Safety and Efficacy of Bispecific Antibody Treatment in Relapsed/Refractory Multiple Myeloma: A Systematic Review and Meta-Analysis of Proportions from Clinical Trials
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Extraction and Effect Estimates
2.2. Statistical Analysis
2.3. Assessment of Study Quality, Risk of Bias and Certainty
3. Results
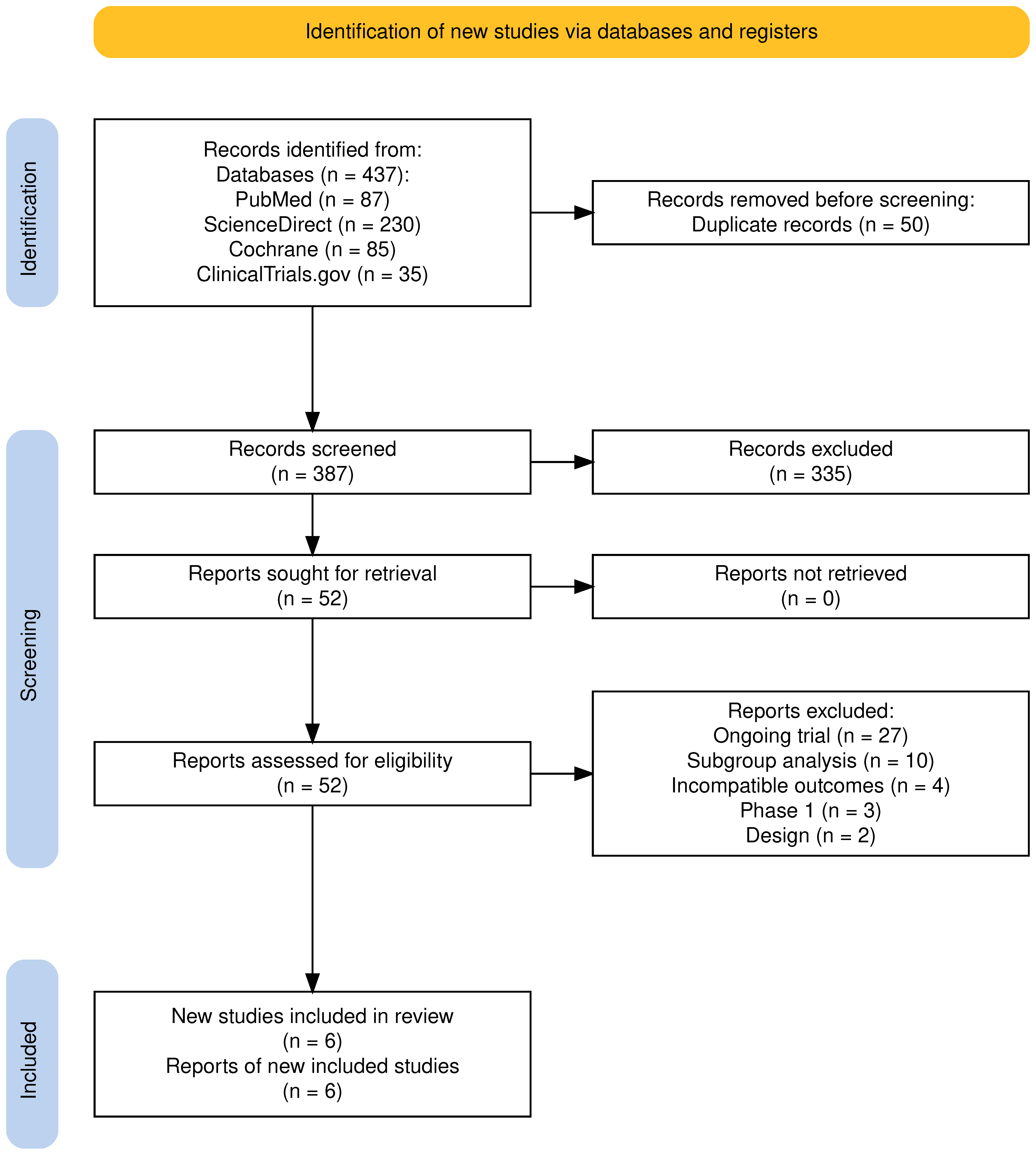
3.1. Overview of Screening and Included Studies
3.2. Efficacy
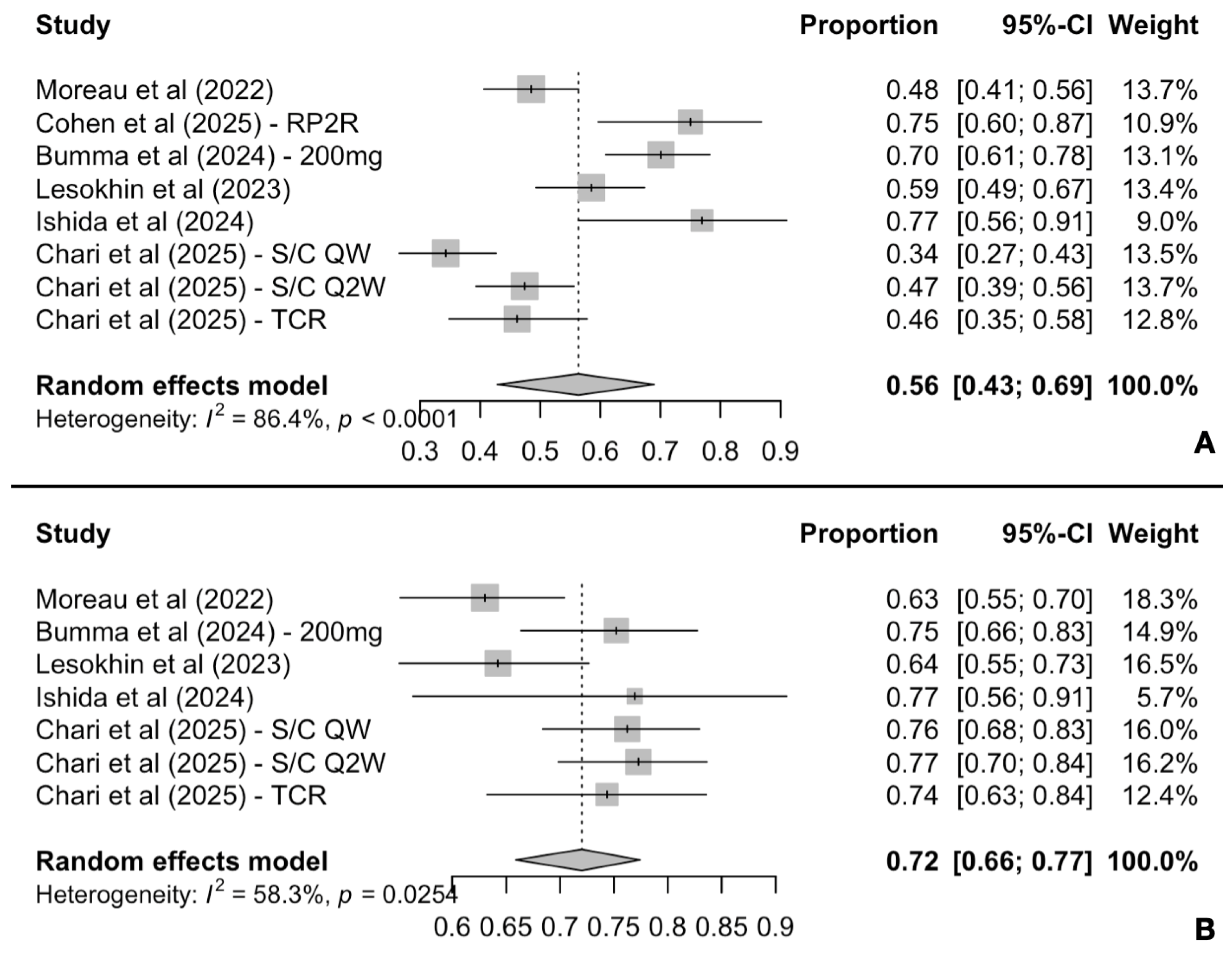
3.2.1. Response Rates and Duration of Response Analysis
3.2.2. One-Year Survival Rates Analysis
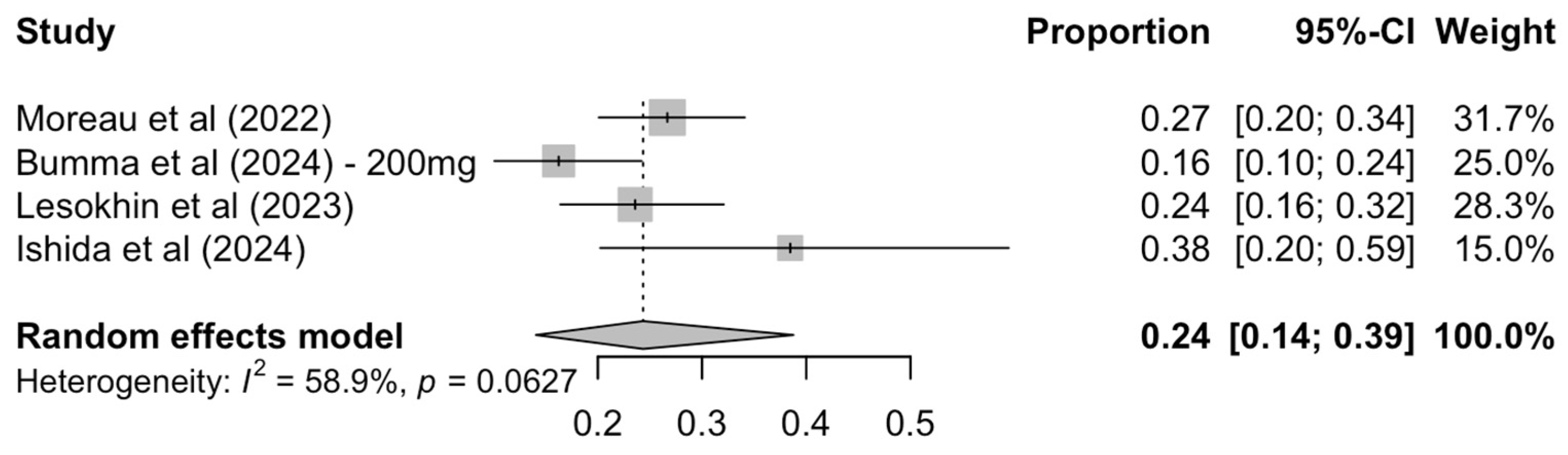
3.2.3. Exploratory Analysis on MRD Negativity Rates
