Etiologies and Outcomes of Diabetic Ketoacidosis in Cancer Patients: A Retrospective Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Settings and Participants
2.2. Data Collection
2.3. Definitions
2.4. Management
2.5. Statistical Analysis
2.6. Confounding Factors
3. Results
3.1. Baseline Characteristics
| Medication Regimen on Admission | Total (n = 91) | Type 1 Diabetes (n = 19) | Type 2 Diabetes (n = 45) | Drug Induced Diabetes (n = 27) |
|---|---|---|---|---|
| None—no. (%) | 30 (33%) | 0 (0%) | 6 (13%) | 25 (93%) |
| Oral/GLP1agonist—no. (%) | 17 (19%) | 0 (0%) | 17 (38%) | 0 (0%) |
| Oral/GLP1 agonist + basal insulin—no. (%) | 9 (10%) | 0 (0%) | 9 (20%) | 0 (0%) |
| Oral/GLP1 agonist + multiple dose insulin—no. (%) | 3 (3%) | 1 (5%) | 1 (2%) | 0 (0%) |
| Multiple dose insulin—no. (%) | 24 (27%) | 13 (68%) | 10 (22%) | 1 (4%) |
| Insulin pump—no. (%) | 8 (9%) | 5 (26%) | 2 (4%) | 1 (4%) |
3.2. Episodes of DKA
3.3. Predictors of In-Hospital Mortality
3.4. 30-Days Post Hospital Mortality
3.5. Provoking Factors for DKA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richardson, L.C.; Pollack, L.A. Therapy insight: Influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat. Clin. Pract. Oncol. 2005, 2, 48–53. [Google Scholar] [CrossRef]
- de Haan-Du, J.; Groenier, K.H.; Wauben-Spaetgens, B.; Jalving, M.; Kleefstra, N.; Landman, G.W.D.; de Bock, G.H. The Value of Glycemic Control Prior to Cancer Diagnosis on All-Cause Mortality among Patients with Type 2 Diabetes in Dutch Primary Care. Cancer Epidemiol. Biomark. Prev. 2023, 32, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Natalicchio, A.; Marrano, N.; Montagnani, M.; Gallo, M.; Faggiano, A.; Zatelli, M.C.; Argentiero, A.; Del Re, M.; D’Oronzo, S.; Fogli, S.; et al. Glycemic control and cancer outcomes in oncologic patients with diabetes: An Italian Association of Medical Oncology (AIOM), Italian Association of Medical Diabetologists (AMD), Italian Society of Diabetology (SID), Italian Society of Endocrinology (SIE), Italian Society of Pharmacology (SIF) multidisciplinary critical view. J. Endocrinol. Investig. 2024, 47, 2915–2928. [Google Scholar] [CrossRef]
- Farsani, S.F.; Brodovicz, K.; Soleymanlou, N.; Marquard, J.; Wissinger, E.; Maiese, B.A. Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): A systematic literature review. BMJ Open 2017, 7, e016587. [Google Scholar] [CrossRef]
- Barski, L.; Nevzorov, R.; Jotkowitz, A.; Rabaev, E.; Zektser, M.; Zeller, L.; Shleyfer, E.; Harman-Boehm, I.; Almog, Y. Comparison of diabetic ketoacidosis in patients with type-1 and type-2 diabetes mellitus. Am. J. Med. Sci. 2013, 345, 326–330. [Google Scholar] [CrossRef]
