Aromatase Inhibitors as Adjuvant Therapy in Early Breast Cancer: Insights into Toxicities and Their Management
Simple Summary
Abstract
1. Introduction
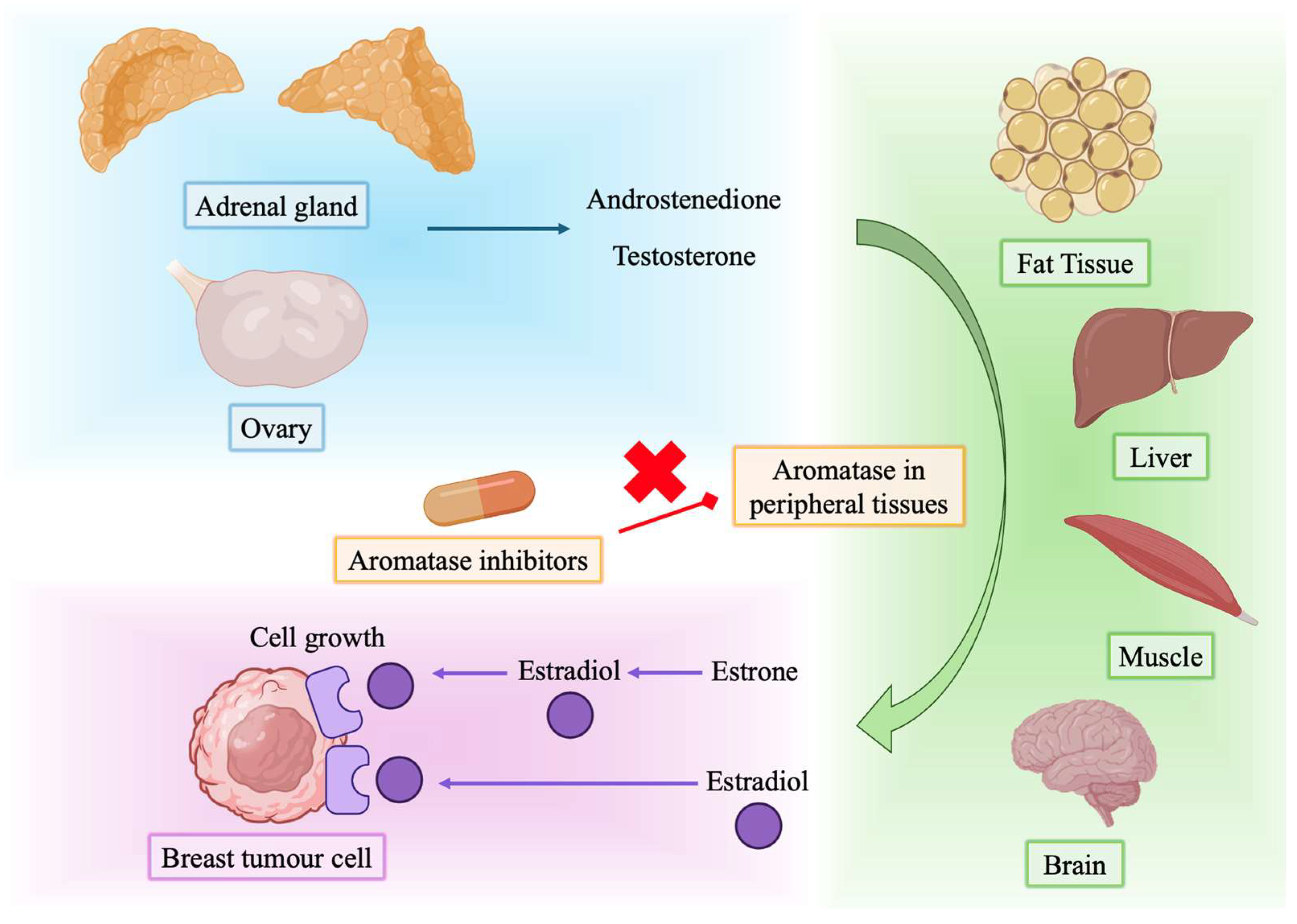
2. Mechanism of Action
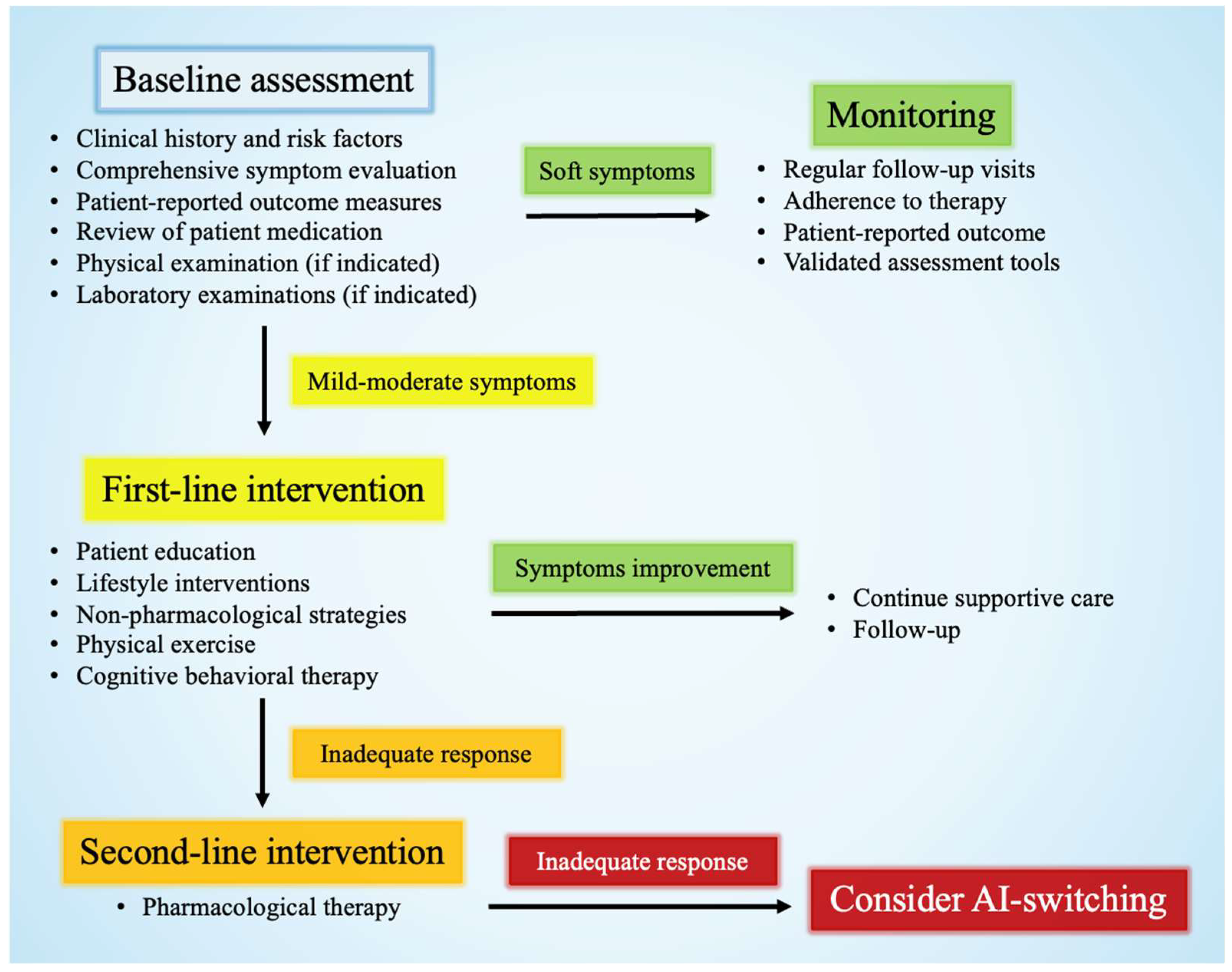
3. General Management of Side Effects
3.1. Vasomotor Symptoms
3.2. Musculoskeletal Symptoms
3.3. Bone Health
3.4. Cognitive Changes and Mood Disorders
3.5. Gynecological and Sexual Dysfunction
3.6. Fatigue
4. Addressing Cardiovascular Risks
4.1. Cardiovascular Complications
4.2. Monitoring and Management Strategies
5. Expert Opinion and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment. JAMA 2019, 321, 288. [Google Scholar] [CrossRef]
- Bradley, R.; Braybrooke, J.; Gray, R.; Hills, R.K.; Liu, Z.; Pan, H.; Peto, R.; Dodwell, D.; McGale, P.; Taylor, C.; et al. Aromatase Inhibitors versus Tamoxifen in Premenopausal Women with Oestrogen Receptor-Positive Early-Stage Breast Cancer Treated with Ovarian Suppression: A Patient-Level Meta-Analysis of 7030 Women from Four Randomised Trials. Lancet Oncol. 2022, 23, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase Inhibitors versus Tamoxifen in Early Breast Cancer: Patient-Level Meta-Analysis of the Randomised Trials. Lancet 2015, 386, 1341–1352. [Google Scholar] [CrossRef]
- Pagani, O.; Walley, B.A.; Fleming, G.F.; Colleoni, M.; Láng, I.; Gomez, H.L.; Tondini, C.; Burstein, H.J.; Goetz, M.P.; Ciruelos, E.M.; et al. Adjuvant Exemestane With Ovarian Suppression in Premenopausal Breast Cancer: Long-Term Follow-Up of the Combined TEXT and SOFT Trials. J. Clin. Oncol. 2023, 41, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Lobo-Martins, S.; Arecco, L.; Cabral, T.P.; Agostinetto, E.; Dauccia, C.; Franzoi, M.A.; del Mastro, L.; Lambertini, M.; Piccart, M.; de Azambuja, E. Extended Adjuvant Endocrine Therapy in Early Breast Cancer: Finding the Individual Balance. ESMO Open 2025, 10, 105057. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.H.; Bergh, J.; Burstein, H.J.; Cardoso, M.J.; Carey, L.A.; Dawood, S.; Del Mastro, L.; et al. Early Breast Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef]
- Franzoi, M.A.; Agostinetto, E.; Perachino, M.; Del Mastro, L.; de Azambuja, E.; Vaz-Luis, I.; Partridge, A.H.; Lambertini, M. Evidence-Based Approaches for the Management of Side-Effects of Adjuvant Endocrine Therapy in Patients with Breast Cancer. Lancet Oncol. 2021, 22, e303–e313. [Google Scholar] [CrossRef]
- Pistilli, B.; Paci, A.; Ferreira, A.R.; Di Meglio, A.; Poinsignon, V.; Bardet, A.; Menvielle, G.; Dumas, A.; Pinto, S.; Dauchy, S.; et al. Serum Detection of Nonadherence to Adjuvant Tamoxifen and Breast Cancer Recurrence Risk. J. Clin. Oncol. 2020, 38, 2762–2772. [Google Scholar] [CrossRef]
- Bernhard, J.; Luo, W.; Ribi, K.; Colleoni, M.; Burstein, H.J.; Tondini, C.; Pinotti, G.; Spazzapan, S.; Ruhstaller, T.; Puglisi, F.; et al. Patient-Reported Outcomes with Adjuvant Exemestane versus Tamoxifen in Premenopausal Women with Early Breast Cancer Undergoing Ovarian Suppression (TEXT and SOFT): A Combined Analysis of Two Phase 3 Randomised Trials. Lancet Oncol. 2015, 16, 848–858. [Google Scholar] [CrossRef]
- Henry, N.L.; Azzouz, F.; Desta, Z.; Li, L.; Nguyen, A.T.; Lemler, S.; Hayden, J.; Tarpinian, K.; Yakim, E.; Flockhart, D.A.; et al. Predictors of Aromatase Inhibitor Discontinuation as a Result of Treatment-Emergent Symptoms in Early-Stage Breast Cancer. J. Clin. Oncol. 2012, 30, 936–942. [Google Scholar] [CrossRef]
- Murphy, C.C.; Bartholomew, L.K.; Carpentier, M.Y.; Bluethmann, S.M.; Vernon, S.W. Adherence to Adjuvant Hormonal Therapy among Breast Cancer Survivors in Clinical Practice: A Systematic Review. Breast Cancer Res. Treat. 2012, 134, 459–478. [Google Scholar] [CrossRef]
- Çetiner, G.; Acar Çevik, U.; Celik, I.; Bostancı, H.E.; Özkay, Y.; Kaplancıklı, Z.A. New Imidazole Derivatives as Aromatase Inhibitor: Design, Synthesis, Biological Activity, Molecular Docking, and Computational ADME-Tox Studies. J. Mol. Struct. 2023, 1278, 134920. [Google Scholar] [CrossRef]
- Gilep, A.A.; Sushko, T.A.; Usanov, S.A. At the Crossroads of Steroid Hormone Biosynthesis: The Role, Substrate Specificity and Evolutionary Development of CYP17. Biochim. Biophys. Acta—Proteins Proteomics 2011, 1814, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Griswold, J.; Erman, M.; Pangborn, W. Structural Basis for Androgen Specificity and Oestrogen Synthesis in Human Aromatase. Nature 2009, 457, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Chumsri, S.; Howes, T.; Bao, T.; Sabnis, G.; Brodie, A. Aromatase, Aromatase Inhibitors, and Breast Cancer. J. Steroid Biochem. Mol. Biol. 2011, 125, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R. Aromatase Inhibitors: Mechanism of Action and Role in the Treatment of Breast Cancer. Semin. Oncol. 2003, 30, 3–11. [Google Scholar] [CrossRef]
- Carpenter, R.; Miller, W.R. Role of Aromatase Inhibitors in Breast Cancer. Br. J. Cancer 2005, 93, S1–S5. [Google Scholar] [CrossRef]
- Serrano, D.; Gandini, S.; Thomas, P.; Crew, K.D.; Kumar, N.B.; Vornik, L.A.; Lee, J.J.; Veronesi, P.; Viale, G.; Guerrieri-Gonzaga, A.; et al. Efficacy of Alternative Dose Regimens of Exemestane in Postmenopausal Women With Stage 0 to II Estrogen Receptor–Positive Breast Cancer. JAMA Oncol. 2023, 9, 664. [Google Scholar] [CrossRef]
- Brueggemeier, R.W. Aromatase Inhibitors in Breast Cancer Therapy. Expert Rev. Anticancer Ther. 2002, 2, 181–191. [Google Scholar] [CrossRef]
- Hertz, D.L.; Speth, K.A.; Kidwell, K.M.; Gersch, C.L.; Desta, Z.; Storniolo, A.M.; Stearns, V.; Skaar, T.C.; Hayes, D.F.; Henry, N.L.; et al. Variable Aromatase Inhibitor Plasma Concentrations Do Not Correlate with Circulating Estrogen Concentrations in Post-Menopausal Breast Cancer Patients. Breast Cancer Res. Treat. 2017, 165, 659–668. [Google Scholar] [CrossRef]
- Freedman, R.R. Menopausal Hot Flashes: Mechanisms, Endocrinology, Treatment. J. Steroid Biochem. Mol. Biol. 2014, 142, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.H. The Role of Serotonin in Hot Flushes. Maturitas 2000, 36, 155–164. [Google Scholar] [CrossRef]
- Henry, N.L.; Giles, J.T.; Ang, D.; Mohan, M.; Dadabhoy, D.; Robarge, J.; Hayden, J.; Lemler, S.; Shahverdi, K.; Powers, P.; et al. Prospective Characterization of Musculoskeletal Symptoms in Early Stage Breast Cancer Patients Treated with Aromatase Inhibitors. Breast Cancer Res. Treat. 2008, 111, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J.; Stricker, C.; Bruner, D.; Xie, S.; Bowman, M.A.; Farrar, J.T.; Greene, B.T.; DeMichele, A. Patterns and Risk Factors Associated with Aromatase Inhibitor-related Arthralgia among Breast Cancer Survivors. Cancer 2009, 115, 3631–3639. [Google Scholar] [CrossRef] [PubMed]
- Crew, K.D.; Greenlee, H.; Capodice, J.; Raptis, G.; Brafman, L.; Fuentes, D.; Sierra, A.; Hershman, D.L. Prevalence of Joint Symptoms in Postmenopausal Women Taking Aromatase Inhibitors for Early-Stage Breast Cancer. J. Clin. Oncol. 2007, 25, 3877–3883. [Google Scholar] [CrossRef]
- Reinbolt, R.E.; Sonis, S.; Timmers, C.D.; Fernández-Martínez, J.L.; Cernea, A.; de Andrés-Galiana, E.J.; Hashemi, S.; Miller, K.; Pilarski, R.; Lustberg, M.B. Genomic Risk Prediction of Aromatase Inhibitor-Related Arthralgia in Patients with Breast Cancer Using a Novel Machine-Learning Algorithm. Cancer Med. 2018, 7, 240–253. [Google Scholar] [CrossRef]