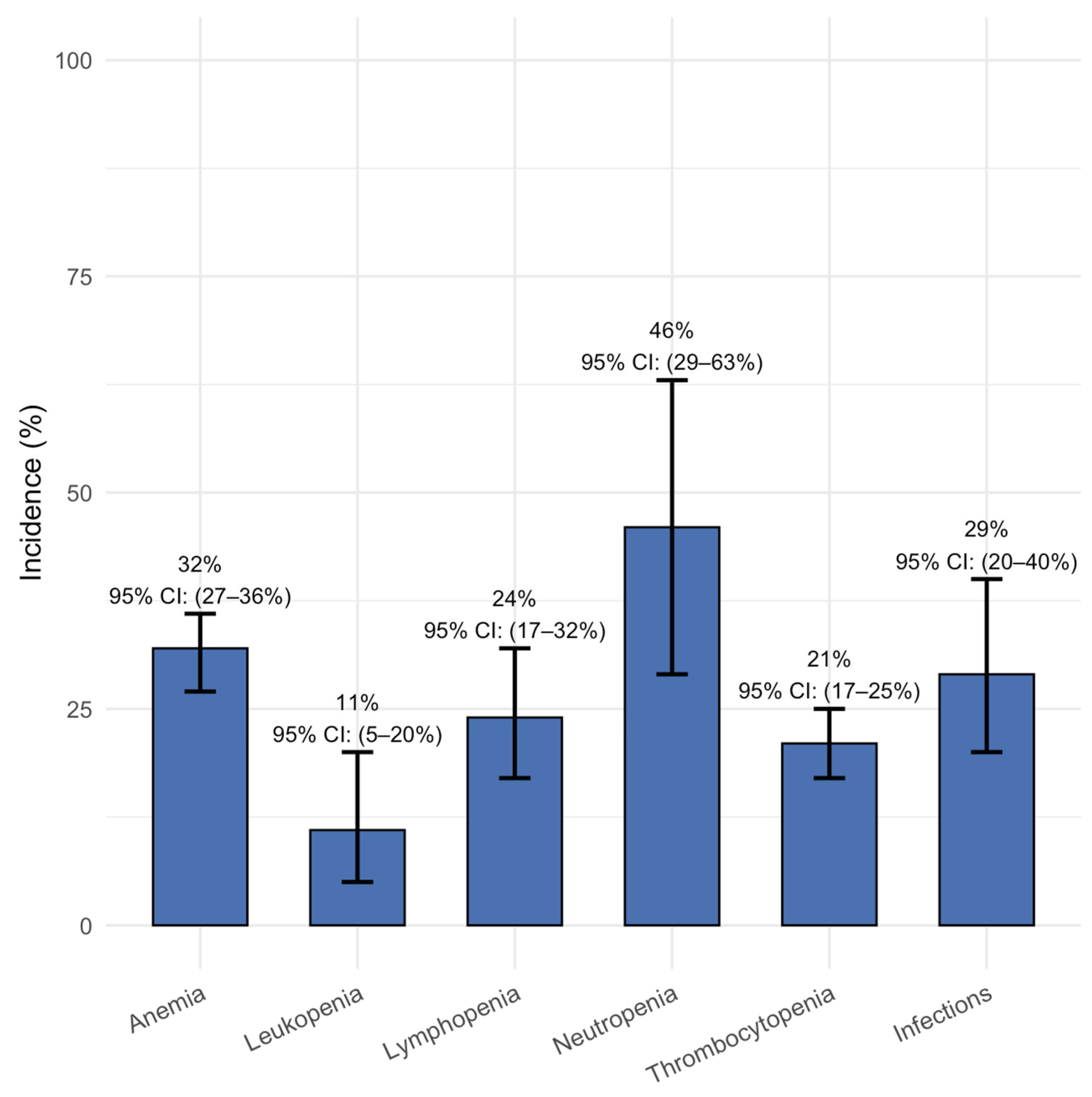
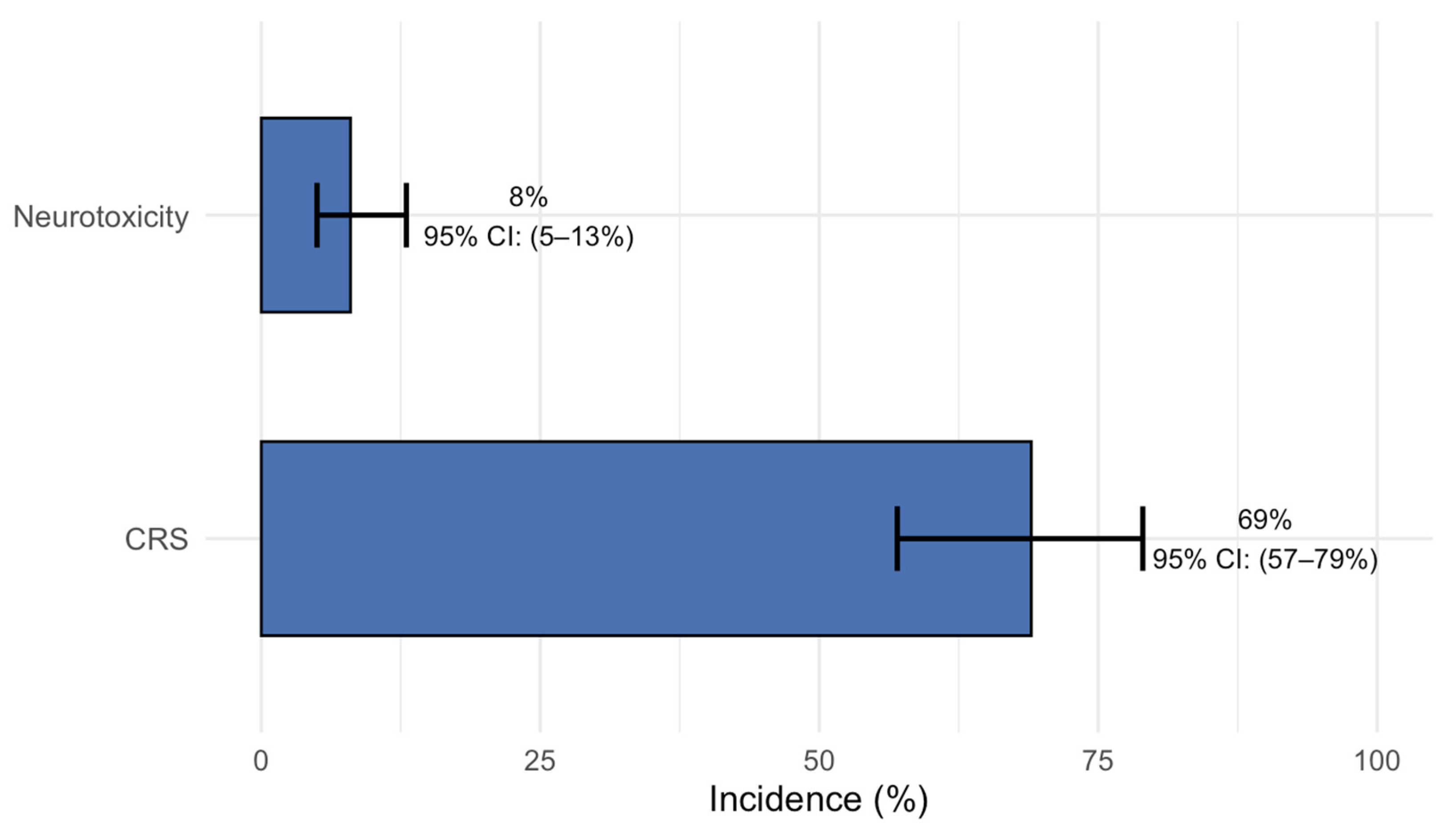
3.3. Safety
3.4. Risk of Bias and Certainty of Evidence Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajkumar, S.V. Multiple myeloma: 2024 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2024, 99, 1802–1824. [Google Scholar] [CrossRef]
- Mafra, A.; Laversanne, M.; Marcos-Gragera, R.; Chaves, H.V.S.; Mcshane, C.; Bray, F.; Znaor, A. The global multiple myeloma incidence and mortality burden in 2022 and predictions for 2045. J. Natl. Cancer Inst. 2025, 117, 907–914. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2024. Available online: https://gco.iarc.who.int/today (accessed on 18 August 2025).
