Role and Therapeutic Targeting of the PI3K/Akt/mTOR Signaling Pathway in Skin Cancer: A Review of Current Status and Future Trends on Natural and Synthetic Agents Therapy
Abstract
1. Introduction
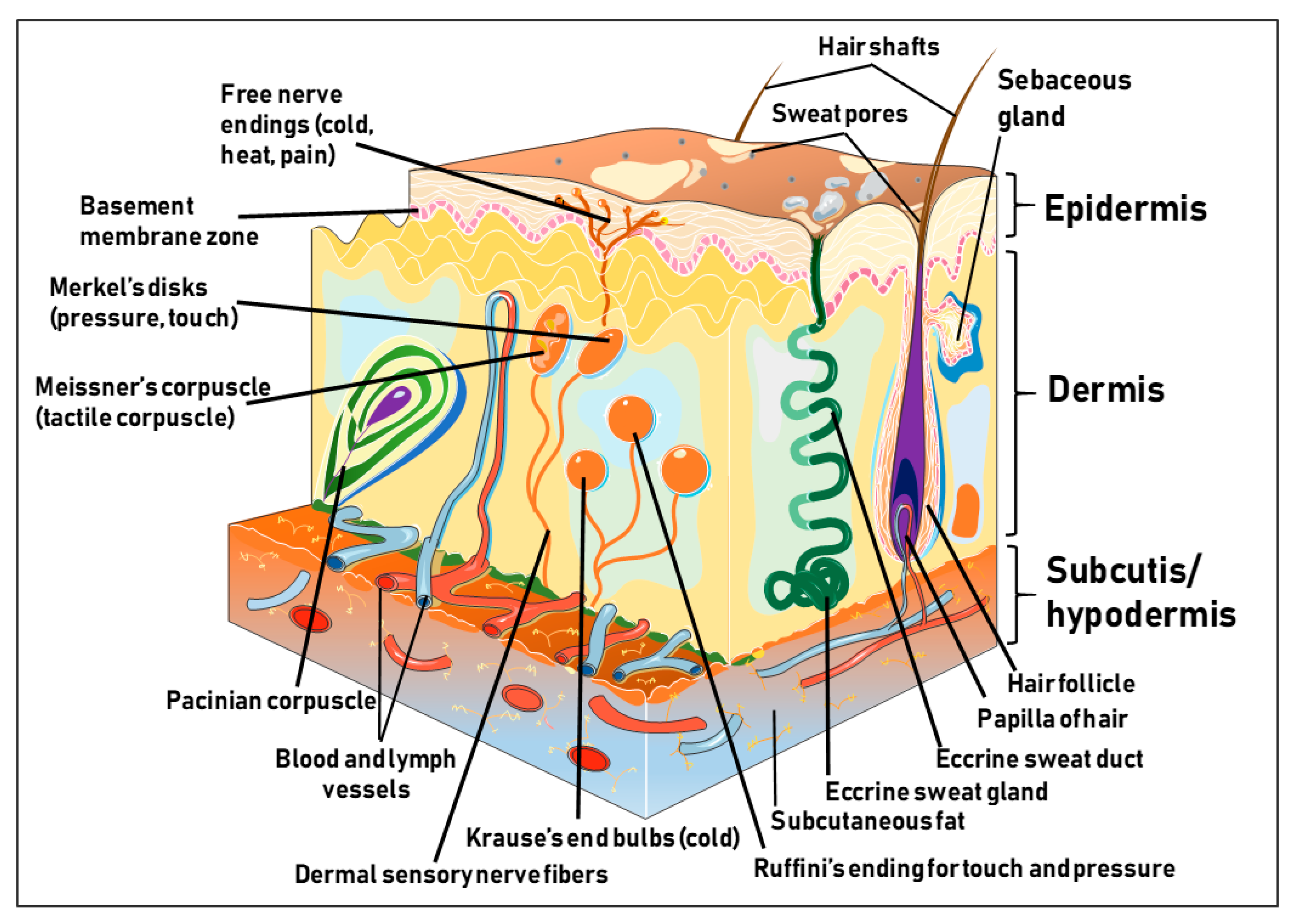
1.1. Structure and Function of the Human Skin
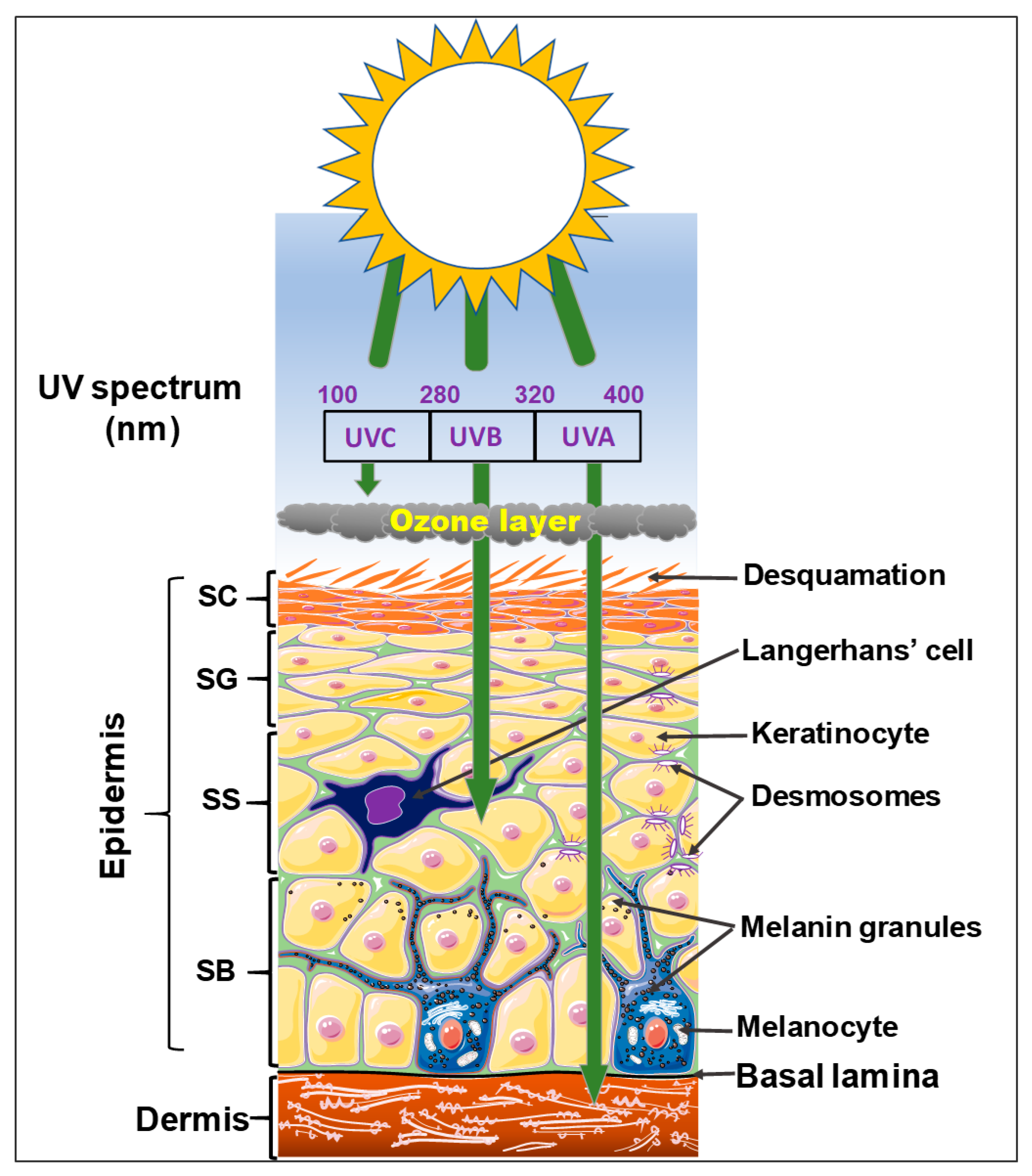
1.2. The Epidermis
1.3. The Dermis
1.4. The Hypodermis
2. Risk Factors Associated with Cutaneous Carcinogenesis
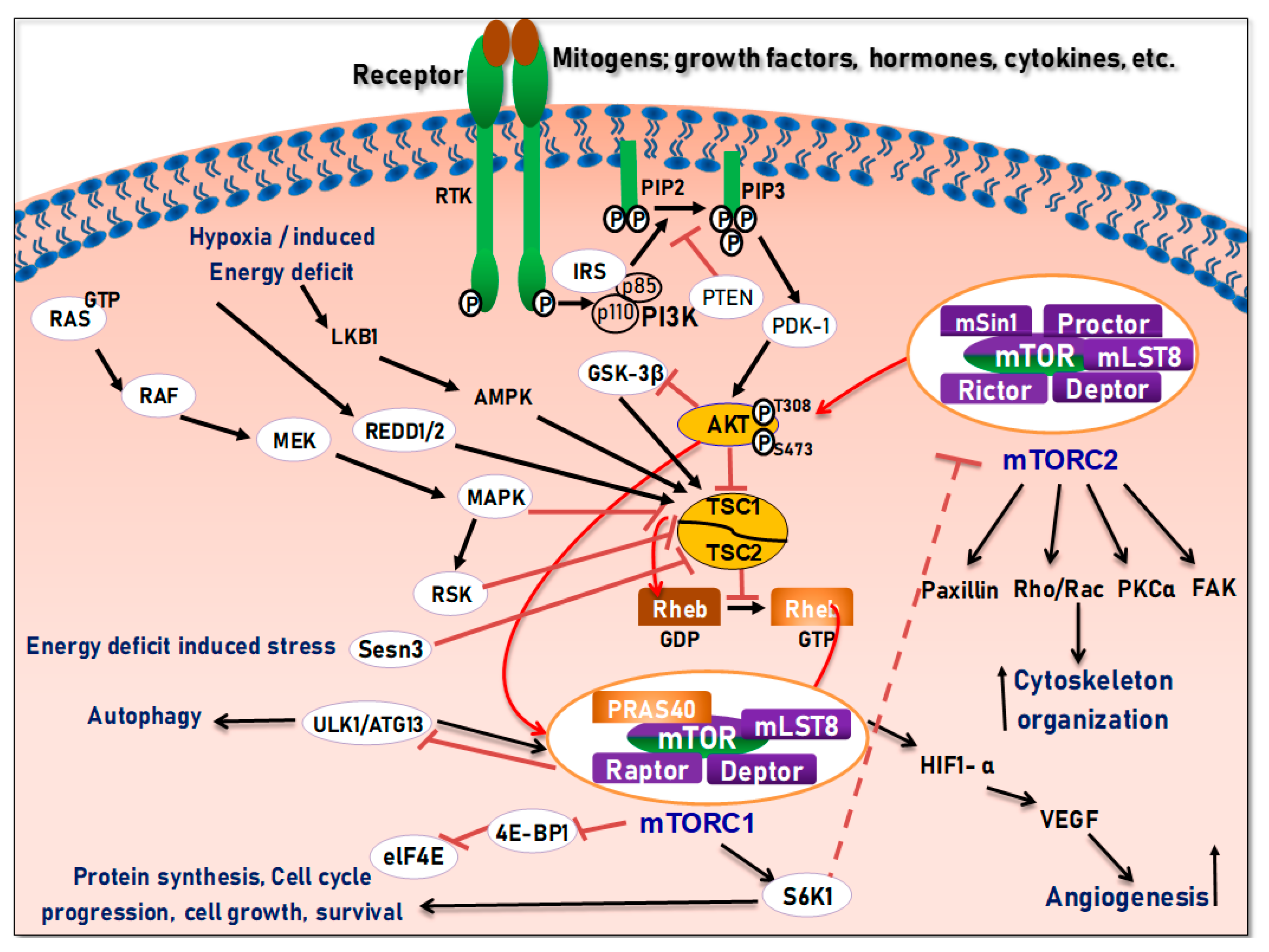
3. The PI3K/Akt/Mtor Signaling and Interrelations in Tissue Development
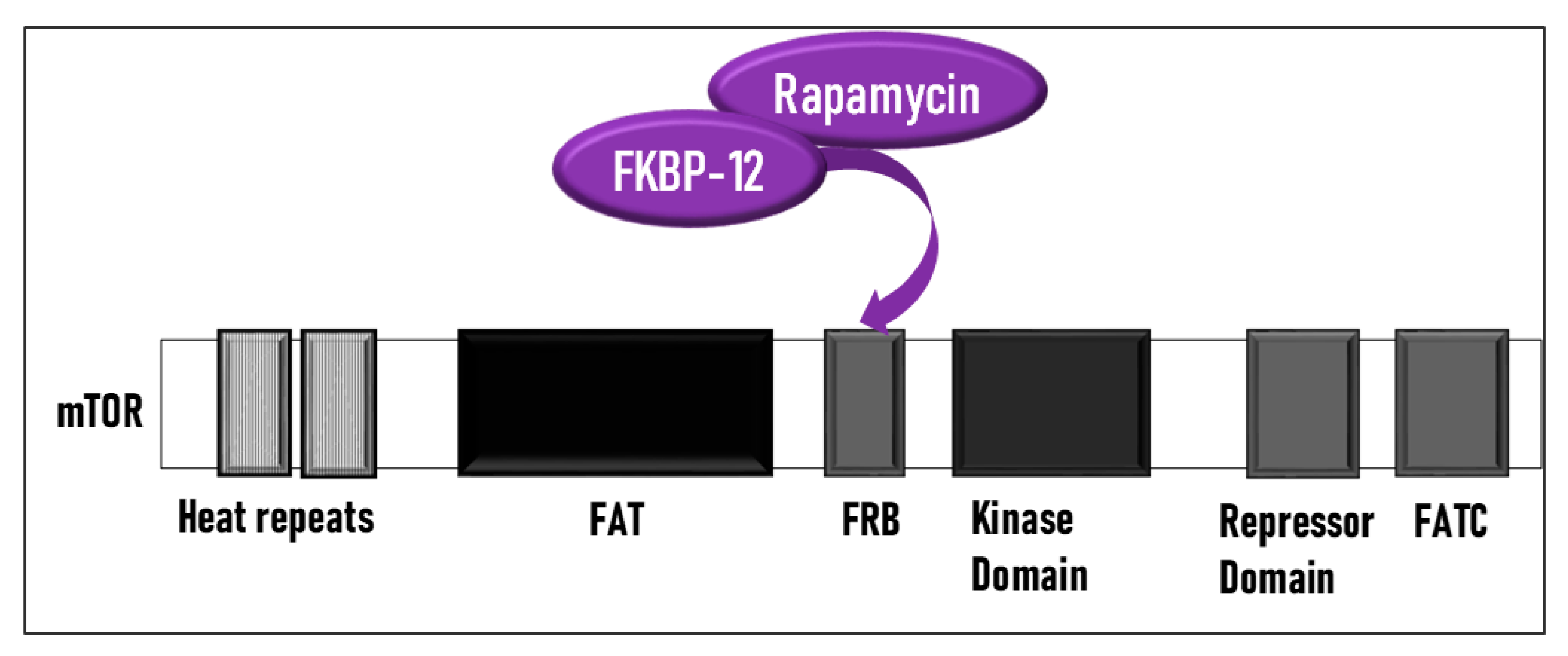
4. Structure and Function of the mTOR Pathway
5. Regulation of the PI3K/Akt/mTOR Pathways in Development and Carcinogenesis
6. Cutaneous Cancers Associated with Dysregulation of the PI3K/Akt/mTOR Pathways
6.1. Role of the PI3K/Akt/mTOR and their Targeting in Melanoma Skin Cancer
6.2. Targeting PI3K/Akt/mTOR and Associated Pathways with Chemotherapeutics, Biologic Drugs, Natural Products, and Synthetic Derivatives in Melanoma
6.2.1. Chemotherapeutic Small Molecules and Biologic Drugs
6.2.2. Natural Plant-Derived Extracts, and Phytochemicals and their Synthetic Derivatives
6.3. Targeting PI3K/Akt/mTOR for Treatment of Basal Cell Carcinoma
6.4. Targeting PI3K/Akt/mTOR for Treatment of Cutaneous Squamous Cell Carcinoma
6.5. Targeting PI3K/Akt/mTOR for Treatment of Merkel Cell Carcinoma
6.6. Targeting PI3K/Akt/mTOR for Treatment of Tuberous Sclerosis
7. Clinical Implications, Conclusions, and Future Prospects
8. Materials and Methods
Funding
Conflicts of Interest
Abbreviations
| PI3K | phosphatidyl-inositiol 3-kinase |
| BMZ | Bbaement membrane zone |
| mTOR | mammalian target of rapamycin |
| UV | ultraviolet |
| EGCG | Epigallocatechin-3-gallate |
| Akt | protein kinase B |
| NMSC | non-melanoma skin cancer |
| SCC | squamous cell carcinoma |
| BCC | basal cell carcinoma |
| MCC | Merkel cell carcinoma |
| K6 | keratin 6 |
| K14 | keratin 14 |
| EMT | epithelial-mesenchymal transition |
| CK15 | cytokeratin 15 |
| CK5 | cytokeratin |
References
- Chamcheu, J.C.; Adhami, V.M.; Siddiqui, I.V.; Mukhtar, H. Cutaneous Cell-and Gene-Based Therapies for Inherited and Acquired Skin Disorders. In Gene and Cell Therapy: Therapeutic Mechanisms and Strategies, 4th ed.; Templeton, N.S., Ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Gonzales, K.A.U.; Fuchs, E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev. Cell 2017, 43, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Magin, T.M.; Vijayaraj, P.; Leube, R.E. Structural and regulatory functions of keratins. Exp. Cell Res. 2007, 313, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Eichenfield, L.F.; Fowler, J.F.; Horowitz, P.; McLeod, R.P. Update on the structure and function of the skin barrier: Atopic dermatitis as an exemplar of clinical implications. Semin. Cutan. Med. Surg. 2013, 32, S21–S24. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Segre, J.A. Epidermal barrier formation and recovery in skin disorders. J. Clin. Investig. 2006, 116, 1150–1158. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Siddiqui, I.A.; Syed, D.N.; Adhami, V.M.; Liovic, M.; Mukhtar, H. Keratin gene mutations in disorders of human skin and its appendages. Arch. Biochem. Biophys. 2011, 508, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Katiyar, S. Green tea and skin cancer: Photoimmunology, angiogenesis and DNA repair. J. Nutr. Biochem. 2007, 18, 287–296. [Google Scholar]
- Eckhart, L.; Lippens, S.; Tschachler, E.; Declercq, W. Cell death by cornification. Biochim. Biophys. Acta 2013, 1833, 3471–3480. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Yang, X.; Wei, J.; Zhou, S.; Zhao, Z.; Cheng, J.; Duan, H.; Jia, T.; Lei, Q.; et al. Characterization of Th17 and FoxP3+ Treg Cells in Paediatric Psoriasis Patients. Scand. J. Immunol. 2016, 83, 174–180. [Google Scholar] [CrossRef]
- Ng, W.L.; Qi, J.T.Z.; Yeong, W.Y.; Naing, M.W. Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication 2018, 10, 25005. [Google Scholar] [CrossRef]
- Tharmarajah, G.; Eckhard, U.; Jain, F.; Marino, G.; Prudova, A.; Urtatiz, O.; Fuchs, H.; De Angelis, M.H.; Overall, C.M.; Van Raamsdonk, C.D. Melanocyte development in the mouse tail epidermis requires the Adamts9 metalloproteinase. Pigment. Cell Melanoma Res. 2018, 31, 693–707. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.-M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef]
- Bertolesi, G.E.; McFarlane, S. Seeing the light to change colour: An evolutionary perspective on the role of melanopsin in neuroendocrine circuits regulating light-mediated skin pigmentation. Pigment. Cell Melanoma Res. 2018, 31, 354–373. [Google Scholar] [CrossRef]
- De Assis, L.V.M.; Moraes, M.N.; Magalhaes-Marques, K.K.; de Lauro Castrucci, A.M. Melanopsin and rhodopsin mediate UVA-induced immediate pigment darkening: Unravelling the photosensitive system of the skin. Eur. J. Cell Biol. 2018, 97, 150–162. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Hearing, V.J. Physiological factors that regulate skin pigmentation. BioFactors 2009, 35, 193–199. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Morita, A.; Maeda, A.; Hearing, V.J. Regulation of skin pigmentation and thickness by dickkopf 1 (DKK1). J. Investig. Dermatol. Symp. Proc. 2009, 14, 73–75. [Google Scholar] [CrossRef]
- Perez-Sanchez, A.; Barrajon-Catalan, E.; Herranz-Lopez, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef]
- Sagi, Z.; Hieronymus, T. The Impact of the Epithelial–Mesenchymal Transition Regulator Hepatocyte Growth Factor Receptor/Met on Skin Immunity by Modulating Langerhans Cell Migration. Front. Immunol. 2018, 9, 517. [Google Scholar] [CrossRef]
- Deckers, J.; Hammad, H.; Hoste, E. Langerhans Cells: Sensing the Environment in Health and Disease. Front. Immunol. 2018, 9, 93. [Google Scholar] [CrossRef]
- Iwamoto, K.; Nümm, T.J.; Koch, S.; Herrmann, N.; Leib, N.; Bieber, T.; Stroisch, T.J. Langerhans and inflammatory dendritic epidermal cells in atopic dermatitis are tolerized toward TLR2 activation. Allergy 2018, 73, 2205–2213. [Google Scholar] [CrossRef]