- Hadji, P. Aromatase Inhibitor-Associated Bone Loss in Breast Cancer Patients Is Distinct from Postmenopausal Osteoporosis. Crit. Rev. Oncol. Hematol. 2009, 69, 73–82. [Google Scholar] [CrossRef]
- Coleman, R.; de Boer, R.; Eidtmann, H.; Llombart, A.; Davidson, N.; Neven, P.; von Minckwitz, G.; Sleeboom, H.P.; Forbes, J.; Barrios, C.; et al. Zoledronic Acid (Zoledronate) for Postmenopausal Women with Early Breast Cancer Receiving Adjuvant Letrozole (ZO-FAST Study): Final 60-Month Results. Ann. Oncol. 2013, 24, 398–405. [Google Scholar] [CrossRef]
- Gnant, M.; Pfeiler, G.; Steger, G.G.; Egle, D.; Greil, R.; Fitzal, F.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger-Zeinitzer, E.; et al. Adjuvant Denosumab in Postmenopausal Patients with Hormone Receptor-Positive Breast Cancer (ABCSG-18): Disease-Free Survival Results from a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2019, 20, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Gallicchio, L.; Visvanathan, K.; Miller, S.R.; Babus, J.; Lewis, L.M.; Zacur, H.; Flaws, J.A. Body Mass, Estrogen Levels, and Hot Flashes in Midlife Women. Am. J. Obstet. Gynecol. 2005, 193, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Faubion, S.S.; Sood, R.; Kapoor, E. Genitourinary Syndrome of Menopause: Management Strategies for the Clinician. Mayo Clin. Proc. 2017, 92, 1842–1849. [Google Scholar] [CrossRef]
- Revenson, T.A.; Temple, L.K.; McClelland, S.I. Improving Sexual Function in Female Cancer Survivors: A Systematic Review of Psychosocial Interventions. J. Clin. Oncol. 2010, 28, e19522. [Google Scholar] [CrossRef]
- Phillips, K.-A.; Ribi, K.; Sun, Z.; Stephens, A.; Thompson, A.; Harvey, V.; Thürlimann, B.; Cardoso, F.; Pagani, O.; Coates, A.S.; et al. Cognitive Function in Postmenopausal Women Receiving Adjuvant Letrozole or Tamoxifen for Breast Cancer in the BIG 1-98 Randomized Trial. The Breast 2010, 19, 388–395. [Google Scholar] [CrossRef]
- Bender, C.M.; Sereika, S.M.; Brufsky, A.M.; Ryan, C.M.; Vogel, V.G.; Rastogi, P.; Cohen, S.M.; Casillo, F.E.; Berga, S.L. Memory Impairments with Adjuvant Anastrozole versus Tamoxifen in Women with Early-Stage Breast Cancer. Menopause 2007, 14, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Reinertsen, K.V.; Engebraaten, O.; Loge, J.H.; Cvancarova, M.; Naume, B.; Wist, E.; Edvardsen, H.; Wille, E.; Bjøro, T.; Kiserud, C.E. Fatigue During and After Breast Cancer Therapy—A Prospective Study. J. Pain Symptom Manage. 2017, 53, 551–560. [Google Scholar] [CrossRef]
- Bower, J.E. Cancer-Related Fatigue—Mechanisms, Risk Factors, and Treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef]
- Amir, E.; Seruga, B.; Niraula, S.; Carlsson, L.; Ocaña, A. Toxicity of Adjuvant Endocrine Therapy in Postmenopausal Breast Cancer Patients: A Systematic Review and Meta-Analysis. JNCI J. Natl. Cancer Inst. 2011, 103, 1299–1309. [Google Scholar] [CrossRef]
- Bérczi, B.; Farkas, N.; Hegyi, P.; Tóth, B.; Csupor, D.; Németh, B.; Lukács, A.; Czumbel, L.M.; Kerémi, B.; Kiss, I.; et al. Aromatase Inhibitors and Plasma Lipid Changes in Postmenopausal Women with Breast Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 1818. [Google Scholar] [CrossRef]
- ATAC Trialists’ Group Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) Trial after Completion of 5 Years’ Adjuvant Treatment for Breast Cancer. Lancet 2005, 365, 60–62. [CrossRef]
- Mann, E.; Smith, M.J.; Hellier, J.; Balabanovic, J.A.; Hamed, H.; Grunfeld, E.A.; Hunter, M.S. Cognitive Behavioural Treatment for Women Who Have Menopausal Symptoms after Breast Cancer Treatment (MENOS 1): A Randomised Controlled Trial. Lancet Oncol. 2012, 13, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Duijts, S.F.A.; van Beurden, M.; Oldenburg, H.S.A.; Hunter, M.S.; Kieffer, J.M.; Stuiver, M.M.; Gerritsma, M.A.; Menke-Pluymers, M.B.E.; Plaisier, P.W.; Rijna, H.; et al. Efficacy of Cognitive Behavioral Therapy and Physical Exercise in Alleviating Treatment-Induced Menopausal Symptoms in Patients With Breast Cancer: Results of a Randomized, Controlled, Multicenter Trial. J. Clin. Oncol. 2012, 30, 4124–4133. [Google Scholar] [CrossRef]
- Wang, X.-P.; Zhang, D.-J.; Wei, X.-D.; Wang, J.-P.; Zhang, D.-Z. Acupuncture for the Relief of Hot Flashes in Breast Cancer Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials and Observational Studies. J. Cancer Res. Ther. 2018, 14, 600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Gao, C.; Guo, Z.; Zhao, W.; Xu, X.; Wen, H.; Li, Y.; Lin, R.; Xu, N.; Cui, S. How Effective Is Acupuncture in Treating Hot Flashes in Breast Cancer Patients? A Systematic Review and Meta-Analysis. Front. Oncol. 2025, 15, 1543938. [Google Scholar] [CrossRef] [PubMed]
- Chien, T.-J.; Hsu, C.-H.; Liu, C.-Y.; Fang, C.-J. Effect of Acupuncture on Hot Flush and Menopause Symptoms in Breast Cancer- A Systematic Review and Meta-Analysis. PLoS One 2017, 12, e0180918. [Google Scholar] [CrossRef]
- Yuanqing, P.; Yong, T.; Haiqian, L.; Gen, C.; Shen, X.; Dong, J.; Qi, C.; Miaomiao, Q. Acupuncture for Hormone Therapy–Related Side Effects in Breast Cancer Patients: A GRADE-Assessed Systematic Review and Updated Meta-Analysis. Integr. Cancer Ther. 2020, 19, 1543735420940394. [Google Scholar] [CrossRef]
- Walker, E.M.; Rodriguez, A.I.; Kohn, B.; Ball, R.M.; Pegg, J.; Pocock, J.R.; Nunez, R.; Peterson, E.; Jakary, S.; Levine, R.A. Acupuncture Versus Venlafaxine for the Management of Vasomotor Symptoms in Patients With Hormone Receptor–Positive Breast Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2010, 28, 634–640. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Kugler, J.W.; Sloan, J.A.; Mailliard, J.A.; LaVasseur, B.I.; Barton, D.L.; Novotny, P.J.; Dakhil, S.R.; Rodger, K.; Rummans, T.A.; et al. Venlafaxine in Management of Hot Flashes in Survivors of Breast Cancer: A Randomised Controlled Trial. Lancet 2000, 356, 2059–2063. [Google Scholar] [CrossRef]
- Carpenter, J.S.; Storniolo, A.M.; Johns, S.; Monahan, P.O.; Azzouz, F.; Elam, J.L.; Johnson, C.S.; Shelton, R.C. Randomized, Double-Blind, Placebo-Controlled Crossover Trials of Venlafaxine for Hot Flashes After Breast Cancer. Oncologist 2007, 12, 124–135. [Google Scholar] [CrossRef]
- Buijs, C.; Mom, C.H.; Willemse, P.H.B.; Marike Boezen, H.; Maurer, J.M.; Wymenga, A.N.M.; de Jong, R.S.; Nieboer, P.; de Vries, E.G.E.; Mourits, M.J.E. Venlafaxine versus Clonidine for the Treatment of Hot Flashes in Breast Cancer Patients: A Double-Blind, Randomized Cross-over Study. Breast Cancer Res. Treat. 2009, 115, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Pandya, K.J.; Morrow, G.R.; Roscoe, J.A.; Zhao, H.; Hickok, J.T.; Pajon, E.; Sweeney, T.J.; Banerjee, T.K.; Flynn, P.J. Gabapentin for Hot Flashes in 420 Women with Breast Cancer: A Randomised Double-Blind Placebo-Controlled Trial. Lancet 2005, 366, 818–824. [Google Scholar] [CrossRef]
- Marsden, J.; Marsh, M.; Rigg, A. British Menopause Society Consensus Statement on the Management of Estrogen Deficiency Symptoms, Arthralgia and Menopause Diagnosis in Women Treated for Early Breast Cancer. Post Reprod. Heal. 2019, 25, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Su, H.I.; Sammel, M.D.; Springer, E.; Freeman, E.W.; DeMichele, A.; Mao, J.J. Weight Gain Is Associated with Increased Risk of Hot Flashes in Breast Cancer Survivors on Aromatase Inhibitors. Breast Cancer Res. Treat. 2010, 124, 205–211. [Google Scholar] [CrossRef]