- Yan, S.H.; Sheu, W.H.; Song, Y.M.; Tseng, L.N. The occurrence of diabetic ketoacidosis in adults. Intern. Med. 2000, 39, 10–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Newton, C.A.; Raskin, P. Diabetic ketoacidosis in type 1 and type 2 diabetes mellitus: Clinical and biochemical differences. Arch. Intern. Med. 2004, 164, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Quintas, J.; Mowatt, K.B.; Mullally, J.A.; Steinberg, A. New-onset persistent hyperglycemia with initiation of brentuximab treatment. J. Oncol. Pharm. Pract. 2023, 138, 10781552231168951. [Google Scholar] [CrossRef]
- Mudd, T.W., Jr.; Fox, A.D.; Ghaly, M.; Keruakous, A. Case report: Hyperosmolar hyperglycemic syndrome secondary to PEG-asparaginase-induced hypertriglyceridemia and pancreatitis. Front. Oncol. 2022, 12, 1094964. [Google Scholar] [CrossRef]
- Shen, S.; Chen, Y.; Carpio, A.; Chang, C.; Iyengar, N.M. Incidence, risk factors, and management of alpelisib-associated hyperglycemia in metastatic breast cancer. Cancer 2023, 129, 3854–3861. [Google Scholar] [CrossRef]
- Jain, A.B.; Lai, V. Medication-Induced Hyperglycemia and Diabetes Mellitus: A Review of Current Literature and Practical Management Strategies. Diabetes Ther. 2024, 15, 2001–2025. [Google Scholar] [CrossRef] [PubMed]
- Umpierrez, G.E.; Davis, G.M.; ElSayed, N.A.; Fadini, G.P.; Galindo, R.J.; Hirsch, I.B.; Klonoff, D.C.; McCoy, R.G.; Misra, S.; Gabbay, R.A.; et al. Hyperglycaemic crises in adults with diabetes: A consensus report. Diabetologia 2024, 67, 1455–1479. [Google Scholar] [CrossRef]
- Lin, E.H.; Katon, W.; Von Korff, M.; Rutter, C.; Simon, G.E.; Oliver, M.; Ciechanowski, P.; Ludman, E.J.; Bush, T.; Young, B. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care 2004, 27, 2154–2160. [Google Scholar] [CrossRef]
- Ehrmann, D.; Kulzer, B.; Roos, T.; Haak, T.; Al-Khatib, M.; Hermanns, N. Risk factors and prevention strategies for diabetic ketoacidosis in people with established type 1 diabetes. Lancet Diabetes Endocrinol. 2020, 8, 436–446. [Google Scholar] [CrossRef]
- Benoit, S.R.; Zhang, Y.; Geiss, L.S.; Gregg, E.W.; Albright, A. Trends in Diabetic Ketoacidosis Hospitalizations and In-Hospital Mortality—United States, 2000–2014. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Shahid, R.K.; Haider, Q.; Yadav, S.; Le, D.; Ahmed, S. Diabetic ketoacidosis and hyperglycaemic hyperosmolar syndrome in patients with cancer: A multicentre study. Clin. Med. 2025, 25, 100262. [Google Scholar] [CrossRef]
- Ose, D.J.; Viskochil, R.; Holowatyj, A.N.; Larson, M.; Wilson, D.; Dunson, W.A.; Deshmukh, V.G.; Butcher, J.R.; Taylor, B.R.; Svoboda, K.; et al. Understanding the Prevalence of Prediabetes and Diabetes in Patients with Cancer in Clinical Practice: A Real-World Cohort Study. J. Natl. Compr. Cancer Netw. 2021, 19, 709–718. [Google Scholar] [CrossRef]
