.; McShane, H. Towards new TB vaccines. Semin. Immunopathol. 2020, 42, 315–331. [Google Scholar] [CrossRef]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F.; et al. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Scriba, T.J.; Mizrahi, V. Renewing the Fight against TB with an Old Vaccine. Cell 2020, 180, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Aronson, N.E.; Santosham, M.; Comstock, G.W.; Howard, R.S.; Moulton, L.H.; Rhoades, E.R.; Harrison, L.H. Long-term Efficacy of BCG Vaccine in American Indians and Alaska Natives: A 60-Year Follow-up Study. JAMA 2004, 291, 2086–2091. [Google Scholar] [CrossRef] [PubMed]
- Mangtani, P.; Nguipdop-Djomo, P.; Keogh, R.H.; Sterne, J.; Abubakar, I.; Smith, P.G.; Fine, P.; Vynnycky, E.; Watson, J.M.; Elliman, D.; et al. The duration of protection of school-aged BCG vaccination in England: A population-based case-control study. Int. J. Epidemiol. 2018, 47, 193–201. [Google Scholar] [CrossRef]
- Schwander, S.; Dheda, K. Human lung immunity against Mycobacterium tuberculosis: Insights into pathogenesis and protection. Am. J. Respir. Crit. Care Med. 2011, 183, 696–707. [Google Scholar] [CrossRef]
- Ferguson, J.S.; Schlesinger, L.S. Pulmonary surfactant in innate immunity and the pathogenesis of tuberculosis. Tuber. Lung Dis. 2000, 80, 173–184. [Google Scholar] [CrossRef]
- Delogu, G.; Provvedi, R.; Sali, M.; Manganelli, R. Mycobacterium tuberculosis virulence: Insights and impact on vaccine development. Future Microbiol. 2015, 10, 1177–1194. [Google Scholar] [CrossRef]
- Schorey, J.S.; Schlesinger, L.S. Innate Immune Responses to Tuberculosis. Microbiol. Spectr. 2016, 4, TBTB2-0010-2016. [Google Scholar] [CrossRef]
- Verrall, A.J.; Netea, M.G.; Alisjahbana, B.; Hill, P.C.; van Crevel, R. Early clearance of Mycobacterium tuberculosis: A new frontier in prevention. Immunology 2014, 141, 506–513. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Yao, Y.; Afkhami, S.; Smaill, F.; Xing, Z. New Tuberculosis Vaccine Strategies: Taking Aim at Un-Natural Immunity. Trends Immunol. 2018, 39, 419–433. [Google Scholar] [CrossRef]
- de Martino, M.; Lodi, L.; Galli, L.; Chiappini, E. Immune Response to Mycobacterium tuberculosis: A Narrative Review. Front. Pediatr. 2019, 7, 350. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.D. The immunological life cycle of tuberculosis. Nat. Rev. Immunol. 2012, 12, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, M.; McCormick, S.; Lai, R.; Afkhami, S.; Shaler, C.R.; Horvath, C.N.; Damjanovic, D.; Zganiacz, A.; Barra, N.; Ashkar, A.; et al. Pulmonary M. tuberculosis infection delays Th1 immunity via immunoadaptor DAP12-regulated IRAK-M and IL-10 expression in antigenpresenting cells. Mucosal Immunol. 2014, 7, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Oe, T.; Kawakami, K.; Osada-Oka, M.; Ozeki, Y.; Terahara, K.; Yasuda, I.; Edwards, T.; Tanaka, T.; Tsunetsugu-Yokota, Y.; et al. CD4+ T Responses Other Than Th1 Type Are Preferentially Induced by Latency-Associated Antigens in the State of Latent Mycobacterium tuberculosis Infection. Front. Immunol. 2019, 10, 2807. [Google Scholar] [CrossRef]