- Kumar, S.K.; Callander, N.S.; Adekola, K.; Anderson, L.D.; Baljevic, M.; Baz, R.; Campagnaro, E.; Costello, C.; D’Angelo, C.; Derman, B.; et al. NCCN Guidelines® Insights: Multiple Myeloma, Version 1.2025. J. Natl. Compr. Cancer Netw. 2025, 23, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Weisel, K.; De Stefano, V.; Goldschmidt, H.; Delforge, M.; Mohty, M.; Dytfeld, D.; Angelucci, E.; Vincent, L.; Perrot, A.; et al. LocoMMotion: A study of real-life current standards of care in triple-class exposed patients with relapsed/refractory multiple myeloma—2-year follow-up (final analysis). Leukemia 2024, 38, 2554–2560. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Unawane, R.; Wang, S.; Ahn, J.; Aleman, A.; Siegel, D.S.; Vesole, D.H.; Parmar, H.; Phull, P.; Biran, N. I-OPen: Inferior outcomes of penta-refractory compared to penta-exposed multiple myeloma patients. Blood Cancer J. 2022, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Terpos, E.; Boccadoro, M.; Delimpasi, S.; Beksac, M.; Katodritou, E.; Moreau, P.; Baldini, L.; Symeonidis, A.; Bila, J.; et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 801–812. [Google Scholar] [CrossRef]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Wudhikarn, K.; Wills, B.; Lesokhin, A.M. Monoclonal antibodies in multiple myeloma: Current and emerging targets and mechanisms of action. Best Pr. Res. Clin. Haematol. 2020, 33, 101143. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Usmani, S.Z. CD38 Antibodies in Multiple Myeloma: Mechanisms of Action and Modes of Resistance. Front. Immunol. 2018, 9, 2134. [Google Scholar] [CrossRef]
- Lancman, G.; Sastow, D.L.; Cho, H.J.; Jagannath, S.; Madduri, D.; Parekh, S.S.; Richard, S.; Richter, J.; Sanchez, L.; Chari, A. Bispecific Antibodies in Multiple Myeloma: Present and Future. Blood Cancer Discov. 2021, 2, 423–433. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, Q.; Liu, X.; Guo, X.; Song, Y. Bispecific antibodies targeting BCMA, GPRC5D, and FcRH5 for multiple myeloma therapy: Latest updates from ASCO 2023 Annual Meeting. J. Hematol. Oncol. 2023, 16, 92. [Google Scholar] [CrossRef]
- Baines, A.C.; Kanapuru, B.; Zhao, J.; Price, L.S.L.; Zheng, N.; Konicki, R.; Manning, M.L.; Gehrke, B.J.; Theoret, M.R.; Gormley, N.J. FDA Approval Summary: Teclistamab-A Bispecific CD3 T-Cell Engager for Patients with Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2024, 30, 5515–5520. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Talquetamab: First Approval. Drugs 2023, 83, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Elranatamab: First Approval. Drugs 2023, 83, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Caraccio, C.; Krishna, S.; Phillips, D.J.; Schürch, C.M. Bispecific Antibodies for Multiple Myeloma: A Review of Targets, Drugs, Clinical Trials, and Future Directions. Front. Immunol. 2020, 11, 501. [Google Scholar] [CrossRef]
- Ntanasis-Stathopoulos, I.; Filippatos, C.; Malandrakis, P.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A.; Gavriatopoulou, M. Observation or treatment for smoldering multiple myeloma? A systematic review and meta-analysis of randomized controlled studies. Blood Cancer J. 2025, 15, 104. [Google Scholar] [CrossRef]
- Ntanasis-Stathopoulos, I.; Filippatos, C.; Malandrakis, P.; Koutoulidis, V.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A.; Gavriatopoulou, M. Upfront Anti-CD38 Monoclonal Antibody-Based Quadruplet Therapy for Multiple Myeloma: A Systematic Review and Meta-Analysis of Clinical Trials. Cancers 2025, 17, 1943. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://handbook-5-1.cochrane.org/ (accessed on 28 July 2025).
