Multi-Observer Study on Diagnostic Accuracy of Pediatric Renal Tumors Imaged with Higher-Harmonic-Generation Microscopy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Handling
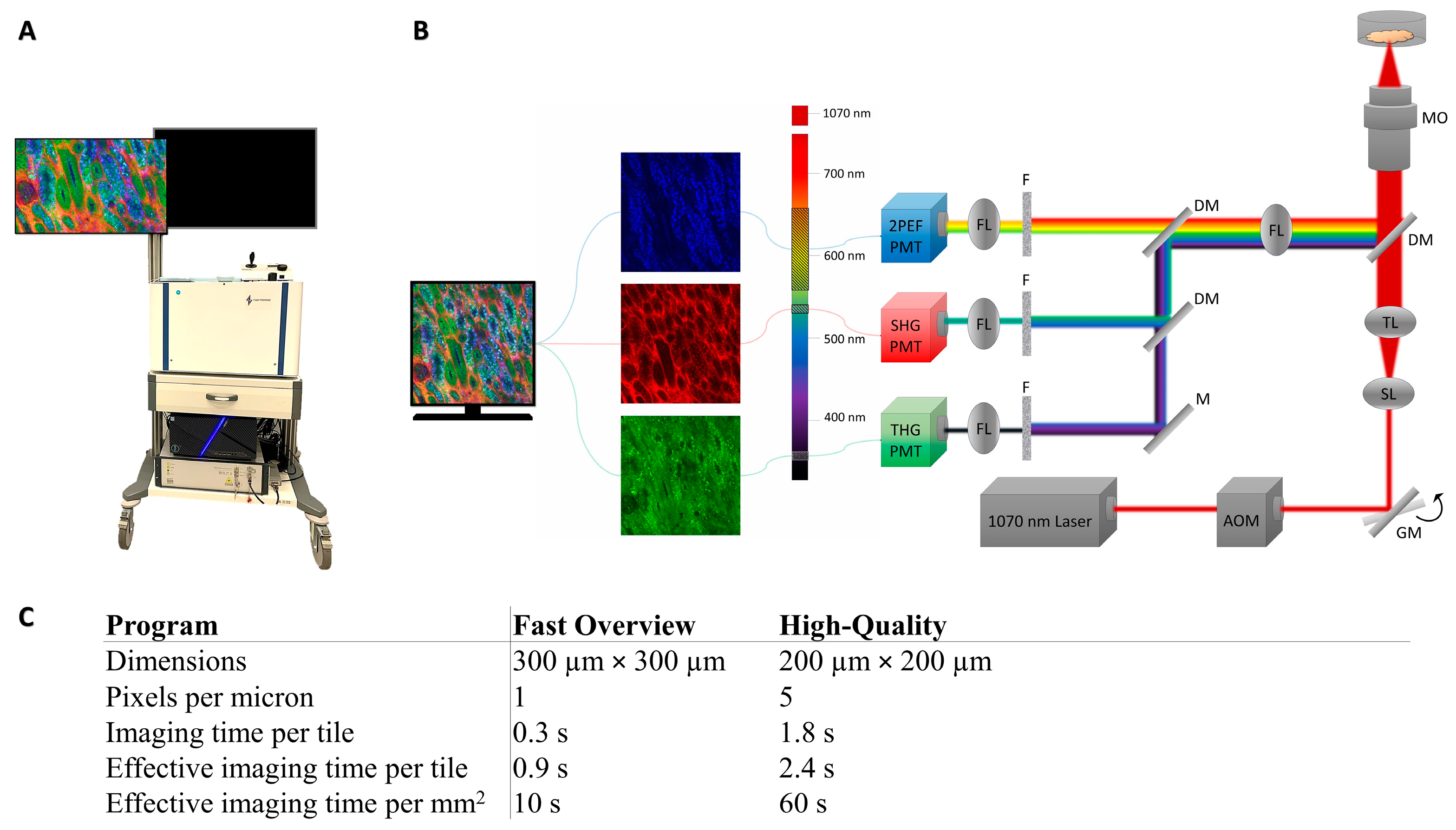
2.3. Image Acquisition
2.4. Pathologists’ Assessment
2.4.1. Slide Score
2.4.2. Training of the Pathologists
2.4.3. Study Design
2.4.4. Data Analysis
3. Results
3.1. Diagnostic Characteristics
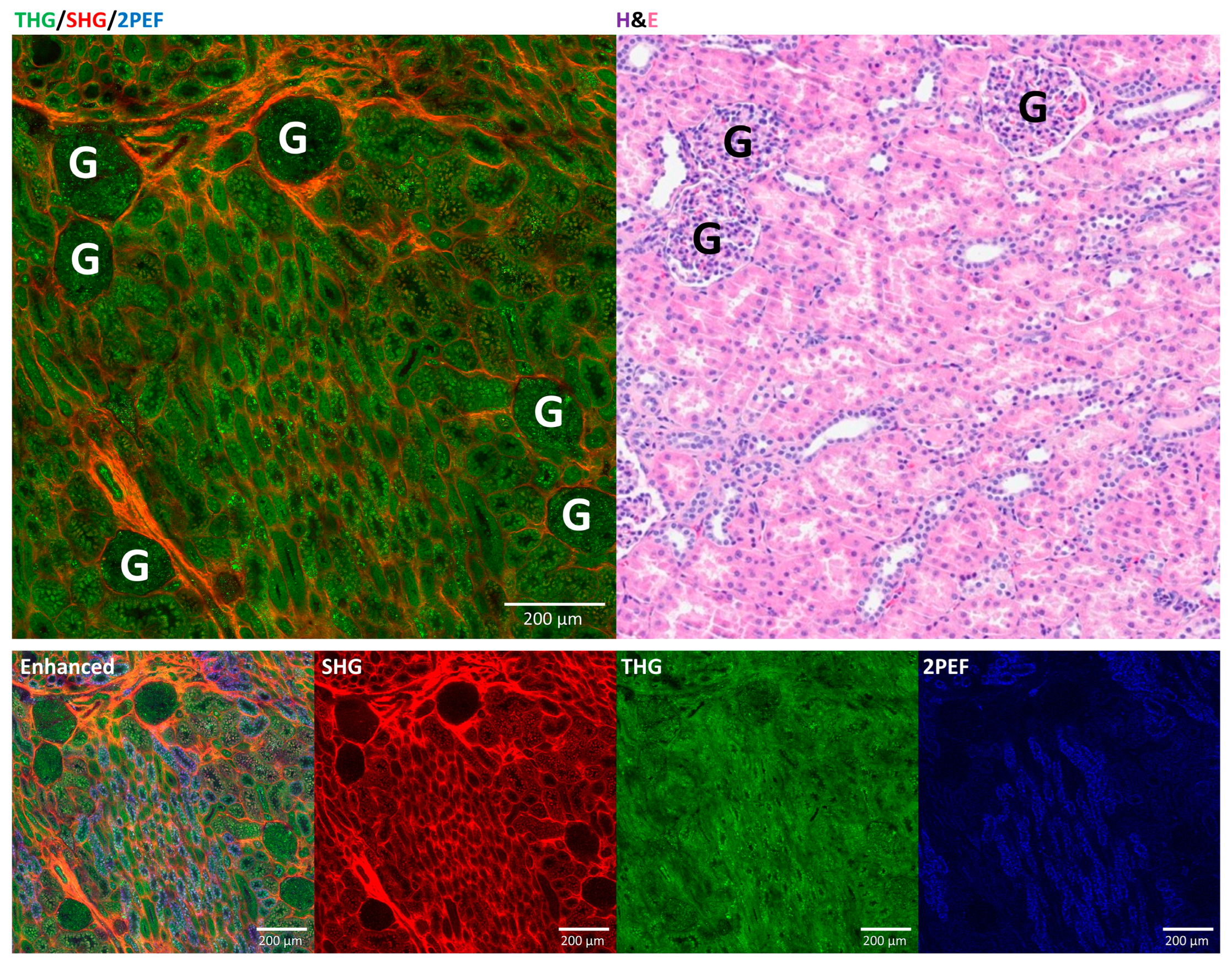
3.2. Comparison of HHGM and H&E for Normal Kidney
3.3. Diagnostic Characteristics for Tumor Diagnosis
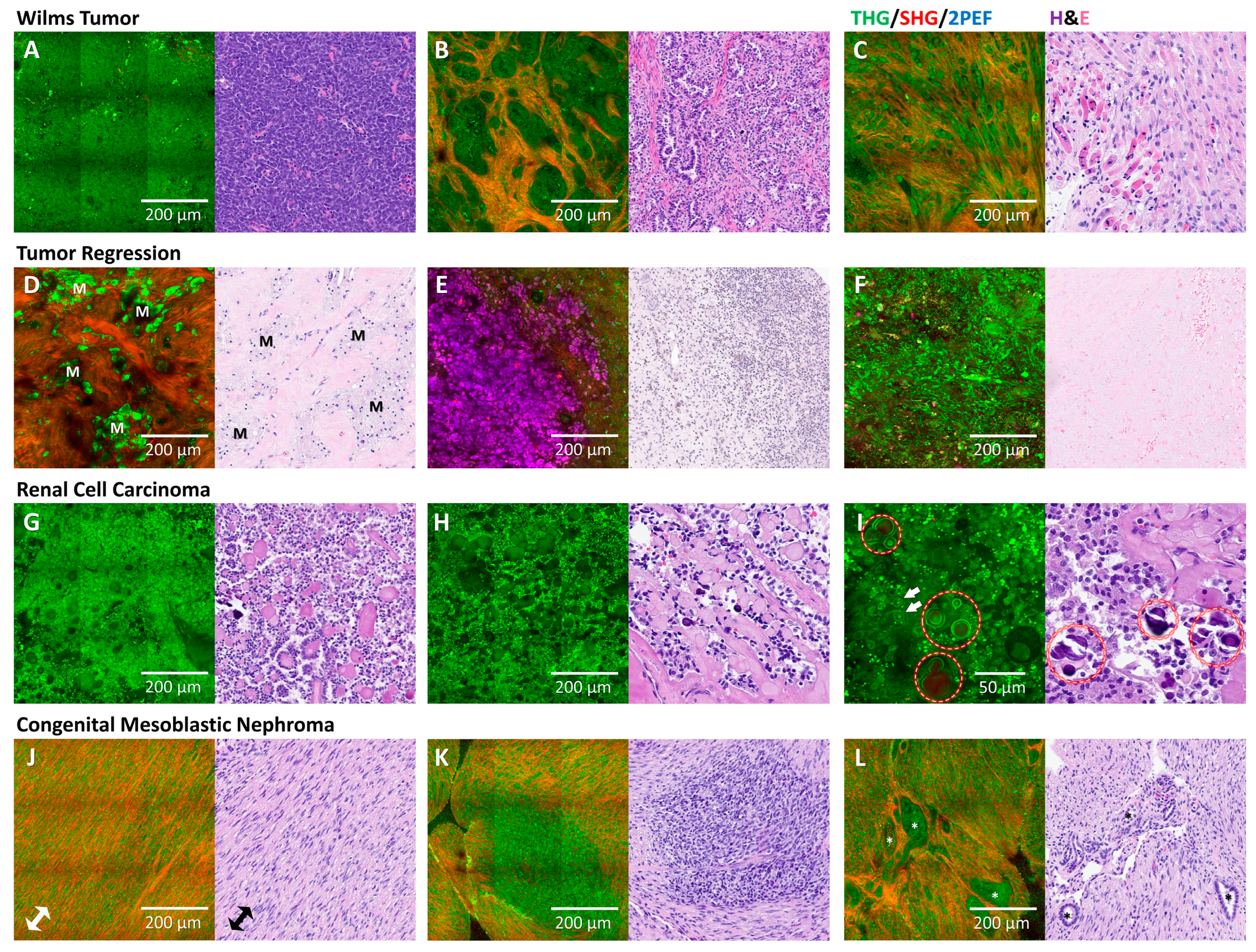
3.4. Comparison of HHGM and H&E for Renal Tumors
3.5. Classification of Viable Wilms Tumor Components
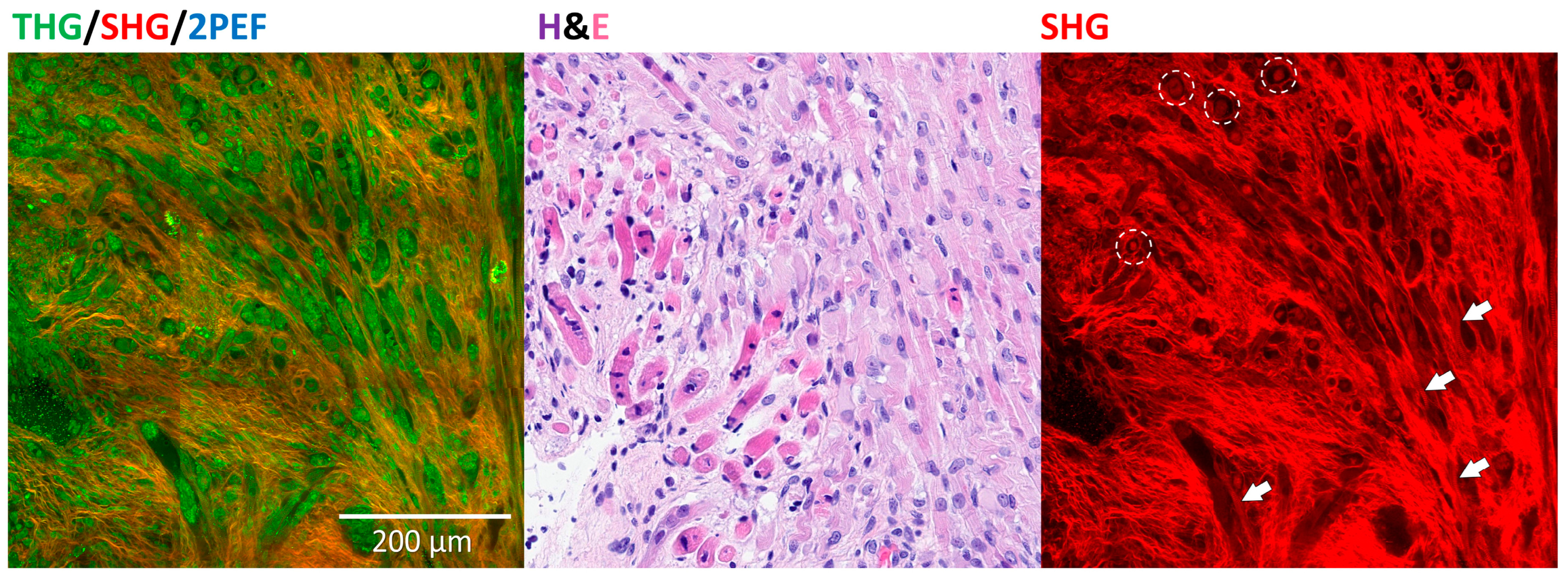
3.6. Rhabdomyoblastic Differentiation and Anaplasia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2PEF | Two-photon excited autofluorescence |
| CMN | Congenital mesoblastic nephroma |
| H&E | Hematoxylin and eosin |
| HHG(M) | Higher-harmonic-generation (microscopy) |
| RCC | Renal cell carcinoma |
| SHG | Second-harmonic generation |
| SIOP-RTSG | The International Society of Paediatric Oncology—Renal Tumor Study Group |
| THG | Third-harmonic generation |
| WT | Wilms tumor |
Appendix A
Higher-Harmonic-Generation Microscopy
References
- Ooms, A.; Vujanić, G.M.; D’Hooghe, E.; Collini, P.; L’Herminé-Coulomb, A.; Vokuhl, C.; Graf, N.; van den Heuvel-Eibrink, M.M.; de Krijger, R.R. Renal Tumors of Childhood-A Histopathologic Pattern-Based Diagnostic Approach. Cancers 2020, 12, 729. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel-Eibrink, M.M.; Grundy, P.; Graf, N.; Pritchard-Jones, K.; Bergeron, C.; Patte, C.; van Tinteren, H.; Rey, A.; Langford, C.; Anderson, J.R.; et al. Characteristics and survival of 750 children diagnosed with a renal tumor in the first seven months of life: A collaborative study by the SIOP/GPOH/SFOP, NWTSG, and UKCCSG Wilms tumor study groups. Pediatr. Blood Cancer 2008, 50, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; van Peer, S.E.; de Witte, M.M.; Tytgat, G.A.M.; Karim-Kos, H.E.; van Grotel, M.; van de Ven, C.P.; Mavinkurve-Groothuis, A.M.C.; Merks, J.H.M.; Kuiper, R.P.; et al. Characteristics and outcome of children with renal tumors in the Netherlands: The first five-year’s experience of national centralization. PLoS ONE 2022, 17, e0261729. [Google Scholar] [CrossRef] [PubMed]
- Vujanić, G.M.; Gessler, M.; Ooms, A.H.A.G.; Collini, P.; Coulomb-l’Hermine, A.; D’Hooghe, E.; de Krijger, R.R.; Perotti, D.; Pritchard-Jones, K.; Vokuhl, C.; et al. The UMBRELLA SIOP–RTSG 2016 Wilms tumour pathology and molecular biology protocol. Nat. Rev. Urol. 2018, 15, 693–701. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel-Eibrink, M.M.; Hol, J.A.; Pritchard-Jones, K.; van Tinteren, H.; Furtwängler, R.; Verschuur, A.C.; Vujanic, G.M.; Leuschner, I.; Brok, J.; Rübe, C.; et al. Position paper: Rationale for the treatment of Wilms tumour in the UMBRELLA SIOP-RTSG 2016 protocol. Nat. Rev. Urol. 2017, 14, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.J.; Brisse, H.J.; Pritchard-Jones, K.; Nakata, K.; Morosi, C.; Oue, T.; Irtan, S.; Vujanic, G.; van