- Thurston, R.C.; Sowers, M.R.; Sternfeld, B.; Gold, E.B.; Bromberger, J.; Chang, Y.; Joffe, H.; Crandall, C.J.; Waetjen, L.E.; Matthews, K.A. Gains in Body Fat and Vasomotor Symptom Reporting Over the Menopausal Transition: The Study of Women’s Health Across the Nation. Am. J. Epidemiol. 2009, 170, 766–774. [Google Scholar] [CrossRef]
- Lederman, S.; Ottery, F.D.; Cano, A.; Santoro, N.; Shapiro, M.; Stute, P.; Thurston, R.C.; English, M.; Franklin, C.; Lee, M.; et al. Fezolinetant for Treatment of Moderate-to-Severe Vasomotor Symptoms Associated with Menopause (SKYLIGHT 1): A Phase 3 Randomised Controlled Study. Lancet 2023, 401, 1091–1102. [Google Scholar] [CrossRef]
- Cardoso, F.; Parke, S.; Brennan, D.J.; Briggs, P.; Donders, G.; Panay, N.; Haseli-Mashhadi, N.; Block, M.; Caetano, C.; Francuski, M.; et al. Elinzanetant for Vasomotor Symptoms from Endocrine Therapy for Breast Cancer. N. Engl. J. Med. 2025, 393, 753–763. [Google Scholar] [CrossRef]
- Elkins, G.; Marcus, J.; Stearns, V.; Perfect, M.; Rajab, M.H.; Ruud, C.; Palamara, L.; Keith, T. Randomized Trial of a Hypnosis Intervention for Treatment of Hot Flashes Among Breast Cancer Survivors. J. Clin. Oncol. 2008, 26, 5022–5026. [Google Scholar] [CrossRef]
- MacLaughlan David, S.; Salzillo, S.; Bowe, P.; Scuncio, S.; Malit, B.; Raker, C.; Gass, J.S.; Granai, C.O.; Dizon, D.S. Randomised Controlled Trial Comparing Hypnotherapy versus Gabapentin for the Treatment of Hot Flashes in Breast Cancer Survivors: A Pilot Study. BMJ Open 2013, 3, e003138. [Google Scholar] [CrossRef]
- Haest, K.; Kumar, A.; Van Calster, B.; Leunen, K.; Smeets, A.; Amant, F.; Berteloot, P.; Wildiers, H.; Paridaens, R.; Van Limbergen, E.; et al. Stellate Ganglion Block for the Management of Hot Flashes and Sleep Disturbances in Breast Cancer Survivors: An Uncontrolled Experimental Study with 24 Weeks of Follow-Up. Ann. Oncol. 2012, 23, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, P.; Imani, F.; Nafissi, N.; Ebrahimi, B.; Faiz, S.H.R. Comparison of the Effects of Stellate Ganglion Block and Paroxetine on Hot Flashes and Sleep Disturbance in Breast Cancer Survivors. Cancer Manag. Res. 2018, 10, 4831–4837. [Google Scholar] [CrossRef]
- Irwin, M.L.; Cartmel, B.; Gross, C.P.; Ercolano, E.; Li, F.; Yao, X.; Fiellin, M.; Capozza, S.; Rothbard, M.; Zhou, Y.; et al. Randomized Exercise Trial of Aromatase Inhibitor–Induced Arthralgia in Breast Cancer Survivors. J. Clin. Oncol. 2015, 33, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.M.; Sereika, S.M.; Gentry, A.L.; Zhu, Y.; Wagner, M.; Cuglewski, C.; Duquette, J.; Grove, G.; Cummings, M.; Cho, M.; et al. Aerobic Exercise and Aromatase Inhibitor–Associated Musculoskeletal Symptoms: Results of a Randomized Clinical Trial. Support. Care Cancer 2025, 33, 244. [Google Scholar] [CrossRef] [PubMed]
- Galantino, M.L.; Desai, K.; Greene, L.; DeMichele, A.; Stricker, C.T.; Mao, J.J. Impact of Yoga on Functional Outcomes in Breast Cancer Survivors With Aromatase Inhibitor–Associated Arthralgias. Integr. Cancer Ther. 2012, 11, 313–320. [Google Scholar] [CrossRef]
- Henry, N.L.; Unger, J.M.; Schott, A.F.; Fehrenbacher, L.; Flynn, P.J.; Prow, D.M.; Sharer, C.W.; Burton, G.V.; Kuzma, C.S.; Moseley, A.; et al. Randomized, Multicenter, Placebo-Controlled Clinical Trial of Duloxetine Versus Placebo for Aromatase Inhibitor–Associated Arthralgias in Early-Stage Breast Cancer: SWOG S1202. J. Clin. Oncol. 2018, 36, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, A.L.; Taylor, M.E.; Gao, F.; Armamento-Villareal, R.; Jamalabadi-Majidi, S.; Napoli, N.; Ellis, M.J. Vitamin D and Aromatase Inhibitor-Induced Musculoskeletal Symptoms (AIMSS): A Phase II, Double-Blind, Placebo-Controlled, Randomized Trial. Breast Cancer Res. Treat. 2011, 129, 107–116. [Google Scholar] [CrossRef]
- Khan, Q.J.; Kimler, B.F.; Reddy, P.S.; Sharma, P.; Klemp, J.R.; Nydegger, J.L.; Yeh, H.-W.; Fabian, C.J. Randomized Trial of Vitamin D3 to Prevent Worsening of Musculoskeletal Symptoms in Women with Breast Cancer Receiving Adjuvant Letrozole. The VITAL Trial. Breast Cancer Res. Treat. 2017, 166, 491–500. [Google Scholar] [CrossRef]
- Shapiro, A.C.; Adlis, S.A.; Robien, K.; Kirstein, M.N.; Liang, S.; Richter, S.A.; Lerner, R.E. Randomized, Blinded Trial of Vitamin D3 for Treating Aromatase Inhibitor-Associated Musculoskeletal Symptoms (AIMSS). Breast Cancer Res. Treat. 2016, 155, 501–512. [Google Scholar] [CrossRef]
- Kadakia, K.C.; Kidwell, K.M.; Seewald, N.J.; Snyder, C.F.; Flockhart, D.A.; Carpenter, J.S.; Otte, J.L.; Hayes, D.F.; Storniolo, A.M.; Stearns, V.; et al. Crossover from One Aromatase Inhibitor (AI) to Another in the Exemestane and Letrozole Pharmacogenetics (ELPh) Trial. J. Clin. Oncol. 2016, 34, 158. [Google Scholar] [CrossRef]
- Hershman, D.L.; Unger, J.M.; Crew, K.D.; Awad, D.; Dakhil, S.R.; Gralow, J.; Greenlee, H.; Lew, D.L.; Minasian, L.M.; Till, C.; et al. Randomized Multicenter Placebo-Controlled Trial of Omega-3 Fatty Acids for the Control of Aromatase Inhibitor–Induced Musculoskeletal Pain: SWOG S0927. J. Clin. Oncol. 2015, 33, 1910–1917. [Google Scholar] [CrossRef]
- Shen, S.; Unger, J.M.; Crew, K.D.; Till, C.; Greenlee, H.; Gralow, J.; Dakhil, S.R.; Minasian, L.M.; Wade, J.L.; Fisch, M.J.; et al. Omega-3 Fatty Acid Use for Obese Breast Cancer Patients with Aromatase Inhibitor-Related Arthralgia (SWOG S0927). Breast Cancer Res. Treat. 2018, 172, 603–610. [Google Scholar] [CrossRef]
- Hines, S.L.; Mincey, B.; Dentchev, T.; Sloan, J.A.; Perez, E.A.; Johnson, D.B.; Schaefer, P.L.; Alberts, S.; Liu, H.; Kahanic, S.; et al. Immediate versus Delayed Zoledronic Acid for Prevention of Bone Loss in Postmenopausal Women with Breast Cancer Starting Letrozole after Tamoxifen-N03CC. Breast Cancer Res. Treat. 2009, 117, 603–609. [Google Scholar] [CrossRef]
- Brufsky, A. Management of Cancer-Treatment–Induced Bone Loss in Postmenopausal Women Undergoing Adjuvant Breast Cancer Therapy: A Z-FAST Update. Semin. Oncol. 2006, 33, 13–17. [Google Scholar] [CrossRef]
- Gnant, M.; Pfeiler, G.; Dubsky, P.C.; Hubalek, M.; Greil, R.; Jakesz, R.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger, E.; et al. Adjuvant Denosumab in Breast Cancer (ABCSG-18): A Multicentre, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2015, 386, 433–443. [Google Scholar] [CrossRef]
- Abdel-Rahman, O. Denosumab versus Zoledronic Acid to Prevent Aromatase Inhibitors-Associated Fractures in Postmenopausal Early Breast Cancer; a Mixed Treatment Meta-Analysis. Expert Rev. Anticancer Ther. 2016, 16, 885–891. [Google Scholar] [CrossRef]
- Coleman, R.; Hadji, P.; Body, J.-J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, Ø.; Flamen, P.; Kurth, A.; et al. Bone Health in Cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Jassim, G.A.; Doherty, S.; Whitford, D.L.; Khashan, A.S. Psychological Interventions for Women with Non-Metastatic Breast Cancer. Cochrane Database Syst. Rev. 2023, 1, CD008729. [Google Scholar] [CrossRef]
- Mao, J.J.; Farrar, J.T.; Bruner, D.; Zee, J.; Bowman, M.; Seluzicki, C.; DeMichele, A.; Xie, S.X. Electroacupuncture for Fatigue, Sleep, and Psychological Distress in Breast Cancer Patients with Aromatase Inhibitor-related Arthralgia: A Randomized Trial. Cancer 2014, 120, 3744–3751. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.L.; Banerjee, M.; Wicha, M.; Van Poznak, C.; Smerage, J.B.; Schott, A.F.; Griggs, J.J.; Hayes, D.F. Pilot Study of Duloxetine for Treatment of Aromatase Inhibitor-associated Musculoskeletal Symptoms. Cancer 2011, 117, 5469–5475. [Google Scholar] [CrossRef]