- Wohlin, C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. In Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering, London, UK, 13–14 May 2014; ACM: London, UK, 2014; pp. 1–10. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Cheema, H.A.; Shahid, A.; Ehsan, M.; Ayyan, M. The misuse of funnel plots in meta-analyses of proportions: Are they really useful? Clin. Kidney J. 2022, 15, 1209–1210. [Google Scholar] [CrossRef]
- Hunter, J.P.; Saratzis, A.; Sutton, A.J.; Boucher, R.H.; Sayers, R.D.; Bown, M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J. Clin. Epidemiol. 2014, 67, 897–903. [Google Scholar] [CrossRef]
- Brozek, J.L.; Canelo-Aybar, C.; Akl, E.A.; Bowen, J.M.; Bucher, J.; Chiu, W.A.; Cronin, M.; Djulbegovic, B.; Falavigna, M.; Guyatt, G.H.; et al. GRADE Guidelines 30: The GRADE approach to assessing the certainty of modeled evidence-An overview in the context of health decision-making. J. Clin. Epidemiol. 2021, 129, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Garfall, A.L.; van de Donk, N.W.C.J.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Bumma, N.; Richter, J.; Jagannath, S.; Lee, H.C.; Hoffman, J.E.; Suvannasankha, A.; Zonder, J.A.; Shah, M.R.; Lentzsch, S.; Baz, R.; et al. Linvoseltamab for Treatment of Relapsed/Refractory Multiple Myeloma. J. Clin. Oncol. 2024, 42, 2702–2712, Erratum in: J. Clin. Oncol. 2024, 42, 4001. https://doi.org/10.1200/JCO-24-02169. [Google Scholar] [CrossRef] [PubMed]
- Lesokhin, A.M.; Tomasson, M.H.; Arnulf, B.; Bahlis, N.J.; Miles Prince, H.; Niesvizky, R.; Rodrίguez-Otero, P.; Martinez-Lopez, J.; Koehne, G.; Touzeau, C.; et al. Elranatamab in relapsed or refractory multiple myeloma: Phase 2 MagnetisMM-3 trial results. Nat. Med. 2023, 29, 2259–2267. [Google Scholar] [CrossRef]
- Ishida, T.; Kuroda, Y.; Matsue, K.; Komeno, T.; Ishiguro, T.; Ishikawa, J.; Ito, T.; Kosugi, H.; Sunami, K.; Nishikawa, K.; et al. A Phase 1/2 study of teclistamab, a humanized BCMA × CD3 bispecific Ab in Japanese patients with relapsed/refractory MM. Int. J. Hematol. 2025, 121, 222–231. [Google Scholar] [CrossRef]
- Chari, A.; Touzeau, C.; Schinke, C.; Minnema, M.C.; Berdeja, J.G.; Oriol, A.; van de Donk, N.W.C.J.; Rodríguez-Otero, P.; Morillo, D.; Martinez-Chamorro, C.; et al. Safety and activity of talquetamab in patients with relapsed or refractory multiple myeloma (MonumenTAL-1): A multicentre, open-label, phase 1-2 study. Lancet Haematol. 2025, 12, e269–e281. [Google Scholar] [CrossRef]
- Cohen, Y.C.; Magen, H.; Gatt, M.; Sebag, M.; Kim, K.; Min, C.K.; Ocio, E.M.; Yoon, S.S.; Chu, M.P.; Rodríguez-Otero, P.; et al. Talquetamab plus teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2025, 392, 138–149. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, A.; Zhu, J.; Niu, T. Efficacy and safety of bispecific antibodies therapy for relapsed or refractory multiple myeloma: A systematic review and meta-analysis of prospective clinical trials. Front. Immunol. 2024, 15, 1348955. [Google Scholar] [CrossRef]
- Lee, H.; Ahn, S.; Maity, R.; Leblay, N.; Ziccheddu, B.; Truger, M.; Chojnacka, M.; Cirrincione, A.; Durante, M.; Tilmont, R.; et al. Mechanisms of antigen escape from BCMA- or GPRC5D-targeted immunotherapies in multiple myeloma. Nat. Med. 2023, 29, 2295–2306. [Google Scholar] [CrossRef]
- Letouzé, E.; Moreau, P.; Munshi, N.; Samur, M.; Minvielle, S.; Touzeau, C. Mechanisms of resistance to bispecific T-cell engagers in multiple myeloma and their clinical implications. Blood Adv. 2024, 8, 2952–2959. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Neri, P.; Bahlis, N.J. BCMA- or GPRC5D-targeting bispecific antibodies in multiple myeloma: Efficacy, safety, and resistance mechanisms. Blood 2024, 143, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- FDA Grants Accelerated Approval to Linvoseltamab-Gcpt for Relapsed or Refractory Multiple Myeloma; USA Food and Drug Administration: Silver Spring, MD, USA, 2025.