den Heuvel-Eibrink, M.M.; Graf, N.; et al. How we approach paediatric renal tumour core needle biopsy in the setting of preoperative chemotherapy: A Review from the SIOP Renal Tumour Study Group. Pediatr. Blood Cancer 2022, 69, e29702. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel-Eibrink, M.M.; van Tinteren, H.; Bergeron, C.; Coulomb-L’Hermine, A.; de Camargo, B.; Leuschner, I.; Sandstedt, B.; Acha, T.; Godzinski, J.; Oldenburger, F.; et al. Outcome of localised blastemal-type Wilms tumour patients treated according to intensified treatment in the SIOP WT 2001 protocol, a report of the SIOP Renal Tumour Study Group (SIOP-RTSG). Eur. J. Cancer 2015, 51, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, N.V.; Wesseling, P.; de Witt Hamer, P.C.; Noske, D.P.; Galgano, G.D.; Mansvelder, H.D.; Baayen, J.C.; Groot, M.L. Third harmonic generation imaging for fast, label-free pathology of human brain tumors. Biomed. Opt. Express 2016, 7, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- van Huizen, L.M.G.; Kuzmin, N.V.; Barbé, E.; van der Velde, S.; te Velde, E.A.; Groot, M.L. Second and third harmonic generation microscopy visualizes key structural components in fresh unprocessed healthy human breast tissue. J. Biophotonics 2019, 12, e201800297. [Google Scholar] [CrossRef] [PubMed]
- van Huizen, L.M.G.; Radonic, T.; van Mourik, F.; Seinstra, D.; Dickhoff, C.; Daniels, J.M.A.; Bahce, I.; Annema, J.T.; Groot, M.L. Compact portable multiphoton microscopy reveals histopathological hallmarks of unprocessed lung tumor tissue in real time. Transl. Biophotonics 2020, 2, e202000009. [Google Scholar] [CrossRef] [PubMed]