- Lami, A.; Alvisi, S.; Baldassarre, M.; Zanella, S.; Amati, V.; Seracchioli, R.; Meriggiola, M.C. Safety and Efficacy of Non-Ablative CO2 Laser Treatment of Vulvo-Vaginal Atrophy in Women with History of Breast Cancer. Arch. Gynecol. Obstet. 2024, 309, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Cruff, J.; Khandwala, S. A Double-Blind Randomized Sham-Controlled Trial to Evaluate the Efficacy of Fractional Carbon Dioxide Laser Therapy on Genitourinary Syndrome of Menopause. J. Sex. Med. 2021, 18, 761–769. [Google Scholar] [CrossRef]
- Massarotti, C.; Asinaro, G.; Schiaffino, M.G.; Ronzini, C.; Vacca, I.; Lambertini, M.; Anserini, P.; Del Mastro, L.; Cagnacci, A. Vaginal Oxygen plus Hyaluronic Acid on Genito-Urinary Symptoms of Breast Cancer Survivors. Climacteric 2023, 26, 129–134. [Google Scholar] [CrossRef]
- Carter, J.; Baser, R.E.; Goldfrank, D.J.; Seidel, B.; Milli, L.; Stabile, C.; Canty, J.; Saban, S.; Goldfarb, S.; Dickler, M.N.; et al. A Single-Arm, Prospective Trial Investigating the Effectiveness of a Non-Hormonal Vaginal Moisturizer Containing Hyaluronic Acid in Postmenopausal Cancer Survivors. Support. Care Cancer 2021, 29, 311–322. [Google Scholar] [CrossRef]
- Taranto, P.; de Brito Sales, D.; Maluf, F.C.; Guendelmann, R.A.K.; de Melo Pompei, L.; Leal, A.; Buzaid, A.C.; Schvartsman, G. Safety and Efficacy of Topical Testosterone in Breast Cancer Patients Receiving Ovarian Suppression and Aromatase Inhibitor Therapy. Breast Cancer Res. 2024, 26, 133. [Google Scholar] [CrossRef] [PubMed]
- Goetsch, M.F.; Lim, J.Y.; Caughey, A.B. A Practical Solution for Dyspareunia in Breast Cancer Survivors: A Randomized Controlled Trial. J. Clin. Oncol. 2015, 33, 3394–3400. [Google Scholar] [CrossRef] [PubMed]
- Biglia, N.; Peano, E.; Sgandurra, P.; Moggio, G.; Panuccio, E.; Migliardi, M.; Ravarino, N.; Ponzone, R.; Sismondi, P. Low-Dose Vaginal Estrogens or Vaginal Moisturizer in Breast Cancer Survivors with Urogenital Atrophy: A Preliminary Study. Gynecol. Endocrinol. 2010, 26, 404–412. [Google Scholar] [CrossRef] [PubMed]
- McVicker, L.; Labeit, A.M.; Coupland, C.A.C.; Hicks, B.; Hughes, C.; McMenamin, Ú.; McIntosh, S.A.; Murchie, P.; Cardwell, C.R. Vaginal Estrogen Therapy Use and Survival in Females With Breast Cancer. JAMA Oncol. 2024, 10, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Le Ray, I.; Dell’Aniello, S.; Bonnetain, F.; Azoulay, L.; Suissa, S. Local Estrogen Therapy and Risk of Breast Cancer Recurrence among Hormone-Treated Patients: A Nested Case–Control Study. Breast Cancer Res. Treat. 2012, 135, 603–609. [Google Scholar] [CrossRef]
- Faltinová, M.; Vehmanen, L.; Lyytinen, H.; Savolainen-Peltonen, H.; Virtanen, A.; Haanpää, M.; Hämäläinen, E.; Tiitinen, A.; Mattson, J. Effects of Vaginal Estrogen on Serum Estradiol during Aromatase Inhibitor Therapy in Breast Cancer Patients with Vulvovaginal Atrophy: A Prospective Trial. Breast Cancer Res. Treat. 2025, 210, 295–305. [Google Scholar] [CrossRef]
- Portman, D.J.; Bachmann, G.A.; Simon, J.A. Ospemifene, a Novel Selective Estrogen Receptor Modulator for Treating Dyspareunia Associated with Postmenopausal Vulvar and Vaginal Atrophy. Menopause 2013, 20, 623–630. [Google Scholar] [CrossRef]
- Cai, B.; Simon, J.; Villa, P.; Biglia, N.; Panay, N.; Djumaeva, S.; Particco, M.; Kanakamedala, H.; Altomare, C. No Increase in Incidence or Risk of Recurrence of Breast Cancer in Ospemifene-Treated Patients with Vulvovaginal Atrophy (VVA). Maturitas 2020, 142, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, A.D.; Marshall, P.W.M.; Lonsdale, C.; Cheema, B.S.; Fiatarone Singh, M.A.; Green, S. Resistance Training Improves Fatigue and Quality of Life in Previously Sedentary Breast Cancer Survivors: A Randomised Controlled Trial. Eur. J. Cancer Care 2016, 25, 784–794. [Google Scholar] [CrossRef]
- Baumann, F.T.; Bieck, O.; Oberste, M.; Kuhn, R.; Schmitt, J.; Wentrock, S.; Zopf, E.; Bloch, W.; Schüle, K.; Reuss-Borst, M. Sustainable Impact of an Individualized Exercise Program on Physical Activity Level and Fatigue Syndrome on Breast Cancer Patients in Two German Rehabilitation Centers. Support. Care Cancer 2017, 25, 1047–1054. [Google Scholar] [CrossRef]
- Cramp, F.; Byron-Daniel, J. Exercise for the Management of Cancer-Related Fatigue in Adults. Cochrane Database Syst. Rev. 2012, 2012. [Google Scholar] [CrossRef]
- Cramer, H.; Rabsilber, S.; Lauche, R.; Kümmel, S.; Dobos, G. Yoga and Meditation for Menopausal Symptoms in Breast Cancer Survivors—A Randomized Controlled Trial. Cancer 2015, 121, 2175–2184. [Google Scholar] [CrossRef]
- Hou, L.; Wang, J.; Mao, M.; Zhang, Z.; Liu, D.; Gao, S.; Liang, K.; Lu, L. Effect of Yoga on Cancer-Related Fatigue in Patients with Breast Cancer: A Systematic Review and Meta-Analysis. Medicine 2024, 103, e36468. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Koukamari, P.; Karimy, M.; Ghaffari, M.; Milajerdi, A. Effect of Cognitive-Behavioral Therapy on Fatigue in Cancer Patients: A Systematic Review and Meta-Analysis. Front. Psychol. 2025, 15, 1435110. [Google Scholar] [CrossRef]
- Poort, H.; Peters, M.E.W.J.; van der Graaf, W.T.A.; Nieuwkerk, P.T.; van de Wouw, A.J.; Nijhuis-van der Sanden, M.W.G.; Bleijenberg, G.; Verhagen, C.A.H.H.V.M.; Knoop, H. Cognitive Behavioral Therapy or Graded Exercise Therapy Compared with Usual Care for Severe Fatigue in Patients with Advanced Cancer during Treatment: A Randomized Controlled Trial. Ann. Oncol. 2020, 31, 115–122. [Google Scholar] [CrossRef]
- Gielissen, M.F.M.; Verhagen, C.A.H.H.V.M.; Bleijenberg, G. Cognitive Behaviour Therapy for Fatigued Cancer Survivors: Long-Term Follow-Up. Br. J. Cancer 2007, 97, 612–618. [Google Scholar] [CrossRef]
- Zick, S.M.; Sen, A.; Wyatt, G.K.; Murphy, S.L.; Arnedt, J.T.; Harris, R.E. Investigation of 2 Types of Self-Administered Acupressure for Persistent Cancer-Related Fatigue in Breast Cancer Survivors. JAMA Oncol. 2016, 2, 1470. [Google Scholar] [CrossRef] [PubMed]
- Yennurajalingam, S.; Lu, Z.; Rozman De Moraes, A.; Tull, N.N.; Kubiak, M.J.; Geng, Y.; Andersen, C.R.; Bruera, E. Meta-Analysis of Pharmacological, Nutraceutical and Phytopharmaceutical Interventions for the Treatment of Cancer Related Fatigue. Cancers 2022, 15, 91. [Google Scholar] [CrossRef]
- Andreas, M.; Ernst, M.; Kusch, M.; Ruffer, J.U.; Csenar, M.; Cryns, N.; Bröckelmann, P.J.; Aldin, A.; Skoetz, N. Pharmacological Interventions to Treat Adults with Cancer-Related Fatigue. Cochrane Database Syst. Rev. 2023, 2023, CD015118. [Google Scholar] [CrossRef]
- Bohlius, J.; Tonia, T.; Nüesch, E.; Jüni, P.; Fey, M.F.; Egger, M.; Bernhard, J. Effects of Erythropoiesis-Stimulating Agents on Fatigue- and Anaemia-Related Symptoms in Cancer Patients: Systematic Review and Meta-Analyses of Published and Unpublished Data. Br. J. Cancer 2014, 111, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.M.; Clemons, M.; Alzahrani, M.; Liu, M.; Larocque, G.; MacDonald, F.; Hutton, B.; Piper, A.; Fernandes, R.; Pond, G.R.; et al. Vasomotor Symptoms in Early Breast Cancer—A “Real World” Exploration of the Patient Experience. Support. Care Cancer 2022, 30, 4437–4446. [Google Scholar] [CrossRef] [PubMed]
- Kligman, L.; Younus, J. Management of Hot Flashes in Women with Breast Cancer. Curr. Oncol. 2010, 17, 81–86. [Google Scholar] [CrossRef]
- Zdenkowski, N.; Tesson, S.; Lombard, J.; Lovell, M.; Hayes, S.; Francis, P.A.; Dhillon, H.M.; Boyle, F.M. Supportive Care of Women with Breast Cancer: Key Concerns and Practical Solutions. Med. J. Aust. 2016, 205, 471–475. [Google Scholar] [CrossRef]