- Gerwer, J.E.; Bacani, G.; Juang, P.S.; Kulasa, K. Electronic Health Record-Based Decision-Making Support in Inpatient Diabetes Management. Curr. Diabetes Rep. 2022, 22, 433–440. [Google Scholar] [CrossRef]
- Wright, J.J.; Powers, A.C.; Johnson, D.B. Endocrine toxicities of immune checkpoint inhibitors. Nat. Rev. Endocrinol. 2021, 17, 389–399. [Google Scholar] [CrossRef]
- Tsang, V.H.M.; McGrath, R.T.; Clifton-Bligh, R.J.; Scolyer, R.A.; Jakrot, V.; Guminski, A.D.; Long, G.V.; Menzies, A.M. Checkpoint Inhibitor-Associated Autoimmune Diabetes Is Distinct From Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 5499–5506. [Google Scholar] [CrossRef] [PubMed]
- Jeun, R.; Iyer, P.C.; Best, C.; Lavis, V.; Varghese, J.M.; Yedururi, S.; Brady, V.; Oliva, I.C.G.; Dadu, R.; Milton, D.R.; et al. Clinical outcomes of immune checkpoint inhibitor diabetes mellitus at a comprehensive cancer center. Immunotherapy 2023, 15, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Stamatouli, A.M.; Quandt, Z.; Perdigoto, A.L.; Clark, P.L.; Kluger, H.; Weiss, S.A.; Gettinger, S.; Sznol, M.; Young, A.; Rushakoff, R.; et al. Collateral Damage: Insulin-Dependent Diabetes Induced with Checkpoint Inhibitors. Diabetes 2018, 67, 1471–1480. [Google Scholar] [CrossRef]
- Rosenstock, J.; Ferrannini, E. Euglycemic Diabetic Ketoacidosis: A Predictable, Detectable, and Preventable Safety Concern with SGLT2 Inhibitors. Diabetes Care 2015, 38, 1638–1642. [Google Scholar] [CrossRef]
- Leslie, B.R.; Gerwin, L.E.; Taylor, S.I. Sodium-Glucose Cotransporter-2 Inhibitors: Lack of a Complete History Delays Diagnosis. Ann. Intern. Med. 2019, 171, 421–426. [Google Scholar] [CrossRef]
- Goldenberg, R.M.; Berard, L.D.; Cheng, A.Y.Y.; Gilbert, J.D.; Verma, S.; Woo, V.C.; Yale, J.F. SGLT2 Inhibitor-associated Diabetic Ketoacidosis: Clinical Review and Recommendations for Prevention and Diagnosis. Clin. Ther. 2016, 38, 2654–2664.e2651. [Google Scholar] [CrossRef]
- Malone, M.L.; Gennis, V.; Goodwin, J.S. Characteristics of diabetic ketoacidosis in older versus younger adults. J. Am. Geriatr. Soc. 1992, 40, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Graves, E.J.; Gillum, B.S. Detailed diagnoses and procedures, National Hospital Discharge Survey, 1995. Vital Health Stat. 1997, 13, 1–146. [Google Scholar]
- Zhong, V.W.; Juhaeri, J.; Mayer-Davis, E.J. Trends in Hospital Admission for Diabetic Ketoacidosis in Adults with Type 1 and Type 2 Diabetes in England, 1998–2013: A Retrospective Cohort Study. Diabetes Care 2018, 41, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.X.; Umpierrez, G.E.; Afessa, B.; Latif, K.A.; Bridges, L.; Kitabchi, A.E. Predictors of intensive care unit and hospital length of stay in diabetic ketoacidosis. J. Crit. Care 2002, 17, 207–211. [Google Scholar] [CrossRef]