- Petersson, M.; Niemann, C. Stem cell dynamics and heterogeneity: Implications for epidermal regeneration and skin cancer. Curr. Med. Chem. 2012, 19, 5984–5992. [Google Scholar] [CrossRef]
- Wang, L.-J.; Wang, Y.-L.; Yang, X. Progress in epidermal stem cells. Yi Chuan 2010, 32, 198–204. [Google Scholar] [CrossRef]
- Flores, E.R.; Halder, G. Stem cell proliferation in the skin: Alpha-catenin takes over the hippo pathway. Sci. Signal. 2011, 4, pe34. [Google Scholar] [CrossRef]
- Uzarska, M.; Porowińska, D.; Bajek, A.; Drewa, T. Epidermal stem cells—Biology and potential applications in regenerative medicine. Postępy Biochem. 2013, 59, 219–227. [Google Scholar]
- Shen, Q.; Jin, H.; Wang, X. Epidermal Stem Cells and Their Epigenetic Regulation. Int. J. Mol. Sci. 2013, 14, 17861–17880. [Google Scholar] [CrossRef]
- Lavker, R.M.; Sun, T.-T. Epidermal stem cells: Properties, markers, and location. Proc. Natl. Acad. Sci. USA 2000, 97, 13473–13475. [Google Scholar] [CrossRef]
- Matsui, T.; Amagai, M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef]
- Osawa, R.; Akiyama, M.; Shimizu, H. Filaggrin Gene Defects and the Risk of Developing Allergic Disorders. Allergol. Int. 2011, 60, 1–9. [Google Scholar] [CrossRef]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H.I. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef]
- Koster, M.I. Making an epidermis. Ann. N. Y. Acad. Sci. 2009, 1170, 7–10. [Google Scholar] [CrossRef]
- Labat-Robert, J. Information exchanges between cells and extracellular matrix. Influence of aging. Biol. Aujourd’hui 2012, 206, 103–109. [Google Scholar] [CrossRef]
- Quan, T.; Fisher, G.J. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Watt, F.M. Mammalian skin cell biology: At the interface between laboratory and clinic. Science 2014, 346, 937–940. [Google Scholar] [CrossRef]
- Gilchrest, B.A.; Geller, A.C.; Yaar, M.; Eller, M.S. The Pathogenesis of Melanoma Induced by Ultraviolet Radiation. N. Engl. J. Med. 1999, 340, 1341–1348. [Google Scholar] [CrossRef]
- Watson, M.; Holman, D.M.; Maguire-Eisen, M. Ultraviolet Radiation Exposure and Its Impact on Skin Cancer Risk. Semin. Oncol. Nurs. 2016, 32, 241–254. [Google Scholar] [CrossRef]
- Miyamura, Y.; Coelho, S.G.; Schlenz, K.; Batzer, J.; Smuda, C.; Choi, W.; Brenner, M.; Passeron, T.; Zhang, G.; Kolbe, L.; et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res. 2011, 24, 136–147. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- McKenzie, R.L.; Aucamp, P.J.; Madronich, S.; Tourpali, K.; Bais, A.; Bernhard, G.; Ilyas, M. Ozone depletion and climate change: Impacts on UV radiation. Photochem. Photobiol. Sci. 2015, 14, 19–52. [Google Scholar] [CrossRef]
- Holick, M.F. Sunlight, UV-radiation, vitamin D and skin cancer: How much sunlight do we need? Adv. Exp. Med. Biol. 2008, 624, 1–15. [Google Scholar]
- Juzeniene, A.; Moan, J. Beneficial effects of UV radiation other than via vitamin D production. Dermato-Endocrinology 2012, 4, 109–117. [Google Scholar] [CrossRef]
- Gupta, A.; Avci, P.; Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Ultraviolet Radiation in Wound Care: Sterilization and Stimulation. Adv. Wound Care 2013, 2, 422–437. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef]
- Diffey, B.L. Solar ultraviolet radiation effects on biological systems. Phys. Med. Boil. 1991, 36, 299–328. [Google Scholar] [CrossRef]
- Abeyama, K.; Eng, W.; Jester, J.V.; Vink, A.A.; Edelbaum, D.; Cockerell, C.J.; Bergstresser, P.R.; Takashima, A. A role for NF-kappaB-dependent gene transactivation in sunburn. J. Clin. Investig. 2000, 105, 1751–1759. [Google Scholar] [CrossRef]
- Leo, M.S.; Sivamani, R.K. Phytochemical modulation of the Akt/mTOR pathway and its potential use in cutaneous disease. Arch. Dermatol. Res. 2014, 306, 861–871. [Google Scholar] [CrossRef]
- Huang, S. Targeting mTOR signaling for cancer therapy. Curr. Opin. Pharmacol. 2003, 3, 371–377. [Google Scholar] [CrossRef]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR Signaling in Growth and Metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef]
- Guertin, D.A.; Sabatini, D.M. Defining the Role of mTOR in Cancer. Cancer Cell 2007, 12, 9–22. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Stephens, L.; Hawkins, P. PI3K signalling: The path to discovery and understanding. Nat. Rev. Mol. Cell Boil. 2012, 13, 195–203. [Google Scholar] [CrossRef]
- Pópulo, H.; Lopes, J.M.; Soares, P. The mTOR Signalling Pathway in Human Cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef]
- Sehgal, S.N.; Baker, H.; Vezina, C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J. Antibiot. 1975, 28, 727–732. [Google Scholar] [CrossRef]
- Sabatini, D.M.; Erdjument-Bromage, H.; Lui, M.; Tempst, P.; Snyder, S.H. RAFT1: A mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 1994, 78, 35–43. [Google Scholar] [CrossRef]
- Buerger, C. Epidermal mTORC1 Signaling Contributes to the Pathogenesis of Psoriasis and Could Serve as a Therapeutic Target. Front. Immunol. 2018, 9, 2786. [Google Scholar] [CrossRef]
- Vahidnezhad, H.; Youssefian, L.; Uitto, J.; Youssefian, L. Molecular Genetics of the PI3K-AKT-mTOR Pathway in Genodermatoses: Diagnostic Implications and Treatment Opportunities. J. Investig. Dermatol. 2016, 136, 15–23. [Google Scholar] [CrossRef]
- Huang, T.; Lin, X.; Meng, X.; Lin, M. Phosphoinositide-3 Kinase/Protein Kinase-B/Mammalian Target of Rapamycin Pathway in Psoriasis Pathogenesis. A Potential Therapeutic Target? Acta Derm. Venereol. 2014, 94, 371–379. [Google Scholar] [CrossRef]
- Kezić, A.; Popovic, L.; Lalic, K. mTOR Inhibitor Therapy and Metabolic Consequences: Where Do We Stand? Oxidative Med. Cell. Longev. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Corti, F.; Nichetti, F.; Raimondi, A.; Niger, M.; Prinzi, N.; Torchio, M.; Tamborini, E.; Perrone, F.; Pruneri, G.; Di Bartolomeo, M.; et al. Targeting the PI3K/AKT/mTOR pathway in biliary tract cancers: A review of current evidences and future perspectives. Cancer Treat. Rev. 2019, 72, 45–55. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural Products: A Continuing Source of Novel Drug Leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Strickland, L.R.; Pal, H.C.; Elmets, C.A.; Afaq, F. Targeting Drivers of Melanoma with Synthetic Small Molecules and Phytochemicals. Cancer Lett. 2015, 359, 20–35. [Google Scholar] [CrossRef]
