Multi-Modal Machine Learning for Evaluating the Predictive Value of Pelvimetric Measurements (Pelvimetry) for Anastomotic Leakage After Restorative Low Anterior Resection
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
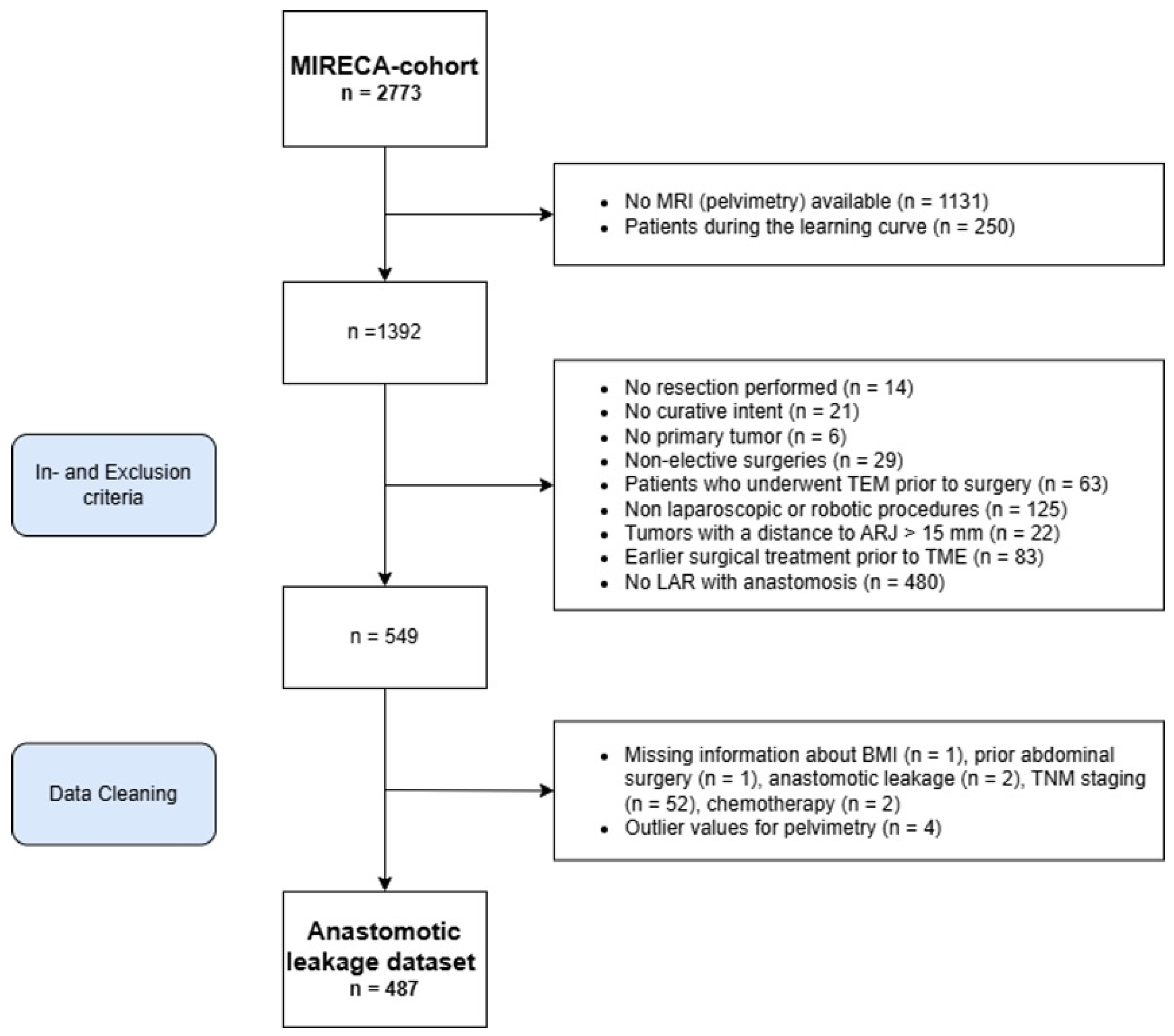
2.2. Inclusion and Exclusion Criteria
2.3. Ethical Considerations
2.4. Outcomes and Definitions
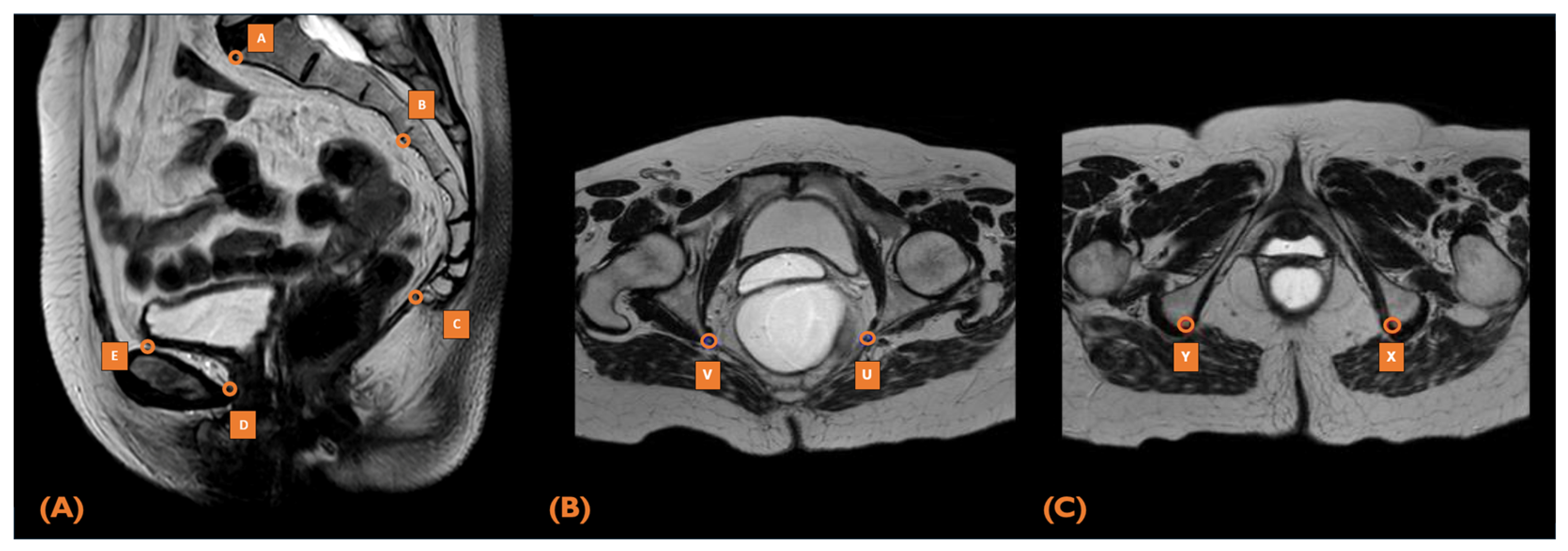
2.5. MRI Pelvimetry
2.6. Statistical Analysis
2.7. Assessment of Predictive Value
3. Results
3.1. Patient Characteristics
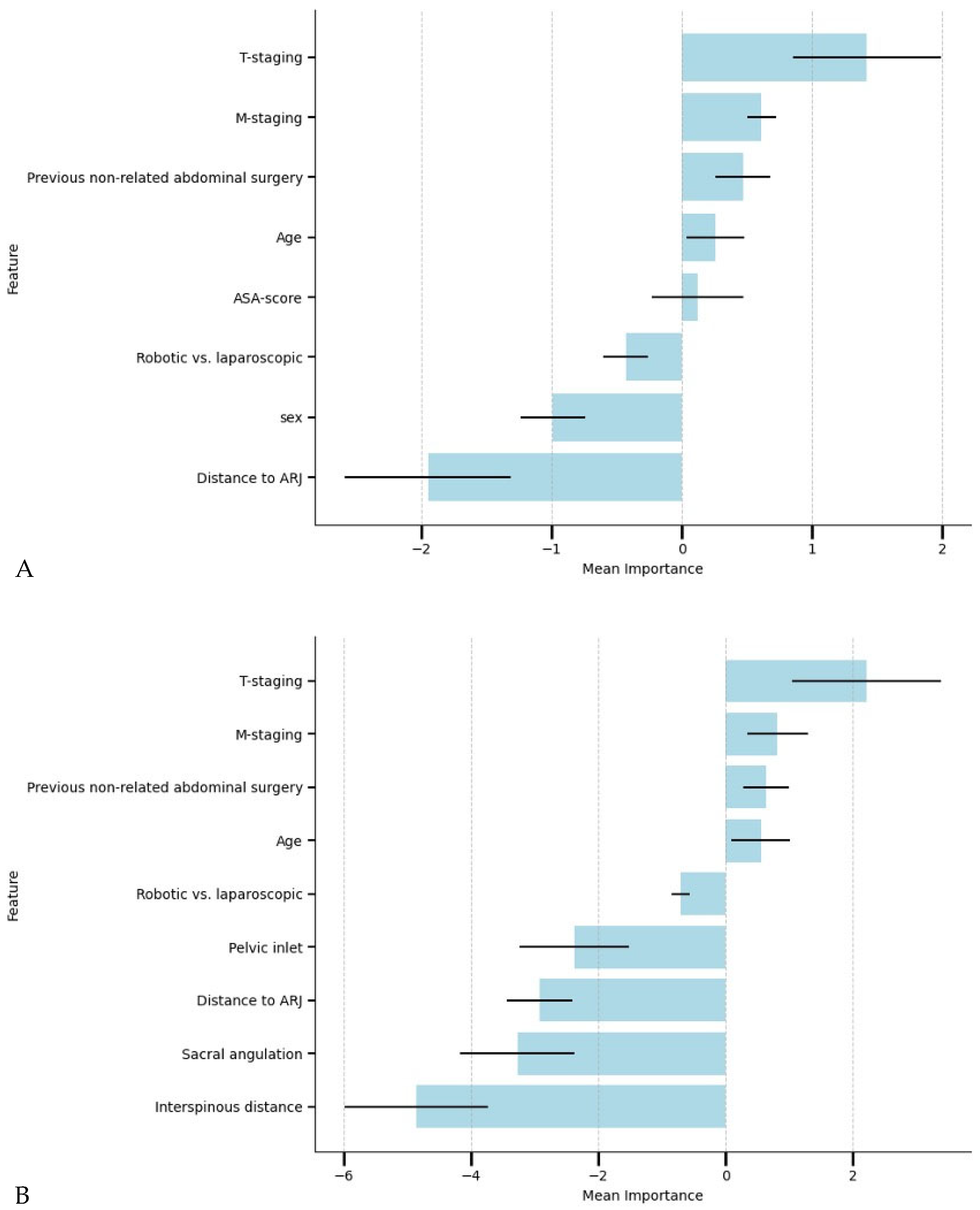
3.2. The Relationship Between the Preoperative Factors and AL
3.3. Performance of Machine Learning Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three-letter acronym |
| LD | Linear dichroism |
| TME | Total Mesorectal Excision |
| AL | Anastomotic Leakage |
| ML | Machine Learning |
| MIRECA | Minimally Invasive Rectal Cancer Taskforce |
| RLAR | Restorative Low Anterior Resection |
| MRI | Magnetic Resonance Imaging |
| DCRA | Dutch ColoRectal Audit |
| ARJ | Anorectal Junction |
| TEM | Transanal Endoscopic Microsurgery |
| NRLAR | Non-Restorative Low Anterior Resection |
| APR | Abdominoperineal Resection |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| MEC-U | Medical Research Ethics Committees United |
| ISREC | International Study Group of Rectal Cancer |
| T2 | T2-Weighted Imaging (MRI Sequence) |
| ICC | Intraclass Correlation Coefficient |
| SD | Standard Deviation |
| IQR | Interquartile Range |
| MAR | Missing at Random |
| MICE | Multiple Imputation by Chained Equations |
| ASA | American Society of Anesthesiologists |
| LR | Logistic Regression |
| RFC | Random Forest Classifier |
| XGB | XGBoost |
| SMOTE | Synthetic Minority Oversampling Technique |
| AUC-PR | Area Under the Precision-Recall Curve |
| AUC-ROC | Area Under the Receiver Operating Characteristic Curve |
Appendix A

References