- Kwan, C.; Ernst, J.D. HIV and tuberculosis: A deadly human syndemic. Clin. Microbiol. Rev. 2011, 24, 351–376. [Google Scholar] [CrossRef]
- Bares, S.H.; Swindells, S. Latent Tuberculosis and HIV Infection. Curr. Infect. Dis. Rep. 2020, 22, 17. [Google Scholar] [CrossRef]
- Harris, R.C.; Sumner, T.; Knight, G.M.; White, R.G. Systematic review of mathematical models exploring the epidemiological impact of future TB vaccines. Hum. Vaccin. Immunother. 2016, 12, 2813–2832. [Google Scholar] [CrossRef]
- Knight, G.M.; Griffiths, U.K.; Sumner, T.; Laurence, Y.V.; Gheorghe, A.; Vassall, A.; Glaziou, P.; White, R.G. Impact and cost-effectiveness of new tuberculosis vaccines in low- and middle-income countries. Proc. Natl. Acad. Sci. USA 2014, 111, 15520–15525. [Google Scholar] [CrossRef]
- World Health Organization. WHO Preferred Product Characteristics for New Tuberculosis Vaccines; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Schrager, L.K.; Harris, R.C.; Vekemans, J. Research and development of new tuberculosis vaccines: A review. F1000Research 2019, 7, 1732. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E.; Weiner, J.; von Reyn, C.F. Novel approaches to tuberculosis vaccine development. Int. J. Infect. Dis. 2017, 56, 263–267. [Google Scholar] [CrossRef]
- Tameris, M.D.; Hatherill, M.; Landry, B.S.; Scriba, T.J.; Snowden, M.A.; Lockhart, S.; Shea, J.E.; McClain, J.B.; Hussey, G.D.; Hanekom, W.A.; et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: A randomised, placebo-controlled phase 2b trial. Lancet 2013, 81, 1021–1028. [Google Scholar] [CrossRef]
- Macleod, M. Learning lessons from MVA85A, a failed booster vaccine for BCG. BMJ 2018, 360, k66. [Google Scholar] [CrossRef] [PubMed]
- Tameris, M.; McShane, H.; McClain, J.B.; Landry, B.; Lockhart, S.; Luabeya, A.K.; Geldenhuys, H.; Shea, J.; Hussey, G.; van der Merwe, L.; et al. Lessons learnt from the first efficacy trial of a new infant tuberculosis vaccine since BCG. Tuberculosis 2013, 93, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Aguilo, N.; Marinova, D.; Gonzalo-Asensio, J. Update on TB vaccine pipeline. Appl. Sci. 2020, 10, 2632. [Google Scholar] [CrossRef]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef]
- Stylianou, E.; Harrington-Kandt, R.; Beglov, J.; Bull, N.; Pinpathomrat, N.; Swarbrick, G.M.; Lewinsohn, D.A.; Lewinsohn, D.M.; McShane, H. Identification and evaluation of novel protective antigens for the development of a candidate tuberculosis subunit vaccine. Infect. Immun. 2018, 86, e00014-18. [Google Scholar] [CrossRef]
- Bettencourt, P.; Müller, J.; Nicastri, A.; Cantillon, D.; Madhavan, M.; Charles, P.D.; Fotso, C.B.; Wittenberg, R.; Bull, N.; Pinpathomrat, N.; et al. Identification of antigens presented by MHC for vaccines against tuberculosis. NPJ Vaccines 2020, 5, 2. [Google Scholar] [CrossRef]
- Kapopoulou, A.; Lew, J.M.; Cole, S.T. The MycoBrowser portal: A comprehensive and manually annotated resource for mycobacterial genomes. Tuberculosis 2011, 91, 8–13. [Google Scholar] [CrossRef]
- Voss, G.; Casimiro, D.; Neyrolles, O.; Williams, A.; Kaufmann, S.; McShane, H.; Hatherill, M.; Fletcher, H.A. Progress and challenges in TB vaccine development. F1000Research 2018, 7, 199. [Google Scholar] [CrossRef]