- Cronin, K.A.; Lake, A.J.; Scott, S.; Sherman, R.L.; Noone, A.-M.; Howlader, N.; Henley, S.J.; Anderson, R.N.; Firth, A.U.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018, 124, 2785–2800. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Qin, H.; Xu, J. The Role of Autophagy in the Resistance to BRAF Inhibition in BRAF-Mutated Melanoma. Target. Oncol. 2018, 13, 437–446. [Google Scholar] [CrossRef]
- Wahid, M.; Jawed, A.; Mandal, R.K.; Dar, S.A.; Akhter, N.; Somvanshi, P.; Khan, F.; Lohani, M.; Areeshi, M.Y.; Haque, S. Recent developments and obstacles in the treatment of melanoma with BRAF and MEK inhibitors. Crit. Rev. Oncol. 2018, 125, 84–88. [Google Scholar] [CrossRef]
- Shao, Z.; Bao, Q.; Jiang, F.; Qian, H.; Fang, Q.; Hu, X. VS-5584, a Novel PI3K-mTOR Dual Inhibitor, Inhibits Melanoma Cell Growth In Vitro and In Vivo. PLoS ONE 2015, 10, e0132655. [Google Scholar] [CrossRef]
- Syed, D.N.; Chamcheu, J.-C.; Khan, M.I.; Sechi, M.; Lall, R.K.; Adhami, V.M.; Mukhtar, H. Fisetin inhibits human melanoma cell growth through direct binding to p70S6K and mTOR: Findings from 3-D melanoma skin equivalents and computational modeling. Biochem. Pharmacol. 2014, 89, 349–360. [Google Scholar] [CrossRef][Green Version]
- Bosbous, M.W.; Dzwierzynski, W.W.; Neuburg, M. Lentigo Maligna: Diagnosis and Treatment. Clin. Plast. Surg. 2010, 37, 35–46. [Google Scholar] [CrossRef]
- Broussard, L.; Howland, A.; Ryu, S.; Song, K.; Norris, D.; Armstrong, C.A.; Song, P.I. Melanoma Cell Death Mechanisms. Chonnam Med. J. 2018, 54, 135–142. [Google Scholar] [CrossRef][Green Version]
- Hersey, P.; Bastholt, L.; Chiarion-Sileni, V.; Cinat, G.; Dummer, R.; Eggermont, A.M.M.; Espinosa, E.; Hauschild, A.; Quirt, I.; Robert, C.; et al. Small molecules and targeted therapies in distant metastatic disease. Ann. Oncol. 2009, 20, vi35–vi40. [Google Scholar] [CrossRef]
- Yu, Y. A novel combination treatment against melanoma with NRAS mutation and therapy resistance. EMBO Mol. Med. 2018, 10, e8573. [Google Scholar] [CrossRef]
- Caenepeel, S.; Cooke, K.; Wadsworth, S.; Huang, G.; Robert, L.; Moreno, B.H.; Parisi, G.; Cajulis, E.; Kendall, R.; Beltran, P.; et al. MAPK pathway inhibition induces MET and GAB1 levels, priming BRAF mutant melanoma for rescue by hepatocyte growth factor. Oncotarget 2017, 8, 17795–17809. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, Q.; Wang, H.; Chang, P.; Tao, K. KCNQ1OT1 promotes melanoma growth and metastasis. Aging 2018, 10, 632–644. [Google Scholar] [CrossRef]
- Luan, W.; Li, L.; Shi, Y.; Bu, X.; Xia, Y.; Wang, J.; Djangmah, H.S.; Liu, X.; You, Y.; Xu, B. Long non-coding RNA MALAT1 acts as a competing endogenous RNA to promote malignant melanoma growth and metastasis by sponging miR-22. Oncotarget 2016, 7, 63901–63912. [Google Scholar] [CrossRef]
- Luan, W.; Li, R.; Liu, L.; Ni, X.; Shi, Y.; Xia, Y.; Wang, J.; Lu, F.; Xu, B. Long non-coding RNA HOTAIR acts as a competing endogenous RNA to promote malignant melanoma progression by sponging miR-152-3p. Oncotarget 2017, 8, 85401–85414. [Google Scholar] [CrossRef]
- Karbowniczek, M.; Spittle, C.S.; Morrison, T.; Wu, H.; Henske, E.P. mTOR Is Activated in the Majority of Malignant Melanomas. J. Investig. Dermatol. 2008, 128, 980–987. [Google Scholar] [CrossRef]
- Molhoek, K.R.; Brautigan, D.L.; Slingluff, C.L. Synergistic inhibition of human melanoma proliferation by combination treatment with B-Raf inhibitor BAY43-9006 and mTOR inhibitor Rapamycin. J. Transl. Med. 2005, 3, 39. [Google Scholar] [CrossRef]
- Vera Aguilera, J.; Rao, R.D.; Allred, J.B.; Suman, V.J.; Windschitl, H.E.; Kaur, J.S.; Maples, W.J.; Lowe, V.J.; Creagan, E.T.; Erickson, L.A.; et al. Phase II Study of Everolimus in Metastatic Malignant Melanoma (NCCTG-N0377, Alliance). Oncologist 2018, 23, 887-e94. [Google Scholar] [CrossRef]
- Niessner, H.; Kosnopfel, C.; Sinnberg, T.; Beck, D.; Krieg, K.; Wanke, I.; Lasithiotakis, K.; Bonin, M.; Garbe, C.; Meier, F. Combined activity of temozolomide and the mTOR inhibitor temsirolimus in metastatic melanoma involves DKK1. Exp. Dermatol. 2017, 26, 598–606. [Google Scholar] [CrossRef]
- Rangwala, R.; Chang, Y.C.; Hu, J.; Algazy, K.M.; Evans, T.L.; Fecher, L.A.; Schuchter, L.M.; Torigian, D.A.; Panosian, J.T.; Troxel, A.B.; et al. Combined MTOR and autophagy inhibition: Phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy 2014, 10, 1391–1402. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Infante, J.R.; Spigel, D.R.; Peyton, J.D.; Thompson, D.S.; Lane, C.M.; Clark, B.L.; Rubin, M.S.; Trent, D.F.; Burris, H.A., III. Bevacizumab and everolimus in the treatment of patients with metastatic melanoma: A phase 2 trial of the Sarah Cannon Oncology Research Consortium. Cancer 2010, 116, 4122–4129. [Google Scholar] [CrossRef]
- Kolev, V.N.; Wright, Q.G.; Vidal, C.M.; Ring, J.E.; Shapiro, I.M.; Ricono, J.; Weaver, D.T.; Padval, M.V.; Pachter, J.A.; Xu, Q. PI3K/mTOR dual inhibitor VS-5584 preferentially targets cancer stem cells. Cancer Res. 2015, 75, 446–455. [Google Scholar] [CrossRef]
- Hutchinson, L. Skin cancer. Golden age of melanoma therapy. Nat. Rev. Clin. Oncol. 2015, 12, 1. [Google Scholar] [CrossRef]
- Webster, R.M.; Mentzer, S.E. The malignant melanoma landscape. Nat. Rev. Drug Discov. Engl. 2014, 13, 491–492. [Google Scholar] [CrossRef]
- Schadendorf, D.; Hauschild, A. Melanoma in 2013: Melanoma—The run of success continues. Nat. Rev. Clin. Oncol. 2014, 11, 75–76. [Google Scholar] [CrossRef]
- Mi, C.; Ma, J.; Shi, H.; Li, J.; Wang, F.; Lee, J.J.; Jin, X. 4′,6-dihydroxy-4-methoxyisoaurone inhibits the HIF-1alpha pathway through inhibition of Akt/mTOR/p70S6K/4E-BP1 phosphorylation. J. Pharmacol. Sci. 2014, 125, 193–201. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Wen, J.; Ma, F.; Wang, F.; Yu, K.; Tang, M.; Wu, W.; Dong, Y.; Cheng, X.; et al. SKLB-M8 induces apoptosis through the AKT/mTOR signaling pathway in melanoma models and inhibits angiogenesis with decrease of ERK1/2 phosphorylation. J. Pharmacol. Sci. 2014, 126, 198–207. [Google Scholar] [CrossRef]
