Metabolic Heterogeneity in Diffuse Large B-Cell Lymphoma Cells Reveals an Innovative Antimetabolic Combination Strategy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Human DLBCL Cell Lines and Culture Conditions
2.2. Antibodies and Reagents
2.3. Measurement of Intracellular ATP Content
2.4. Apoptosis Assays
2.5. Measurement of Mitochondrial Transmembrane Potential
2.6. Cell Viability Assay
2.7. Immunoblotting
2.8. NMR-Based Metabolomics
2.9. Antimetabolic Therapy
2.10. Tissue Processing and Hematoxylin and Eosin Staining of DLBCL Patient Biopsies
2.11. Statistics
3. Results
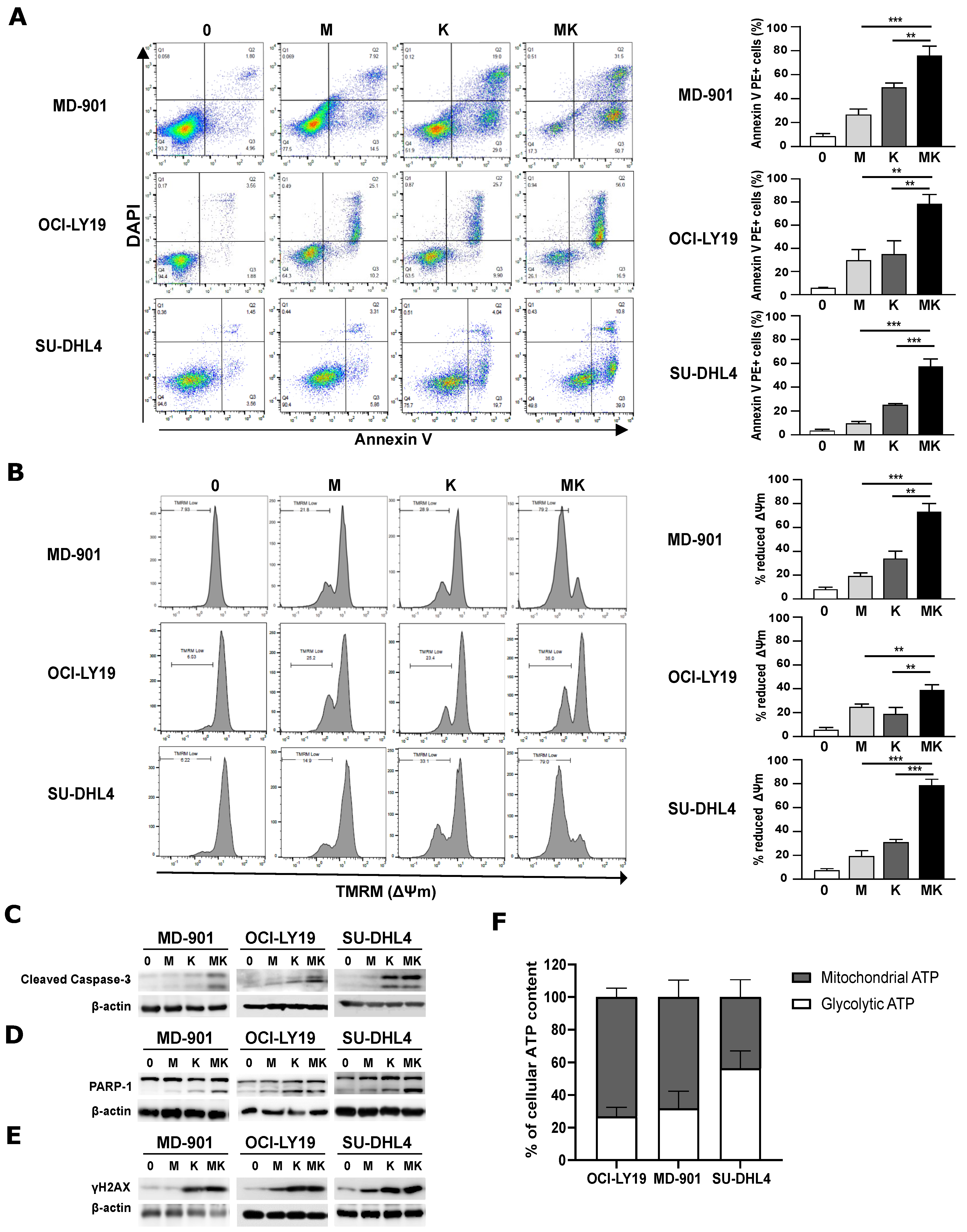
3.1. Combining Metformin and L-Asparaginase Strongly Enhances Apoptosis in DLBCL Cell Lines Irrespective of Their OxPhos or BCR/Glycolytic Subtypes
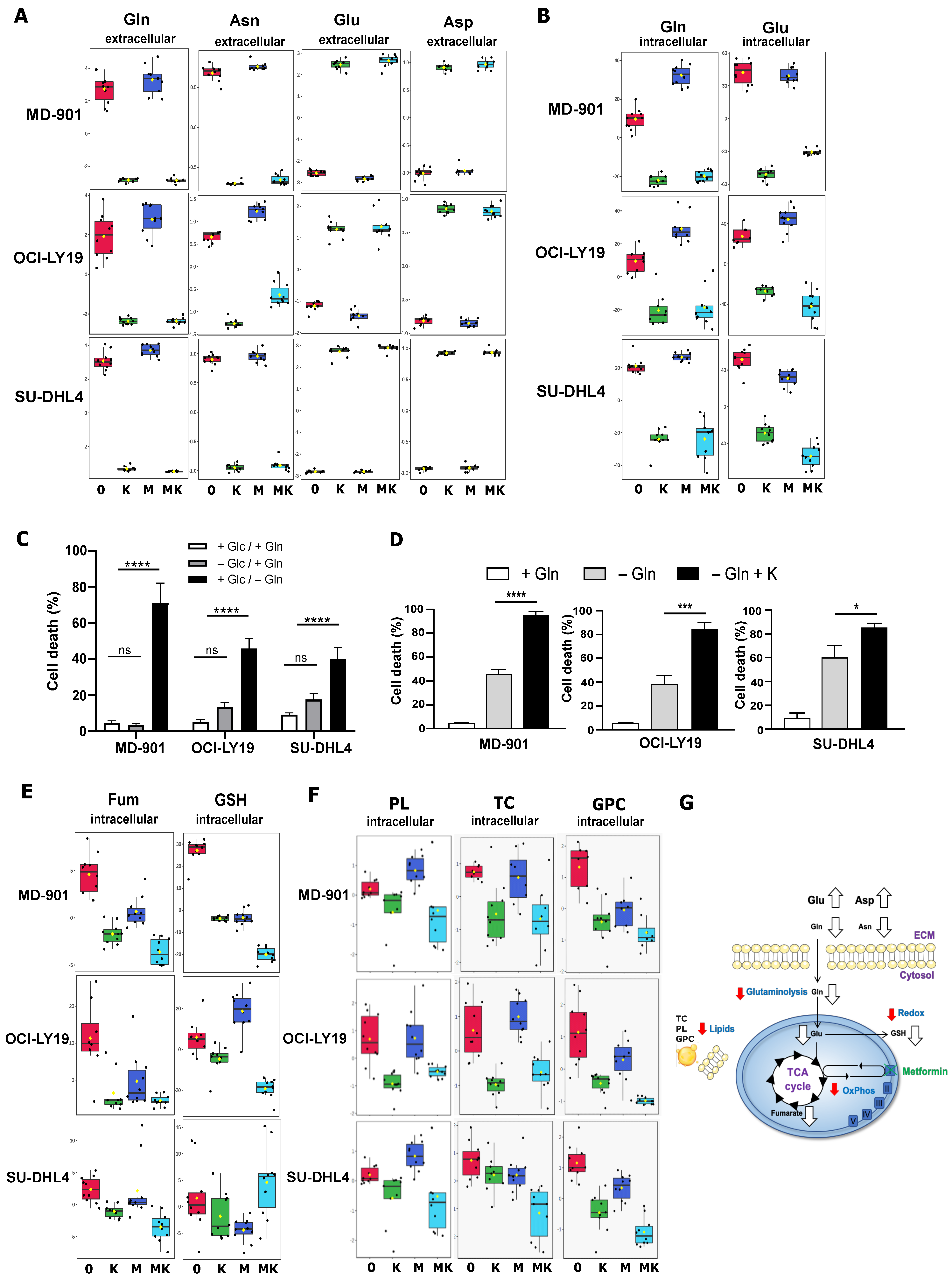
3.2. Metabolic Alteration Landscape of Metformin and L-Asparaginase Combination in DLBCL Cells
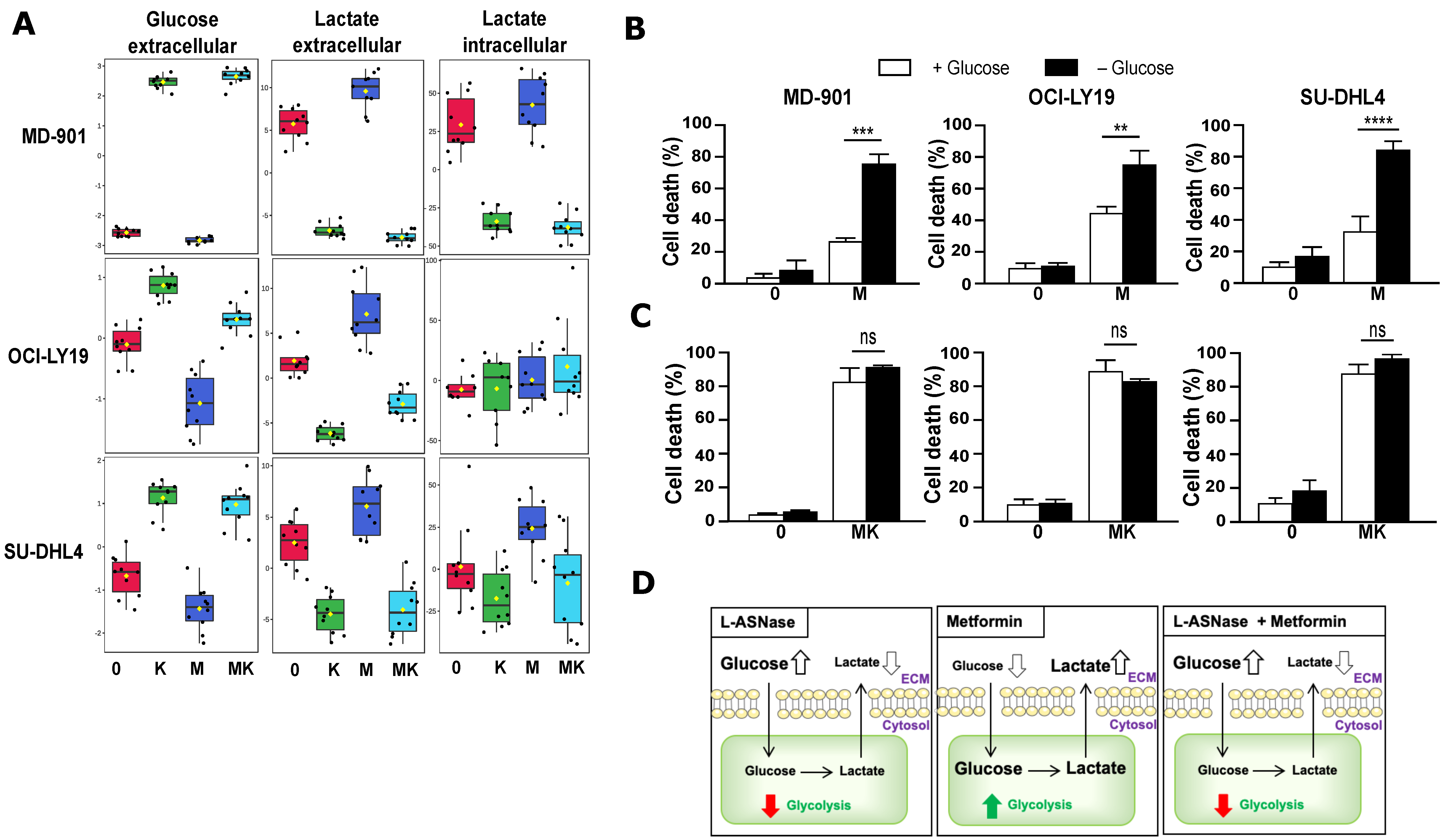
3.3. Combination of Metformin and L-Asparaginase Strongly Inhibits Glycolysis
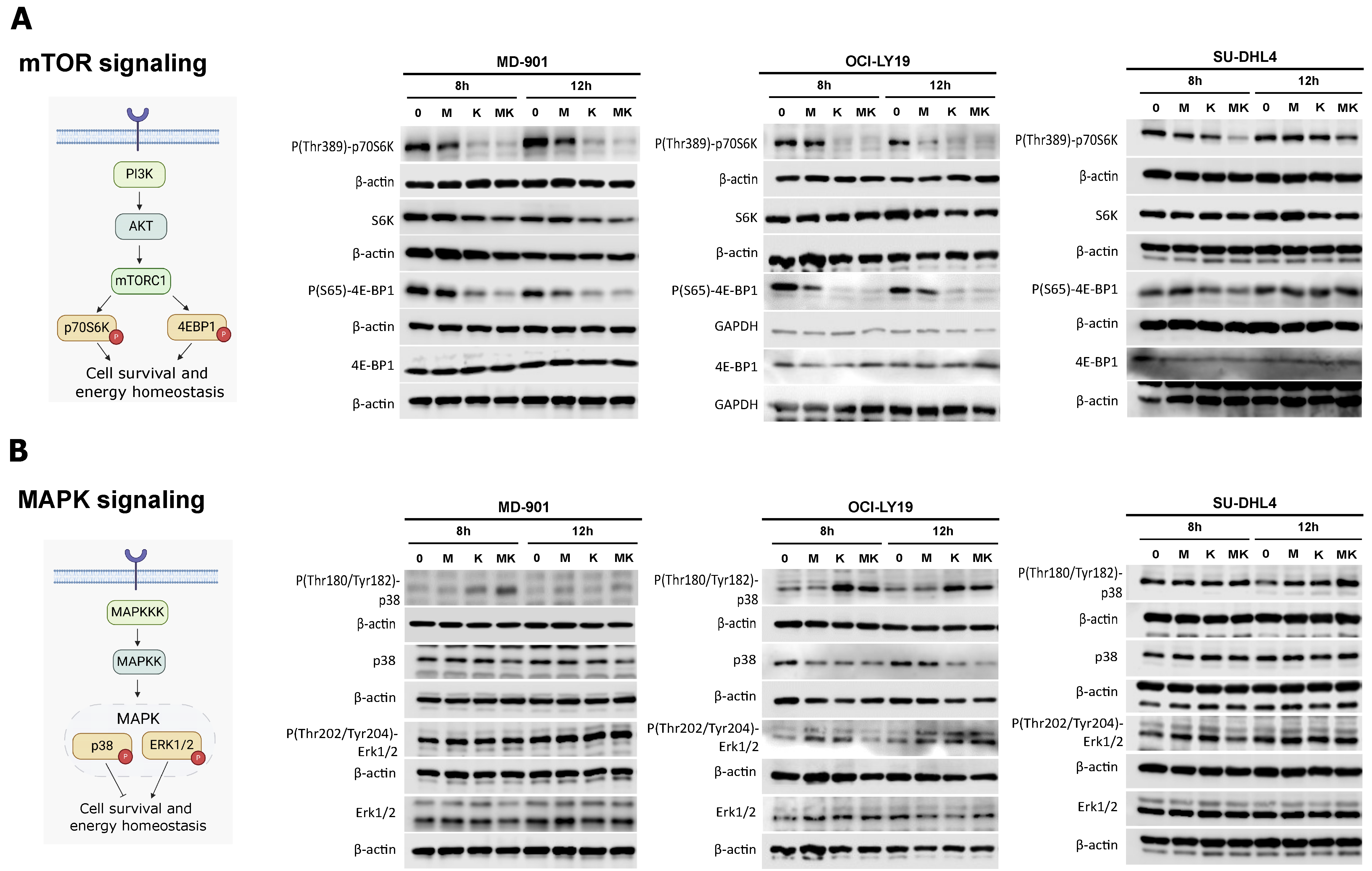
3.4. Metformin Cooperates with L-Asparaginase to Modulate mTOR and MAPK Signaling Pathways
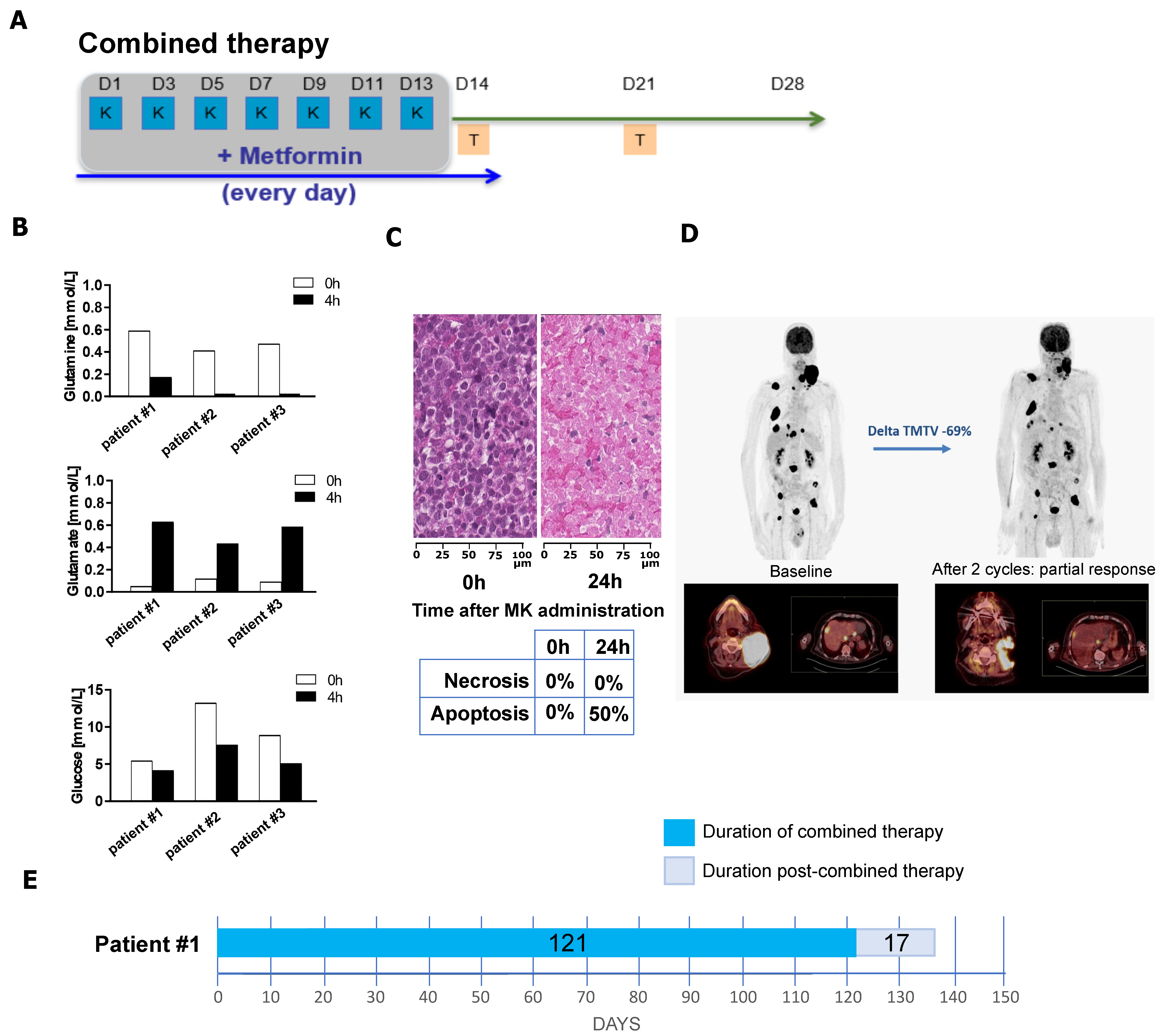
3.5. Combination of Metformin and L-Asparaginase Has a Clinical Benefit in Refractory/Relapsed DLBCL Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sehn, L.H.; Salles, G. Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 Revision of the World Health Organization Classification of Lymphoid Neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Lepage, E.; Brière, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; Van Den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Houot, R.; Bachy, E.; Cartron, G.; Gros, F.-X.; Morschhauser, F.; Oberic, L.; Gastinne, T.; Feugier, P.; Duléry, R.; Thieblemont, C.; et al. Axicabtagene Ciloleucel as Second-Line Therapy in Large B Cell Lymphoma Ineligible for Autologous Stem Cell Transplantation: A Phase 2 Trial. Nat. Med. 2023, 29, 2593–2601. [Google Scholar] [CrossRef]