- Uplekar, S.; Heym, B.; Friocourt, V.; Rougemont, J.; Cole, S.T. Comparative genomics of ESX genes from clinical isolates of Mycobacterium tuberculosis provides evidence for gene conversion and epitope variation. Infect. Immun. 2011, 79, 4042–4049. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.J. The enigmatic PE/PPE multigene family of mycobacteria and tuberculosis vaccination. Infect. Immun. 2017, 85, e00969-16. [Google Scholar] [CrossRef] [PubMed]
- Schrager, L.K.; Chandrasekaran, P.; Fritzell, B.H.; Hatherill, M.; Lambert, P.H.; McShane, H.; Tornieporth, N.; Vekemans, J. WHO preferred product characteristics for new vaccines against tuberculosis. Lancet Infect. Dis. 2018, 18, 828–829. [Google Scholar] [CrossRef]
- Lewinsohn, D.A.; Swarbrick, G.M.; Park, B.; Cansler, M.E.; Null, M.D.; Toren, K.G.; Baseke, J.; Zalwango, S.; Mayanja-Kizza, H.; Malone, L.L.; et al. Comprehensive definition of human immunodominant CD8 antigens in tuberculosis. NPJ Vaccines 2017, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Joosten, S.A.; Ottenhoff, T.H.M.; Lewinsohn, D.M.; Hoft, D.F.; Moody, D.B.; Seshadri, C. Harnessing donor unrestricted T-cells for new vaccines against tuberculosis. Vaccine 2019, 37, 3022–3030. [Google Scholar] [CrossRef]
- Zhang, L. Multi-epitope vaccines: A promising strategy against tumors and viral infections. Cell. Mol. Immunol. 2018, 15, 182–184. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2016, 7, 842–854. [Google Scholar] [CrossRef]
- Oleszycka, E.; McCluskey, S.; Sharp, F.A.; Muñoz-Wolf, N.; Hams, E.; Gorman, A.L.; Fallon, P.G.; Lavelle, E.C. The vaccine adjuvant alum promotes IL-10 production that suppresses Th1 responses. Eur. J. Immunol. 2018, 48, 705–715. [Google Scholar] [CrossRef]
- Khandhar, A.P.; Liang, H.; Simpson, A.C.; Reed, S.G.; Carter, D.; Fox, C.B.; Orr, M.T. Physicochemical structure of a polyacrylic acid stabilized nanoparticle alum (nanoalum) adjuvant governs TH1 differentiation of CD4+ T cells. Nanoscale 2020, 12, 2515–2523. [Google Scholar] [CrossRef]
- Orr, M.T.; Khandhar, A.P.; Seydoux, E.; Liang, H.; Gage, E.; Mikasa, T.; Beebe, E.L.; Rintala, N.D.; Persson, K.H.; Ahniyaz, A.; et al. Reprogramming the adjuvant properties of aluminum oxyhydroxide with nanoparticle technology. NPJ Vaccines 2019, 4, 1. [Google Scholar] [CrossRef]
- Davidsen, J.; Rosenkrands, I.; Christensen, D.; Vangala, A.; Kirby, D.; Perrie, Y.; Agger, E.M.; Andersen, P. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)—A novel adjuvant inducing both strong CMI and antibody responses. Biochim. Biophys. Acta 2005, 1718, 22–31. [Google Scholar] [CrossRef]
- Pedersen, G.K.; Andersen, P.; Christensen, D. Immunocorrelates of CAF family adjuvants. Semin. Immunol. 2018, 39, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, J.S.; Christensen, D.; Cassidy, J.P.; Agger, E.M.; Mortensen, R.; Andersen, P. Mucosal boosting of H56:CAF01 immunization promotes lung-localized T cells and an accelerated pulmonary response to Mycobacterium tuberculosis infection without enhancing vaccine protection. Mucosal Immunol. 2019, 12, 816–826. [Google Scholar] [CrossRef]
