Multi-Observer Study on the Assessment of Pediatric Gonadal Tumors Using Higher Harmonic Generation Microscopy as Compared to Conventional Histology
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Handling
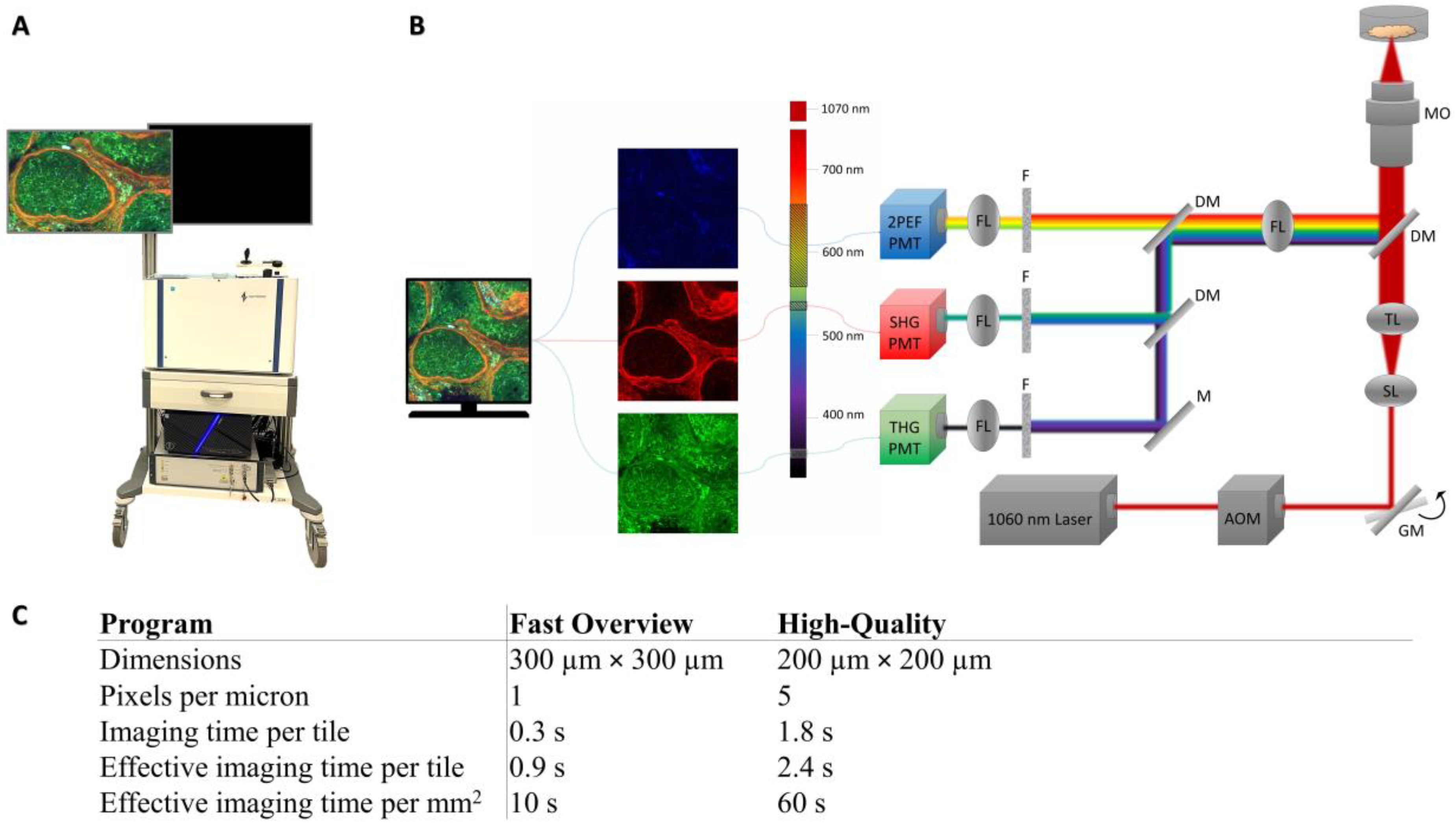
2.3. Image Acquisition
2.4. Pathologists Assessment
2.4.1. Slide Score
2.4.2. Training of the Pathologists
2.4.3. Study Design
2.4.4. Data Analysis
3. Results
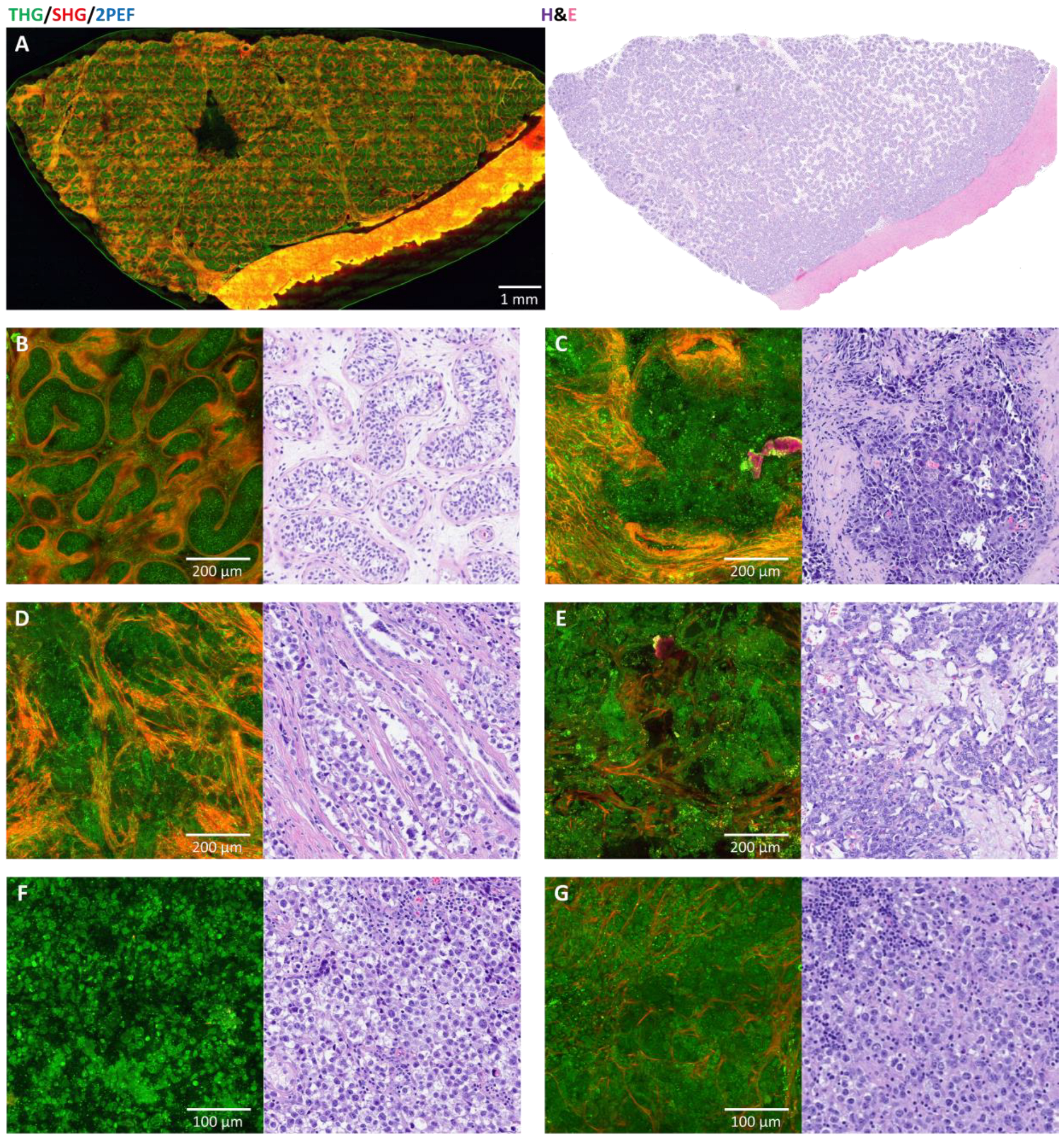
3.1. Normal Versus Tumor
3.2. Comparison with Histology
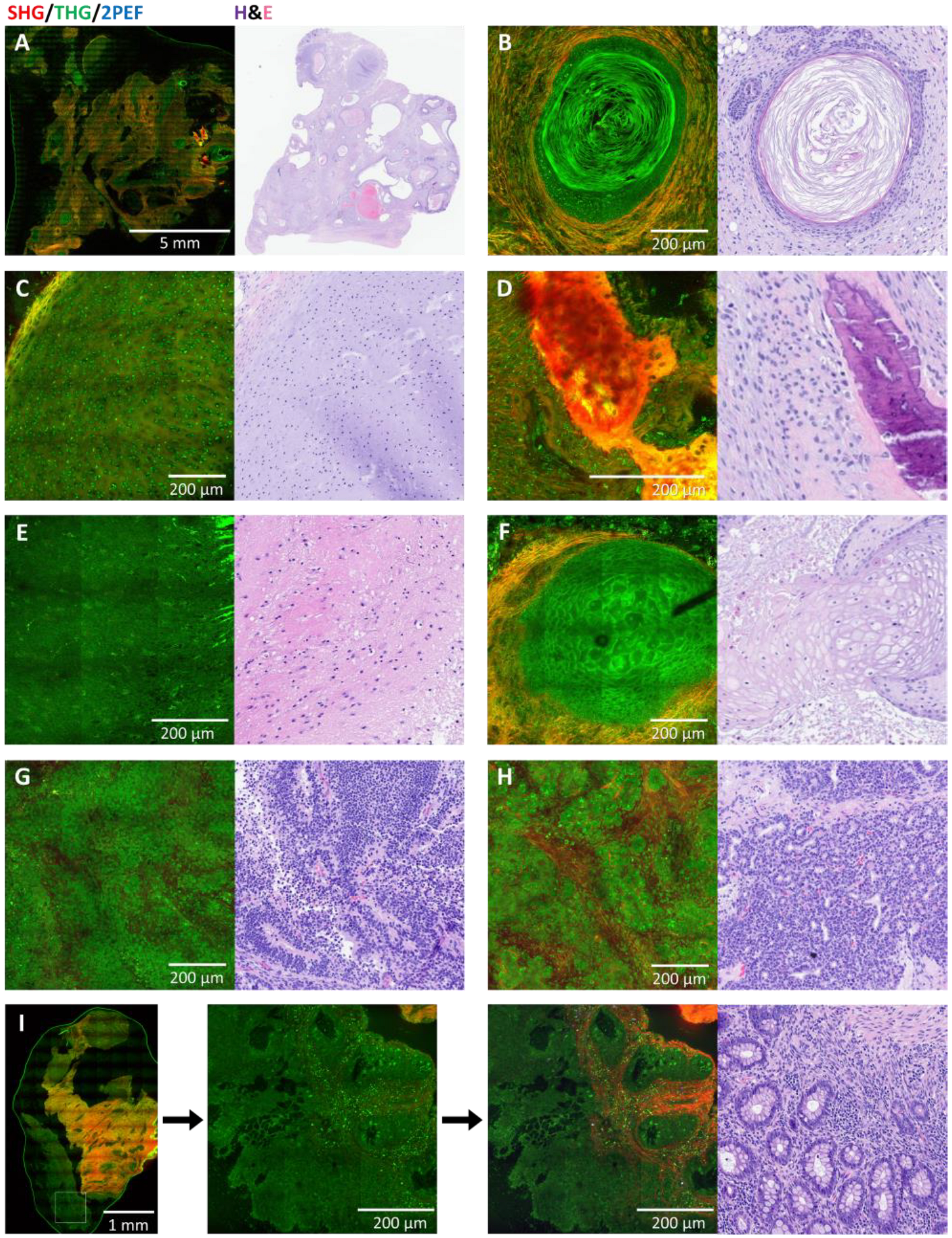
3.3. Germ Cell Tumors: Teratomas
3.4. Other Germ Cell Tumors
3.5. Non-Germ Cell Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2PEF | Two-photon excited autofluorescence |
| CC | Choriocarcinoma |
| EC | Embryonal carcinoma |
| GCNIS | Germ cell neoplasia in situ |
| GCT(s) | Germ cell tumor(s) |
| H&E | Hematoxylin and eosin |
| HHG(M) | Higher harmonic generation (microscopy) |
| MaGIC | Malignant Germ Cell International Consortium |
| MGCT | Mixed germ cell tumor |
| SHG | Second harmonic generation |
| THG | Third harmonic generation |
| YST | Yolk sac tumor |
Appendix A. Higher Harmonic Generation Microscopy
References
- Lobo, J.; Costa, A.L.; Vilela-Salgueiro, B.; Rodrigues, Â.; Guimarães, R.; Cantante, M.; Lopes, P.; Antunes, L.; Jerónimo, C.; Henrique, R. Testicular germ cell tumors: Revisiting a series in light of the new WHO classification and AJCC staging systems, focusing on challenges for pathologists. Hum. Pathol. 2018, 82, 113–124. [Google Scholar] [CrossRef]