- Heald, R.J.; Husband, E.M.; Ryall, R.D.H. The Mesorectum in Rectal Cancer Surgery—The Clue to Pelvic Recurrence? Br. J. Surg. 1982, 69, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.; Freitag, M.; Hellmich, G.; Ludwig, K. Anastomotic Leakage: Impact on Local Recurrence and Survival in Surgery of Colorectal Cancer. Int. J. Color. Dis. 1998, 13, 160–163. [Google Scholar]
- McDermott, F.D.; Heeney, A.; Kelly, M.E.; Steele, R.J.; Carlson, G.L.; Winter, D.C. Systematic Review of Preoperative, Intraoperative and Postoperative Risk Factors for Colorectal Anastomotic Leaks. Br. J. Surg. 2015, 102, 462–479. [Google Scholar]
- Pommergaard, H.C.; Gessler, B.; Burcharth, J.; Angenete, E.; Haglind, E.; Rosenberg, J. Preoperative Risk Factors for Anastomotic Leakage after Resection for Colorectal Cancer: A Systematic Review and Meta-Analysis. Color. Dis. 2014, 16, 662–671. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, X.R.; Liu, X.H.; Chen, Y.F.; Ke, J.; He, X.W.; He, X.S.; Hu, T.; Zou, Y.F.; Zheng, X.B.; et al. Male Gender Is Associated with an Increased Risk of Anastomotic Leak in Rectal Cancer Patients after Total Mesorectal Excision. Gastroenterol. Rep. 2018, 6, 137–143. [Google Scholar] [CrossRef]
- Parthasarathy, M.; Greensmith, M.; Bowers, D.; Groot-Wassink, T. Risk Factors for Anastomotic Leakage after Colorectal Resection: A Retrospective Analysis of 17,518 Patients. Color. Dis. 2017, 19, 288–298. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, Y.W.; Kim, N.K.; Hur, H.; Lee, K.; Min, B.S.; Cho, H.J. Pelvic Anatomy as a Factor in Laparoscopic Rectal Surgery: A Prospective Study. Surg. Laparosc. Endosc. Percutan Tech. 2011, 21, 334–339. [Google Scholar]
- zur Hausen, G.; Gröne, J.; Kaufmann, D.; Niehues, S.M.; Aschenbrenner, K.; Stroux, A.; Hamm, B.; Kreis, M.E.; Lauscher, J.C. Influence of Pelvic Volume on Surgical Outcome after Low Anterior Resection for Rectal Cancer. Int. J. Color. Dis. 2017, 32, 1125–1135. [Google Scholar] [CrossRef]
- Targarona, E.M.; Balague, C.; Pernas, J.C.; Martinez, C.; Berindoague, R.; Gich, I.; Trias, M. Can We Predict Immediate Outcome after Laparoscopic Rectal Surgery? Multivariate Analysis of Clinical, Anatomic, and Pathologic Features after 3-Dimensional Reconstruction of the Pelvic Anatomy. Ann. Surg. 2008, 247, 642–649. [Google Scholar] [CrossRef]