- Westin, J.R.; Kersten, M.J.; Salles, G.; Abramson, J.S.; Schuster, S.J.; Locke, F.L.; Andreadis, C. Efficacy and Safety of CD19-Directed CAR-T Cell Therapies in Patients with Relapsed/Refractory Aggressive B-Cell Lymphomas: Observations from the JULIET, ZUMA-1, and TRANSCEND Trials. Am. J. Hematol. 2021, 96, 1295–1312. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The Hallmarks of Cancer Metabolism: Still Emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Demicco, M.; Liu, X.-Z.; Leithner, K.; Fendt, S.-M. Metabolic Heterogeneity in Cancer. Nat. Metab. 2024, 6, 18–38. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct Types of Diffuse Large B-Cell Lymphoma Identified by Gene Expression Profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular Subtypes of Diffuse Large B Cell Lymphoma Are Associated with Distinct Pathogenic Mechanisms and Outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Ennishi, D.; Takata, K.; Béguelin, W.; Duns, G.; Mottok, A.; Farinha, P.; Bashashati, A.; Saberi, S.; Boyle, M.; Meissner, B.; et al. Molecular and Genetic Characterization of MHC Deficiency Identifies EZH2 as Therapeutic Target for Enhancing Immune Recognition. Cancer Discov. 2019, 9, 546–563. [Google Scholar] [CrossRef]
- Reddy, A.; Zhang, J.; Davis, N.S.; Moffitt, A.B.; Love, C.L.; Waldrop, A.; Leppa, S.; Pasanen, A.; Meriranta, L.; Karjalainen-Lindsberg, M.-L.; et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell 2017, 171, 481–494.e15. [Google Scholar] [CrossRef] [PubMed]