- Oudart, J.-B.; Doue, M.; Vautrin, A.; Brassart, B.; Sellier, C.; Dupont-Deshorgue, A.; Monboisse, J.C.; Maquart, F.X.; Brassart-Pasco, S.; Ramont, L. The anti-tumor NC1 domain of collagen XIX inhibits the FAK/PI3K/Akt/mTOR signaling pathway through alphavbeta3 integrin interaction. Oncotarget 2016, 7, 1516–1528. [Google Scholar] [CrossRef]
- Lee, B.Y.; Timpson, P.; Horvath, L.G.; Daly, R.J. FAK signaling in human cancer as a target for therapeutics. Pharmacol. Ther. 2015, 146, 132–149. [Google Scholar] [CrossRef]
- Sulzmaier, F.J.; Jean, C.; Schlaepfer, D.D. FAK in cancer: Mechanistic findings and clinical applications. Nat. Rev. Cancer 2014, 14, 598–610. [Google Scholar] [CrossRef]
- Si, L.; Xu, X.; Kong, Y.; Flaherty, K.T.; Chi, Z.; Cui, C.; Sheng, X.; Li, S.; Dai, J.; Yu, W.; et al. Major Response to Everolimus in Melanoma With Acquired Imatinib Resistance. J. Clin. Oncol. 2012, 30, e37–e40. [Google Scholar] [CrossRef]
- Pierard-Franchimont, C.; Hermanns-Lê, T.; Paquet, P.; Herfs, M.; Delvenne, P.; Piérard, G.E. Hedgehog-and mTOR-targeted therapies for advanced basal cell carcinomas. Future Oncol. 2015, 11, 2997–3002. [Google Scholar] [CrossRef]
- Kapoor, V.; Zaharieva, M.M.; Das, S.N.; Berger, M.R. Erufosine simultaneously induces apoptosis and autophagy by modulating the Akt–mTOR signaling pathway in oral squamous cell carcinoma. Cancer Lett. 2012, 319, 39–48. [Google Scholar] [CrossRef]
- Ding, L.-T.; Zhao, P.; Yang, M.-L.; Lv, G.-Z.; Zhao, T.-L. GDC-0084 inhibits cutaneous squamous cell carcinoma cell growth. Biochem. Biophys. Res. Commun. 2018, 503, 1941–1948. [Google Scholar] [CrossRef]
- Nguyen, N.; Sharma, A.; Nguyen, N.; Sharma, A.K.; Desai, D.; Huh, S.J.; Amin, S.; Meyers, C.; Robertson, G.P. Melanoma chemoprevention in skin reconstructs and mouse xenografts using isoselenocyanate-4. Cancer Prev. Res. 2011, 4, 248–258. [Google Scholar] [CrossRef]
- Kannan, A.; Lin, Z.; Shao, Q.; Zhao, S.; Fang, B.; Moreno, M.A.; Vural, E.; Stack, B.C., Jr.; Suen, J.Y.; Kannan, K.; et al. Dual mTOR inhibitor MLN0128 suppresses Merkel cell carcinoma (MCC) xenograft tumor growth. Oncotarget 2016, 7, 6576–6592. [Google Scholar] [CrossRef]
- Cassler, N.M.; Merrill, D.; Bichakjian, C.K.; Brownell, I. Merkel Cell Carcinoma Therapeutic Update. Curr. Treat. Options Oncol. 2016, 17, 36. [Google Scholar] [CrossRef]
- Villani, A.; Fabbrocini, G.; Costa, C.; Annunziata, M.C.; Scalvenzi, M. Merkel Cell Carcinoma: Therapeutic Update and Emerging Therapies. Dermatol. Ther. 2019, 9, 209–222. [Google Scholar] [CrossRef]
- Calero, R.; Morchon, E.; Martinez-Argudo, I.; Serrano, R. Synergistic anti-tumor effect of 17AAG with the PI3K/mTOR inhibitor NVP-BEZ235 on human melanoma. Cancer Lett. 2017, 406, 1–11. [Google Scholar] [CrossRef]
- Lin, Z.; Mei, H.; Fan, J.; Yin, Z.; Wu, G. Effect of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 against human Merkel cell carcinoma MKL-1 cells. Oncol. Lett. 2015, 10, 3663–3667. [Google Scholar] [CrossRef][Green Version]
- Chung, C.-Y.; Madhunapantula, S.V.; Desai, D.; Amin, S.; Robertson, G.P. Melanoma prevention using topical PBISe. Cancer Prev. Res. 2011, 4, 935–948. [Google Scholar] [CrossRef]
- Hou, G.; Xue, L.; Lu, Z.; Fan, T.; Tian, F.; Xue, Y. An activated mTOR/p70S6K signaling pathway in esophageal squamous cell carcinoma cell lines and inhibition of the pathway by rapamycin and siRNA against mTOR. Cancer Lett. 2007, 253, 236–248. [Google Scholar] [CrossRef]
- Werzowa, J.; Koehrer, S.; Strommer, S.; Cejka, D.; Fuereder, T.; Zebedin, E.; Wacheck, V. Vertical Inhibition of the mTORC1/mTORC2/PI3K Pathway Shows Synergistic Effects against Melanoma In Vitro and In Vivo. J. Investig. Dermatol. 2011, 131, 495–503. [Google Scholar] [CrossRef]
- Chong, K.; Daud, A.; Ortiz-Urda, S.; Arron, S.T. Cutting Edge in Medical Management of Cutaneous Oncology. Semin. Cutan. Med. Surg. 2012, 31, 140–149. [Google Scholar] [CrossRef][Green Version]
- So, P.-L.; Wang, G.Y.; Wang, K.; Chuang, M.; Chiueh, V.C.; Kenny, P.A.; Epstein, E.H. PI3K-AKT signaling is a downstream effector of retinoid prevention of murine basal cell carcinogenesis. Cancer Prev. Res. 2014, 7, 407–417. [Google Scholar] [CrossRef]
- Wu, C.-S.; Chen, G.-S.; Lin, P.-Y.; Pan, I.-H.; Wang, S.-T.; Lin, S.H.; Yu, H.-S.; Lin, C.-C. Tazarotene Induces Apoptosis in Human Basal Cell Carcinoma via Activation of Caspase-8/t-Bid and the Reactive Oxygen Species-Dependent Mitochondrial Pathway. DNA Cell Boil. 2014, 33, 652–666. [Google Scholar] [CrossRef]
- Velho, T.R. Metastatic melanoma—A review of current and future drugs. Drugs Context 2012, 2012, 1–17. [Google Scholar] [CrossRef]
- Lin, Z.; McDermott, A.; Shao, L.; Kannan, A.; Morgan, M.; Stack, B.C., Jr.; Moreno, M.; Davis, D.A.; Cornelius, L.A.; Gao, L. Chronic mTOR activation promotes cell survival in Merkel cell carcinoma. Cancer Lett. 2014, 344, 272–281. [Google Scholar] [CrossRef]
- Liang, G.; Liu, M.; Wang, Q.; Shen, Y.; Mei, H.; Li, D.; Liu, W. Itraconazole exerts its anti-melanoma effect by suppressing Hedgehog, Wnt, and PI3K/mTOR signaling pathways. Oncotarget 2017, 8, 28510–28525. [Google Scholar] [CrossRef]
- Head, S.A.; Shi, W.; Zhao, L.; Gorshkov, K.; Pasunooti, K.; Chen, Y.; Deng, Z.; Li, R.-J.; Shim, J.S.; Tan, W.; et al. Antifungal drug itraconazole targets VDAC1 to modulate the AMPK/mTOR signaling axis in endothelial cells. Proc. Natl. Acad. Sci. USA 2015, 112, E7276–E7285. [Google Scholar] [CrossRef]
- Zou, Y.; Ge, M.; Wang, X. Targeting PI3K-AKT-mTOR by LY3023414 inhibits human skin squamous cell carcinoma cell growth in vitro and in vivo. Biochem. Biophys. Res. Commun. 2017, 490, 385–392. [Google Scholar] [CrossRef]
- Sweetlove, M.; Wrightson, E.; Kolekar, S.; Rewcastle, G.W.; Baguley, B.C.; Shepherd, P.R.; Jamieson, S.M.F. Inhibitors of pan-PI3K Signaling Synergize with BRAF or MEK Inhibitors to Prevent BRAF-Mutant Melanoma Cell Growth. Front. Oncol. 2015, 5, 135. [Google Scholar] [CrossRef]