- Shimada, T.; Tsuruta, M.; Hasegawa, H.; Okabayashi, K.; Ishida, T.; Asada, Y.; Suzumura, H.; Kitagawa, Y. Pelvic Inlet Shape Measured by Three-Dimensional Pelvimetry Is a Predictor of the Operative Time in the Anterior Resection of Rectal Cancer. Surg. Today 2018, 48, 51–57. [Google Scholar] [CrossRef]
- Akiyoshi, T.; Kuroyanagi, H.; Oya, M.; Konishi, T.; Fukuda, M.; Fujimoto, Y.; Ueno, M.; Miyata, S.; Yamaguchi, T. Factors Affecting the Difficulty of Laparoscopic Total Mesorectal Excision with Double Stapling Technique Anastomosis for Low Rectal Cancer. Surgery 2009, 146, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Knol, J.; Keller, D.S. Total Mesorectal Excision Technique-Past, Present, and Future. Clin. Colon. Rectal Surg. 2020, 33, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Elfanagely, O.; Toyoda, Y.; Othman, S.; Mellia, J.A.; Basta, M.; Liu, T.; Kording, K.; Ungar, L.; Fischer, J.P. Machine Learning and Surgical Outcomes Prediction: A Systematic Review. J. Surg. Res. 2021, 264, 346–361. [Google Scholar]
- Stam, W.T.; Goedknegt, L.K.; Ingwersen, E.W.; Schoonmade, L.J.; Bruns, E.R.J.; Daams, F. The Prediction of Surgical Complications Using Artificial Intelligence in Patients Undergoing Major Abdominal Surgery: A Systematic Review. Surgery 2022, 171, 1014–1021. [Google Scholar] [PubMed]
- Bektaş, M.; Tuynman, J.B.; Costa Pereira, J.; Burchell, G.L.; van der Peet, D.L. Machine Learning Algorithms for Predicting Surgical Outcomes after Colorectal Surgery: A Systematic Review. World J. Surg. 2022, 46, 3100–3110. [Google Scholar]
- Wen, R.; Zheng, K.; Zhang, Q.; Zhou, L.; Liu, Q.; Yu, G.; Gao, X.; Hao, L.; Lou, Z.; Zhang, W. Machine Learning-Based Random Forest Predicts Anastomotic Leakage after Anterior Resection for Rectal Cancer. J. Gastrointest. Oncol. 2021, 12, 921–932. [Google Scholar] [CrossRef]