- Pasqualucci, L.; Trifonov, V.; Fabbri, G.; Ma, J.; Rossi, D.; Chiarenza, A.; Wells, V.A.; Grunn, A.; Messina, M.; Elliot, O.; et al. Analysis of the Coding Genome of Diffuse Large B-Cell Lymphoma. Nat. Genet. 2011, 43, 830–837. [Google Scholar] [CrossRef]
- Eluard, B.; Nuan-Aliman, S.; Faumont, N.; Collares, D.; Bordereaux, D.; Montagne, A.; Martins, I.; Cagnard, N.; Caly, M.; Taoui, O.; et al. The Alternative RelB NF-κB Subunit Is a Novel Critical Player in Diffuse Large B-Cell Lymphoma. Blood 2022, 139, 384–398. [Google Scholar] [CrossRef]
- Ngo, V.N.; Young, R.M.; Schmitz, R.; Jhavar, S.; Xiao, W.; Lim, K.-H.; Kohlhammer, H.; Xu, W.; Yang, Y.; Zhao, H.; et al. Oncogenically Active MYD88 Mutations in Human Lymphoma. Nature 2011, 470, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Lenz, G.; Wright, G.; Dave, S.S.; Xiao, W.; Powell, J.; Zhao, H.; Xu, W.; Tan, B.; Goldschmidt, N.; Iqbal, J.; et al. Stromal Gene Signatures in Large-B-Cell Lymphomas. N. Engl. J. Med. 2008, 359, 2313–2323. [Google Scholar] [CrossRef]
- Melani, C.; Lakhotia, R.; Pittaluga, S.; Phelan, J.D.; Huang, D.W.; Wright, G.; Simard, J.; Muppidi, J.; Thomas, C.J.; Ceribelli, M.; et al. Combination Targeted Therapy in Relapsed Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2024, 390, 2143–2155. [Google Scholar] [CrossRef]
- Monti, S.; Savage, K.J.; Kutok, J.L.; Feuerhake, F.; Kurtin, P.; Mihm, M.; Wu, B.; Pasqualucci, L.; Neuberg, D.; Aguiar, R.C.T.; et al. Molecular Profiling of Diffuse Large B-Cell Lymphoma Identifies Robust Subtypes Including One Characterized by Host Inflammatory Response. Blood 2005, 105, 1851–1861. [Google Scholar] [CrossRef]