- van Dissel, J.T.; Joosten, S.A.; Hoff, S.T.; Soonawala, D.; Prins, C.; Hokey, D.A.; O’Dee, D.M.; Graves, A.; Thierry-Carstensen, B.; Andreasen, L.V.; et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine 2014, 32, 7098–7107. [Google Scholar] [CrossRef] [PubMed]
- Coler, R.N.; Bertholet, S.; Moutaftsi, M.; Guderian, J.A.; Windish, H.P.; Baldwin, S.L.; Laughlin, E.M.; Duthie, M.S.; Fox, C.B.; Carter, D.; et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS ONE 2011, 6, e16333. [Google Scholar] [CrossRef] [PubMed]
- Penn-Nicholson, A.; Tameris, M.; Smit, E.; Day, T.A.; Musvosvi, M.; Jayashankar, L.; Vergara, J.; Mabwe, S.; Bilek, N.; TBVPX-114 Study Team; et al. Safety and immunogenicity of the novel tuberculosis vaccine ID93 + GLA-SE in BCG-vaccinated healthy adults in South Africa: A randomised, double-blind, placebo-controlled phase 1 trial. Lancet Respir. Med. 2018, 6, 287–298. [Google Scholar] [CrossRef]
- Coler, R.N.; Day, T.A.; Ellis, R.; Piazza, F.M.; Beckmann, A.M.; Vergara, J.; Rolf, T.; Lu, L.; Alter, G.; TBVPX-113 Study Team; et al. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: First-in-human trial. NPJ Vaccines 2018, 3, 34. [Google Scholar] [CrossRef]
- Van Der Meeren, O.; Hatherill, M.; Nduba, V.; Wilkinson, R.J.; Muyoyeta, M.; Van Brakel, E.; Ayles, H.M.; Henostroza, G.; Thienemann, F.; Scriba, T.J.; et al. Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N. Engl. J. Med. 2018, 379, 1621–1634. [Google Scholar] [CrossRef]
- Tait, D.R.; Hatherill, M.; Van Der Meeren, O.; Ginsberg, A.M.; Van Brakel, E.; Salaun, B.; Scriba, T.J.; Akite, E.J.; Ayles, H.M.; Bollaerts, A.; et al. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N. Engl. J. Med. 2019, 381, 2429–2439. [Google Scholar] [CrossRef]
- Marciani, D.J. Elucidating the Mechanisms of Action of Saponin-Derived Adjuvants. Trends Pharmacol. Sci. 2018, 39, 573–585. [Google Scholar] [CrossRef]
- Agger, E.M. Novel adjuvant formulations for delivery of anti-tuberculosis vaccine candidates. Adv. Drug Del. Rev. 2016, 102, 73–82. [Google Scholar] [CrossRef]
- Geldenhuys, H.; Mearns, H.; Miles, D.J.; Tameris, M.; Hokey, D.; Shi, Z.; Bennett, S.; Andersen, P.; Kromann, I.; Hoff, S.T.; et al. The tuberculosis vaccine H4: IC31 is safe and induces a persistent polyfunctional CD4 T cell response in South African adults: A randomized controlled trial. Vaccine 2015, 33, 3592–3599. [Google Scholar] [CrossRef]
- Suliman, S.; Luabeya, A.; Geldenhuys, H.; Tameris, M.; Hoff, S.T.; Shi, Z.; Tait, D.; Kromann, I.; Ruhwald, M.; H56-035 Trial Group; et al. Dose optimization of H56:IC31 vaccine for tuberculosis-endemic populations a double-blind, placebo-controlled, dose-selection trial. Am. J. Respir. Crit. Care Med. 2019, 199, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Vasina, D.V.; Kleymenov, D.A.; Manuylov, V.A.; Mazunina, E.P.; Koptev, E.Y.; Tukhovskaya, E.A.; Murashev, A.N.; Gintsburg, A.L.; Gushchin, V.A.; Tkachuk, A.P. First-in-human trials of gamtbvac, a recombinant subunit tuberculosis vaccine candidate: Safety and immunogenicity assessment. Vaccines 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.; Triccas, J.A.; Petrovsky, N. Adjuvant strategies for more effective tuberculosis vaccine immunity. Microorganisms 2019, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Counoupas, C.; Pinto, R.; Nagalingam, G.; Britton, W.J.; Petrovsky, N.; Triccas, J.A. Delta inulin-based adjuvants promote the generation of polyfunctional CD4+ T cell responses and protection against Mycobacterium tuberculosis infection. Sci. Rep. 2017, 7, 8582. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Chandrudu, S.; Rigau-Planella, B.; Islam, M.T.; Cheong, Y.S.; Liu, G.; Wang, X.; Toth, I.; Hussein, W.M. Progress in the development of subunit vaccines against malaria. Vaccines 2020, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Azuar, A.; Jin, W.; Mukaida, S.; Hussein, W.M.; Toth, I.; Skwarczynski, M. Recent advances in the development of peptide vaccines and their delivery systems against group a streptococcus. Vaccines 2019, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Kalli, K.R.; Block, M.S.; Kasi, P.M.; Erskine, C.L.; Hobday, T.J.; Dietz, A.; Padley, D.; Gustafson, M.P.; Shreeder, B.; Puglisi-Knutson, D.; et al. Folate receptor alpha peptide vaccine generates immunity in breast and ovarian cancer patients. Clin. Cancer Res. 2018, 24, 3014–3025. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Lu, B.; Melisko, M.; Price Hiller, J.; Bondarenko, I.; Brunt, A.M.; Sergii, G.; Petrakova, K.; Peoples, G.E. Efficacy and safety analysis of nelipepimut-S vaccine to prevent breast cancer recurrence: A randomized, multicenter, phase III clinical trial. Clin. Cancer Res. 2019, 25, 4248–4254. [Google Scholar] [CrossRef]
- Dakappagari, N.K.; Douglas, D.B.; Triozzi, P.L.; Stevens, V.C.; Kaumaya, P.T.P. Prevention of mammary tumors with a chimeric HER-2 B-cell epitope peptide vaccine. Cancer Res. 2000, 60, 3782–3789, PMID:10919651. [Google Scholar] [PubMed]
- Purcell, A.W.; McCluskey, J.; Rossjohn, J. More than one reason to rethink the use of peptides in vaccine design. Nat. Rev. Drug Discov. 2007, 6, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Coppola, M.; Van Den Eeden, S.J.F.; Wilson, L.; Franken, K.L.M.C.; Ottenhoff, T.H.M.; Geluk, A. Synthetic long peptide derived from Mycobacterium tuberculosis latency antigen Rv1733c protects against tuberculosis. Clin. Vaccine Immunol. 2015, 22, 1060–1069. [Google Scholar] [CrossRef]
- Ashhurst, A.S.; McDonald, D.M.; Hanna, C.C.; Stanojevic, V.A.; Britton, W.J.; Payne, R.J. Mucosal Vaccination with a Self-Adjuvanted Lipopeptide Is Immunogenic and Protective against Mycobacterium tuberculosis. J. Med. Chem. 2019, 62, 8080–8089. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, U.; Singh, V.; Zeng, W.; Jain, S.; Siddiqui, K.F.; Chodisetti, S.B.; Gurram, R.K.; Parihar, P.; Gupta, P.; Gupta, U.D.; et al. Promiscuous peptide of 16 kDa antigen linked to Pam2Cys protects against mycobacterium tuberculosis by evoking enduring memory T-cell response. J. Infect. Dis. 2011, 204, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Chodisetti, S.B.; Zeng, W.; Nadeem, S.; Maurya, S.K.; Pahari, S.; Janmeja, A.K.; Jackson, D.C.; Agrewala, J.N. A lipidated peptide of Mycobacterium tuberculosis resuscitates the protective efficacy of BCG vaccine by evoking memory T cell immunity. J. Transl. Med. 2017, 15, 201. [Google Scholar] [CrossRef]
