Breast Lesions of Uncertain Malignant Potential (B3) and the Risk of Breast Cancer Development: A Long-Term Follow-Up Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Upgrade Rates
3.3. Risk of Future Breast Cancer after B3 Diagnosis
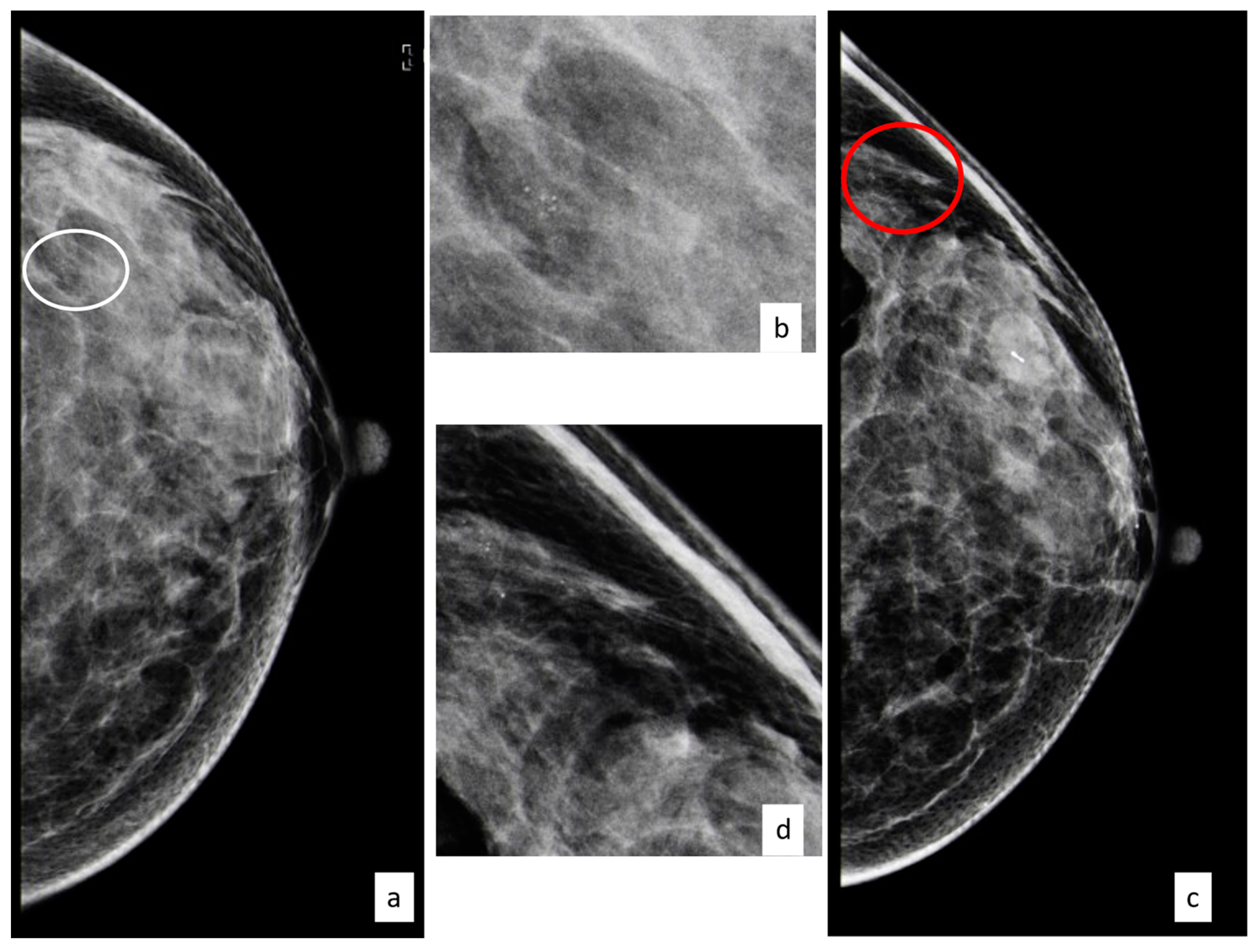
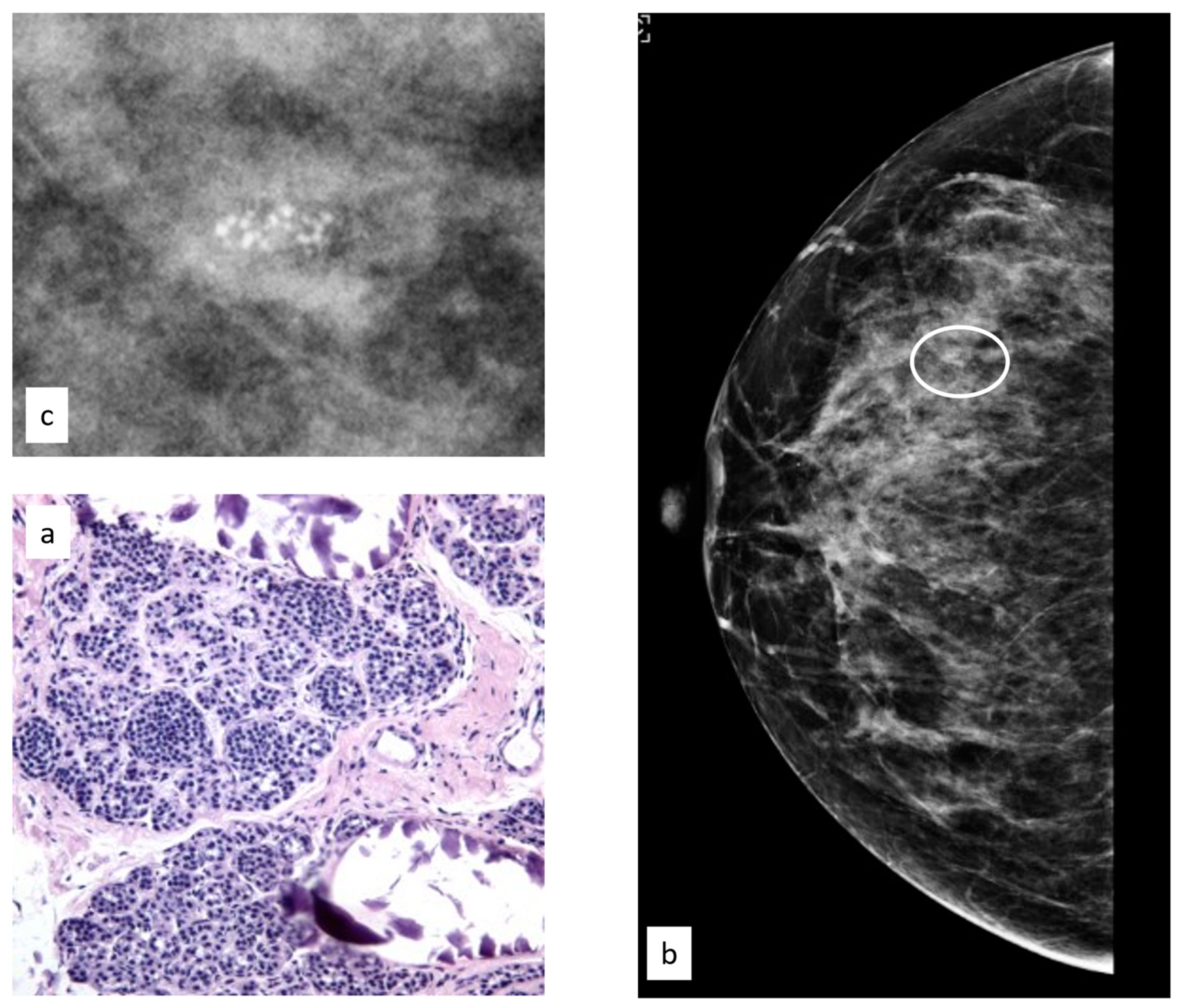
3.3.1. Flat Epithelial Atypia
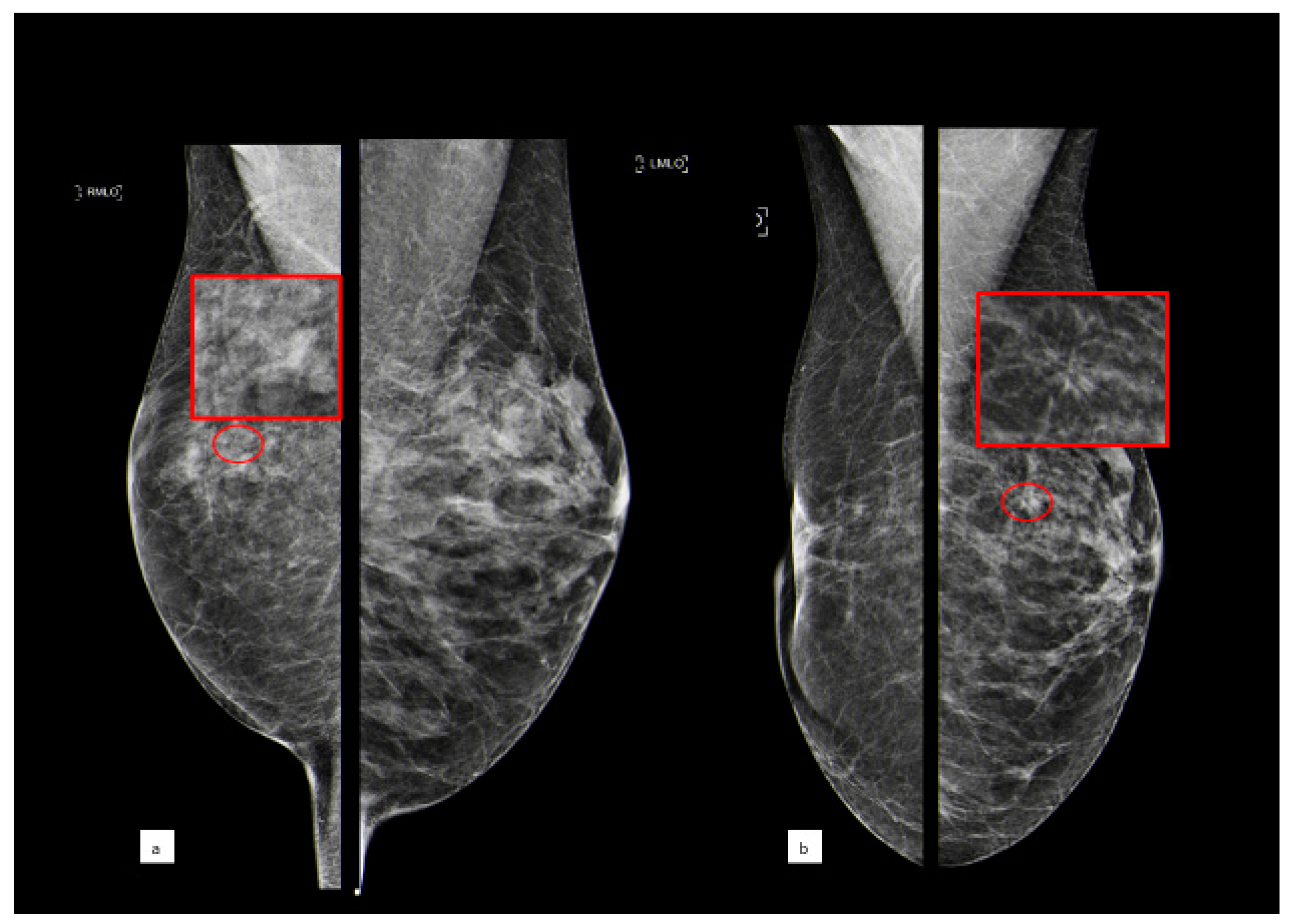
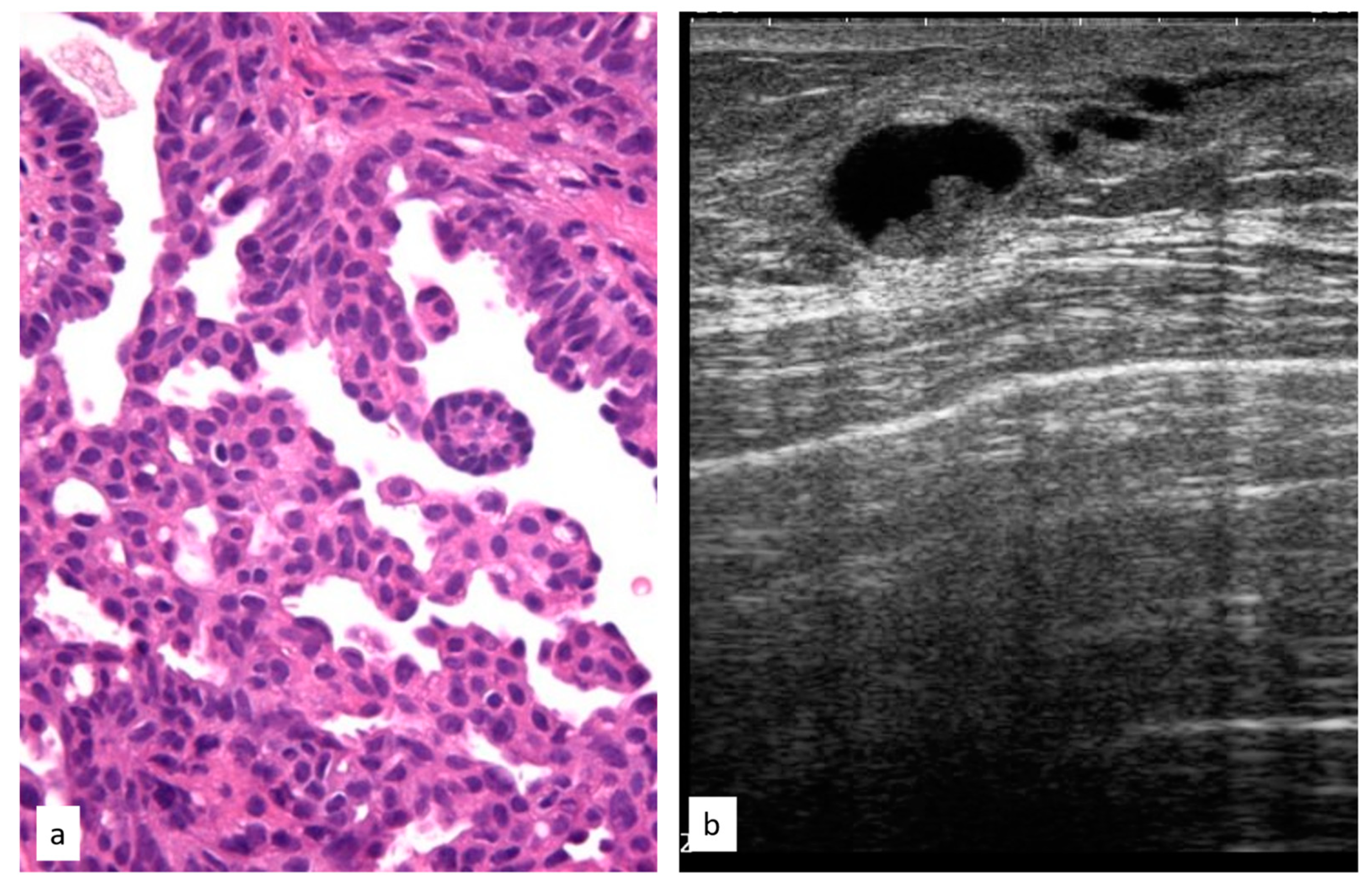
3.3.2. Atypical Ductal Hyperplasia
3.3.3. Lobular Intraepithelial Neoplasia
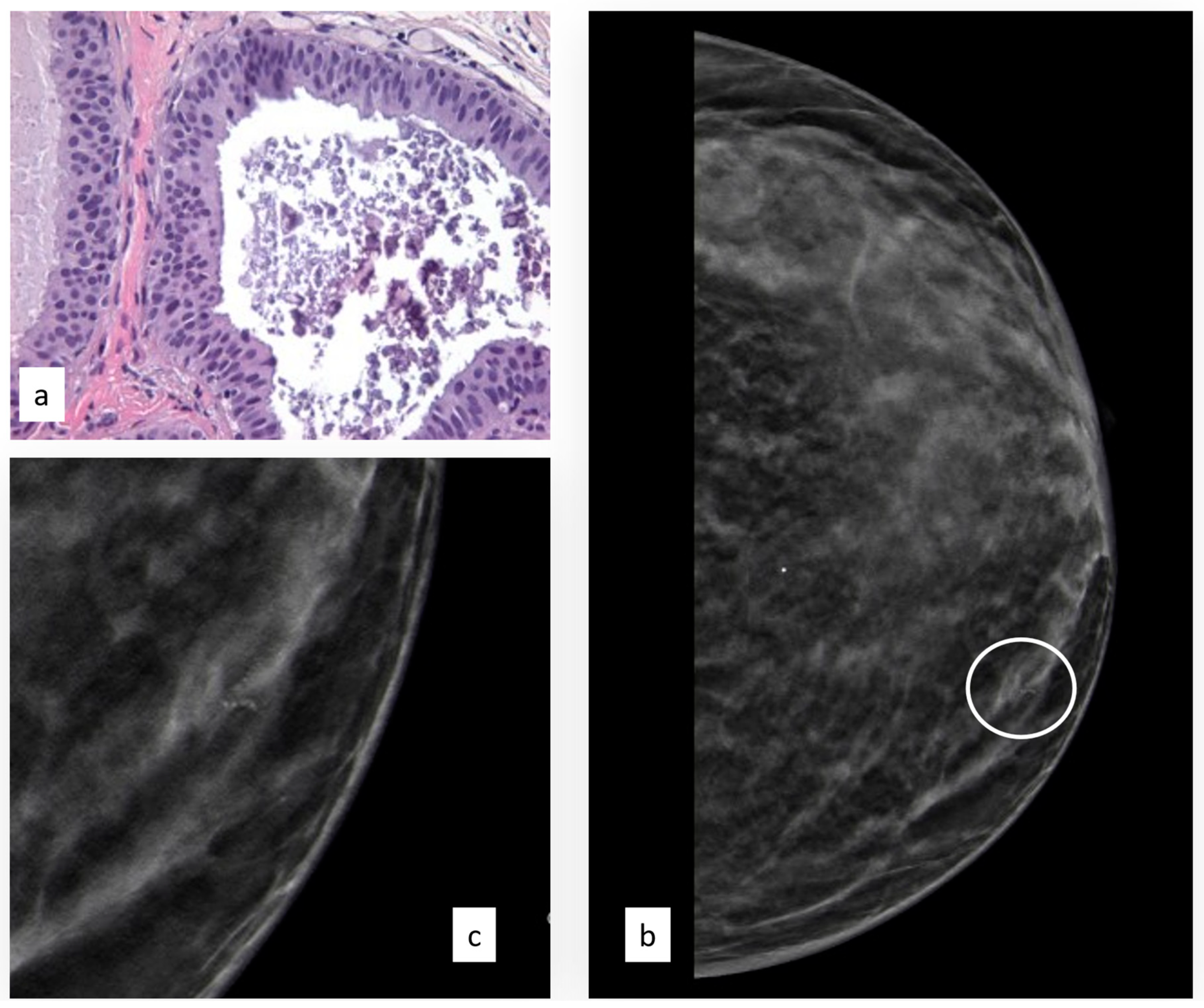
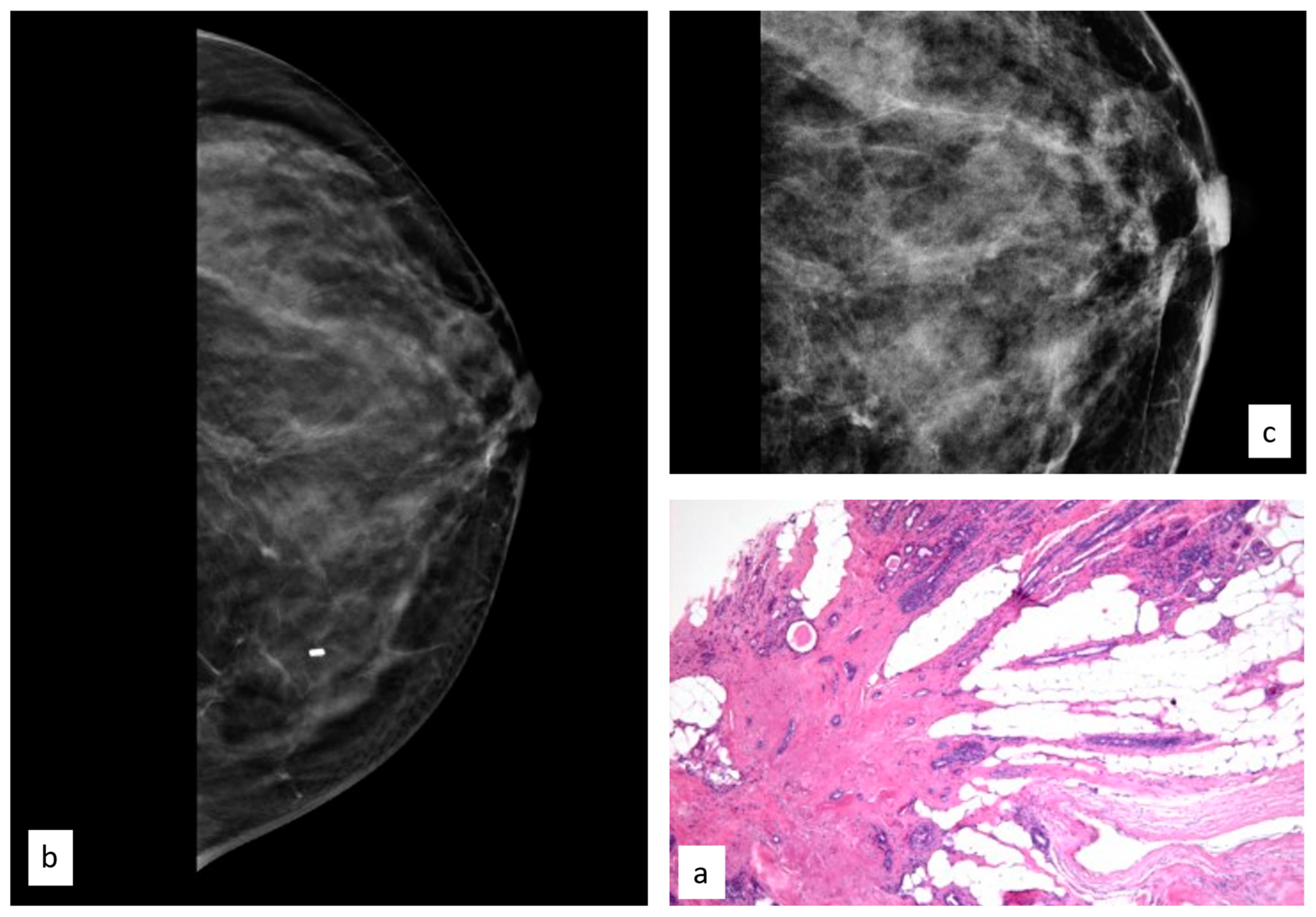
3.3.4. Atypical Papillary Lesion