- Caro, P.; Kishan, A.U.; Norberg, E.; Stanley, I.A.; Chapuy, B.; Ficarro, S.B.; Polak, K.; Tondera, D.; Gounarides, J.; Yin, H.; et al. Metabolic Signatures Uncover Distinct Targets in Molecular Subsets of Diffuse Large B Cell Lymphoma. Cancer Cell 2012, 22, 547–560. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Maggs, D.G.; Spollett, G.R.; Page, S.L.; Rife, F.S.; Walton, V.; Shulman, G.I. Efficacy and Metabolic Effects of Metformin and Troglitazone in Type II Diabetes Mellitus. N. Engl. J. Med. 1998, 338, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, J.H.; Maggi, M.; Tarasco, E.; Scotti, C.; Avramis, V.I.; Mittelman, S.D. Glutaminase Activity Determines Cytotoxicity of L-Asparaginases on Most Leukemia Cell Lines. Leuk. Res. 2015, 39, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Inoue, J.; Sakaguchi, K.; Takagi, M.; Mizutani, S.; Inazawa, J. Autophagy Is Required for Cell Survival under L-Asparaginase-Induced Metabolic Stress in Acute Lymphoblastic Leukemia Cells. Oncogene 2017, 36, 4267–4276. [Google Scholar] [CrossRef]
- Derudder, E.; Dejardin, E.; Pritchard, L.L.; Green, D.R.; Körner, M.; Baud, V. RelB/P50 Dimers Are Differentially Regulated by Tumor Necrosis Factor-α and Lymphotoxin-β Receptor Activation: CRITICAL ROLES FOR P100 *. J. Biol. Chem. 2003, 278, 23278–23284. [Google Scholar] [CrossRef] [PubMed]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.D.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic Profiling, Metabolomic and Metabonomic Procedures for NMR Spectroscopy of Urine, Plasma, Serum and Tissue Extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Jacob, D.; Deborde, C.; Lefebvre, M.; Maucourt, M.; Moing, A. NMRProcFlow: A Graphical and Interactive Tool Dedicated to 1D Spectra Processing for NMR-Based Metabolomics. Metabolomics 2017, 13, 36. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a Unified Platform for Metabolomics Data Processing, Analysis and Interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]