- Rai, P.K.; Chodisetti, S.B.; Nadeem, S.; Maurya, S.K.; Gowthaman, U.; Zeng, W.; Janmeja, A.K.; Jackson, D.C.; Agrewala, J.N. A novel therapeutic strategy of lipidated promiscuous peptide against Mycobacterium tuberculosis by eliciting Th1 and Th17 immunity of host. Sci. Rep. 2016, 6, 23917. [Google Scholar] [CrossRef]
- Chesson, C.B.; Huante, M.; Nusbaum, R.J.; Walker, A.G.; Clover, T.M.; Chinnaswamy, J.; Endsley, J.J.; Rudra, J.S. Nanoscale Peptide Self-assemblies Boost BCG-primed Cellular Immunity against Mycobacterium tuberculosis. Sci. Rep. 2018, 8, 12519. [Google Scholar] [CrossRef]
- Horváti, K.; Pályi, B.; Henczkó, J.; Balka, G.; Szabó, E.; Farkas, V.; Biri-Kovács, B.; Szeder, B.; Fodor, K. A convenient synthetic method to improve immunogenicity of mycobacterium tuberculosis related T-cell epitope peptides. Vaccines 2019, 7, 101. [Google Scholar] [CrossRef]
- Rai, P.K.; Chodisetti, S.B.; Maurya, S.K.; Nadeem, S.; Zeng, W.; Janmeja, A.K.; Jackson, D.C.; Agrewala, J.N. A lipidated bi-epitope vaccine comprising of MHC-I and MHC-II binder peptides elicits protective CD4 T cell and CD8 T cell immunity against Mycobacterium tuberculosis. J. Transl. Med. 2018, 16, 279. [Google Scholar] [CrossRef]
- Chatterjee, N.; Ojha, R.; Khatoon, N.; Prajapati, V.K. Scrutinizing Mycobacterium tuberculosis membrane and secretory proteins to formulate multiepitope subunit vaccine against pulmonary tuberculosis by utilizing immunoinformatic approaches. Int. J. Biol. Macromol. 2018, 118, 180–188. [Google Scholar] [CrossRef]
- Shah, P.; Mistry, J.; Reche, P.A.; Gatherer, D.; Flower, D.R. In silico design of Mycobacterium tuberculosis epitope ensemble vaccines. Mol. Immunol. 2018, 97, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; da Silva Antunes, R.; Sidney, J.; Lindestam Arlehamn, C.S.; Grifoni, A.; Dhanda, S.K.; Paul, S.; Peters, B.; Weiskopf, D.; Sette, A. A review on T Cell epitopes identified using prediction and cell-mediated immune models for mycobacterium tuberculosisand bordetella pertussis. Front. Immunol. 2018, 9, 2778. [Google Scholar] [CrossRef] [PubMed]
- Garnica, O.; Das, K.; Devasundaram, S.; Dhandayuthapani, S. Enhanced delivery of Mycobacterium tuberculosis antigens to antigen presenting cells using RVG peptide. Tuberculosis 2019, 116S, S34–S41. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.W.; Hansen, P.R.; Holm, A.; Andersen, P. Efficient protection against Mycobacterium tuberculosis by vaccination with a single subdominant epitope from the ESAT-6 antigen. Eur. J. Immunol. 2000, 30, 1724–1732. [Google Scholar] [CrossRef]
- Aagaard, C.S.; Hoang, T.T.; Vingsbo-Lundberg, C.; Dietrich, J.; Andersen, P. Quality and Vaccine Efficacy of CD4 + T Cell Responses Directed to Dominant and Subdominant Epitopes in ESAT-6 from Mycobacterium tuberculosis. J. Immunol. 2009, 183, 2659–2668. [Google Scholar] [CrossRef]
- Geluk, A.; van den Eeden, S.J.; van Meijgaarden, K.E.; Dijkman, K.; Franken, K.L.; Ottenhoff, T.H. A multistage-polyepitope vaccine protects against Mycobacterium tuberculosis infection in HLA-DR3 transgenic mice. Vaccine 2012, 30, 7513–7521. [Google Scholar] [CrossRef]