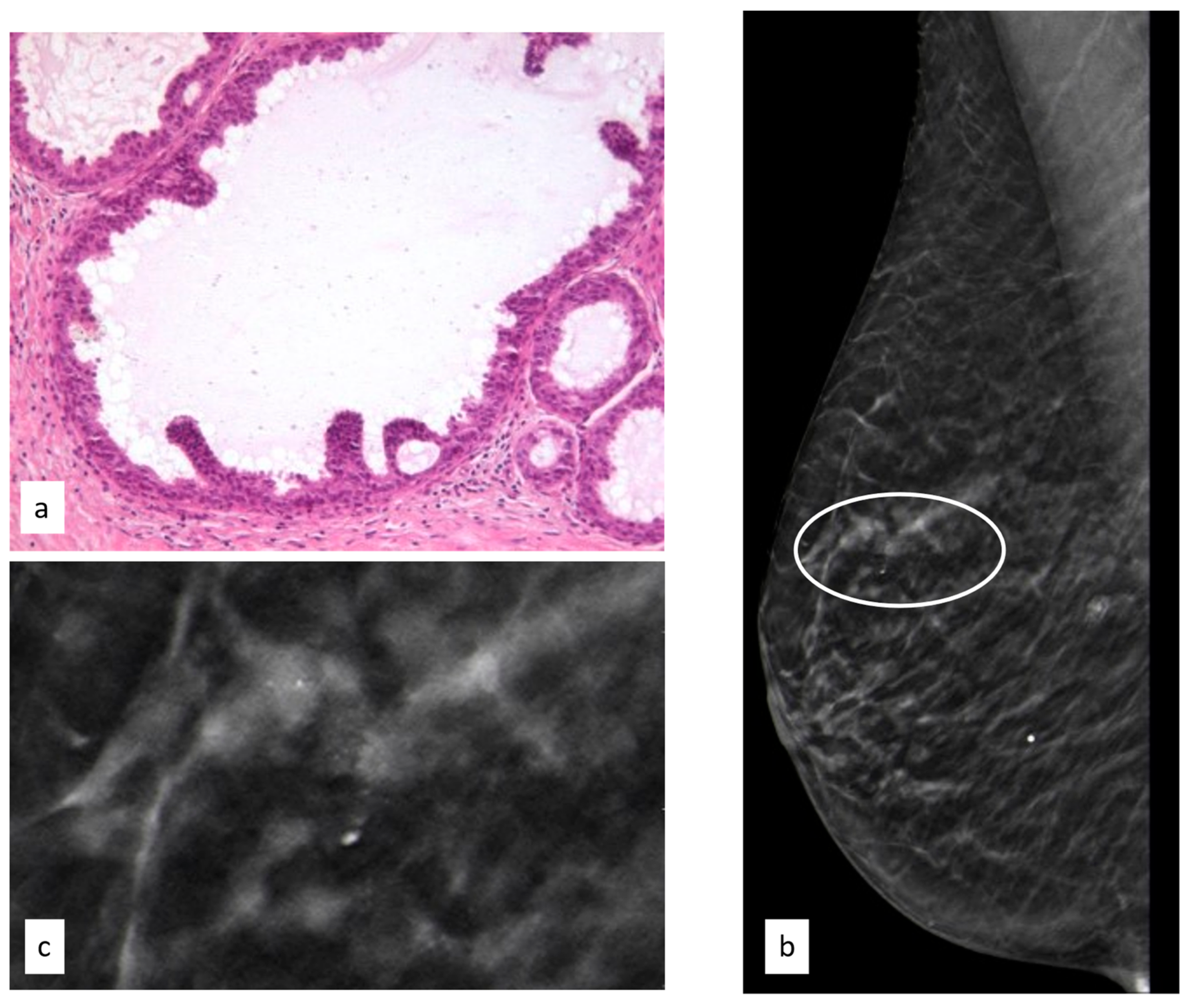
3.3.5. Phyllodes Tumors
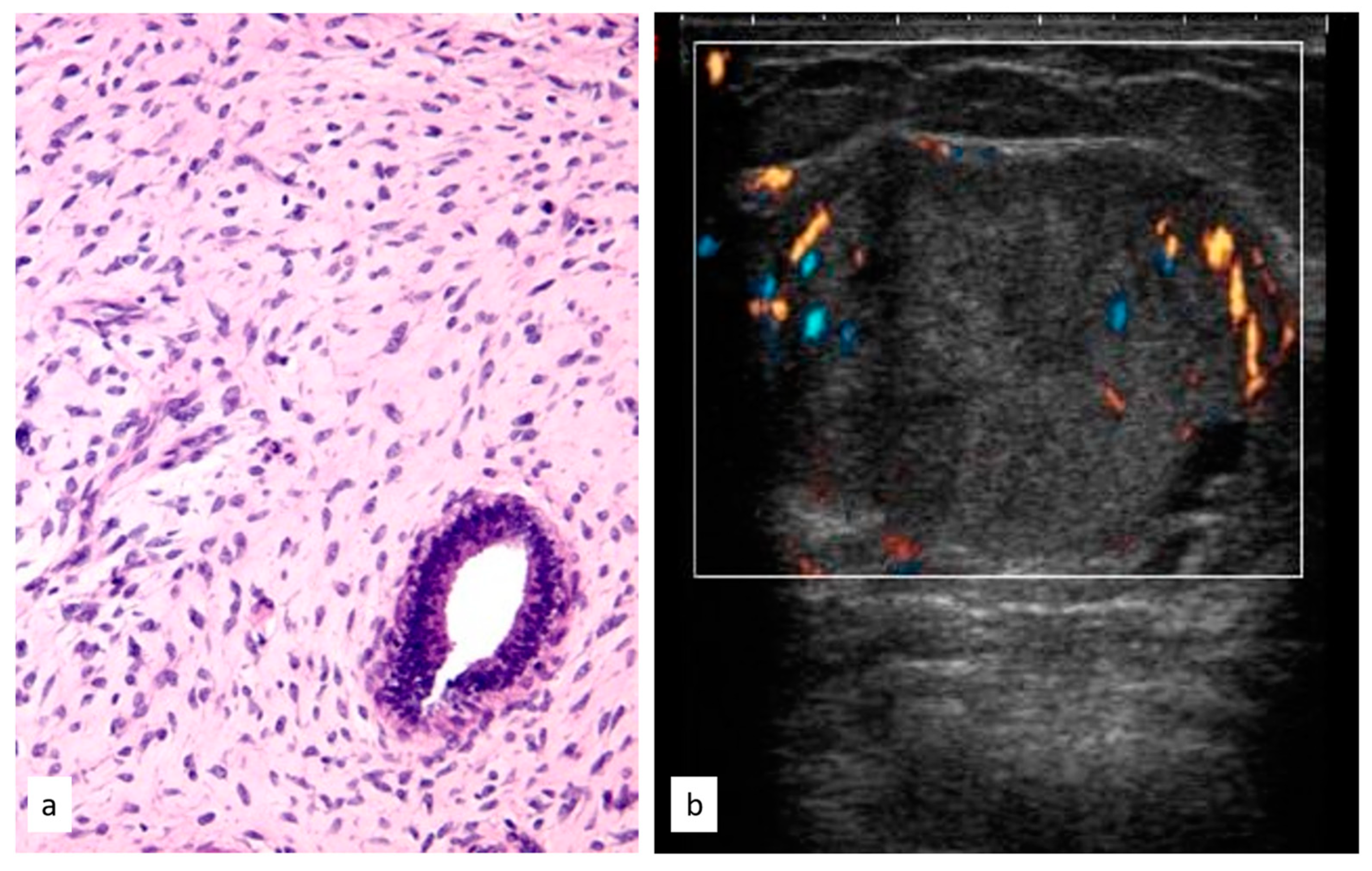
3.3.6. Radial Scar
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Schopper, D.; de Wolf, C. How Effective Are Breast Cancer Screening Programmes by Mammography? Review of the Current Evidence. Eur. J. Cancer 2009, 45, 1916–1923. [Google Scholar] [CrossRef]
- Poorolajal, J.; Heidarimoghis, F.; Karami, M.; Cheraghi, Z.; Gohari-Ensaf, F.; Shahbazi, F.; Zareie, B.; Ameri, P.; Sahraee, F. Factors for the Primary Prevention of Breast Cancer: A Meta-Analysis of Prospective Cohort Studies. J. Res. Health Sci. 2021, 21, e00520. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Pinder, S.E.; Shaaban, A.; Deb, R.; Desai, A.; Gandhi, A.; Lee, A.H.S.; Pain, S.; Wilkinson, L.; Sharma, N. NHS Breast Screening Multidisciplinary Working Group Guidelines for the Diagnosis and Management of Breast Lesions of Uncertain Malignant Potential on Core Biopsy (B3 Lesions). Clin. Radiol. 2018, 73, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Caini, S.; Renne, G.; Cassano, E.; Ambrogetti, D.; Cattani, M.G.; Saguatti, G.; Chiaramondia, M.; Bellotti, E.; Bottiglieri, R.; et al. Positive Predictive Value for Malignancy on Surgical Excision of Breast Lesions of Uncertain Malignant Potential (B3) Diagnosed by Stereotactic Vacuum-Assisted Needle Core Biopsy (VANCB): A Large Multi-Institutional Study in Italy. Breast 2011, 20, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Rageth, C.J.; O’Flynn, E.A.M.; Pinker, K.; Kubik-Huch, R.A.; Mundinger, A.; Decker, T.; Tausch, C.; Dammann, F.; Baltzer, P.A.; Fallenberg, E.M.; et al. Second International Consensus Conference on Lesions of Uncertain Malignant Potential in the Breast (B3 Lesions). Breast Cancer Res. Treat. 2019, 174, 279–296. [Google Scholar] [CrossRef]