- Sidana, S.; Moreau, P.; Garfall, A.; Bhutani, M.; Oriol, A.; Nooka, A.; Martin, T.; Rosiñol Dachs, L.; Mateos, M.V.; Bahlis, N.J.; et al. P879: Long-Term Follow-Up from MajesTEC-1 of Teclistamab, a B-Cell Maturation Antigen (BCMA) X CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Hemasphere 2023, 7 (Suppl. S3), e62475d0. [Google Scholar] [CrossRef]
- Waldschmidt, J.M.; Rasche, L.; Kortüm, K.M.; Einsele, H. Comprehensive Review of Bispecific Antibody Constructs In Multiple Myeloma: Affinities, Dosing Strategies and Future Perspectives. Clin. Lymphoma Myeloma Leuk. 2025, 25, 309–315. [Google Scholar] [CrossRef]
- Merz, M.; Dima, D.; Hashmi, H.; Ahmed, N.; Stölzel, F.; Holderried, T.A.W.; Fenk, R.; Müller, F.; Tovar, N.; Oliver-Cáldes, A.; et al. Bispecific antibodies targeting BCMA or GPRC5D are highly effective in relapsed myeloma after CAR T-cell therapy. Blood Cancer J. 2024, 14, 214. [Google Scholar] [CrossRef]
- Liang, X.; Wang, Y.; Luo, B.; Lin, B.; Lu, W.; Tian, S.; Liu, D.; Wang, L. Comparison of CAR T-cell and bispecific antibody as third-line or later-line treatments for multiple myeloma: A meta-analysis. J. ImmunoTher. Cancer 2024, 12, e010064. [Google Scholar] [CrossRef]
- Shaji, Κ.; Bachier, C.R.; Cavo, M.; Corradini, P.; Delforge, M.; Janowski, W.; Lesokhin, A.M.; Mina, R.; Paris, L.; Rosiñol, L.; et al. CAMMA 2: A phase I/II trial evaluating the efficacy and safety of cevostamab in patients with relapsed/refractory multiple myeloma (RRMM) who have triple-class refractory disease and have received a prior anti-B-cell maturation antigen (BCMA) agent. J. Clin. Oncol. 2023, 41, 16. [Google Scholar] [CrossRef]
- Richter, J.; Thomas, S.K.; Krishnan, A.Y.; Laubach, J.P.; Cohen, A.D.; Trudel s Costa, L.J.; Bahlis, N.J.; Forsberg, P.A.; Kaedbey, R.; Fonseca, R.; et al. Cevostamab in Patients with Heavily Pretreated Relapsed/Refractory Multiple Myeloma (RRMM): Updated Results from an Ongoing Phase I Study Demonstrate Clinically Meaningful Activity and Manageable Safety and Inform the Doses and Regimen for Combination Studies. Blood 2024, 144 (Suppl. S1), 1021. [Google Scholar] [CrossRef]
- Swan, D.; Murphy, P.; Glavey, S.; Quinn, J. Bispecific Antibodies in Multiple Myeloma: Opportunities to Enhance Efficacy and Improve Safety. Cancers 2023, 15, 1819. [Google Scholar] [CrossRef]
- Dhakal, B.; Akhtar, O.S.; Fandrei, D.; Jensen, A.; Banerjee, R.; Pan, D.; Richard, S.; Friend, R.; Rees, M.J.; Costello, P.; et al. Sequential Targeting in Multiple Myeloma: Talquetamab, a GPRC5D bispecific antibody, as a Bridge to BCMA CAR-T cell therapy. Blood 2025. [Google Scholar] [CrossRef]
- Chakraborty, R.; Cheruvalath, H.; Patwari, A.; Szabo, A.; Schinke, C.; Dhakal, B.; Lentzsch, S.; D’Souza, A.; Mohyuddin, G.R.; Julian, K.; et al. Sustained remission following finite duration bispecific antibody therapy in patients with relapsed/refractory myeloma. Blood Cancer J. 2024, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Ntanasis-Stathopoulos, I.; Filippatos, C.; Malandrakis, P.; Koutoulidis, V.; Trapali, M.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A.; Gavriatopoulou, M. The impact of high-risk cytogenetics on treatment efficacy and outcomes of patients with relapsed/refractory multiple myeloma: A systematic review and meta-analysis of randomized controlled trials. Leukemia 2025. [Google Scholar] [CrossRef] [PubMed]