- Tauschmann, M.; Hermann, J.M.; Freiberg, C.; Papsch, M.; Thon, A.; Heidtmann, B.; Placzeck, K.; Agena, D.; Kapellen, T.M.; Schenk, B.; et al. Reduction in Diabetic Ketoacidosis and Severe Hypoglycemia in Pediatric Type 1 Diabetes During the First Year of Continuous Glucose Monitoring: A Multicenter Analysis of 3553 Subjects From the DPV Registry. Diabetes Care 2020, 43, e40–e42. [Google Scholar] [CrossRef]
- Roussel, R.; Riveline, J.P.; Vicaut, E.; de Pouvourville, G.; Detournay, B.; Emery, C.; Levrat-Guillen, F.; Guerci, B. Important Drop in Rate of Acute Diabetes Complications in People with Type 1 or Type 2 Diabetes After Initiation of Flash Glucose Monitoring in France: The RELIEF Study. Diabetes Care 2021, 44, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total (n = 91) | Type 1 Diabetes (n = 19, 21%) | Type 2 Diabetes (n = 45, 49%) | Drug- Induced Diabetes (n = 27, 30%) | p-Value |
|---|---|---|---|---|---|
| Age, years—Median (IQR) | 63 (44–69) | 62 (40–67) | 63 (49–69) | 62 (41.5–70.5) | 0.77 |
| Female sex—no. (%) | 42 (46%) | 9 (47%) | 21 (47%) | 12 (44%) | 1.00 |
| HbA1C checked—no. | 74 (81%) | 14 (74%) | 38 (84%) | 22 (81%) | — |
| Baseline HbA1C %– Median (IQR) | 8.2 (7.3–10.4) | 8.1 (7.3–9.7) | 8.1 (7.1–12) | 8.4 (7.3–9.7) | 0.82 |
| HbA1C > 9%—no. (%) (n = 74) | 29 (39%) | 5 (36%) | 16 (42%) | 8 (36%) | 0.81 |
| Duration of diabetes, years—Median (IQR) | 5 (0–18) | 25 (12–35) | 11 (1–19) | 0 (0–0) | <0.001 |
| Lack of diabetes survival skills—no. (%) | 22 (24%) | 12 (63%) | 8 (18%) | 2 (7%) | <0.001 |
| CGM use before presentation | 4 (4%) | 3 (16%) | 1 (2%) | 0 (0%) | - |
| Characteristic | Total (n = 91) | Type 1 Diabetes (n = 19, 21%) | Type 2 Diabetes (n = 45, 49%) | Drug-Induced Diabetes (n = 27, 30%) | p-Value |
|---|---|---|---|---|---|
| Cancer Diagnosis | 0.053 | ||||
| Gastrointestinal Malignancy | 18 (20%) | 3 (16%) | 9 (20%) | 6 (22%) | |
| Dermatological Malignancy | 14 (15%) | 3 (16%) | 4 (9%) | 7 (26%) | |
| Hematological Malignancy | 13 (14%) | 3 (16%) | 9 (20%) | 1 (4%) | |
| Genitourinary Malignancy | 10 (11%) | 1 (5%) | 4 (9%) | 5 (19%) | |
| Breast Cancer | 7 (8%) | 1 (5%) | 5 (11%) | 1 (4%) | |
| Thoracic Malignancy | 7 (8%) | 0 (0%) | 5 (11%) | 2 (7%) | |
| Head & Neck Malignancy | 5 (5%) | 4 (21%) | 0 (0%) | 1 (4%) | |
| Gynecological Malignancy | 5 (5%) | 0 (0%) | 3 (7%) | 2 (7%) | |
| Central Nervous System Malignancy | 3 (3%) | 1 (5%) | 1 (2%) | 1 (4%) | |
| Sarcoma | 2 (2%) | 1 (5%) | 1 (2%) | 0 (0%) | |
| Endocrine Malignancy | 1 (1%) | 0 (0%) | 0 (0%) | 1 (4%) | |