- El-Sayed, M.E.; Rakha, E.A.; Reed, J.; Lee, A.H.S.; Evans, A.J.; Ellis, I.O. Predictive Value of Needle Core Biopsy Diagnoses of Lesions of Uncertain Malignant Potential (B3) in Abnormalities Detected by Mammographic Screening. Histopathology 2008, 53, 650–657. [Google Scholar] [CrossRef]
- Houssami, N.; Ciatto, S.; Ellis, I.; Ambrogetti, D. Underestimation of Malignancy of Breast Core-Needle Biopsy: Concepts and Precise Overall and Category-Specific Estimates. Cancer 2007, 109, 487–495. [Google Scholar] [CrossRef]
- Giannotti, E.; James, J.J.; Chen, Y.; Sun, R.; Karuppiah, A.; Yemm, J.; Lee, A.H.S. Effectiveness of Percutaneous Vacuum-Assisted Excision (VAE) of Breast Lesions of Uncertain Malignant Potential (B3 Lesions) as an Alternative to Open Surgical Biopsy. Eur. Radiol. 2021, 31, 9540–9547. [Google Scholar] [CrossRef]
- Hennessy, G.; Boland, M.R.; Bambrick, M.; Crone, L.; Lloyd, A.; Abdelwahab, S.; Downey, E.; Staunton, M.; Hambly, N.; Mhuircheartaigh, N.N.; et al. Value of Long-Term Follow-up in Surgically Excised Lesions of Uncertain Malignant Potential in the Breast—Is 5 Years Necessary? Clin. Breast Cancer 2022, 22, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.R.; Ellis, I.O.; Schnitt, S.J.; Tan, P.H.; van de Vijver, M.J. WHO Classification of Tumours of the Breast; WHO: Geneva, Switzerland, ISBN 978-92-832-2433-4.