- Kok, S.D.; Rodriguez Schaap, P.M.; van Dommelen, L.; van Huizen, L.M.G.; Dickhoff, C.; Nieveen-van Dijkum, E.M.; Engelsman, A.F.; van der Valk, P.; Groot, M.L. Compact portable higher harmonic generation microscopy for the real time assessment of unprocessed thyroid tissue. J. Biophotonics 2024, 17, e202300079. [Google Scholar] [CrossRef] [PubMed]
- Blokker, M.; de Witt Hamer, P.C.; Wesseling, P.; Groot, M.L.; Veta, M. Fast intraoperative histology-based diagnosis of gliomas with third harmonic generation microscopy and deep learning. Sci. Rep. 2022, 12, 11334. [Google Scholar] [CrossRef] [PubMed]
- van Huizen, L.M.G.; Blokker, M.; Daniels, J.M.A.; Radonic, T.; von der Thüsen, J.H.; Veta, M.; Annema, J.T.; Groot, M.L. Rapid On-Site Histology of Lung and Pleural Biopsies Using Higher Harmonic Generation Microscopy and Artificial Intelligence Analysis. Mod. Pathol. 2025, 38, 100633. [Google Scholar] [CrossRef] [PubMed]
- Strupler, M.; Hernest, M.; Fligny, C.; Martin, J.-L.; Tharaux, P.-L.; Schanne-Klein, M.-C. Second harmonic microscopy to quantify renal interstitial fibrosis and arterial remodeling. J. Biomed. Opt. 2008, 13, 054041. [Google Scholar] [CrossRef] [PubMed]
- Best, S.L.; Liu, Y.; Keikhosravi, A.; Drifka, C.R.; Woo, K.M.; Mehta, G.S.; Altwegg, M.; Thimm, T.N.; Houlihan, M.; Bredfeldt, J.S.; et al. Collagen organization of renal cell carcinoma differs between low and high grade tumors. BMC Cancer 2019, 19, 490. [Google Scholar] [CrossRef] [PubMed]