- Chiche, J.; Reverso-Meinietti, J.; Mouchotte, A.; Rubio-Patiño, C.; Mhaidly, R.; Villa, E.; Bossowski, J.P.; Proics, E.; Grima-Reyes, M.; Paquet, A.; et al. GAPDH Expression Predicts the Response to R-CHOP, the Tumor Metabolic Status, and the Response of DLBCL Patients to Metabolic Inhibitors. Cell Metab. 2019, 29, 1243–1257.e10. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A.; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; et al. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Jiang, W.; Lu, W.; Yang, J.; Yang, W. B Cell Lymphoma with Different Metabolic Characteristics Show Distinct Sensitivities to Metabolic Inhibitors. J. Cancer 2018, 9, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Monti, S.; Juszczynski, P.; Daley, J.; Chen, W.; Witzig, T.E.; Habermann, T.M.; Kutok, J.L.; Shipp, M.A. SYK-Dependent Tonic B-Cell Receptor Signaling Is a Rational Treatment Target in Diffuse Large B-Cell Lymphoma. Blood 2008, 111, 2230–2237. [Google Scholar] [CrossRef]
- Nuan-Aliman, S.; Bordereaux, D.; Thieblemont, C.; Baud, V. The Alternative RelB NF-kB Subunit Exerts a Critical Survival Function upon Metabolic Stress in Diffuse Large B-Cell Lymphoma-Derived Cells. Biomedicines 2022, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Cluntun, A.A.; Lukey, M.J.; Cerione, R.A.; Locasale, J.W. Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer 2017, 3, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Soleja, M.; Mims, M.; Rivero, G. Uncovering Molecular Abnormalities Leading to the Warburg Effect in Primary Refractory Diffuse Large B-Cell Lymphoma. Blood Cancer J. 2016, 6, e502. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted Role of mTOR (Mammalian Target of Rapamycin) Signaling Pathway in Human Health and Disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.P.; Blenis, J. ERK and P38 MAPK-Activated Protein Kinases: A Family of Protein Kinases with Diverse Biological Functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef]