- Breast Imaging Reporting & Data System. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads (accessed on 27 January 2020).
- Schiaffino, S.; Calabrese, M.; Melani, E.F.; Trimboli, R.M.; Cozzi, A.; Carbonaro, L.A.; Di Leo, G.; Sardanelli, F. Upgrade Rate of Percutaneously Diagnosed Pure Atypical Ductal Hyperplasia: Systematic Review and Meta-Analysis of 6458 Lesions. Radiology 2020, 294, 76–86. [Google Scholar] [CrossRef]
- Preibsch, H.; Wanner, L.K.; Staebler, A.; Hahn, M.; Siegmann-Luz, K.C. Malignancy Rates of B3-Lesions in Breast Magnetic Resonance Imaging—Do All Lesions Have to Be Excised? BMC Med. Imaging 2018, 18, 27. [Google Scholar] [CrossRef]
- Dillon, M.F.; McDermott, E.W.; Hill, A.D.; O’Doherty, A.; O’Higgins, N.; Quinn, C.M. Predictive Value of Breast Lesions of “Uncertain Malignant Potential” and “Suspicious for Malignancy” Determined by Needle Core Biopsy. Ann. Surg. Oncol. 2007, 14, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Bendinelli, B.; Saladino, V.; Vezzosi, V.; Brancato, B.; Nori, J.; Palli, D. Non-Malignant Breast Papillary Lesions—B3 Diagnosed on Ultrasound--Guided 14-Gauge Needle Core Biopsy: Analysis of 114 Cases from a Single Institution and Review of the Literature. Pathol. Oncol. Res. 2015, 21, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Girardi, V.; Guaragni, M.; Ruzzenenti, N.; Palmieri, F.; Fogazzi, G.; Cozzi, A.; Lucchini, D.; Buffoli, A.; Schiaffino, S.; Sardanelli, F. B3 Lesions at Vacuum-Assisted Breast Biopsy under Ultrasound or Mammography Guidance: A Single-Center Experience on 3634 Consecutive Biopsies. Cancers 2021, 13, 5443. [Google Scholar] [CrossRef]
- Forester, N.D.; Lowes, S.; Mitchell, E.; Twiddy, M. High Risk (B3) Breast Lesions: What Is the Incidence of Malignancy for Individual Lesion Subtypes? A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2019, 45, 519–527. [Google Scholar] [CrossRef]
- My Personalized Breast Screening—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03672331 (accessed on 16 June 2023).
- Schiaffino, S.; Massone, E.; Gristina, L.; Fregatti, P.; Rescinito, G.; Villa, A.; Friedman, D.; Calabrese, M. Vacuum Assisted Breast Biopsy (VAB) Excision of Subcentimeter Microcalcifications as an Alternative to Open Biopsy for Atypical Ductal Hyperplasia. Br. J. Radiol. 2018, 91, 20180003. [Google Scholar] [CrossRef]
- Rahman, W.T.; Helvie, M.A. Breast Cancer Screening in Average and High-Risk Women. Best. Pract. Res. Clin. Obstet. Gynaecol. 2022, 83, 3–14. [Google Scholar] [CrossRef]
- Evans, A.; Trimboli, R.M.; Athanasiou, A.; Balleyguier, C.; Baltzer, P.A.; Bick, U.; Camps Herrero, J.; Clauser, P.; Colin, C.; Cornford, E.; et al. Breast Ultrasound: Recommendations for Information to Women and Referring Physicians by the European Society of Breast Imaging. Insights Imaging 2018, 9, 449–461. [Google Scholar] [CrossRef]
- Mann, R.M.; Balleyguier, C.; Baltzer, P.A.; Bick, U.; Colin, C.; Cornford, E.; Evans, A.; Fallenberg, E.; Forrai, G.; Fuchsjäger, M.H.; et al. Breast MRI: EUSOBI Recommendations for Women’s Information. Eur. Radiol. 2015, 25, 3669–3678. [Google Scholar] [CrossRef] [PubMed]
- Pashayan, N.; Antoniou, A.C.; Lee, A.; Wolfson, M.; Chiquette, J.; Eloy, L.; Eisen, A.; Stockley, T.L.; Nabi, H.; Brooks, J.D.; et al. Should Age-Dependent Absolute Risk Thresholds Be Used for Risk Stratification in Risk-Stratified Breast Cancer Screening? J. Pers. Med. 2021, 11, 916. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L.C.; Sellers, T.A.; Frost, M.H.; Lingle, W.L.; Degnim, A.C.; Ghosh, K.; Vierkant, R.A.; Maloney, S.D.; Pankratz, V.S.; Hillman, D.W.; et al. Benign Breast Disease and the Risk of Breast Cancer. N. Engl. J. Med. 2005, 353, 229–237. [Google Scholar] [CrossRef]
- Collins, L.C.; Baer, H.J.; Tamimi, R.M.; Connolly, J.L.; Colditz, G.A.; Schnitt, S.J. Magnitude and Laterality of Breast Cancer Risk According to Histologic Type of Atypical Hyperplasia: Results from the Nurses’ Health Study. Cancer 2007, 109, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Wilkinson, L.S.; Pinder, S.E. The B3 Conundrum—The Radiologists’ Perspective. Br. J. Radiol. 2017, 90, 20160595. [Google Scholar] [CrossRef]