- Hol, J.C.; Burghgraef, T.A.; Rutgers, M.L.W.; Crolla, R.M.P.H.; van Geloven, N.A.W.; Hompes, R.; Leijtens, J.W.A.; Polat, F.; Pronk, A.; Smits, A.B.; et al. Comparison of Laparoscopic versus Robot-Assisted versus Transanal Total Mesorectal Excision Surgery for Rectal Cancer: A Retrospective Propensity Score-Matched Cohort Study of Short-Term Outcomes. Br. J. Surg. 2021, 108, 1380–1387. [Google Scholar] [CrossRef]
- Burghgraef, T.A.; Sikkenk, D.J.; Verheijen, P.M.; Moumni, M.E.; Hompes, R.; Consten, E.C.J. The Learning Curve of Laparoscopic, Robot-Assisted and Transanal Total Mesorectal Excisions: A Systematic Review. Surg. Endosc. 2022, 36, 6337–6360. [Google Scholar]
- Burghgraef, T.A.; Sikkenk, D.J.; Crolla, R.M.P.H.; Fahim, M.; Melenhorst, J.; Moumni, M.E.; van der Schelling, G.; Smits, A.B.; Stassen, L.P.S.; Verheijen, P.M.; et al. Assessing the Learning Curve of Robot-Assisted Total Mesorectal Excision: A Multicenter Study Considering Procedural Safety, Pathological Safety, and Efficiency. Int. J. Color. Dis. 2023, 38, 9. [Google Scholar] [CrossRef]
- Tanis, P.; Beets, G.; Verhoef, C.; van Westreenen, H.; Punt, C.; de Groot, D. Dutch Colorectal Cancer Guideline. Available online: https://www.mdl.nl/files/richlijnen/Richtlijn%20Colorectaal%20Carcinoom.pdf (accessed on 19 September 2023).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (StroBE) Statement: Guidelines for Reporting Observational Studies. BMJ 2017, 7624, 806–808. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and Grading of Anastomotic Leakage Following Anterior Resection of the Rectum: A Proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.S.Y.; Brown, K.G.M.; Waller, J.; Young, C.J.; Solomon, M.J. The Role of MRI Pelvimetry in Predicting Technical Difficulty and Outcomes of Open and Minimally Invasive Total Mesorectal Excision: A Systematic Review. Tech. Coloproctol. 2020, 24, 991–1000. [Google Scholar] [PubMed]