- Goedeke, J.; Schreiber, P.; Seidmann, L.; Li, G.; Birkenstock, J.; Simon, F.; König, J.; Muensterer, O.J. Multiphoton microscopy in the diagnostic assessment of pediatric solid tissue in comparison to conventional histopathology: Results of the first international online interobserver trial. Cancer Manag. Res. 2019, 11, 3655–3667. [Google Scholar] [CrossRef] [PubMed]
- Muensterer, O.J.; Waldron, S.; Boo, Y.J.; Ries, C.; Sehls, L.; Simon, F.; Seidmann, L.; Birkenstock, J.; Gödeke, J. Multiphoton microscopy: A novel diagnostic method for solid tumors in a prospective pediatric oncologic cohort, an experimental study. Int. J. Surg. 2017, 48, 128–133. [Google Scholar] [CrossRef] [PubMed]
- MedCalc Software Ltd. Diagnostic Test Evaluation Calculator (Version 22.032). Available online: https://www.medcalc.org/calc/diagnostic_test.php (accessed on 23 August 2024).
- Mercaldo, N.D.; Lau, K.F.; Zhou, X.H. Confidence intervals for predictive values with an emphasis to case-control studies. Stat. Med. 2007, 26, 2170–2183. [Google Scholar] [CrossRef] [PubMed]
- Marzi, G.; Balzano, M.; Marchiori, D. K-Alpha Calculator–Krippendorff’s Alpha Calculator: A user-friendly tool for computing Krippendorff’s Alpha inter-rater reliability coefficient. MethodsX 2024, 12, 102545. [Google Scholar] [CrossRef] [PubMed]
- Krippendorff, K. Content Analysis: An Introduction to Its Methodology; Sage Publications: Thousand Oaks, CA, USA, 2019. [Google Scholar] [CrossRef]
- Luciano, R.L.; Moeckel, G.W. Update on the Native Kidney Biopsy: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 73, 404–415. [Google Scholar] [CrossRef] [PubMed]