- Rakha, E.A.; Lee, A.H.S.; Jenkins, J.A.; Murphy, A.E.; Hamilton, L.J.; Ellis, I.O. Characterization and Outcome of Breast Needle Core Biopsy Diagnoses of Lesions of Uncertain Malignant Potential (B3) in Abnormalities Detected by Mammographic Screening. Int. J. Cancer 2011, 129, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Elfgen, C.; Leo, C.; Kubik-Huch, R.A.; Muenst, S.; Schmidt, N.; Quinn, C.; McNally, S.; van Diest, P.J.; Mann, R.M.; Bago-Horvath, Z.; et al. Third International Consensus Conference on Lesions of Uncertain Malignant Potential in the Breast (B3 Lesions). Virchows Arch. 2023. [Google Scholar] [CrossRef]
- Cullinane, C.; Byrne, J.; Kelly, L.; O Sullivan, M.; Antony Corrigan, M.; Paul Redmond, H. The Positive Predictive Value of Vacuum Assisted Biopsy (VAB) in Predicting Final Histological Diagnosis for Breast Lesions of Uncertain Malignancy (B3 Lesions): A Systematic Review & Meta-Analysis. Eur. J. Surg. Oncol. 2022, 48, 1464–1474. [Google Scholar] [CrossRef]

| Histology | Nr. Patients | Percentage % |
|---|---|---|
| FEA | 146 | 15.1 |
| ADH | 259 | 26.8 |
| LIN | 222 | 23 |
| PL | 124 | 12.8 |
| PT | 53 | 5.5 |
| RS | 145 | 15 |
| Others/mucocele-like, myofibroblastoma, spindle cells proliferation | 17 | 1.8 |
| Total | 966 | 100.0 |
| Histology | Open Excision | Vacuum-Assisted Excision | Follow-Up |
|---|---|---|---|
| FEA (n = 146) | 123 (84.2%) | 9 (6.2%) | 14 (9.6%) |
| ADH (n = 259) | 238 (91.9%) | 6 (2.3%) | 15 (5.8%) |
| LIN (n = 222) | 207 (93.2%) | 6 (2.7%) | 9 (4.1%) |
| PL (n = 124) | 115 (92.7%) | 5 (4.0%) | 4 (3.2%) |
| PT (n = 53) | 53 (100%) | 0 (-) | 0 (-) |
| RS (n = 145) | 128 (88.3%) | 12 (8.3%) | 5 (3.4%) |
| Others (n = 17) | 15 (88.2%) | 0 (-) | 2 (11.8%) |
| Histology | Upgrade to In Situ Carcinoma | Upgrade to Invasive Carcinoma | Total Upgrade Rate |
|---|---|---|---|
| FEA (n = 146) | 3 (2.1%) | 14 (9.6%) | 17 (11.6%) |
| ADH (n = 259) | 67 (25.9%) | 36 (13.9%) | 103 (39.8%) |
| LIN (n = 222) | 18 (8.1%) | 26 (11.8%) | 44 (19.9%) |
| PL (n = 124) | 9 (7.3%) | 21 (17.1%) | 30 (24.4%) |
| PT 1 (n = 53) | 0 (-) | 7 (13.2%) | 7 (13.2%) |
| RS (n = 145) | 5 (3.4%) | 2 (1.4%) | 7 (4.8%) |
| Others (n = 17) | 1 (5.9%) | 1 (5.9%) | 2 (11.8%) |
| Total (n = 966) | 103 (10.6%) | 107 (11.1%) | 210 (22.7%) |
| Type of Treatment for the B3 Lesion | No Future Cancer during Follow-Up | Diagnosis of New Cancer during Follow-Up | Total |
|---|---|---|---|
| Open surgical excision | 592 (90.7%) | 61 (9.3%) | 653 |
| Vacuum-assisted excision | 29 (90.6%) | 3 (9.4%) | 32 |
| Follow-up | 43 (93.5%) | 3 (6.5%) | 46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, C.; Nori Cucchiari, J.; Di Naro, F.; De Benedetto, D.; Bicchierai, G.; Franconeri, A.; Renda, I.; Bianchi, S.; Susini, T. Breast Lesions of Uncertain Malignant Potential (B3) and the Risk of Breast Cancer Development: A Long-Term Follow-Up Study. Cancers 2023, 15, 3521. https://doi.org/10.3390/cancers15133521
Bellini C, Nori Cucchiari J, Di Naro F, De Benedetto D, Bicchierai G, Franconeri A, Renda I, Bianchi S, Susini T. Breast Lesions of Uncertain Malignant Potential (B3) and the Risk of Breast Cancer Development: A Long-Term Follow-Up Study. Cancers. 2023; 15(13):3521. https://doi.org/10.3390/cancers15133521
Chicago/Turabian StyleBellini, Chiara, Jacopo Nori Cucchiari, Federica Di Naro, Diego De Benedetto, Giulia Bicchierai, Andrea Franconeri, Irene Renda, Simonetta Bianchi, and Tommaso Susini. 2023. "Breast Lesions of Uncertain Malignant Potential (B3) and the Risk of Breast Cancer Development: A Long-Term Follow-Up Study" Cancers 15, no. 13: 3521. https://doi.org/10.3390/cancers15133521
APA StyleBellini, C., Nori Cucchiari, J., Di Naro, F., De Benedetto, D., Bicchierai, G., Franconeri, A., Renda, I., Bianchi, S., & Susini, T. (2023). Breast Lesions of Uncertain Malignant Potential (B3) and the Risk of Breast Cancer Development: A Long-Term Follow-Up Study. Cancers, 15(13), 3521. https://doi.org/10.3390/cancers15133521