- Ataikiru, U.O.; Iacob, E.R.; Heredea, E.R.; Stănciulescu, M.C.; Galinescu, M.C.; Popoiu, C.M.; Boia, E.S. A 10-year retrospective study of the risks and peculiarities in pediatric patients with (para)gonadal tumors and cysts. Rom. J. Morphol. Embryol. 2024, 65, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Birbas, E.; Kanavos, T.; Gkrozou, F.; Skentou, C.; Daniilidis, A.; Vatopoulou, A. Ovarian Masses in Children and Adolescents: A Review of the Literature with Emphasis on the Diagnostic Approach. Children 2023, 10, 1114. [Google Scholar] [CrossRef] [PubMed]
- Ergun-Longmire, B.; Greydanus, D.E. Ovarian tumors in the pediatric population: An update. Dis.-a-Mon. 2024, 70, 101691. [Google Scholar] [CrossRef] [PubMed]
- Calaminus, G.; Schneider, D.T.; von Schweinitz, D.; Jürgens, H.; Infed, N.; Schönberger, S.; Olson, T.A.; Albers, P.; Vokuhl, C.; Stein, R.; et al. Age-Dependent Presentation and Clinical Course of 1465 Patients Aged 0 to Less than 18 Years with Ovarian or Testicular Germ Cell Tumors; Data of the MAKEI 96 Protocol Revisited in the Light of Prenatal Germ Cell Biology. Cancers 2020, 12, 611. [Google Scholar] [CrossRef] [PubMed]
- von Allmen, D. Malignant lesions of the ovary in childhood. Semin. Pediatr. Surg. 2005, 14, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Fakhralddin, S.S.; Ali, R.M.; Omar, S.S.; Ali, R.M.; Mohammed, B.O.; Karim, R.A.; Abdullah, H.O.; Mohammed, K.K.; Mohammed, S.H.; Ahmed, S.M.; et al. Intratesticular prepubertal-type mature teratoma in an infant: A case report and mini-review of the literature. Med. Int. 2024, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, H.; Cost, N.G.; Saltzman, A.F. Testicular tumors in the pediatric patient. Semin. Pediatr. Surg. 2021, 30, 151079. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, K.M.; Young, R.H. Sex cord-stromal tumors of the testis. Diagn. Histopathol. 2019, 25, 398–407. [Google Scholar] [CrossRef]