- Santen, R.J.; Stuenkel, C.A.; Davis, S.R.; Pinkerton, J.V.; Gompel, A.; Lumsden, M.A. Managing Menopausal Symptoms and Associated Clinical Issues in Breast Cancer Survivors. J. Clin. Endocrinol. Metab. 2017, 102, 3647–3661. [Google Scholar] [CrossRef]
- dos Santos, B.S.; Bordignon, C.; Rosa, D.D. Managing Common Estrogen Deprivation Side Effects in HR+ Breast Cancer: An Evidence-Based Review. Curr. Oncol. Rep. 2021, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- Lammerink, E.A.G.; de Bock, G.H.; Schröder, C.P.; Mourits, M.J.E. The Management of Menopausal Symptoms in Breast Cancer Survivors: A Case-Based Approach. Maturitas 2012, 73, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Caan, B.J.; Emond, J.A.; Su, H.I.; Patterson, R.E.; Flatt, S.W.; Gold, E.B.; Newman, V.A.; Rock, C.L.; Thomson, C.A.; Pierce, J.P. Effect of Postdiagnosis Weight Change on Hot Flash Status Among Early-Stage Breast Cancer Survivors. J. Clin. Oncol. 2012, 30, 1492–1497. [Google Scholar] [CrossRef]
- Gold, E.B.; Flatt, S.W.; Pierce, J.P.; Bardwell, W.A.; Hajek, R.A.; Newman, V.A.; Rock, C.L.; Stefanick, M.L. Dietary Factors and Vasomotor Symptoms in Breast Cancer Survivors. Menopause 2006, 13, 423–433. [Google Scholar] [CrossRef]
- Wen, H.; Deng, G.; Shi, X.; Liu, Z.; Lin, A.; Cheng, Q.; Zhang, J.; Luo, P. Body Mass Index, Weight Change, and Cancer Prognosis: A Meta-Analysis and Systematic Review of 73 Cohort Studies. ESMO Open 2024, 9, 102241. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Vieira, R.; Abar, L.; Aune, D.; Balducci, K.; Cariolou, M.; Greenwood, D.C.; Markozannes, G.; Nanu, N.; Becerra-Tomás, N.; et al. Postdiagnosis Body Fatness, Weight Change and Breast Cancer Prognosis: Global Cancer Update Program (CUP Global) Systematic Literature Review and Meta-analysis. Int. J. Cancer 2023, 152, 572–599. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Hu, W.-Y.; Chang, Y.-M. Cognitive-Behavioral Therapy to Alleviate Treatment-Induced Menopausal Symptoms in Women With Breast Cancer. Cancer Nurs. 2021, 44, 411–418. [Google Scholar] [CrossRef]
- Cramer, H.; Peng, W.; Lauche, R. Yoga for Menopausal Symptoms—A Systematic Review and Meta-Analysis. Maturitas 2018, 109, 13–25. [Google Scholar] [CrossRef] [PubMed]
- The North American Menopause Society The 2022 Hormone Therapy Position Statement of The North American Menopause Society. Menopause 2022, 29, 767–794. [CrossRef] [PubMed]
- The North American Menopause Society Nonhormonal Management of Menopause-Associated Vasomotor Symptoms. Menopause 2015, 22, 1155–1174. [CrossRef]
- Biglia, N.; Torta, R.; Roagna, R.; Maggiorotto, F.; Cacciari, F.; Ponzone, R.; Kubatzki, F.; Sismondi, P. Evaluation of Low-Dose Venlafaxine Hydrochloride for the Therapy of Hot Flushes in Breast Cancer Survivors. Maturitas 2005, 52, 78–85. [Google Scholar] [CrossRef]
- Pandya, K.J.; Raubertas, R.F.; Flynn, P.J.; Hynes, H.E.; Rosenbluth, R.J.; Kirshner, J.J.; Pierce, H.I.; Dragalin, V.; Morrow, G.R. Oral Clonidine in Postmenopausal Patients with Breast Cancer Experiencing Tamoxifen-Induced Hot Flashes: A University of Rochester Cancer Center Community Clinical Oncology Program Study. Ann. Intern. Med. 2000, 132, 788–793. [Google Scholar] [CrossRef]
- Bordeleau, L.; Pritchard, K.I.; Loprinzi, C.L.; Ennis, M.; Jugovic, O.; Warr, D.; Haq, R.; Goodwin, P.J. Multicenter, Randomized, Cross-Over Clinical Trial of Venlafaxine Versus Gabapentin for the Management of Hot Flashes in Breast Cancer Survivors. J. Clin. Oncol. 2010, 28, 5147–5152. [Google Scholar] [CrossRef] [PubMed]
- Leon-Ferre, R.A.; Novotny, P.J.; Wolfe, E.G.; Faubion, S.S.; Ruddy, K.J.; Flora, D.; Dakhil, C.S.R.; Rowland, K.M.; Graham, M.L.; Le-Lindqwister, N.; et al. Oxybutynin vs Placebo for Hot Flashes in Women With or Without Breast Cancer: A Randomized, Double-Blind Clinical Trial (ACCRU SC-1603). JNCI Cancer Spectr. 2020, 4, pkz088. [Google Scholar] [CrossRef]
- Fahlén, M.; Fornander, T.; Johansson, H.; Johansson, U.; Rutqvist, L.-E.; Wilking, N.; von Schoultz, E. Hormone Replacement Therapy after Breast Cancer: 10 Year Follow up of the Stockholm Randomised Trial. Eur. J. Cancer 2013, 49, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Poggio, F.; Del Mastro, L.; Bruzzone, M.; Ceppi, M.; Razeti, M.G.; Fregatti, P.; Ruelle, T.; Pronzato, P.; Massarotti, C.; Franzoi, M.A.; et al. Safety of Systemic Hormone Replacement Therapy in Breast Cancer Survivors: A Systematic Review and Meta-Analysis. Breast Cancer Res. Treat. 2022, 191, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Lacchetti, C.; Andersen, B.L.; Barton, D.L.; Bolte, S.; Damast, S.; Diefenbach, M.A.; DuHamel, K.; Florendo, J.; Ganz, P.A.; et al. Interventions to Address Sexual Problems in People With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Adaptation of Cancer Care Ontario Guideline. J. Clin. Oncol. 2018, 36, 492–511. [Google Scholar] [CrossRef]
- Gupta, A.; Henry, N.L.; Loprinzi, C.L. Management of Aromatase Inhibitor–Induced Musculoskeletal Symptoms. JCO Oncol. Pract. 2020, 16, 733–739. [Google Scholar] [CrossRef]
- Briot, K.; Tubiana-Hulin, M.; Bastit, L.; Kloos, I.; Roux, C. Effect of a Switch of Aromatase Inhibitors on Musculoskeletal Symptoms in Postmenopausal Women with Hormone-Receptor-Positive Breast Cancer: The ATOLL (Articular Tolerance of Letrozole) Study. Breast Cancer Res. Treat. 2010, 120, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.; Rickett, K.; Greer, R.; Woodward, N. Management of Aromatase Inhibitor Induced Musculoskeletal Symptoms in Postmenopausal Early Breast Cancer: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2017, 111, 66–80. [Google Scholar] [CrossRef]
- Lash, B.W.; Katz, J.; Gilman, P. Feasibility of a Defined Exercise Program for Aromatase Inhibitor–Related Arthralgia (AIRA) in Breast Cancer Survivors. J. Clin. Oncol. 2011, 29, e19725. [Google Scholar] [CrossRef]
- DeNysschen, C.A.; Burton, H.; Ademuyiwa, F.; Levine, E.; Tetewsky, S.; O’Connor, T. Exercise Intervention in Breast Cancer Patients with Aromatase Inhibitor-Associated Arthralgia: A Pilot Study. Eur. J. Cancer Care 2014, 23, 493–501. [Google Scholar] [CrossRef]
- Fields, J.; Richardson, A.; Hopkinson, J.; Fenlon, D. Nordic Walking as an Exercise Intervention to Reduce Pain in Women With Aromatase Inhibitor–Associated Arthralgia: A Feasibility Study. J. Pain Symptom Manag. 2016, 52, 548–559. [Google Scholar] [CrossRef]
- Cantarero-Villanueva, I.; Fernández-Lao, C.; Cuesta-Vargas, A.I.; Del Moral-Avila, R.; Fernández-de-las-Peñas, C.; Arroyo-Morales, M. The Effectiveness of a Deep Water Aquatic Exercise Program in Cancer-Related Fatigue in Breast Cancer Survivors: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2013, 94, 221–230. [Google Scholar] [CrossRef]
- Jacobsen, P.B.; Muchnick, S.; Marcus, S.; Amheiser, P.; Reiersen, P.; Gonzalez, B.; Gomez, M.; Jim, H.S.L.; Thompson, L.M.A.; Minton, S.; et al. Pilot Study of Iyengar Yoga for Management of Aromatase Inhibitor-Associated Arthralgia in Women with Breast Cancer. Psychooncology. 2015, 24, 1578–1580. [Google Scholar] [CrossRef] [PubMed]
- Crew, K.D.; Capodice, J.L.; Greenlee, H.; Apollo, A.; Jacobson, J.S.; Raptis, G.; Blozie, K.; Sierra, A.; Hershman, D.L. Pilot Study of Acupuncture for the Treatment of Joint Symptoms Related to Adjuvant Aromatase Inhibitor Therapy in Postmenopausal Breast Cancer Patients. J. Cancer Surviv. 2007, 1, 283–291. [Google Scholar] [CrossRef]