- Ntanasis-Stathopoulos, I.; Filippatos, C.; Ntanasis-Stathopoulos, A.; Malandrakis, P.; Kastritis, E.; Tsitsilonis, O.E.; Dimopoulos, M.A.; Terpos, E.; Gavriatopoulou, M. Evaluating Minimal Residual Disease Negativity as a Surrogate Endpoint for Treatment Efficacy in Multiple Myeloma: A Meta-Analysis of Randomized Controlled Trials. Am. J. Hematol. 2025, 100, 427–438. [Google Scholar] [CrossRef] [PubMed]


| Study | Phase | BsAb Target | Drug Name | n | FUP (Median) | Age (Median) | Prior LoT (Median) | Triple Class Refractory |
|---|---|---|---|---|---|---|---|---|
| MajesTEC-1 Moreau et al., (2022) [24] | I/II | BCMA × CD3 | teclistamab | 165 | 14.10 | 64.00 | 5.00 | 128 (77.6) |
| LINKER-MM1 (200 mg arm) Bumma et al., (2024) [25] | I/II | BCMA × CD3 | linvoseltamab | 117 | 14.30 | 70.00 | 5.00 | 96 (82.1) |
| MagnetisMM-3 Lesokhin et al., (2023) [26] | II | BCMA × CD3 | elranatamab | 123 | 14.70 | 68.00 | 5.00 | 119 (96.7) |
| NCT04696809 Ishida et al., (2024) [27] | I/II | BCMA × CD3 | teclistamab | 26 | 14.30 | 67.50 | 4.50 | 17 (65.4) |
| MonumenTAL-1 (S/C QW arm) Chari et al., (2025) [28] | Ia/Ib | GPRC5D × CD3 | talquetamab | 143 | 25.60 | 67.00 | 5.00 | 107 (74.8) |
| MonumenTAL-1 (S/C Q2W arm) Chari et al., (2025) [28] | Ia/Ib | GPRC5D × CD3 | talquetamab | 154 | 19.40 | 67.00 | 4.50 | 110 (71.4) |
| MonumenTAL-1 (TCR arm) Chari et al., (2025) [28] | Ia/Ib | GPRC5D × CD3 | talquetamab | 78 | 16.80 | 61.00 | 6.00 | 66 (84.6) |
| RedirecTT-1 (RP2R arm) Cohen et al., (2025) [29] | Ib/II | GPRC5D × CD3 + BCMA × CD3 | teclistamab + talquetamab | 44 | 18.20 | 63.00 | 4.00 | 37 (84.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakogeorgou, S.; Filippatos, C.; Malandrakis, P.; Tentolouris, A.; Terpos, E.; Gavriatopoulou, M.; Ntanasis-Stathopoulos, I. Safety and Efficacy of Bispecific Antibody Treatment in Relapsed/Refractory Multiple Myeloma: A Systematic Review and Meta-Analysis of Proportions from Clinical Trials. Cancers 2025, 17, 2727. https://doi.org/10.3390/cancers17172727
Bakogeorgou S, Filippatos C, Malandrakis P, Tentolouris A, Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I. Safety and Efficacy of Bispecific Antibody Treatment in Relapsed/Refractory Multiple Myeloma: A Systematic Review and Meta-Analysis of Proportions from Clinical Trials. Cancers. 2025; 17(17):2727. https://doi.org/10.3390/cancers17172727
Chicago/Turabian StyleBakogeorgou, Sabrina, Charalampos Filippatos, Panagiotis Malandrakis, Anastasios Tentolouris, Evangelos Terpos, Maria Gavriatopoulou, and Ioannis Ntanasis-Stathopoulos. 2025. "Safety and Efficacy of Bispecific Antibody Treatment in Relapsed/Refractory Multiple Myeloma: A Systematic Review and Meta-Analysis of Proportions from Clinical Trials" Cancers 17, no. 17: 2727. https://doi.org/10.3390/cancers17172727
APA StyleBakogeorgou, S., Filippatos, C., Malandrakis, P., Tentolouris, A., Terpos, E., Gavriatopoulou, M., & Ntanasis-Stathopoulos, I. (2025). Safety and Efficacy of Bispecific Antibody Treatment in Relapsed/Refractory Multiple Myeloma: A Systematic Review and Meta-Analysis of Proportions from Clinical Trials. Cancers, 17(17), 2727. https://doi.org/10.3390/cancers17172727