- Trinh, A.; Khamari, R.; Fovez, Q.; Mahon, F.-X.; Turcq, B.; Bouscary, D.; Maboudou, P.; Joncquel, M.; Coiteux, V.; Germain, N.; et al. Antimetabolic Cooperativity with the Clinically Approved L-Asparaginase and Tyrosine Kinase Inhibitors to Eradicate CML Stem Cells. Mol. Metab. 2021, 55, 101410. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-N.; Zhang, Y.; Wang, W.-G.; Sheng, D.; Zhou, X.-Y.; Li, X.-Q. Alteration of Cholesterol Metabolism by Metformin Is Associated With Improved Outcome in Type II Diabetic Patients With Diffuse Large B-Cell Lymphoma. Front. Oncol. 2021, 11, 608238. [Google Scholar] [CrossRef] [PubMed]
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.-M. Lipid Metabolism in Cancer: New Perspectives and Emerging Mechanisms. Dev. Cell 2021, 56, 1363–1393. [Google Scholar] [CrossRef]
- Pera, B.; Krumsiek, J.; Assouline, S.E.; Marullo, R.; Patel, J.; Phillip, J.M.; Román, L.; Mann, K.K.; Cerchietti, L. Metabolomic Profiling Reveals Cellular Reprogramming of B-Cell Lymphoma by a Lysine Deacetylase Inhibitor through the Choline Pathway. EBioMedicine 2018, 28, 80–89. [Google Scholar] [CrossRef]
- Peeters, R.; Cuenca-Escalona, J.; Zaal, E.A.; Hoekstra, A.T.; Balvert, A.C.G.; Vidal-Manrique, M.; Blomberg, N.; van Deventer, S.J.; Stienstra, R.; Jellusova, J.; et al. Fatty Acid Metabolism in Aggressive B-Cell Lymphoma Is Inhibited by Tetraspanin CD37. Nat. Commun. 2022, 13, 5371. [Google Scholar] [CrossRef] [PubMed]
- Gifford, G.K.; Gifford, A.J.; Chen, Q.; Shen, Y.; Gabrielli, S.; Gill, A.J.; Stevenson, W.S.; Best, O.G. Fatty Acid Synthase and Adenosine Monophosphate-Activated Protein Kinase Regulate Cell Survival and Drug Sensitivity in Diffuse Large B-Cell Lymphoma. Leuk. Lymphoma 2020, 61, 1810–1822. [Google Scholar] [CrossRef] [PubMed]