- Kuzmin, N.V.; Wesseling, P.; de Witt Hamer, P.C.; Noske, D.P.; Galgano, G.D.; Mansvelder, H.D.; Baayen, J.C.; Groot, M.L. Third harmonic generation imaging for fast, label-free pathology of human brain tumors. Biomed. Opt. Express 2016, 7, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- van Huizen, L.M.G.; Kuzmin, N.V.; Barbé, E.; van der Velde, S.; te Velde, E.A.; Groot, M.L. Second and third harmonic generation microscopy visualizes key structural components in fresh unprocessed healthy human breast tissue. J. Biophotonics 2019, 12, e201800297. [Google Scholar] [CrossRef] [PubMed]
- van Huizen, L.M.G.; Radonic, T.; van Mourik, F.; Seinstra, D.; Dickhoff, C.; Daniels, J.M.A.; Bahce, I.; Annema, J.T.; Groot, M.L. Compact portable multiphoton microscopy reveals histopathological hallmarks of unprocessed lung tumor tissue in real time. Transl. Biophotonics 2020, 2, e202000009. [Google Scholar] [CrossRef] [PubMed]
- Kok, S.D.; Rodriguez Schaap, P.M.; van Dommelen, L.; van Huizen, L.M.G.; Dickhoff, C.; Nieveen-van Dijkum, E.M.; Engelsman, A.F.; van der Valk, P.; Groot, M.L. Compact portable higher harmonic generation microscopy for the real time assessment of unprocessed thyroid tissue. J. Biophotonics 2024, 17, e202300079. [Google Scholar] [CrossRef] [PubMed]
- Blokker, M.; de Witt Hamer, P.C.; Wesseling, P.; Groot, M.L.; Veta, M. Fast intraoperative histology-based diagnosis of gliomas with third harmonic generation microscopy and deep learning. Sci. Rep. 2022, 12, 11334. [Google Scholar] [CrossRef] [PubMed]
- van Huizen, L.M.G.; Blokker, M.; Daniels, J.M.A.; Radonic, T.; von der Thüsen, J.H.; Veta, M.; Annema, J.T.; Groot, M.L. Rapid On-Site Histology of Lung and Pleural Biopsies Using Higher Harmonic Generation Microscopy and Artificial Intelligence Analysis. Mod. Pathol. 2025, 38, 100633. [Google Scholar] [CrossRef] [PubMed]