- Polini, B.; Carpi, S.; Romanini, A.; Breschi, M.C.; Nieri, P.; Podestà, A. Circulating cell-free microRNAs in cutaneous melanoma staging and recurrence or survival prognosis. Pigment. Cell Melanoma Res. 2019, 32, 486–499. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Li, C.; Wang, W. miR-224-5p inhibits proliferation, migration, and invasion by targeting PIK3R3/AKT3 in uveal melanoma. J. Cell. Biochem. 2019, 120, 12412–12421. [Google Scholar] [CrossRef]
- Jiang, Q.-Q.; Liu, W.-B. miR-25 Promotes Melanoma Progression by regulating RNA binding motif protein 47. Med. Sci. 2018, 34, 59–65. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, Y.; Li, X.; Wang, J. Clinical significance of miR-138 in patients with malignant melanoma through targeting of PDK1 in the PI3K/AKT autophagy signaling pathway. Oncol. Rep. 2017, 38, 1655–1662. [Google Scholar] [CrossRef]
- Micevic, G.; Muthusamy, V.; Damsky, W.; Theodosakis, N.; Liu, X.; Meeth, K.; Wingrove, E.; Santhanakrishnan, M.; Bosenberg, M. DNMT3b modulates melanoma growth by controlling levels of mTORC2 component RICTOR. Cell Rep. 2016, 14, 2180–2192. [Google Scholar] [CrossRef]
- Schmidt, K.M.; Dietrich, P.; Hackl, C.; Guenzle, J.; Bronsert, P.; Wagner, C.; Fichtner-Feigl, S.; Schlitt, H.J.; Geissler, E.K.; Hellerbrand, C.; et al. Inhibition of mTORC2/RICTOR Impairs Melanoma Hepatic Metastasis12. Neoplasia 2018, 20, 1198–1208. [Google Scholar] [CrossRef]
- Damsky, W.; Micevic, G.; Meeth, K.; Muthusamy, V.; Curley, D.P.; Santhankrishnan, M.; Erdélyi, I.; Platt, J.T.; Huang, L.; Theodosakis, N.; et al. mTORC1 activation blocks BrafV600E-induced growth-arrest, but is insufficient for melanoma formation. Cancer Cell 2015, 27, 41–56. [Google Scholar] [CrossRef]
- Ciołczyk-Wierzbicka, D.; Gil, D.; Laidler, P. Treatment of melanoma with selected inhibitors of signaling kinases effectively reduces proliferation and induces expression of cell cycle inhibitors. Med. Oncol. 2017, 35, 7. [Google Scholar] [CrossRef]
- Sikora, A.G.; Gelbard, A.; Davies, M.A.; Sano, D.; Ekmekcioglu, S.; Kwon, J.; Hailemichael, Y.; Jayaraman, P.; Myers, J.N.; Grimm, E.A.; et al. Targeted inhibition of inducible nitric oxide synthase inhibits growth of human melanoma in vivo and synergizes with chemotherapy. Clin. Cancer Res. 2010, 16, 1834–1844. [Google Scholar] [CrossRef]
- Ernst, D.S.; Eisenhauer, E.; Wainman, N.; Davis, M.; Lohmann, R.; Baetz, T.; Bélanger, K.; Smylie, M. Phase II Study of Perifosine in Previously Untreated Patients with Metastatic Melanoma. Investig. New Drugs 2005, 23, 569–575. [Google Scholar] [CrossRef]
- Jung, S.K.; Kim, J.E.; Lee, S.-Y.; Lee, M.H.; Byun, S.; Kim, Y.A.; Lim, T.G.; Reddy, K.; Huang, Z.; Bode, A.M.; et al. The P110 subunit of PI3-K is a therapeutic target of acacetin in skin cancer. Carcinogenesis 2014, 35, 123–130. [Google Scholar] [CrossRef]
- Chien, S.-T.; Lin, S.-S.; Wang, C.-K.; Lee, Y.-B.; Chen, K.-S.; Fong, Y.; Shih, Y.W. Acacetin inhibits the invasion and migration of human non-small cell lung cancer A549 cells by suppressing the p38alpha MAPK signaling pathway. Mol. Cell. Biochem. 2011, 350, 135–148. [Google Scholar] [CrossRef]
- Shin, D.-H.; Kim, O.-H.; Jun, H.-S.; Kang, M.-K. Inhibitory effect of capsaicin on B16-F10 melanoma cell migration via the phosphatidylinositol 3-kinase/Akt/Rac1 signal pathway. Exp. Mol. Med. 2008, 40, 486–494. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.; Wang, M. Evodiamine-induced human melanoma A375-S2 cell death was mediated by PI3K/Akt/caspase and Fas-L/NF-kappaB signaling pathways and augmented by ubiquitin-proteasome inhibition. Toxicol. In Vitro 2010, 24, 898–904. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Li, D.-F.; Han, J.-C.; Wang, B.; Dong, Z.-P.; Yu, L.-N.; Pan, Z.H.; Qu, C.J.; Chen, Y.; Sun, S.G.; et al. Reprogramming induced by isoliquiritigenin diminishes melanoma cachexia through mTORC2-AKT-GSK3beta signaling. Oncotarget 2017, 8, 34565–34575. [Google Scholar]
- Lim, H.N.; Baek, S.B.; Jung, H.J. Bee Venom and Its Peptide Component Melittin Suppress Growth and Migration of Melanoma Cells via Inhibition of PI3K/AKT/mTOR and MAPK Pathways. Molecules 2019, 24, 929. [Google Scholar] [CrossRef]
- Lai, S.-L.; Mustafa, M.R.; Wong, P.-F.; Mr, M. Panduratin A induces protective autophagy in melanoma via the AMPK and mTOR pathway. Phytomedicine 2018, 42, 144–151. [Google Scholar] [CrossRef]
- Zou, N.; Wei, Y.; Li, F.; Yang, Y.; Cheng, X.; Wang, C. The inhibitory effects of compound Muniziqi granule against B16 cells and harmine induced autophagy and apoptosis by inhibiting Akt/mTOR pathway. BMC Complement. Altern. Med. 2017, 17, 517. [Google Scholar] [CrossRef]
- Sun, Z.; Zheng, L.; Liu, X.; Xing, W.; Liu, X. Sinomenine inhibits the growth of melanoma by enhancement of autophagy via PI3K/AKT/mTOR inhibition. Drug Des. Dev. Ther. 2018, 12, 2413–2421. [Google Scholar] [CrossRef]
- Espona-Fiedler, M.; Soto-Cerrato, V.; Hosseini, A.; Lizcano, J.M.; Guallar, V.; Quesada, R.; Gao, T.; Pérez-Tomás, R. Identification of dual mTORC1 and mTORC2 inhibitors in melanoma cells: Prodigiosin vs. obatoclax. Biochem. Pharmacol. 2012, 83, 489–496. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Meyskens, F. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol. Nutr. Food Res. 2016, 60, 1264–1274. [Google Scholar] [CrossRef]
- Pal, H.C.; Hunt, K.M.; Diamond, A.; Elmets, C.A.; Afaq, F. Phytochemicals for the Management of Melanoma. Mini-Rev. Med. Chem. 2016, 16, 953–979. [Google Scholar]
- Adhami, V.M.; Syed, D.N.; Khan, N.; Mukhtar, H. Dietary flavonoid fisetin: A novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem. Pharmacol. 2012, 84, 1277–1281. [Google Scholar] [CrossRef]
- Syed, D.N.; Mukhtar, H. Botanicals for the prevention and treatment of cutaneous melanoma. Pigment. Cell Melanoma Res. 2011, 24, 688–702. [Google Scholar] [CrossRef]