- McDonald, G.; Chubukov, V.; Coco, J.; Truskowski, K.; Narayanaswamy, R.; Choe, S.; Steadman, M.; Artin, E.; Padyana, A.K.; Jin, L.; et al. Selective Vulnerability to Pyrimidine Starvation in Hematologic Malignancies Revealed by AG-636, a Novel Clinical-Stage Inhibitor of Dihydroorotate Dehydrogenase. Mol. Cancer Ther. 2020, 19, 2502–2515. [Google Scholar] [CrossRef] [PubMed]
- Ricci, J.-E.; Chiche, J. Metabolic Reprogramming of Non-Hodgkin’s B-Cell Lymphomas and Potential Therapeutic Strategies. Front. Oncol. 2018, 8, 556. [Google Scholar] [CrossRef]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting Cancer Metabolism in the Era of Precision Oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef]
- Lemberg, K.M.; Gori, S.S.; Tsukamoto, T.; Rais, R.; Slusher, B.S. Clinical Development of Metabolic Inhibitors for Oncology. J. Clin. Investig. 2022, 132, e148550. [Google Scholar] [CrossRef] [PubMed]
- Cunha Júnior, A.D.; Pericole, F.V.; Carvalheira, J.B.C. Metformin and Blood Cancers. Clinics 2018, 73, e412s. [Google Scholar] [CrossRef]
- Singh, A.R.; Gu, J.J.; Zhang, Q.; Torka, P.; Sundaram, S.; Mavis, C.; Hernandez-Ilizaliturri, F.J. Metformin Sensitizes Therapeutic Agents and Improves Outcome in Pre-Clinical and Clinical Diffuse Large B-Cell Lymphoma. Cancer Metab. 2020, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.; Blunt, D.N.; Gatov, E.; Nagamuthu, C.; Croxford, R.; Mozessohn, L.; Cheung, M.C. Statin and Cyclooxygenase-2 Inhibitors Improve Survival in Newly Diagnosed Diffuse Large B-Cell Lymphoma: A Large Population-Based Study of 4913 Subjects. Br. J. Haematol. 2020, 191, 396–404. [Google Scholar] [CrossRef]