- Yu, Z.-L.; Liu, X.H.; Liu, H.-S.; Ke, J.; Zou, Y.-F.; Cao, W.-T.; Xiao, J.; Zhou, Z.-Y.; Lan, P.; Wu, X.-J.; et al. Impact of Pelvic Dimensions on Anastomotic Leak after Anterior Resection for Patients with Rectal Cancer. Surg. Endosc. 2021, 35, 2134–2143. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, G.; Arslan, N.C.; Elibol, F.D.; Sagol, O.; Obuz, F.; Sokmen, S. Magnetic Resonance-Based Pelvimetry and Tumor Volumetry Can Predict Surgical Difficulty and Oncologic Outcome in Locally Advanced Mid–Low Rectal Cancer. Surg. Today 2018, 48, 1040–1051. [Google Scholar] [CrossRef]
- Baik, S.H.; Kim, N.K.; Lee, K.Y.; Sohn, S.K.; Cho, C.H.; Kim, M.J.; Kim, H.; Shinn, R.K. Factors Influencing Pathologic Results after Total Mesorectal Excision for Rectal Cancer: Analysis of Consecutive 100 Cases. Ann. Surg. Oncol. 2008, 15, 721–728. [Google Scholar] [CrossRef]
- Hoek, V.T.; Buettner, S.; Sparreboom, C.L.; Detering, R.; Menon, A.G.; Kleinrensink, G.J.; Wouters, M.W.J.M.; Lange, J.F.; Wiggers, J.K.; Dutch ColoRectal Audit Group. A preoperative prediction model for anastomotic leakage after rectal cancer resection based on 13,175 patients. Eur. J. Surg. Oncol. 2022, 48, 2495–2501. [Google Scholar] [CrossRef]
- Zhou, J.T.; Wang, Y.N.; Zhang, N.; Wu, S.F. Establishment and application of three predictive models of anastomotic leakage after rectal cancer sphincter-preserving surgery. World J. Gastrointest. Surg. 2023, 15, 2201–2210. [Google Scholar] [CrossRef]
- Kourou, K.; Exarchos, T.P.; Exarchos, K.P.; Karazoumis, M.V.; Fotiadis, D.I. Machine learning applications in cancer prognosis and prediction. Comput. Struct. Biotechnol. J. 2015, 13, 8–17. [Google Scholar] [CrossRef]
- Baltus, S.C.; Geitenbeek, R.T.J.; Frieben, M.; Thibeau-Sutre, E.; Wolterink, J.M.; Tan, C.O.; Vermeulen, M.C.; Consten, E.C.J.; Broeders, I.A.M.J. Deep Learning-Based Pelvimetry in Pelvic MRI Volumes for Pre-Operative Difficulty Assessment of Total Mesorectal Excision. Surg. Endosc. 2025, 39, 1536–1543. [Google Scholar] [CrossRef]
- Kristensen, H.; Thyø, A.; Emmertsen, K.J.; Smart, N.J.; Pinkney, T.; Warwick, A.M.; Pang, D.; Elfeki, H.; Shalaby, M.; Emile, S.H.; et al. Surviving Rectal Cancer at the Cost of a Colostomy: Global Survey of Long-Term Health-Related Quality of Life in 10 Countries. BJS Open 2022, 6, zrac085. [Google Scholar] [CrossRef]