- Govindarajan, B.; Sligh, J.E.; Vincent, B.J.; Li, M.; Canter, J.A.; Nickoloff, B.J.; Rodenburg, R.J.; Smeitink, J.A.; Oberley, L.; Zhang, Y.; et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J. Clin. Investig. 2007, 117, 719–729. [Google Scholar] [CrossRef]
- Pearce, L.R.; Alton, G.R.; Richter, D.T.; Kath, J.C.; Lingardo, L.; Chapman, J.; Hwang, C.; Alessi, D.R. Characterization of PF-4708671, a novel and highly specific inhibitor of p70 ribosomal S6 kinase (S6K1). Biochem. J. 2010, 431, 245–255. [Google Scholar] [CrossRef]
- Sechi, M.; Lall, R.K.; Afolabi, S.O.; Singh, A.; Joshi, D.C.; Chiu, S.-Y.; Mukhtar, H.; Syed, D.N. Fisetin targets YB-1/RSK axis independent of its effect on ERK signaling: Insights from in vitro and in vivo melanoma models. Sci. Rep. 2018, 8, 15726. [Google Scholar] [CrossRef]
- Pal, H.C.; Baxter, R.D.; Hunt, K.M.; Agarwal, J.; Elmets, C.A.; Athar, M.; Afaq, F. Fisetin, a phytochemical, potentiates sorafenib-induced apoptosis and abrogates tumor growth in athymic nude mice implanted with BRAF-mutated melanoma cells. Oncotarget 2015, 6, 28296–28311. [Google Scholar] [CrossRef]
- Zhao, G.; Han, X.; Zheng, S.; Li, Z.; Sha, Y.; Ni, J.; Sun, Z.; Qiao, S.; Song, Z. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol. Rep. 2016, 35, 1065–1074. [Google Scholar] [CrossRef]
- Rozzo, C.; Fanciulli, M.; Fraumene, C.; Corrias, A.; Cubeddu, T.; Sassu, I.; Cossu, S.; Nieddu, V.; Galleri, G.; Azara, E.; et al. Molecular changes induced by the curcumin analogue D6 in human melanoma cells. Mol. Cancer 2013, 12, 37. [Google Scholar] [CrossRef]
- Wang, M.; Yu, T.; Zhu, C.; Sun, H.; Qiu, Y.; Zhu, X.; Li, J. Resveratrol Triggers Protective Autophagy Through the Ceramide/Akt/mTOR Pathway in Melanoma B16 Cells. Nutr. Cancer 2014, 66, 435–440. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Darjatmoko, S.R.; Polans, A.S. Resveratrol modulates the malignant properties of cutaneous melanoma through changes in the activation and attenuation of the antiapoptotic protooncogenic protein Akt/PKB. Melanoma Res. 2011, 21, 180–187. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar]
- Kaushik, G.; Ramalingam, S.; Subramaniam, D.; Rangarajan, P.; Protti, P.; Rammamoorthy, P.; Anant, S.; Mammen, J.M.V. Honokiol induces cytotoxic and cytostatic effects in malignant melanoma cancer cells. Am. J. Surg. 2012, 204, 868–873. [Google Scholar] [CrossRef]
- Prieto, J.M.; Alqathama, A. Natural products with therapeutic potential in melanoma metastasis. Nat. Prod. Rep. 2015, 32, 1170–1182. [Google Scholar]
- Hambright, H.G.; Meng, P.; Kumar, A.P.; Ghosh, R. Inhibition of PI3K/AKT/mTOR axis disrupts oxidative stress-mediated survival of melanoma cells. Oncotarget 2015, 6, 7195–7208. [Google Scholar] [CrossRef]
- Gong, J.; Munoz, A.R.; Chan, D.; Ghosh, R.; Kumar, A.P. STAT3 down regulates LC3 to inhibit autophagy and pancreatic cancer cell growth. Oncotarget 2014, 5, 2529–2541. [Google Scholar] [CrossRef]
- Huang, K.-J.; Kuo, C.-H.; Chen, S.-H.; Lin, C.-Y.; Lee, Y.-R. Honokiol inhibits in vitro and in vivo growth of oral squamous cell carcinoma through induction of apoptosis, cell cycle arrest and autophagy. J. Cell. Mol. Med. 2018, 22, 1894–1908. [Google Scholar] [CrossRef]
- Surdu, S. Non-melanoma skin cancer: Occupational risk from UV light and arsenic exposure. Rev. Environ. Health 2014, 29, 255–264. [Google Scholar] [CrossRef]
- Burton, K.A.; Ashack, K.A.; Khachemoune, A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am. J. Clin. Dermatol. 2016, 17, 491–508. [Google Scholar] [CrossRef]
- Cohen, B.J.; Cohen, E.S.; Cohen, P.R. Basal Cell Carcinoma: A Patient and Physician’s Experience. Dermatol. Ther. 2018, 8, 329–337. [Google Scholar] [CrossRef]
- Wong, C.S.M.; Strange, R.C.; Lear, J.T. Basal cell carcinoma. BMJ 2003, 327, 794–798. [Google Scholar] [CrossRef]
- Marzuka, A.G.; Book, S.E. Basal Cell Carcinoma: Pathogenesis, Epidemiology, Clinical Features, Diagnosis, Histopathology, and Management. Yale J. Boil. Med. 2015, 88, 167–179. [Google Scholar]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef]
- Eisemann, N.; Waldmann, A.; Geller, A.C.; Weinstock, M.A.; Volkmer, B.; Greinert, R.; Breitbart, E.W.; Katalinic, A. Non-Melanoma Skin Cancer Incidence and Impact of Skin Cancer Screening on Incidence. J. Investig. Dermatol. 2014, 134, 43–50. [Google Scholar] [CrossRef]
- Guy, G.P.; Thomas, C.C.; Thompson, T.; Watson, M.; Massetti, G.M.; Richardson, L.C. Vital Signs: Melanoma Incidence and Mortality Trends and Projections—United States, 1982–2030. MMWR. Morb. Mortal. Wkly. Rep. 2015, 64, 591–596. [Google Scholar]
- Rigel, D.S. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J. Am. Acad. Dermatol. 2008, 58, S129–S132. [Google Scholar] [CrossRef]
- Tarallo, M.; Cigna, E.; Frati, R.; Delfino, S.; Innocenzi, D.; Fama, U.; Corbianco, A.; Scuderi, N. Metatypical basal cell carcinoma: A clinical review. J. Exp. Clin. Cancer Res. 2008, 27, 65. [Google Scholar] [CrossRef]
- Athar, M.; Li, C.; Kim, A.L.; Spiegelman, V.S.; Bickers, D.R. Sonic Hedgehog Signaling in Basal Cell Nevus Syndrome. Cancer Res. 2014, 74, 4967–4975. [Google Scholar] [CrossRef]
- Noubissi, F.K.; Kim, T.; Kawahara, T.N.; Aughenbaugh, W.D.; Berg, E.; Longley, B.J.; Athar, M.; Spiegelman, V.S. Role of CRD-BP in the growth of human Basal Cell Carcinoma Cells. J. Investig. Dermatol. 2014, 134, 1718–1724. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Rady, I.; Chamcheu, R.-C.N.; Siddique, A.B.; Bloch, M.B.; Mbeumi, S.B.; Babatunde, A.S.; Uddin, M.B.; Noubissi, F.K.; Jurutka, P.W.; et al. Graviola (Annona muricata) Exerts Anti-Proliferative, Anti-Clonogenic and Pro-Apoptotic Effects in Human Non-Melanoma Skin Cancer UW-BCC1 and A431 Cells In Vitro: Involvement of Hedgehog Signaling. Int. J. Mol. Sci. 2018, 19, 1791. [Google Scholar] [CrossRef]
- Kasper, M.; Jaks, V.; Hohl, D.; Toftgård, R. Basal cell carcinoma—Molecular biology and potential new therapies. J. Clin. Investig. 2012, 122, 455–463. [Google Scholar] [CrossRef]
- Bakshi, A.; Chaudhary, S.C.; Rana, M.; Elmets, C.A.; Athar, M. Basal cell carcinoma pathogenesis and therapy involving hedgehog signaling and beyond. Mol. Carcinog. 2017, 56, 2543–2557. [Google Scholar] [CrossRef]