| A | ||||
| Normal vs. Abnormal | H&E Consensus | |||
| Abnormal | Normal | |||
| HHGM Consensus | Abnormal | 23 * | 0 | PPV = 100% [85–100%] |
| Normal | 0 | 6 | NPV = 100% [54–100%] | |
| Sensitivity = 100% [85–100%] | Specificity = 100% [54–100%] | Accuracy = 100% [88–100%] | ||
| B | ||||
| Non-Tumor vs. Tumor | H&E Consensus | |||
| Tumor | Non-Tumor | |||
| HHGM Consensus | Tumor | 18 | 1 | PPV = 95% [74–99%] |
| Non-Tumor | 0 | 10 ** | NPV = 100% [69–100%] | |
| Sensitivity = 100% [81–100%] | Specificity = 91% [59–100%] | Accuracy = 97% [82–100%] | ||
| C | ||||
| Krippendorff’s Alpha [95% confidence interval, 1000 bootstrap iterations] | HHGM | H&E | ||
| Classification Normal versus Abnormal | 0.82 [0.66, 0.93] | 0.89 [0.72, 1.00] | ||
| Classification Non-Tumor versus Tumor | 0.45 [0.25, 0.59] | 0.81 [0.64, 0.92] | ||
| Gold Standard Diagnosis | ||||
|---|---|---|---|---|
| CMN | RCC | WT | ||
| HHGM Consensus | CMN | 1 | 0 | 0 |
| RCC | 0 | 1 | 0 | |
| WT | 0 | 0 | 15 | |
| No consensus | 0 | 1 * | 0 | |
| Gold Standard Diagnosis | ||||
| CMN | RCC | WT | ||
| H&E Consensus | CMN | 1 | 0 | 0 |
| RCC | 0 | 2 | 0 | |
| WT | 0 | 0 | 14 | |
| No consensus | 0 | 0 | 1 ** | |
| H&E Majority Opinion | |||||
|---|---|---|---|---|---|
| >66% Blastema | >66% Epithelium | >66% Stroma | Mixed | ||
| HHGM Majority Opinion | >66% Blastema | 2 | 0 | 0 | 0 |
| >66% Epithelium | 0 | 0 | 0 | 0 | |
| >66% Stroma | 0 | 0 | 7 | 0 | |
| Mixed | 0 | 2 | 0 | 4 | |