- Goedeke, J.; Schreiber, P.; Seidmann, L.; Li, G.; Birkenstock, J.; Simon, F.; König, J.; Muensterer, O.J. Multiphoton microscopy in the diagnostic assessment of pediatric solid tissue in comparison to conventional histopathology: Results of the first international online interobserver trial. Cancer Manag. Res. 2019, 11, 3655–3667. [Google Scholar] [CrossRef] [PubMed]
- Muensterer, O.J.; Waldron, S.; Boo, Y.J.; Ries, C.; Sehls, L.; Simon, F.; Seidmann, L.; Birkenstock, J.; Gödeke, J. Multiphoton microscopy: A novel diagnostic method for solid tumors in a prospective pediatric oncologic cohort, an experimental study. Int. J. Surg. 2017, 48, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Diagnostic Test Evaluation Calculator. Version 22.032. MedCalc Software Ltd.: Ostend, Belgium, 2024. Available online: https://www.medcalc.org/calc/diagnostic_test.php (accessed on 23 August 2024).
- Mercaldo, N.D.; Lau, K.F.; Zhou, X.H. Confidence intervals for predictive values with an emphasis to case-control studies. Stat. Med. 2007, 26, 2170–2183. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.; Lobo, J.; Hazard, F.K.; Gell, J.; Nicholls, P.K.; Weiss, R.S.; Klosterkemper, L.; Volchenboum, S.L.; Nicholson, J.C.; Frazier, A.L.; et al. Advancing clinical and translational research in germ cell tumours (GCT): Recommendations from the Malignant Germ Cell International Consortium. Br. J. Cancer 2022, 127, 1577–1583. [Google Scholar] [CrossRef] [PubMed]

| (A) | |||||
| Gold Standard Diagnosis | |||||
| Tumor | Non-Tumor | ||||
| HHGM Consensus | Tumor | 15 | 1 | PPV = 94% [71–99%] | |
| Non-Tumor | 6 * | 6 | NPV = 50% [32–68%] | ||
| Sensitivity = 71% [48–89%] | Specificity = 86% [42–100%] | Accuracy = 75% [55–89%] | |||