| No Malignancy | 6 (7%) | 2 (11%) | 4 (9%) | 0 (0%) | |
| Cancer Status | |||||
| No Evidence of Disease (NED) | 8 (9%) | 5 (26%) | 3 (7%) | 0 (0%) | 0.009 |
| Stage I | 2 (2%) | 1 (5%) | 1 (2%) | 0 (0%) | |
| Stage II | 4 (4%) | 0 (0%) | 4 (9%) | 0 (0%) | |
| Stage III | 2 (2%) | 0 (0%) | 1 (2%) | 1 (4%) | |
| Stage IV or Metastatic | 61 (67%) | 11 (58%) | 25 (56%) | 25 (93%) | |
| Unknown Stage/Staging not available | 14 (15%) | 2 (11%) | 11 (24%) | 1 (4%) |
| Drug Class | Total (n = 91) | Type 1 Diabetes (n = 19, 21%) | Type 2 Diabetes (n = 45, 49%) | Drug Induced Diabetes (n = 27, 30%) | p-Value |
|---|---|---|---|---|---|
| Immunotherapy use—no. (%) | 26 (29%) | 0 (0%) | 3 (7%) | 23 (85%) | <0.001 |
| Steroid use (n = 88)—no. (%) | 10 (11%) | 1 (6%) | 7 (16%) | 2 (7%) | 0.49 |
| SGLT2 Inhibitor use—no. (%) | 12 (13%) | 0 (0%) | 12 (27%) | 0 (0%) | <0.001 |
| Antidepressant use—no. (%) | 21 (23%) | 4 (21%) | 9 (20%) | 8 (30%) | 0.80 |
| Characteristic | Total (n = 94) | Type 1 Diabetes (n = 20) | Type 2 Diabetes (n = 46) | Drug- Induced Diabetes (n = 28) | p-Value |
|---|---|---|---|---|---|
| DKA Severity—no. (%) | |||||
| Mild | 39 (41%) | 7 (35%) | 23 (50%) | 9 (32%) | 0.55 |
| Moderate | 26 (28%) | 7 (35%) | 9 (20%) | 10 (36%) | |
| Severe | 29 (31%) | 6 (30%) | 14 (30%) | 9 (32%) | |
| Length of Hospitalization (days)—Median (IQR) | 6 (3–13) | 4 (3–6) | 8 (4–16) | 6 (4–10) | 0.019 |
| Characteristics | Total (n = 94) | Type 1 Diabetes (n = 20) | Type 2 Diabetes (n = 46) | Drug-Induced Diabetes (n = 28) | p-Value |
|---|---|---|---|---|---|
| In-Hospital Death—no. (%) | 15 (16%) | 0 (0%) | 11 (24%) | 4 (14%) | 0.055 |
| Death within 30 Days After Discharge—no. (%) | 5 (5%) | 1 (5%) | 3 (7%) | 1 (4%) | 0.861 |
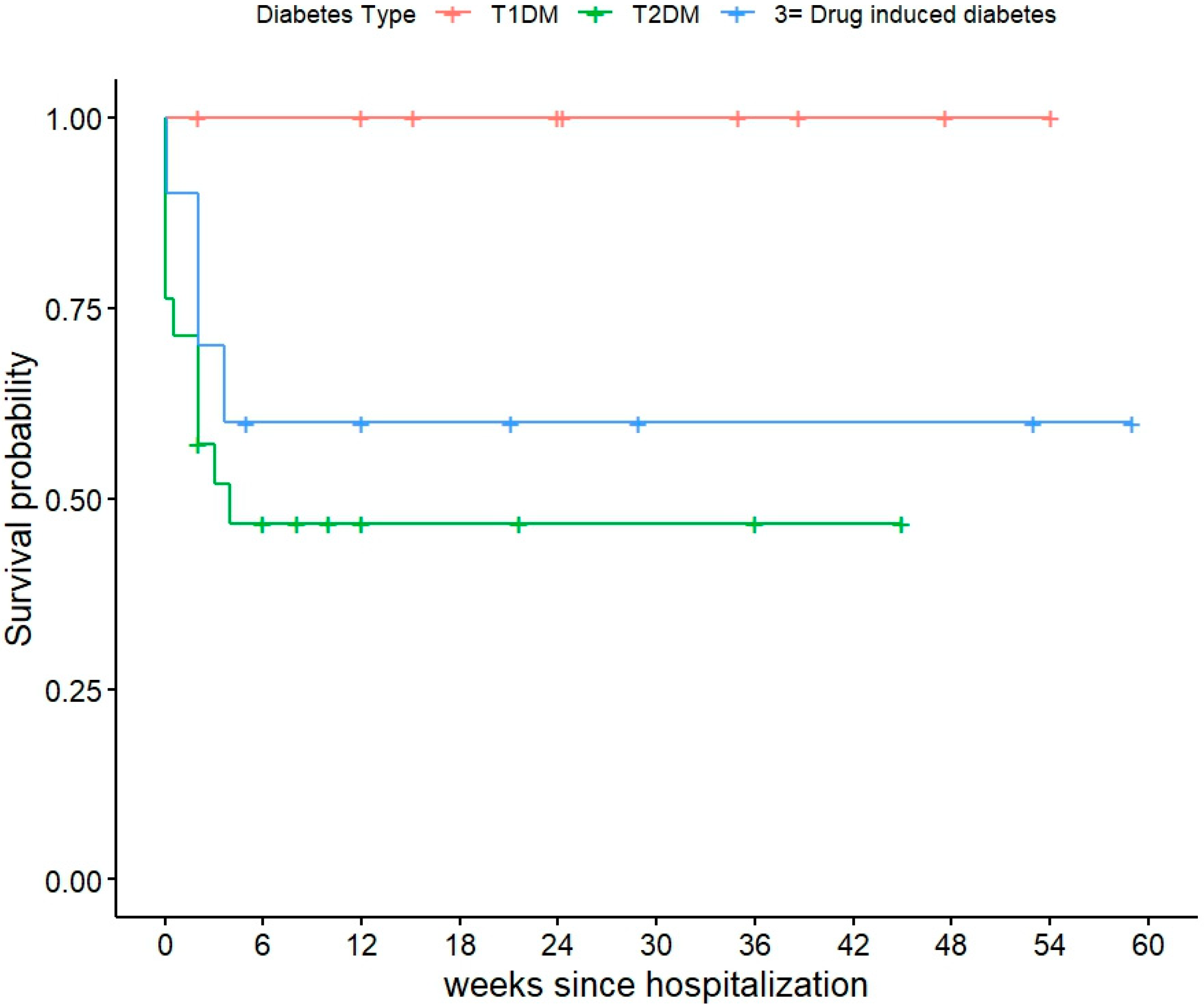
| Death During Study Period—no. (%) | 40 (44%) | 9 (47%) | 21 (46%) | 10 (36%) | 0.60 |
| Time to Death (Weeks) Among patients who died—Median (IQR) | 9.05 (2–24.15) | 24.3 (15.1–38.7) | 3 (0.5–12.0) | 8.5 (2.0–28.9) | 0.009 |
| Measure | Total Events | p-Value | Hazard Ratio (95% CI) |
|---|---|---|---|
| Length of the hospital stay | 40:15 | 0.79 | 1.00 (0.98–1.02) |
| Cancer staging | 0.39 * | ||
| NED | 3:0 | ||
| Stage II | 1:0 | ||
| Stage IV | 4:1 | ||
| Metastatic | 28:13 | ||
| Cancer staging | |||
| Non-Metastatic | 8:1 | 0.10 | Reference level |
| Metastatic | 28:13 | 4.4 (0.6–33.9) | |
| Age | 40:15 | 0.36 | 1.02 (0.98–1.05) |
| DM type | 0.035 * | ||
| T1DM | 9:0 | Ref | |
| T2DM | 21:11 | 0.99 | 1.7 × 107 (0–NE) |
| Drug induced diabetes | 10:4 | 0.99 | 1.1 × 107 (0–NE) |
| Classification of Provoking fact | |||
| Drug induced | |||
| No | 19:6 | 0.52 * | |
| Yes | 21:9 | ||