- Algie, J.P.A.; van Kooten, R.T.; Tollenaar, R.A.E.M.; Wouters, M.W.J.M.; Peeters, K.C.M.J.; Dekker, J.W.T. Stoma versus Anastomosis after Sphincter-Sparing Rectal Cancer Resection; the Impact on Health-Related Quality of Life. Int. J. Color. Dis. 2022, 37, 2197–2205. [Google Scholar] [CrossRef]
- Burghgraef, T.A.; Geitenbeek, R.T.J.; Broekman, M.; Hol, J.C.; Hompes, R.; Consten, E.C.J. Permanent Stoma Rate and Long-Term Stoma Complications in Laparoscopic, Robot-Assisted, and Transanal Total Mesorectal Excisions: A Retrospective Cohort Study. Surg. Endosc. 2024, 38, 105–115. [Google Scholar] [CrossRef] [PubMed]
| Variable | Overall (n = 487) | No AL (n = 417) | AL (n = 70) | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|---|
| p | c | OR [CI] | p | ||||
| Patient and clinical factors | |||||||
| Sex (%) | 0.002 | 0.140 | 0.38 [0.21–0.70] | ||||
| Male | 298 (61.2) | 243 (58.3) | 55 (78.6) | ||||
| Female | 189 (38.8) | 174 (41.7) | 15 (21.4) | ||||
| Age (year) | 65.6 [12.0] | 65.3 [12.0] | 67.0 [12.7] | 0.144 | 0.066 | ||
| Height (cm) | 174.3 [13.0] | 174.1 [13.0] | 175.7 [12.0] | 0.140 | 0.067 | ||
| Weight (kg) | 79.7 [21.0] | 79.5 [22.0] | 80.9 [15.5] | 0.438 | 0.035 | ||
| BMI (kg/m2) | 26.2 [5.2] | 26.2 [5.3] | 26.1 [4.7] | 0.992 | −0.000 | ||
| ASA-score | 0.864 | 0.039 | |||||
| 1 | 111 (22.8) | 97 (23.3) | 14 (20.0) | ||||
| 2 | 300 (61.6) | 254 (60.9) | 46 (65.7) | ||||
| 3 | 75 (15.4) | 65 (15.6) | 10 (14.3) | ||||
| 4 | 1 (0.2) | 1 (0.2) | 0 (0.0) | ||||
| Previous non-related abdominal surgery | 124 (25.5) | 104 (24.9) | 20 (28.6) | 0.619 | 0.023 | ||
| Tumor factors | |||||||
| T-stage | 0.037 | 0.132 | 1.79 [1.16–2.76] | ||||
| 1 | 21 (4.3) | 21 (5.0) | 0 (0.0) | ||||
| 2 | 173 (35.5) | 155 (37.2) | 18 (25.7) | ||||
| 3 | 274 (56.3) | 225 (54.0) | 49 (70.0) | ||||
| 4 | 19 (3.9) | 16 (3.8) | 3 (4.3) | ||||
| N-stage | 0.384 | 0.063 | |||||
| 0 | 243 (49.9) | 213 (51.1) | 30 (42.9) | ||||