| (B) | |||||
| Gold Standard Diagnosis | |||||
| Tumor | Non-Tumor | ||||
| H&E Consensus | Tumor | 18 | 0 | PPV = 100% [81–100%] | |
| Non-Tumor | 3 ** | 7 | NPV = 70% [45–87%] | ||
| Sensitivity = 86% [64–97%] | Specificity = 100% [59–100%] | Accuracy = 89% [72–98%] | |||
| (C) | |||||
| H&E Diagnosis | |||||
| Tumor | Non-Tumor | ||||
| HHGM Consensus | Tumor | 14 | 2 | PPV = 88% [66–96%] | |
| Non-Tumor | 4 | 8 | NPV = 67% [44–83%] | ||
| Sensitivity = 78% [52–94%] | Specificity = 80% [44–97%] | Accuracy = 79% [59–92%] | |||
| H&E Consensus | |||||
|---|---|---|---|---|---|
| Tumor, Teratoma | Tumor, Else | Non-Tumor | Total | ||
| HHGM Consensus | Tumor, teratoma | 3 | 0 | 0 | 3 |
| Tumor, else | 0 | 0 | 1 * | 1 | |
| Non-tumor | 3 ** | 0 | 2 ** | 5 | |
| Total | 6 | 0 | 3 | 9 | |
| Gold Standard Diagnosis | HHGM Consensus | H&E Consensus |
|---|---|---|
| Embryonal carcinoma (testis) | Tumor, no consensus | Tumor, EC |
| Dysgerminoma metastasis (lymph node) | Tumor, no consensus | Tumor, dysgerminoma |
| Seminoma (testis) | Tumor, seminoma or EC | Tumor, seminoma |
| YST (testis) | Tumor, YST | Tumor, YST |
| MGCT (testis) | Tumor, no consensus | Tumor, MGCT: EC/YST |
| MGCT, predominantly teratoma (abdomen) | Tumor, no consensus | Tumor, teratoma |
| MGCT (testis) | Tumor, no consensus | Tumor, MGCT: EC/YST |
| Gold Standard Diagnosis | HHGM Consensus | H&E Consensus |
|---|---|---|
| Mucinous cystadenoma (ovary) | Tumor, no consensus | Tumor, (serous/mucinous) cystadenoma |
| Leydig cell tumor (testis) | Tumor, no consensus | Tumor, Leydig cell tumor |
| Sertoli cell tumor (testis) | Non-tumor * | Tumor, Sertoli cell tumor |
| Rhabdomyosarcoma (testis) | Tumor, no consensus | Tumor, Rhabdomyosarcoma |