- Crew, K.D.; Capodice, J.L.; Greenlee, H.; Brafman, L.; Fuentes, D.; Awad, D.; Yann Tsai, W.; Hershman, D.L. Randomized, Blinded, Sham-Controlled Trial of Acupuncture for the Management of Aromatase Inhibitor–Associated Joint Symptoms in Women With Early-Stage Breast Cancer. J. Clin. Oncol. 2010, 28, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Unger, J.M.; Greenlee, H.; Capodice, J.L.; Lew, D.L.; Darke, A.K.; Kengla, A.T.; Melnik, M.K.; Jorgensen, C.W.; Kreisle, W.H.; et al. Effect of Acupuncture vs Sham Acupuncture or Waitlist Control on Joint Pain Related to Aromatase Inhibitors Among Women With Early-Stage Breast Cancer. JAMA 2018, 320, 167. [Google Scholar] [CrossRef]
- Colleoni, M.; Luo, W.; Karlsson, P.; Chirgwin, J.; Aebi, S.; Jerusalem, G.; Neven, P.; Hitre, E.; Graas, M.-P.; Simoncini, E.; et al. Extended Adjuvant Intermittent Letrozole versus Continuous Letrozole in Postmenopausal Women with Breast Cancer (SOLE): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 127–138. [Google Scholar] [CrossRef]
- Hadji, P.; Aapro, M.S.; Body, J.J.; Bundred, N.J.; Brufsky, A.; Coleman, R.E.; Gnant, M.; Guise, T.; Lipton, A. Management of Aromatase Inhibitor-Associated Bone Loss in Postmenopausal Women with Breast Cancer: Practical Guidance for Prevention and Treatment. Ann. Oncol. 2011, 22, 2546–2555. [Google Scholar] [CrossRef]
- Eastell, R.; Adams, J.E.; Coleman, R.E.; Howell, A.; Hannon, R.A.; Cuzick, J.; Mackey, J.R.; Beckmann, M.W.; Clack, G. Effect of Anastrozole on Bone Mineral Density: 5-Year Results From the Anastrozole, Tamoxifen, Alone or in Combination Trial 18233230. J. Clin. Oncol. 2008, 26, 1051–1057. [Google Scholar] [CrossRef]
- Liu, P.-H.; Tsai, C.-F.; Hsu, Y.-C.; Wu, C.-Y.; Yang, H.-Y. Aromatase Inhibitors Therapy and Major Osteoporotic Fracture Risk in Postmenopausal Breast Cancer Patients: A Nationwide Real-World Cohort Study. Breast Cancer Res. 2025, 27, 95. [Google Scholar] [CrossRef]
- Lambertini, M.; Arecco, L.; Woodard, T.L.; Messelt, A.; Rojas, K.E. Advances in the Management of Menopausal Symptoms, Fertility Preservation, and Bone Health for Women With Breast Cancer on Endocrine Therapy. Am. Soc. Clin. Oncol. Educ. B 2023, 43, e390442. [Google Scholar] [CrossRef] [PubMed]
- Bassatne, A.; Bou Khalil, A.; Chakhtoura, M.; Arabi, A.; Van Poznak, C.; El-Hajj Fuleihan, G. Effect of Antiresorptive Therapy on Aromatase Inhibitor Induced Bone Loss in Postmenopausal Women with Early-Stage Breast Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Metabolism 2022, 128, 154962. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Adjuvant Bisphosphonate Treatment in Early Breast Cancer: Meta-Analyses of Individual Patient Data from Randomised Trials. Lancet 2015, 386, 1353–1361. [CrossRef] [PubMed]
- Bhattoa, H.P.; Vasikaran, S.; Trifonidi, I.; Kapoula, G.; Lombardi, G.; Jørgensen, N.R.; Pikner, R.; Miura, M.; Chapurlat, R.; Hiligsmann, M.; et al. Update on the Role of Bone Turnover Markers in the Diagnosis and Management of Osteoporosis: A Consensus Paper from The European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), International. Osteoporos. Int. 2025, 36, 579–608. [Google Scholar] [CrossRef]
- Eastell, R.; Szulc, P. Use of Bone Turnover Markers in Postmenopausal Osteoporosis. Lancet Diabetes Endocrinol. 2017, 5, 908–923. [Google Scholar] [CrossRef]
- Francis, P.A.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.; Ciruelos, E.; Burstein, H.J.; et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N. Engl. J. Med. 2018, 379, 122–137. [Google Scholar] [CrossRef]
- Bryl, K.; Chimonas, S.; Li, X.; Li, S.Q.; Mao, J.J. The Relationship between Anxiety and Vaginal-Related Sexual Health in Postmenopausal Breast Cancer Survivors on Aromatase Inhibitors Therapies: A Cross Sectional Study. Breast Cancer Res. Treat. 2023, 200, 257–264. [Google Scholar] [CrossRef]
- Andersen, B.L.; Lacchetti, C.; Ashing, K.; Berek, J.S.; Berman, B.S.; Bolte, S.; Dizon, D.S.; Given, B.; Nekhlyudov, L.; Pirl, W.; et al. Management of Anxiety and Depression in Adult Survivors of Cancer: ASCO Guideline Update. J. Clin. Oncol. 2023, 41, 3426–3453. [Google Scholar] [CrossRef]
- Valachis, A.; Garmo, H.; Weinman, J.; Fredriksson, I.; Ahlgren, J.; Sund, M.; Holmberg, L. Effect of Selective Serotonin Reuptake Inhibitors Use on Endocrine Therapy Adherence and Breast Cancer Mortality: A Population-Based Study. Breast Cancer Res. Treat. 2016, 159, 293–303. [Google Scholar] [CrossRef]
- Mok, K.; Juraskova, I.; Friedlander, M. The Impact of Aromatase Inhibitors on Sexual Functioning: Current Knowledge and Future Research Directions. Breast 2008, 17, 436–440. [Google Scholar] [CrossRef]
- Moskalewicz, A.; Di Tomaso, A.; Kachura, J.J.; Scime, S.; Nisenbaum, R.; Lee, R.; Haq, R.; Derzko, C.; Brezden-Masley, C. Gynecologic Symptoms among Hormone Receptor-Positive Breast Cancer Patients on Oral Endocrine Therapy: A Cross-Sectional Study. Curr. Oncol. 2022, 29, 1813–1827. [Google Scholar] [CrossRef] [PubMed]
- Ros, C.; Mension, E.; Rius, M.; Munmany, M.; De Guirior, C.; Espuña-Pons, M.; Anglès-Acedo, S.; Castelo-Branco, C. Assessing Vaginal Wall Thickness by Transvaginal Ultrasound in Breast Cancer Survivors: A Pilot Study. Maturitas 2023, 171, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Prasanchit, P.; Pongchaikul, P.; Lertsittichai, P.; Tantitham, C.; Manonai, J. Vaginal Microbiomes of Breast Cancer Survivors Treated with Aromatase Inhibitors with and without Vulvovaginal Symptoms. Sci. Rep. 2024, 14, 7417. [Google Scholar] [CrossRef] [PubMed]
- Advani, P.; Brewster, A.M.; Baum, G.P.; Schover, L.R. A Pilot Randomized Trial to Prevent Sexual Dysfunction in Postmenopausal Breast Cancer Survivors Starting Adjuvant Aromatase Inhibitor Therapy. J. Cancer Surviv. 2017, 11, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Cyr, M.-P.; Jones, T.; Colombage, U.N.; Frawley, H.C. Effectiveness of Pelvic Floor Muscle and Education-Based Therapies on Bladder, Bowel, Vaginal, Sexual, Psychological Function, Quality of Life, and Pelvic Floor Muscle Function in Females Treated for Breast Cancer: A Systematic Review. Curr. Oncol. Rep. 2025, 27, 168–189. [Google Scholar] [CrossRef]
- Lubián-López, D.M.; Butrón-Hinojo, C.A.; Menjón-Beltrán, S.; González-Mesa, E.; Tapiador-Albertos, S.; Rodríguez-Jiménez, B.; Fiol-Ruiz, G. Effects of Non-Ablative Solid-State Vaginal Laser (SSVL) for the Treatment of Vulvovaginal Atrophy in Breast Cancer Survivors after Adjuvant Aromatase Inhibitor Therapy: Preliminary Results. J. Clin. Med. 2023, 12, 5669. [Google Scholar] [CrossRef]
- Cucciniello, L.; Garufi, G.; Di Rienzo, R.; Martinelli, C.; Pavone, G.; Giuliano, M.; Arpino, G.; Montemurro, F.; Del Mastro, L.; De Laurentiis, M.; et al. Estrogen Deprivation Effects of Endocrine Therapy in Breast Cancer Patients: Incidence, Management and Outcome. Cancer Treat. Rev. 2023, 120, 102624. [Google Scholar] [CrossRef]
- Mao, H.; Bao, T.; Shen, X.; Li, Q.; Seluzicki, C.; Im, E.-O.; Mao, J.J. Prevalence and Risk Factors for Fatigue among Breast Cancer Survivors on Aromatase Inhibitors. Eur. J. Cancer 2018, 101, 47–54. [Google Scholar] [CrossRef]
- Abrahams, H.J.G.; Gielissen, M.F.M.; Donders, R.R.T.; Goedendorp, M.M.; van der Wouw, A.J.; Verhagen, C.A.H.H.V.M.; Knoop, H. The Efficacy of Internet-based Cognitive Behavioral Therapy for Severely Fatigued Survivors of Breast Cancer Compared with Care as Usual: A Randomized Controlled Trial. Cancer 2017, 123, 3825–3834. [Google Scholar] [CrossRef]