- Alter, M.; Hillen, U.; Leiter, U.; Sachse, M.; Gutzmer, R. Current diagnosis and treatment of basal cell carcinoma. J. Dtsch. Dermatol. Ges. 2015, 13, 863–875. [Google Scholar] [CrossRef]
- Tsubamoto, H.; Ueda, T.; Inoue, K.; Sakata, K.; Shibahara, H.; Sonoda, T. Repurposing itraconazole as an anticancer agent. Oncol. Lett. 2017, 14, 1240–1246. [Google Scholar] [CrossRef]
- Alam, M.; Ratner, D. Cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef]
- Lansbury, L.; Bath-Hextall, F.; Perkins, W.; Stanton, W.; Leonardi-Bee, J. Interventions for non-metastatic squamous cell carcinoma of the skin: Systematic review and pooled analysis of observational studies. BMJ 2013, 347, f6153. [Google Scholar] [CrossRef]
- Agamia, N.F.; Abdallah, D.M.; Sorour, O.; Mourad, B.; Younan, D.N. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br. J. Dermatol. 2016, 174, 1299–1307. [Google Scholar] [CrossRef]
- Gurney, B.; Newlands, C. Management of regional metastatic disease in head and neck cutaneous malignancy.1. Cutaneous squamous cell carcinoma. Br. J. Oral Maxillofac. Surg. 2014, 52, 294–300. [Google Scholar] [CrossRef]
- Azimi, A.; Kaufman, K.L.; Ali, M.; Arthur, J.; Kossard, S.; Fernandez-Penas, P. Differential proteomic analysis of actinic keratosis, Bowen’s disease and cutaneous squamous cell carcinoma by label-free LC–MS/MS. J. Dermatol. Sci. 2018, 91, 69–78. [Google Scholar] [CrossRef]
- Voß, M.; Plasmeijer, E.I.; Van Bemmel, B.C.; Van Der Bij, W.; Klaver, N.S.; Erasmus, M.E.; De Bock, G.H.; Verschuuren, E.A.; Rácz, E. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J. Hear. Lung Transplant. 2018, 37, 853–859. [Google Scholar] [CrossRef]
- Lewis, C.M.; Glisson, B.S.; Feng, L.; Wan, F.; Tang, X.; Wistuba, I.I.; El-Naggar, A.K.; Rosenthal, D.I.; Chambers, M.S.; Lustig, R.A.; et al. A Phase II Study of Gefitinib for Aggressive Cutaneous Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 2012, 18, 1435–1446. [Google Scholar] [CrossRef]
- Karayannopoulou, G.; Euvrard, S.; Kanitakis, J. Differential expression of p-mTOR in cutaneous basal and squamous cell carcinomas likely explains their different response to mTOR inhibitors in organ-transplant recipients. Anticancer Res. 2013, 33, 3711–3714. [Google Scholar]
- Wu, N.; Du, Z.; Zhu, Y.; Song, Y.; Pang, L.; Chen, Z. The Expression and Prognostic Impact of the PI3K/AKT/mTOR Signaling Pathway in Advanced Esophageal Squamous Cell Carcinoma. Technol. Cancer Res. Treat. 2018, 17, 1533033818758772. [Google Scholar] [CrossRef]
- Euvrard, S.; Claudy, A.; Kanitakis, J. Skin Cancers after Organ Transplantation. N. Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef]
- Chen, S.-J.; Nakahara, T.; Takahara, M.; Kido, M.; Dugu, L.; Uchi, H.; Takeuchi, S.; Tu, Y.-T.; Moroi, Y.; Furue, M. Activation of the mammalian target of rapamycin signalling pathway in epidermal tumours and its correlation with cyclin-dependent kinase 2. Br. J. Dermatol. 2009, 160, 442–445. [Google Scholar] [CrossRef]
- Monaco, A.P. The Role of mTOR Inhibitors in the Management of Posttransplant Malignancy. Transplant. 2009, 87, 157–163. [Google Scholar] [CrossRef]
- Einspahr, J.G.; Calvert, V.; Alberts, D.S.; Curiel-Lewandrowski, C.; Warneke, J.; Krouse, R.; Stratton, S.P.; Liotta, L.; Longo, C.; Pellicani, G.; et al. Functional protein pathway activation mapping of the progression of normal skin to squamous cell carcinoma. Cancer Prev. Res. 2012, 5, 403–413. [Google Scholar] [CrossRef]
- Massarelli, E.; Lin, H.; Ginsberg, L.E.; Tran, H.T.; Lee, J.J.; Canales, J.R.; Williams, M.D.; Blumenschein, G.R.; Lu, C.; Heymach, J.V.; et al. Phase II trial of everolimus and erlotinib in patients with platinum-resistant recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2015, 26, 1476–1480. [Google Scholar] [CrossRef]
- Schrama, D.; Ugurel, S.; Becker, J.C. Merkel cell carcinoma: Recent insights and new treatment options. Curr. Opin. Oncol. 2012, 24, 141–149. [Google Scholar] [CrossRef]
- Shuda, M.; Kwun, H.J.; Feng, H.; Chang, Y.; Moore, P.S. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J. Clin. Investig. 2011, 121, 3623–3634. [Google Scholar] [CrossRef]
- Fahmy, M.D.; Gupta, A.; Padilla, R.J.; Segura, A.; Brookes, C.D. Desmoplastic fibroma associated with tuberous sclerosis: Case report and literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, e92–e99. [Google Scholar] [CrossRef]
- Ekure, E.N.; Addissie, Y.A.; Sokunbi, O.J.; Kruszka, P.; Muenke, M.; Adeyemo, A.A. Tuberous sclerosis in a patient from Nigeria. Am. J. Med. Genet. Part A 2019, 179, 1423–1425. [Google Scholar] [CrossRef]
- Curatolo, P.; Bombardieri, R.; Jozwiak, S. Tuberous sclerosis. Lancet 2008, 372, 657–668. [Google Scholar] [CrossRef]
- Islam, M.P.; Roach, E.S. Tuberous sclerosis complex. In Handbook of Clinical Neurology; Oxford University Press: Oxford, UK, 2015; Volume 132, pp. 97–109. [Google Scholar]
- Northrup, H.; Koenig, M.K.; Pearson, D.A.; Au, K.S. Tuberous Sclerosis Complex; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Eds.; Oxford University Press: Seattle, WA, USA, 1993. [Google Scholar]
- Wei, C.-C.; Sheu, J.-N.; Liu, J.-T.; Yang, S.-H.; Chou, I.-C.; Tsai, J.-D. Trend of seizure remission in patients with tuberous sclerosis complex: A retrospective medical review. J. Chin. Med. Assoc. 2018, 81, 724–728. [Google Scholar] [CrossRef]
- Volpi, A.; Sala, G.; Lesma, E.; Labriola, F.; Righetti, M.; Alfano, R.M.; Cozzolino, M. Tuberous sclerosis complex: New insights into clinical and therapeutic approach. J. Nephrol. 2019, 32, 355–363. [Google Scholar] [CrossRef]
- Crino, P.B.; Nathanson, K.L.; Henske, E.P. The tuberous sclerosis complex. N. Engl. J. Med. 2006, 355, 1345–1356. [Google Scholar] [CrossRef]
- Curatolo, P.; Moavero, R. mTOR Inhibitors in Tuberous Sclerosis Complex. Curr. Neuropharmacol. 2012, 10, 404–415. [Google Scholar] [CrossRef]