- Wang, Y.; Maurer, M.J.; Larson, M.C.; Allmer, C.; Feldman, A.L.; Bennani, N.N.; Thompson, C.A.; Porrata, L.F.; Habermann, T.M.; Witzig, T.E.; et al. Impact of Metformin Use on the Outcomes of Newly Diagnosed Diffuse Large B-Cell Lymphoma and Follicular Lymphoma. Br. J. Haematol. 2019, 186, 820–828. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lordello, L.; Nuan-Aliman, S.; Kielbassa-Elkadi, K.; Montagne, A.; Kotta, K.; Martins, I.; Pinto Jurado, E.; Caradeuc, C.; Lehmann-Che, J.; Martínez-Climent, J.Á.; et al. Metabolic Heterogeneity in Diffuse Large B-Cell Lymphoma Cells Reveals an Innovative Antimetabolic Combination Strategy. Cancers 2025, 17, 394. https://doi.org/10.3390/cancers17030394
Lordello L, Nuan-Aliman S, Kielbassa-Elkadi K, Montagne A, Kotta K, Martins I, Pinto Jurado E, Caradeuc C, Lehmann-Che J, Martínez-Climent JÁ, et al. Metabolic Heterogeneity in Diffuse Large B-Cell Lymphoma Cells Reveals an Innovative Antimetabolic Combination Strategy. Cancers. 2025; 17(3):394. https://doi.org/10.3390/cancers17030394
Chicago/Turabian StyleLordello, Leonardo, Stéphanie Nuan-Aliman, Karoline Kielbassa-Elkadi, Aurélie Montagne, Konstantina Kotta, Isabelle Martins, Eva Pinto Jurado, Cédric Caradeuc, Jacqueline Lehmann-Che, José Ángel Martínez-Climent, and et al. 2025. "Metabolic Heterogeneity in Diffuse Large B-Cell Lymphoma Cells Reveals an Innovative Antimetabolic Combination Strategy" Cancers 17, no. 3: 394. https://doi.org/10.3390/cancers17030394
APA StyleLordello, L., Nuan-Aliman, S., Kielbassa-Elkadi, K., Montagne, A., Kotta, K., Martins, I., Pinto Jurado, E., Caradeuc, C., Lehmann-Che, J., Martínez-Climent, J. Á., Meignin, V., Giraud, N., Kroemer, G., Bertho, G., Thieblemont, C., & Baud, V. (2025). Metabolic Heterogeneity in Diffuse Large B-Cell Lymphoma Cells Reveals an Innovative Antimetabolic Combination Strategy. Cancers, 17(3), 394. https://doi.org/10.3390/cancers17030394