- Foglietta, J.; Inno, A.; de Iuliis, F.; Sini, V.; Duranti, S.; Turazza, M.; Tarantini, L.; Gori, S. Cardiotoxicity of Aromatase Inhibitors in Breast Cancer Patients. Clin. Breast Cancer 2017, 17, 11–17. [Google Scholar] [CrossRef]
- Matthews, A.; Stanway, S.; Farmer, R.E.; Strongman, H.; Thomas, S.; Lyon, A.R.; Smeeth, L.; Bhaskaran, K. Long Term Adjuvant Endocrine Therapy and Risk of Cardiovascular Disease in Female Breast Cancer Survivors: Systematic Review. BMJ 2018, 363, k3845. [Google Scholar] [CrossRef]
- Yoo, J.-J.; Jung, E.-A.; Kim, Z.; Kim, B.-Y. Risk of Cardiovascular Events and Lipid Profile Change in Patients with Breast Cancer Taking Aromatase Inhibitor: A Systematic Review and Meta-Analysis. Curr. Oncol. 2023, 30, 1831–1843. [Google Scholar] [CrossRef]
- Sund, M.; Garcia-Argibay, M.; Garmo, H.; Ahlgren, J.; Wennstig, A.-K.; Fredriksson, I.; Lindman, H.; Valachis, A. Aromatase Inhibitors Use and Risk for Cardiovascular Disease in Breast Cancer Patients: A Population-Based Cohort Study. Breast 2021, 59, 157–164. [Google Scholar] [CrossRef]
- Khosrow-Khavar, F.; Filion, K.B.; Bouganim, N.; Suissa, S.; Azoulay, L. Aromatase Inhibitors and the Risk of Cardiovascular Outcomes in Women With Breast Cancer. Circulation 2020, 141, 549–559. [Google Scholar] [CrossRef]
- Elisaf, M.S.; Bairaktari, E.T.; Nicolaides, C.; Kakaidi, B.; Tzallas, C.S.; Katsaraki, A.; Pavlidis, N.A. Effect of Letrozole on the Lipid Profile in Postmenopausal Women with Breast Cancer. Eur. J. Cancer 2001, 37, 1510–1513. [Google Scholar] [CrossRef]
- Node, K.; Kitakaze, M.; Kosaka, H.; Minamino, T.; Funaya, H.; Hori, M. Amelioration of Ischemia- and Reperfusion-Induced Myocardial Injury by 17β-Estradiol. Circulation 1997, 96, 1953–1963. [Google Scholar] [CrossRef]
- Anderson, S.E.; Kirkland, D.M.; Beyschau, A.; Cala, P.M. Acute Effects of 17β-Estradiol on Myocardial PH, Na+, and Ca2+ and Ischemia-Reperfusion Injury. Am. J. Physiol. Physiol. 2005, 288, C57–C64. [Google Scholar] [CrossRef] [PubMed]
- Kwaah, P.A.; Mensah, S.A.; Agyemang, E.A.; Kekrebesi, J.S.; Katkov, D.; Carboo, A.; Appah, G.; Rashid, H.A.; Kwan, J.M. Atrial Fibrillation in Breast Cancer Therapy: Does Tamoxifen Confer a Lower Risk than Aromatase Inhibitors? Cardio-Oncology 2025, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.; Wong, C.K.; Wong, Y.K.; Lam, T.H.; Sze-Him Leung, I.; Lin, M.; Tak-Wai Lui, D.; Kwok, W.C.; Tam, C.C.; Chan, Y.H.; et al. Aromatase Inhibitor Therapy Increases the Risk of New-Onset Atrial Fibrillation in Patients With Breast Cancer. JACC Asia 2024, 4, 150–160. [Google Scholar] [CrossRef]
- Nardin, M.; Verdoia, M.; Barbieri, L.; De Luca, G. Novara Atherosclerosis Study Group (NAS) Impact of Metabolic Syndrome on Mean Platelet Volume and Its Relationship with Coronary Artery Disease. Platelets 2019, 30, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Cassetti, E.; Nardin, M.; Bellomo, G.; Marino, P.; Sinigaglia, F.; De Luca, G. Diabetes, Glucose Control and Mean Platelet Volume: A Single-Centre Cohort Study. Diabetes Res. Clin. Pract. 2014, 104, 288–294. [Google Scholar] [CrossRef]
- Rillamas-Sun, E.; Kwan, M.L.; Iribarren, C.; Cheng, R.; Neugebauer, R.; Rana, J.S.; Nguyen-Huynh, M.; Shi, Z.; Laurent, C.A.; Lee, V.S.; et al. Development of Cardiometabolic Risk Factors Following Endocrine Therapy in Women with Breast Cancer. Breast Cancer Res. Treat. 2023, 201, 117–126. [Google Scholar] [CrossRef]
- McCloskey, E.V.; Hannon, R.A.; Lakner, G.; Fraser, W.D.; Clack, G.; Miyamoto, A.; Finkelman, R.D.; Eastell, R. Effects of Third Generation Aromatase Inhibitors on Bone Health and Other Safety Parameters: Results of an Open, Randomised, Multi-Centre Study of Letrozole, Exemestane and Anastrozole in Healthy Postmenopausal Women. Eur. J. Cancer 2007, 43, 2523–2531. [Google Scholar] [CrossRef]
- Polter, E.J.; E. Prizment, A.; Walker, R.F.; Ryan, Z.; Wang, W.; Blaes, A.H.; Lutsey, P.L. Cardiovascular Disease With Hormone Therapy and Ovarian Suppression in Premenopausal Breast Cancer Survivors. JACC CardioOncol. 2024, 6, 907–918. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-Term Secondary Prevention of Cardiovascular Disease with a Mediterranean Diet and a Low-Fat Diet (CORDIOPREV): A Randomised Controlled Trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Celentano, E.; Cristiano, E.; Schena, S.; Gasparri, M.; Ignatiuk, B.; Renda, M.; Bia, E.; Rainone, R.; Graniero, A.; Giroletti, L.; et al. Local Epicardial Robotic-Enhanced Hybrid Ablation Efficacy Predictors for Persistent Atrial Fibrillation. Hear. Rhythm O2 2025, 6, 280–289. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the Management of Elevated Blood Pressure and Hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef]
- Nardin, S.; Ruelle, T.; Giannubilo, I.; Del Mastro, L. Adjuvant Treatment in Hormone Receptor-Positive Early Breast Cancer: New Approaches of Endocrine Therapy. Tumori J. 2024, 110, 162–167. [Google Scholar] [CrossRef]
- Crea, F. A Focus on Two Rapidly Expanding Fields: Cardio-Oncology and Genetics. Eur. Heart J. 2024, 45, 3095–3099. [Google Scholar] [CrossRef] [PubMed]
- Conte, B.; Boni, L.; Bisagni, G.; Durando, A.; Sanna, G.; Gori, S.; Garrone, O.; Tamberi, S.; De Placido, S.; Schettini, F.; et al. SNP of Aromatase Predict Long-Term Survival and Aromatase Inhibitor Toxicity in Patients with Early Breast Cancer: A Biomarker Analysis of the GIM4 and GIM5 Trials. Clin. Cancer Res. 2023, 29, 5217–5226. [Google Scholar] [CrossRef] [PubMed]
| Side Effect | Intervention | Recommendation Level | Evidence Strength | Key References |
|---|---|---|---|---|
| Vasomotor symptoms | CBT | First-line | High | Mann et al., 2012 [42]; Duijts et al., 2012 [43] |
| Acupuncture | First-line | High | Wang et al., 2018 [44]; Zhang et al., 2025 [45]; Chien et al., 2017 [46]; Yuanqing et al., 2020 [47]; Walker et al., 2010 [48] | |
| Venlafaxine, gabapentin, clonidine | First-line | High | Loprinzi et al., 2000 [49]; Carpenter et al., 2007 [50]; Buijs et al., 2009 [51]; Pandya et al., 2005 [52] | |
| Lifestyle modification, dietary interventions, weight loss | Second-line | Moderate | Marsden et al., 2019 [53]; Su et al., 2010 [54]; Thurston et al., 2009 [55] | |
| Fezolinetant, elinzanetant | Second-line | Moderate | Lederman et al., 2023 [56]; Cardoso et al., 2025 [57] | |
| Hypnosis | Second-line | Moderate–low | Elkins et al., 2008 [58]; MacLaughlan David et al., 2013 [59] | |
| SGB | Second-line | Moderate–low | Haest et al., 2012 [60]; Rahimzadeh et al., 2018 [61] | |
| Musculoskeletal symptoms | Exercise, yoga | First-line | High | Irwin et al., 2015 [62]; Bender et al., 2025 [63]; Galantino et al., 2012 [64] |
| Duloxetine | First-line | High | Henry et al., 2018 [65] | |
| Vitamin D | Second-line | Moderate | Rastelli et al., 2011 [66]; Khan et al., 2017 [67]; Shapiro et al., 2016 [68] | |
| Switch AI | Second-line | Moderate | Kadakia et al., 2016 [69] | |
| Omega−3 | Second-line | Low | Hershman et al., 2015 [70]; Shen et al., 2018 [71] | |
| Bone health | Bisphosphonates (Zoledronic acid) | First-line | High | Coleman et al., 2013 [30]; Hines et al., 2009 [72]; Brufsky et al. 2006 [73] |
| Denosumab | First-line | High | Gnant et al., 2015 [74]; Abdel-Rahman et al., 2016 [75] | |
| Calcium, vitamin D | First-line | High | Coleman et al., 2020 [76] | |
| Cognitive changes and mood disorders | Psychological therapy | Second-line | Moderate | Jassim et al., 2023 [77] |
| Electroacupuncture | Second-line | Moderate | Mao et al., 2014 [78] | |