| 1 | 156 (32.0) | 129 (30.9) | 27 (38.6) | ||||
| 2 | 88 (18.1) | 75 (18.0) | 13 (18.6) | ||||
| M-stage | 0.115 | 0.072 | |||||
| 0 | 466 (95.7) | 402 (96.4) | 64 (91.4) | ||||
| 1 | 21 (4.3) | 15 (3.6) | 6 (8.6) | ||||
| Distance to ARJ (cm) | 8.3 [4.0] | 8.6 [4.5] | 7.0 [4.0] | 0.000 | −0.173 | <0.001 | |
| Treatment factors | |||||||
| Radiotherapy | 0.132 | 0.107 | |||||
| None | 247 (50.7) | 219 (52.5) | 28 (40.0) | ||||
| Short | 140 (28.7) | 112 (26.9) | 28 (40.0) | ||||
| Long | 5 (1.0) | 4 (1.0) | 1 (1.4) | ||||
| Chemoradiation | 95 (19.5) | 82 (19.7) | 13 (18.6) | ||||
| Surgical approach | 0.084 | 0.078 | |||||
| Robotic | 299 (61.4) | 249 (59.7) | 50 (71.4) | ||||
| Laparoscopic | 188 (38.6) | 168 (40.3) | 20 (28.6) | ||||
| Variable | Overall (n = 487) | No AL (n = 417) | AL (n = 70) | Univariate | Multivariate | |
|---|---|---|---|---|---|---|
| p | c | p | ||||
| Sacrococcygeal depth | 127.1 ± 13.5 | 127.3 ± 13.5 | 125.6 ± 13.4 | 0.318 | −0.045 | |
| Pelvic inlet | 114.5 ± 11.0 | 115.2 ± 11.2 | 110.5 ± 9.1 | 0.001 | −0.152 | 0.004 |
| Pelvic outlet | 87.7 ± 8.8 | 87.9 ± 8.8 | 87.0 ± 8.8 | 0.463 | −0.033 | |
| Pelvic depth | 157.5 ± 15.4 | 157.5 ± 15.6 | 157.4 ± 14.4 | 0.949 | −0.003 | |
| Intertuberous distance | 101.0 [20.7] | 101.4 [20.0] | 98.9 [20.1] | 0.119 | −0.071 | |
| Interspinous distance | 105.7 [15.8] | 106.5 [15.4] | 101.1 [13.9] | 0.000 | −0.165 | 0.013 |
| Sacral angulation | 111.6 [13.9] | 111.9 [14.5] | 109.7 [11.4] | 0.076 | −0.080 | |
| Pelvic area (A–E) | 225.7 ± 26.8 | 226.4 ± 26.9 | 221.7 ± 25.9 | 0.175 | −0.062 | |
| Pelvic volume | 687.5 [201.4] | 694.1 [211.6] | 647.6 [151.5] | 0.014 | −0.111 | |
| Model | F1-Score | ROC-PR | ROC-AUC | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| LR | +pelvimetry | 0.34 ± 0.06 | 0.32 ± 0.10 | 0.70 ± 0.09 | 0.64 ± 0.29 | 0.65 ± 0.11 |
| −pelvimetry | 0.34± 0.07 | 0.27± 0.08 | 0.68± 0.04 | 0.57± 0.07 | 0.70 ±0.08 | |
| RFC | +pelvimetry | 0.33 ± 0.12 | 0.25 ± 0.08 | 0.67 ± 0.12 | 0.51 ± 0.13 | 0.72 ± 0.15 |