| Infection related | |||
| No | 27:11 | 0.48 * | |
| Yes | 13:4 | ||
| Inadequate exogenous insulin | |||
| No | 31:14 | 0.08 * | |
| Yes | 9:1 | ||
| Other | |||
| No | 39:14 | 0.008 * | |
| Yes | 1:1 |
| Measure | Total Events | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|
| Overall | 25:5 | ||
| Length of the hospital stay | 1.00 (0.98–1.03) | 0.90 | |
| Cancer staging | |||
| NED | 3:0 | 0.12 * | |
| Stage II | 1:0 | ||
| Stage IV | 4:2 | ||
| Metastatic | 28:2 | ||
| Age | 25:5 | 1.01 (0.95–1.08) | 0.75 |
| DM type | |||
| T1DM | 9:1 | Ref | 0.61 * |
| T2DM | 10:3 | 2.82 (0.29–27.18) | 0.81 |
| Drug induced diabetes | 6:1 | 1.50 (0.09–24.06) | 0.08 |
| Classification of Provoking fact | |||
| Drug induced | |||
| No | 13:3 | 0.66 * | |
| Yes | 12:2 | ||
| Infection related | |||
| No | 16:2 | 0.23 * | |
| Yes | 9:3 | ||
| Inadequate exogenous insulin | |||
| No | 17:4 | 0.55 * | |
| Yes | 8:1 | ||
| Other | |||
| No | 25:5 |
| Provoking Factors | Total (n = 94) | Type I Diabetes (n = 20) | Type II Diabetes (n = 45) | Drug-Induced Diabetes (n = 29) |
|---|---|---|---|---|
| Drug Induced | 50 (53%) | 1 (5%) | 20 (44%) | 29 (100%) |
| Inadequate Exogenous Insulin | 34 (36%) | 17 (85%) | 15 (33%) | 2 (7%) |
| Infection Related | 20 (21%) | 4 (20%) | 14 (31%) | 2 (7%) |
| New DM unrelated to cancer treatment | 1 (1%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Unknown (Misc.) | 3 (3%) | 0 (0%) | 3 (7%) | 0 (0%) |
| Number of Provoking Factors—1 | 79 (84%) | 18 (90%) | 37 (82%) | 24 (83%) |
| Number of Provoking Factors—2 | 14 (15%) | 2 (10%) | 7 (15%) | 5 (17%) |
| Number of Provoking Factors—3 | 1 (1%) | 0 (0%) | 1 (2%) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, A.; Jeun, R.; Wang, Z.; Khan, S.; Best, C.; Lavis, V.; Thosani, S. Etiologies and Outcomes of Diabetic Ketoacidosis in Cancer Patients: A Retrospective Analysis. Cancers 2025, 17, 2728. https://doi.org/10.3390/cancers17172728
Gandhi A, Jeun R, Wang Z, Khan S, Best C, Lavis V, Thosani S. Etiologies and Outcomes of Diabetic Ketoacidosis in Cancer Patients: A Retrospective Analysis. Cancers. 2025; 17(17):2728. https://doi.org/10.3390/cancers17172728
Chicago/Turabian StyleGandhi, Ayush, Rebecca Jeun, Zhongya Wang, Sonya Khan, Conor Best, Victor Lavis, and Sonali Thosani. 2025. "Etiologies and Outcomes of Diabetic Ketoacidosis in Cancer Patients: A Retrospective Analysis" Cancers 17, no. 17: 2728. https://doi.org/10.3390/cancers17172728
APA StyleGandhi, A., Jeun, R., Wang, Z., Khan, S., Best, C., Lavis, V., & Thosani, S. (2025). Etiologies and Outcomes of Diabetic Ketoacidosis in Cancer Patients: A Retrospective Analysis. Cancers, 17(17), 2728. https://doi.org/10.3390/cancers17172728