| Rhabdomyosarcoma (testis) | Tumor, no consensus | Tumor, Rhabdomyosarcoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spies, S.; Nazarian, E.; Bremmer, F.; Gonzalez, I.A.; Lobo, J.; Reyes-Múgica, M.; Zambrano, E.; Hulsker, C.C.C.; Mavinkurve-Groothuis, A.M.C.; de Krijger, R.R.; et al. Multi-Observer Study on the Assessment of Pediatric Gonadal Tumors Using Higher Harmonic Generation Microscopy as Compared to Conventional Histology. Cancers 2025, 17, 1636. https://doi.org/10.3390/cancers17101636
Spies S, Nazarian E, Bremmer F, Gonzalez IA, Lobo J, Reyes-Múgica M, Zambrano E, Hulsker CCC, Mavinkurve-Groothuis AMC, de Krijger RR, et al. Multi-Observer Study on the Assessment of Pediatric Gonadal Tumors Using Higher Harmonic Generation Microscopy as Compared to Conventional Histology. Cancers. 2025; 17(10):1636. https://doi.org/10.3390/cancers17101636
Chicago/Turabian StyleSpies, Sylvia, Elina Nazarian, Felix Bremmer, Ivan A. Gonzalez, João Lobo, Miguel Reyes-Múgica, Eduardo Zambrano, Caroline C. C. Hulsker, Annelies M. C. Mavinkurve-Groothuis, Ronald R. de Krijger, and et al. 2025. "Multi-Observer Study on the Assessment of Pediatric Gonadal Tumors Using Higher Harmonic Generation Microscopy as Compared to Conventional Histology" Cancers 17, no. 10: 1636. https://doi.org/10.3390/cancers17101636
APA StyleSpies, S., Nazarian, E., Bremmer, F., Gonzalez, I. A., Lobo, J., Reyes-Múgica, M., Zambrano, E., Hulsker, C. C. C., Mavinkurve-Groothuis, A. M. C., de Krijger, R. R., & Groot, M. L. (2025). Multi-Observer Study on the Assessment of Pediatric Gonadal Tumors Using Higher Harmonic Generation Microscopy as Compared to Conventional Histology. Cancers, 17(10), 1636. https://doi.org/10.3390/cancers17101636