| −pelvimetry | 0.29± 0.06 | 0.25± 0.09 | 0.63± 0.05 | 0.64± 0.29 | 0.55 ± 0.16 | |
| XGBoost | +pelvimetry | 0.30 ± 0.08 | 0.22 ± 0.07 | 0.63 ± 0.11 | 0.57 ± 0.36 | 0.65 ± 0.25 |
| −pelvimetry | 0.28 ± 0.10 | 0.22 ± 0.08 | 0.61 ± 0.07 | 0.47 ± 0.26 | 0.69 ± 0.12 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geitenbeek, R.T.J.; Baltus, S.C.; Broekman, M.; Barendsen, S.N.; Frieben, M.C.; Asaggau, I.; Thibeau-Sutre, E.; Wolterink, J.M.; Vermeulen, M.C.; Tan, C.O.; et al. Multi-Modal Machine Learning for Evaluating the Predictive Value of Pelvimetric Measurements (Pelvimetry) for Anastomotic Leakage After Restorative Low Anterior Resection. Cancers 2025, 17, 1051. https://doi.org/10.3390/cancers17061051
Geitenbeek RTJ, Baltus SC, Broekman M, Barendsen SN, Frieben MC, Asaggau I, Thibeau-Sutre E, Wolterink JM, Vermeulen MC, Tan CO, et al. Multi-Modal Machine Learning for Evaluating the Predictive Value of Pelvimetric Measurements (Pelvimetry) for Anastomotic Leakage After Restorative Low Anterior Resection. Cancers. 2025; 17(6):1051. https://doi.org/10.3390/cancers17061051
Chicago/Turabian StyleGeitenbeek, Ritch T. J., Simon C. Baltus, Mark Broekman, Sander N. Barendsen, Maike C. Frieben, Ilias Asaggau, Elina Thibeau-Sutre, Jelmer M. Wolterink, Matthijs C. Vermeulen, Can O. Tan, and et al. 2025. "Multi-Modal Machine Learning for Evaluating the Predictive Value of Pelvimetric Measurements (Pelvimetry) for Anastomotic Leakage After Restorative Low Anterior Resection" Cancers 17, no. 6: 1051. https://doi.org/10.3390/cancers17061051
APA StyleGeitenbeek, R. T. J., Baltus, S. C., Broekman, M., Barendsen, S. N., Frieben, M. C., Asaggau, I., Thibeau-Sutre, E., Wolterink, J. M., Vermeulen, M. C., Tan, C. O., Broeders, I. A. M. J., & Consten, E. C. J., on behalf of the MIRECA Study Group. (2025). Multi-Modal Machine Learning for Evaluating the Predictive Value of Pelvimetric Measurements (Pelvimetry) for Anastomotic Leakage After Restorative Low Anterior Resection. Cancers, 17(6), 1051. https://doi.org/10.3390/cancers17061051