- Doi, T.; Yamada, H.; Yajima, T.; Wajjwalku, W.; Hara, T.; Yoshikai, Y. H2-M3-Restricted CD8 + T Cells Induced by Peptide-Pulsed Dendritic Cells Confer Protection against Mycobacterium tuberculosis. J. Immunol. 2007, 178, 3806–3813. [Google Scholar] [CrossRef]
- Covián, C.; Fernández-Fierro, A.; Retamal-Díaz, A.; Díaz, F.E.; Vasquez, A.E.; Lay, M.K.; Riedel, C.A.; González, P.A.; Bueno, S.M.; Kalergis, A.M. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front. Immunol. 2019, 10, 2806. [Google Scholar] [CrossRef]
- Covián, C.; Retamal-Díaz, A.; Bueno, S.M.; Kalergis, A.M. Could BCG Vaccination Induce Protective Trained Immunity for SARS-CoV-2? Front. Immunol. 2020, 11, 970. [Google Scholar] [CrossRef]
- Netea, M.G.; Giamarellos-Bourboulis, E.J.; Domínguez-Andrés, J.; Curtis, N.; van Crevel, R.; van de Veerdonk, F.L.; Bonten, M. Trained Immunity: A Tool for Reducing Susceptibility to and the Severity of SARS-CoV-2 Infection. Cell 2020, 181, 969–977. [Google Scholar] [CrossRef]
- Curtis, N.; Sparrow, A.; Ghebreyesus, T.A.; Netea, M.G. Considering BCG vaccination to reduce the impact of COVID-19. Lancet 2020, 395, 1545–1546. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Netea, M.G. BCG-induced trained immunity: Can it offer protection against COVID-19? Nat. Rev. Immunol. 2020, 20, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Klinger, D.; Blass, I.; Rappoport, N.; Linial, M. Significantly improved COVID-19 outcomes in countries with higher bcg vaccination coverage: A multivariable analysis. Vaccines 2020, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus disease 2019–COVID-19. Clin. Microbiol. Rev. 2020, 33, e0028-20. [Google Scholar] [CrossRef]
- O’Connor, E.; Teh, J.; Kamat, A.M.; Lawrentschuk, N. Bacillus Calmette Guérin (BCG) vaccination use in the fight against COVID-19—What’s old is new again? Future Oncol. 2020, 16, 1323–1325. [Google Scholar] [CrossRef]
- Hamiel, U.; Kozer, E.; Youngster, I. SARS-CoV-2 Rates in BCG-Vaccinated and Unvaccinated Young Adults. JAMA 2020, 232, 2340–2341. [Google Scholar] [CrossRef]
- Hunter, R.; Actor, J. The pathogenesis of post-primary tuberculosis. A game for vaccine development. Tuberculosis 2019, 116S, S114–S117. [Google Scholar] [CrossRef]
- Lewinsohn, D.A.; Lewinsohn, D.M.; Scriba, T.J. Polyfunctional CD4+ T Cells as Targets for Tuberculosis Vaccination. Front. Immunol. 2017, 8, 1262. [Google Scholar] [CrossRef]

| Gene Name | Protein Name | TB Database 1 | UniProt Entry 2 | Protein Length (aa) |
|---|---|---|---|---|
| pepA | MTB32A | Rv0125 | O07175 | 355 |
| PPE18 | MTB39A | Rv1196 | L7N675 | 391 |
| PPE42 | PPE42 | Rv2608 | P9WHZ5 | 580 |
| fbpA | Ag85A | Rv3804c | P9WQP3 | 338 |
| fbpB | Ag85B | Rv1886c | P9WQP1 | 325 |
| esxA | ESAT6 | Rv3875 | P9WNK7 | 95 |
| esxB | CFP10 | Rv3874 | P9WNK5 | 100 |
| esxH | TB10.4 | Rv0288 | P9WNK3 | 96 |
| esxV | EsxV | Rv3619c | P0DOA7 | 94 |
| esxW | EsxW | Rv3620c | P9WNI3 | 98 |
| Rv1813 | Rv1813 | Rv1813 | P9WLS1 | 143 |
| Rv2660c | Rv2660c | Rv2660c | I6Y1F5 | 75 |
| Protein * (Rv Code) Position | Peptide Sequence | Formulation | Immunization | Efficacy | Ref. |
|---|---|---|---|---|---|