- Adil, A.; Singh, A.K. Neurofibromatosis Type 1 (Von Recklinghausen); StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Treichel, A.M.; Hamieh, L.; Nathan, N.R.; Tyburczy, M.E.; Wang, J.-A.; Oyerinde, O.; Raiciulescu, S.; Julien-Williams, P.; Jones, A.M.; Gopalakrishnan, V.; et al. Phenotypic distinctions between mosaic forms of tuberous sclerosis complex. Genet. Med. 2019, 1. [Google Scholar] [CrossRef]
- Giannikou, K.; Lasseter, K.D.; Grevelink, J.M.; Tyburczy, M.E.; Dies, K.A.; Zhu, Z.; Hamieh, L.; Wollison, B.M.; Thorner, A.R.; Ruoss, S.J.; et al. Low-level mosaicism in tuberous sclerosis complex: Prevalence, clinical features, and risk of disease transmission. Genet. Med. 2019, 1. [Google Scholar] [CrossRef]
- Leducq, S.; Giraudeau, B.; Tavernier, E.; Maruani, A. Topical use of mammalian target of rapamycin inhibitors in dermatology: A systematic review with meta-analysis. J. Am. Acad. Dermatol. 2019, 80, 735–742. [Google Scholar] [CrossRef]
- Combes, F.P.; Baneyx, G.; Coello, N.; Zhu, P.; Sallas, W.; Yin, H.; Nedelman, J. Population pharmacokinetics–pharmacodynamics of oral everolimus in patients with seizures associated with tuberous sclerosis complex. J. Pharmacokinet. Pharmacodyn. 2018, 45, 707–719. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Boehncke, W.-H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Desmet, S.J.; De Bosscher, K. Glucocorticoid receptors: Finding the middle ground. J. Clin. Investig. 2017, 127, 1136–1145. [Google Scholar] [CrossRef]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef]
- Proksch, E.; Schunck, M.; Zague, V.; Segger, D.; Degwert, J.; Oesser, S. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin Pharmacol. Physiol. 2014, 27, 113–119. [Google Scholar] [CrossRef]
| Lead Compound(s) | Protein Target(s) | Skin Cancer Type | Compound Structure | References |
|---|---|---|---|---|
| Everolimus (RAD-001) | mTOR | Melanoma, Basal Cell Carcinoma | 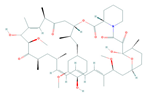 | [92,93] |
| Erufosine | mTOR | Oral Squamous Cell Carcinoma | [94] | |
| GDC-0084 | PI3K, mTOR, Akt | cutaneous Squamous Cell Carcinoma | [95] | |
| Isoselenocyanate-4 | Akt | Melanoma | 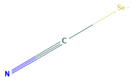 | [96] |
| MLN0128 (Sapanisertib) | mTOR | Melanoma Merkel Cell Carcinoma | 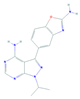 | [97,98,99] |
| NVP-BEZ235 | PI3K, Akt, mTOR | Melanoma Merkel Cell Carcinoma | 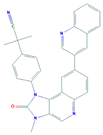 | [100,101] |
| NC1 domain of collagen Type XIX [NC1(XIX)] | PI3K, Akt, mTOR, FAK | Melanoma | [89,90,91] | |
| PBISe | Akt3 | Invasive metastatic Melanoma | 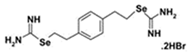 | [102] |
| Rapamycin | PI3k, Akt, mTOR | Melanoma, Esophageal squamous cell carcinoma | 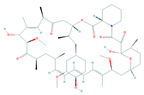 | [77,78,103] |
| SKLB-M8 | Akt, mTOR | Melanoma | [87,88] | |
| PI-103 | PI3K, mTOR | Melanoma | [104] | |
| Perifosine | Akt | Metastatic Melanoma | [105] | |
| Tazarotene | IGFR, PI3K, Akt, mTOR | Basal cell carcinoma | [106,107] | |
| Temsirolimus | mTOR | Metastatic melanoma | 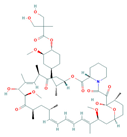 | [108] |
| WYE-354 | mTOR | Merkel cell carcinoma | [109] | |
| VS-5584 | PI3K and mTOR | Melanoma | [67,84,85,86] | |
| Itraconazole | PI3K and mTOR | Melanoma Basal Cell Carcinoma | [110,111] | |
| LY3023414 | PI3K/mTOR | Cutaneous Basal Cell Carcinoma, cutaneous Squamous Cell Carcinoma | [112] | |
| Ku-0063794 | mTORC1 and mTORC2 | BRAF-Mutant Melanoma in combination with MEK inhibitory agents Merkel cell carcinoma | 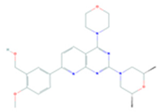 | [113] |
| Lead Compound (s) | Protein Targets | Skin Cancer Type | Compound Structure | References |
|---|---|---|---|---|
| Acacetin | PI3K, Akt, mTOR | Malignant Melanoma |  | [124] |
| Bee Venom Melittin | PI3K, Akt, mTOR | Melanoma | [129] | |
| Capsaicin | PI3K, Akt, Rac1 | Melanoma | 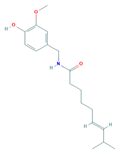 | [126] |
| Curcumin | PI3K, Akt, mTOR | Melanoma | 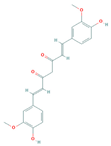 | [142,143] |
| Epigallocatechin-3 (EGCG) | mTOR | Melanoma |  | [148] |
| Evodiamine | PI3K, Akt | Melanoma |  | [127] |
| Fisetin | PI3K, Akt, mTOR | Melanoma | 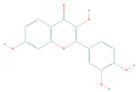 | [68,139,140,141] |
| Isoliquiritigenin | mTORC2, Akt, GSK-3β | Melanoma cachexia | 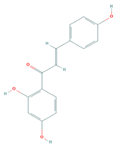 | [128] |
| Harmine | Akt, mTOR and ERK1/2 | Melanoma |  | [131] |
| Obatoclax | Akt, mTOR | Melanoma | [133] | |
| Panduratin A | mTOR | Melanoma |  | [130] |
| Prodigiosin | Akt, mTOR | Melanoma | [133] | |
| Resveratrol | Akt, mTOR | Melanoma |  | [46,144,145,146] |
| Sinomenine | PI3K, Akt, mTOR | Melanoma |  | [132] |
| Honokiol | mTOR | Melanoma, Oral squamous cell carcinoma |  | [63,147,151] |
| NexrutineR | PI3K/Akt/mTOR | Melanoma | [149,150] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamcheu, J.C.; Roy, T.; Uddin, M.B.; Banang-Mbeumi, S.; Chamcheu, R.-C.N.; Walker, A.L.; Liu, Y.-Y.; Huang, S. Role and Therapeutic Targeting of the PI3K/Akt/mTOR Signaling Pathway in Skin Cancer: A Review of Current Status and Future Trends on Natural and Synthetic Agents Therapy. Cells 2019, 8, 803. https://doi.org/10.3390/cells8080803
Chamcheu JC, Roy T, Uddin MB, Banang-Mbeumi S, Chamcheu R-CN, Walker AL, Liu Y-Y, Huang S. Role and Therapeutic Targeting of the PI3K/Akt/mTOR Signaling Pathway in Skin Cancer: A Review of Current Status and Future Trends on Natural and Synthetic Agents Therapy. Cells. 2019; 8(8):803. https://doi.org/10.3390/cells8080803
Chicago/Turabian StyleChamcheu, Jean Christopher, Tithi Roy, Mohammad Burhan Uddin, Sergette Banang-Mbeumi, Roxane-Cherille N. Chamcheu, Anthony L. Walker, Yong-Yu Liu, and Shile Huang. 2019. "Role and Therapeutic Targeting of the PI3K/Akt/mTOR Signaling Pathway in Skin Cancer: A Review of Current Status and Future Trends on Natural and Synthetic Agents Therapy" Cells 8, no. 8: 803. https://doi.org/10.3390/cells8080803
APA StyleChamcheu, J. C., Roy, T., Uddin, M. B., Banang-Mbeumi, S., Chamcheu, R.-C. N., Walker, A. L., Liu, Y.-Y., & Huang, S. (2019). Role and Therapeutic Targeting of the PI3K/Akt/mTOR Signaling Pathway in Skin Cancer: A Review of Current Status and Future Trends on Natural and Synthetic Agents Therapy. Cells, 8(8), 803. https://doi.org/10.3390/cells8080803