| Duloxetine | Second-line | Low | Herny et al., 2011 [79] | |
| Gynecological and sexual disfunction | CO2 laser | Second-line | Moderate | Lami et al., 2024 [80]; Cruff et al., 2021 [81] |
| Vaginal oxygenation and hyaluronic acid | Second-line | Moderate | Massarotti et al., 2023 [82]; Carter et al., 2021 [83] | |
| Topical testosterone | Second-line | Moderate | Taranto et al., 2024 [84] | |
| Topical lidocaine | Second-line | Moderate | Goetsch et al., 2015 [85] | |
| Local estrogen | Investigational | Moderate | Biglia et al., 2010 [86]; McVicker et al., 2024 [87]; Le Ray et al., 2024 [88]; Faltinová et al., 2025 [89] | |
| Ospemifene | Investigational | Moderate | Portman et al., 2013 [90]; Cai et al., 2020 [91] | |
| Fatigue | Physical exercise | First-line | High | Hagstrom et al., 2016 [92]; Baumann et al., 2017 [93]; Cramp et al., 2012 [94] |
| Yoga, meditation, CBT | First-line | High | Cramer et al., 2015 [95]; Hou et al., 2024 [96]; Hosseini Koukamari et al., 2025 [97]; Poort et al., 2020 [98]; Gielissen et al., 2007 [99] | |
| Acupressure | First-line | High | Zick et al., 2016 [100] | |
| Ginseng, L-carnitine, Coenzyme Q10 | Second-line | Low | Yennurajalingam et al., 2022 [101] | |
| Psychostimulants | Investigational | Moderate | Andreas et al., 2023 [102] | |
| Hematopoietic agents | Investigational | Moderate | Bohlius et al., 2014 [103] |
| Baseline | Frequency | Intervention | |
|---|---|---|---|
| CV risk assessment | All patients | ||
| Complete lipid profile | All patients | Annually | Dietary +/− pharmacological |
| Blood pressure measurement | All patients | Every 3–6 months | Lowering pharmacological according to general population threshold |
| Lifestyle modification counseling | All patients | Every 3–6 months | |
| Electrocardiogram | All patients | Annually | Timely atrial fibrillation treatment, including anticoagulation and rhythm and/or rate control |
| Echocardiogram | High or very high CV risk | According to CV diagnosis | Referral to cardiologist |
| Carotid ultrasound | High or very high CV risk | According to CV diagnosis | Referral to vascular surgeon |
| Study | AI Therapy | Study Type | Key Endpoint | Main Toxicity | Key Findings |
|---|---|---|---|---|---|
| Walker et al., 2010 [48] | Anastrozole | RCT | Hot flashes frequency | VMS | Acupuncture matched venlafaxine in reducing hot flash frequency |
| Loprinzi et al., 2000 [49] | Mixed | RCT | Hot flashes frequency and severity | VMS | Venlafaxine alleviates hot flashes; 75 mg daily is optimal. |
| Su et al., 2010 [54] | Mixed | Cross-sectional survey | Presence of hot flashes | VMS | Weight gain is linked to hot flash risk |
| Duijts et al., 2012 [43] | Mixed | RCT | Endocrine symptoms | VMS | CBT and/or PE improved menopausal symptoms |
| Cardoso et al., 2025 [57] | Mixed | RCT | Hot flashes frequency | VMS | Elinzanetant significantly reduced VMS frequency |
| Rastelli et al., 2011 [66] | Anastrozole | RCT | Pain reduction | AIMSS | Vitamin D has a beneficial effect on musculoskeletal pain |
| Briot et al., 2010 [126] | Anastrozole → letrozole | Prospective non-randomized | Percentage of discontinuation of letrozole due to AIMSS | AIMSS | Switching between AIs enabled 70% of patients to continue treatment >6 months |
| Crew et al., 2010 [134] | Mixed | RCT | Pain reduction | AIMSS | True acupuncture reduced joint pain and stiffness vs. sham acupuncture |
| DeNysschen et al., 2014 [129] | Mixed | Pilot | AIMSS2 scale variation | AIMSS | Home-based exercise reduced joint pain |
| Irwin et al., 2015 [62] | Mixed | RCT | Pain score | AIMSS | Exercise reduced AI-related arthralgia pain scores by approximately 30% |
| Kadakia et al., 2016 [69] | Mixed | Prospective | Adherence | AIMSS | Switching AI allowed 2/3 of patients to continue therapy |
| Henry et al., 2018 [65] | Mixed | RCT | Pain reduction | AIMSS | Duloxetine significantly improved pain vs. placebo |
| Hershman et al., 2018 [135] | Mixed | RCT | Pain reduction | AIMSS | True acupuncture significantly reduced joint pain vs. sham |
| Bender et al., 2025 [63] | Mixed | RCT | Pain reduction | AIMSS | Aerobic exercise prevents pain increase |
| Mao et al., 2014 [78] | Mixed | RCT | Pain score, fatigue, psychological distress | AIMSS, fatigue, mood changes | Electro-acupuncture improved fatigue, anxiety, and depression in BC patients who experienced arthralgia related to AI use |
| Gnant et al., 2015 [74] | Anastrozole, Letrozole | RCT | Fracture risk | Bone loss | Denosumab reduced the rate of clinical fractures. |
| Coleman et al., 2013 [30] | Letrozole | RCT | BMD change | Bone loss | Zoledronate preserved BMD and is associated with improved DFS vs. letrozole alone. |
| Advani et al., 2017 [153] | Mixed | Pilot study | FSFI score | Gynecological symptoms | Active intervention resulted in better outcomes at 6 months |
| Carter et al., 2021 [83] | Mixed | Prospective | VAS and VuAS changes | Gynecological symptoms | HLA moisturization improved vulvovaginal health/sexual function |
| Lubián-López et al., 2023 [155] | Mixed | Pilot study | Vulvo-vaginal atrophy | Gynecological symptoms | Non-ablative SSVL improved vaginal atrophy, vaginal pH, dyspareunia, and sexual function |
| Taranto et al., 2024 [84] | Mixed | Pilot study | Serum estradiol elevation, sexual function improvement | Gynecological symptoms | Topical testosterone seems to be safe and effective in improving sexual function |
| Faltinová et al., 2025 [89] | Letrozole | Prospective | Changes in serum E2 levels, menopausal symptoms | Gynecological symptoms | Intravaginal estradiol therapy during adjuvant letrozole resulted in transient increases in systemic E2 levels |
| Hagstrom et al., 2016 [92] | Mixed | RCT | Fatigue | Fatigue | 16 weeks of high intensity RT significantly improved upper and lower body strength, and reduced perceived fatigue |
| Zick et al., 2016 [100] | Mixed | RCT | Change in fatigue score | Fatigue | Acupressure reduced fatigue and improved sleep quality and quality of life |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardin, S.; Ruffilli, B.; Landolfo, T.L.; Isingrini, G.; Taglialatela, I.; Delbarba, A.; D’Avanzo, F.; Rossi, V.; Celentano, E.; Conte, B.; et al. Aromatase Inhibitors as Adjuvant Therapy in Early Breast Cancer: Insights into Toxicities and Their Management. Cancers 2025, 17, 2726. https://doi.org/10.3390/cancers17172726
Nardin S, Ruffilli B, Landolfo TL, Isingrini G, Taglialatela I, Delbarba A, D’Avanzo F, Rossi V, Celentano E, Conte B, et al. Aromatase Inhibitors as Adjuvant Therapy in Early Breast Cancer: Insights into Toxicities and Their Management. Cancers. 2025; 17(17):2726. https://doi.org/10.3390/cancers17172726
Chicago/Turabian StyleNardin, Simone, Beatrice Ruffilli, Tommaso Lupo Landolfo, Giulia Isingrini, Ida Taglialatela, Andrea Delbarba, Francesca D’Avanzo, Valentina Rossi, Eduardo Celentano, Benedetta Conte, and et al. 2025. "Aromatase Inhibitors as Adjuvant Therapy in Early Breast Cancer: Insights into Toxicities and Their Management" Cancers 17, no. 17: 2726. https://doi.org/10.3390/cancers17172726
APA StyleNardin, S., Ruffilli, B., Landolfo, T. L., Isingrini, G., Taglialatela, I., Delbarba, A., D’Avanzo, F., Rossi, V., Celentano, E., Conte, B., Nardin, M., & Gennari, A. (2025). Aromatase Inhibitors as Adjuvant Therapy in Early Breast Cancer: Insights into Toxicities and Their Management. Cancers, 17(17), 2726. https://doi.org/10.3390/cancers17172726