| TB10.4 (Rv0288) 4–11 and Ag85B (Rv1886c) 280–294 | IMYNYPAM and FQDAYNAAGGHNAVF | Self-assembling peptide nanofibres with KFE8 (FKFEFKFE) + Pam2Cys adjuvant | Intranasal boosting of BCG-primed C57BL6 mice | 8-fold expansion in multifunctional CD8+ T cell populations in the lungs and 1.3 log10 CFU reduction in lung bacterial burden. | [79] |
| Ag85B (Rv1886c) 96–111 and Ag85B (Rv1886c) 241–256 | QDAYNAAGGHNAVFN and PAFEWYYQSGLSIVMP | Covalently conjugated to RVG peptide | C57BL/6 mice, s.c. or intranasal immunization | Enhances antigen presentation and in vivo immunogenicity. | [85] |
| ESAT6 (Rv3875) 51–70 | YQGVQQKWDATATELNNALQ | DDA/MPL-A/IL2 emulsion | B6CBAF1 mice, s.c. at the back 3x | Protective immunity against Mtb (around 1 log reduction in bacterial numbers). | [86] |
| ESAT6 (Rv3875) (1–15) | MTEQQWNFAGIEAAA | CAF01 | CB6F1 mice, s.c. at the base of the tail | Vaccine-promoted polyfunctional T cell response. | [87] |
| acr (Rv2031c) 91–110 | SEFAYGSFVRTVSLPVGADE | Covalently attached to Pam2Cys | BALB/c mice or Duncan-Hartley guinea pigs, i.p. or s.c. 2x | Significant protection against Mtb infection in guinea pigs and mice. | [76] |
| (Rv1733c) 57–84 | IPFAAAAGTAVQDSRSHVYAHQAQTRHP | Synthetic long peptide (SLP) with CpG ODN1826 adjuvant | HLA-DR3 transgenic mice injected 3x s.c. | Induces protection against live Mtb challenge and has therapeutic effect when used as a boost a prior BCG vaccination. | [74] |
| Ag85B (Rv1886c) 239–247 and IniB (Rv0341) 33–45 and PPE68 (Rv3873) 127–136 | KLVANNTRL and GLIDIAPHQISSV and FFGINTIPIA | Branched chain palmitoyl-peptide conjugate on Tuftsin (TKPKG) carrier | BALB/cmice injected 3-times s.c. | Significantly lower number of bacteria in the spleen after i.p. challenge with Mtb. | [80] |
| hsp65 (Rv0440) 3–13 and Ag85B (Rv1886c) 56–64 and 19 kDa (Rv3763) 51–61 and hsp16 (Rv2031c) 31–50 and (Rv1733c) 63–77 | KTIAYDEEARR and PSMGRDIKV and KVVIDGKDQNV and LRPTFDTRLMRLEDEMKEGR and AGTAVQDSRSHVYAH | Recompinant polyepitope with CpG ODN1826 adjuvant | HLA-DR3 transgenic mice injected 3-times s.c. | Induces significant numbers (12.7%) of IFN- +/IL-2+/TNF+ CD4+ T-cells and reduce CFU in lungs of Mtb infected HLA-DR3 mice. | [88] |
| MPT64 (Rv1980c) 190–198 And (Rv0476) 1–6 | FAVTNDGVI and f-MLVLLV | Peptide-pulsed BMDCs | C57BL/6 mice injected iv with 106 peptide-pulsed BMDC | Elicited an expansion of peptide-specific CD8+ T-cells in the spleen and the lung, and a significant protection against an intratracheal challenge with Mtb. | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, C.; Horváti, K. Recent Advances in the Development of Protein- and Peptide-Based Subunit Vaccines against Tuberculosis. Cells 2020, 9, 2673. https://doi.org/10.3390/cells9122673
Bellini C, Horváti K. Recent Advances in the Development of Protein- and Peptide-Based Subunit Vaccines against Tuberculosis. Cells. 2020; 9(12):2673. https://doi.org/10.3390/cells9122673
Chicago/Turabian StyleBellini, Chiara, and Kata Horváti. 2020. "Recent Advances in the Development of Protein- and Peptide-Based Subunit Vaccines against Tuberculosis" Cells 9, no. 12: 2673. https://doi.org/10.3390/cells9122673
APA StyleBellini, C., & Horváti, K. (2020). Recent Advances in the Development of Protein- and Peptide-Based Subunit Vaccines against Tuberculosis. Cells, 9(12), 2673. https://doi.org/10.3390/cells9122673