| Agreement: 87% (13/15). | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spies, S.; Nazarian, E.; Annavarapu, S.; Collini, P.; Coulomb L’Hermine, A.; D’Hooghe, E.; Kobos, J.; Morcrette, G.; Morini, M.A.; Popov, S.D.; et al. Multi-Observer Study on Diagnostic Accuracy of Pediatric Renal Tumors Imaged with Higher-Harmonic-Generation Microscopy. Cancers 2025, 17, 1693. https://doi.org/10.3390/cancers17101693
Spies S, Nazarian E, Annavarapu S, Collini P, Coulomb L’Hermine A, D’Hooghe E, Kobos J, Morcrette G, Morini MA, Popov SD, et al. Multi-Observer Study on Diagnostic Accuracy of Pediatric Renal Tumors Imaged with Higher-Harmonic-Generation Microscopy. Cancers. 2025; 17(10):1693. https://doi.org/10.3390/cancers17101693
Chicago/Turabian StyleSpies, Sylvia, Elina Nazarian, Srinivas Annavarapu, Paola Collini, Aurore Coulomb L’Hermine, Ellen D’Hooghe, Jozef Kobos, Guillaume Morcrette, Mariana A. Morini, Sergey D. Popov, and et al. 2025. "Multi-Observer Study on Diagnostic Accuracy of Pediatric Renal Tumors Imaged with Higher-Harmonic-Generation Microscopy" Cancers 17, no. 10: 1693. https://doi.org/10.3390/cancers17101693
APA StyleSpies, S., Nazarian, E., Annavarapu, S., Collini, P., Coulomb L’Hermine, A., D’Hooghe, E., Kobos, J., Morcrette, G., Morini, M. A., Popov, S. D., Shukla, R., Werneck da Cunha, I., van de Ven, C. P., van den Heuvel-Eibrink, M. M., de Krijger, R. R., & Groot, M. L. (2025). Multi-Observer Study on Diagnostic Accuracy of Pediatric Renal Tumors Imaged with Higher-Harmonic-Generation Microscopy. Cancers, 17(10), 1693. https://doi.org/10.3390/cancers17101693








